CHAPTER 31 Vascularized Fibular Grafting for Osteonecrosis of the Femoral Head
Indications
Contraindications
Surgical Technique
Overview
The procedure is performed with the patient under general anesthesia and with the adjunct of an epidural block, which remains in place for 24 to 48 hours postoperatively. The patient is placed in the lateral decubitus position and supported by a pegboard. The entire lower extremity to the level of the iliac crest proximally is prepared and draped. The leg is covered with an impervious stocking up to the mid thigh, over which a sterile tourniquet is placed just proximal to the knee; this is to be used during the fibular graft harvest (Figure 31-1). A Betadine-impregnated occlusive drape is used for both the hip and the leg. The operative procedure on the hip and the harvest of the fibular graft occur contemporaneously and, as such, require cooperation between the surgeons.
Fibular Graft Harvest
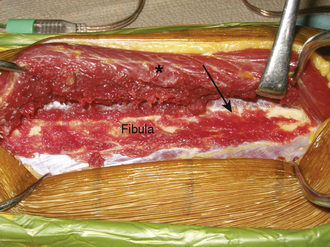
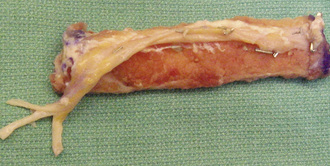
With the use of tourniquet control, a straight, lateral, 15-cm longitudinal incision is made coincident with the natural sulcus between the lateral and posterior compartments of the leg. The incision is begun at least 10 cm distal to the fibular head, and it ends at least 10 cm proximal to the lateral malleolus. The peroneal muscles are reflected in an extraperiosteal fashion off of the lateral aspect of the fibula, working from posterior to anterior and stopping when the anterior intermuscular septum is visualized (Figure 31-2).
Fibular Osteotomy
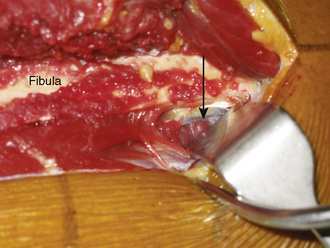
Directly beneath the distal aspect of the flexor hallucis longus muscle, the distal pedicle of the peroneal vessels is identified, and malleable retractors are passed between this pedicle and the fibula. Diligent care is critical during the placement of the retractors to ensure the protection of the pedicle during the osteotomy. After reconfirming that the planned osteotomy is at least 10 cm proximal to the distal tip of the fibula, an oscillating saw is used to cut the fibula. Irrigation during the osteotomy is vital to prevent thermal osteonecrosis. Next, the proximal pedicle is identified deep to the soleus muscle along the posterior aspect of the fibula (Figure 31-3). It is protected, and the proximal fibular osteotomy is performed in a manner similar to that of the distal osteotomy. The fibular cuts are made 15 cm apart to ensure an adequate pedicle length. It is important when performing the proximal osteotomy to identify and protect the superficial peroneal nerve, which is exposed proximally on the deep surface of the peroneus longus muscle.
Preparation of the Fibular Graft
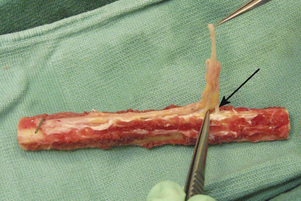
On a back table, the artery and the two veins of the fibular pedicle are delineated from one another and separated with the use of microscissors and jewelers’ forceps. All three vessels are then irrigated with a heparin-impregnated lactated Ringer’s solution and visually inspected for any major leaks. Neither vein will fill over the entire length of the graft because of the valves, but the artery should insufflate throughout its entire course during the injection of the heparin solution. Some oozing from the attached muscle and the periosteum is anticipated and considered normal; however, we consider any leaks from the main vessels that form a stream to be major and worthy of repair with either 8-0 suture or micro hemostatic clips. Such attention to detail is imperative to minimize the risk of the patient’s developing a vascular steal and thus to ensure adequate endosteal blood flow after the anastomosis. The vein with the better size match is chosen as the recipient, whereas the other vein is ligated with a hemostatic clip. The diameter of the fibula is reported to the hip surgeon to determine the endpoint for the core reaming of the hip; this is discussed in detail later in this chapter. Next, the proximal pedicle is reflected in a subperiosteal fashion from the fibula until a nutrient vessel is seen entering the cortex (Figure 31-4). The length of the pedicle at this point should be approximately 4 cm to 5 cm. The proximal fibula is then cut with an oscillating saw at the level of the most proximal nutrient vessel, with the pedicle being protected during the osteotomy. Again, copious irrigation should accompany all osteotomies to prevent thermal necrosis. After the exact length of fibula required has been determined by the preparation of the proximal femur (see Operative Procedure on the Hip Section, p. 254), this length is measured and marked on the fibular graft. The distal pedicle and a small cuff of evaginated periosteum are secured to the distal extent of the fibula with a 4–0 absorbable suture to prevent the stripping of the pedicle and the periosteum during insertion into the femoral core (Figure 31-5).
Operative Procedure on the Hip
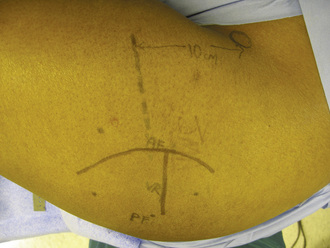
Certain anatomic landmarks are marked to assist with the placement and design of the surgical incision (Figure 31-6). The lateral aspect of the femur is approached through an interval between the tensor fascia lata and the gluteus medius. The vastus lateralis is then encountered, and the donor vessels (i.e., the ascending branch of the lateral femoral circumflex artery and two veins) are identified as they lie between the rectus femoris and the vastus intermedius. After the vessels are identified, the origin of the vastus lateralis is reflected sharply from the vastus ridge and then in a posterior direction for approximately 5 cm. The origin of the vastus intermedius is then carefully detached with a right-angle clamp and knife from its anterior position on the proximal femur. A specially designed four-quadrant retractor is introduced to provide better visibility for the dissection of the donor vessels.
Preparation of the Femoral Head
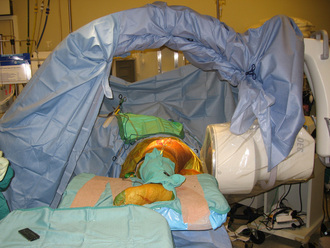
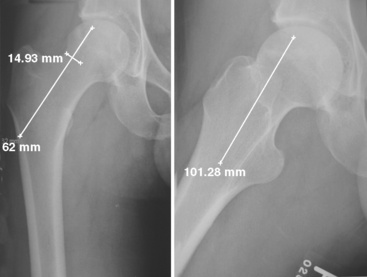
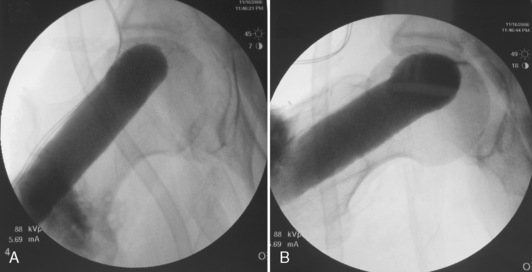
A C-arm fluoroscope is draped with a sterile sleeve and then positioned over the patient’s hip region like an arch (Figure 31-7); this provides for the obtaining of anteroposterior and frog-leg lateral views of the proximal femur with relative ease. Starting no lower than the middle of the lesser trochanter and at the junction of the middle and posterior third of the lateral femur, a 3-mm guide pin is inserted under fluoroscopic control into the center of the necrotic nidus within the femoral head. Pin position must be checked on both anteroposterior and lateral views. The pin must not only be positioned within the center of the necrotic bone, but it must also be spaced appropriately between the cortices of the femoral neck to allow for the passage of a large reamer (Figure 31-8). With correct pin placement confirmed by fluoroscopy, sequential reaming ensues over the guide pin; the procedure starts with a 10-mm reamer, the reamers are then increased in size, and the increasing then stops at the measured diameter of the harvested fibula. The reaming should extend to within 3 mm to 5 mm of the femoral head subchondral plate. This portion of the reaming is best performed with the use of live fluoroscopic guidance. Necrotic bone removed during the reaming process is discarded, whereas healthy appearing bone is saved for later grafting (Figure 31-9). Additional bone is captured with a filtered suction tip (KAM Super Sucker, Anspach, Palm Beach Gardens, FL) during the reaming process, when bone slurry is expressed from the core. Bone from this process is emptied onto a surgical sponge, dried, and fashioned by the scrub nurse into rectangular “bullets” to be used later for grafting. After the final straight reamer is passed, the guide pin is removed, and a special ball-tip reamer is introduced into the femoral core. With the use of fluoroscopic control, additional necrotic bone is excavated from the femoral head to create a bulbous cavity, usually in the anterior and superior quadrants. This step is performed with a water-soluble radiographic contrast medium injected into the femoral core to assess the adequacy and amount of the necrotic bone removed (Figure 31-10, A and B).
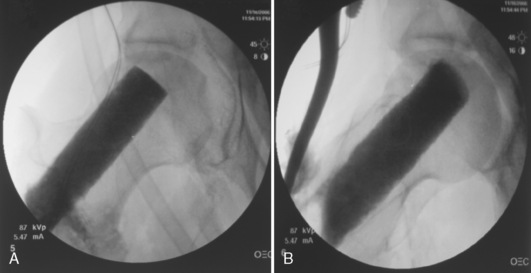
Next, with the use of a large curette, cancellous bone is taken from the greater trochanteric region, with the use of the proximal femoral core as an access point. Some of the cancellous graft is then placed into the femoral head cavity with DeBakey forceps and impacted with the use of a custom-made cancellous bone impaction instrument. This instrument is particularly helpful for elevating the sunken subchondral floor in cases of femoral head collapse. At its inserted end, the device has several windows through which additional cancellous bone is extruded with the help of a specialized drill bit, thus filling voids in the subchondral bone (Figure 31-11). With the impactor fully inserted, the length of the fibular graft is determined by reading the circumferential markings (units in millimeters) on the impactor’s side. After the cancellous bone is inserted, the bone “bullets” created from the reamings are inserted and extruded likewise into the femoral head cavities. Finally, contrast material is reinjected to confirm the adequate filling of the femoral subchondral voids (Figure 31-12, A and B). The femoral head is now ready to receive the fibular graft.
Vessel Anastomosis
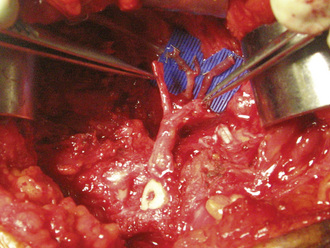
The four-quadrant hip retractor is replaced to optimize the exposure of the harvested vessels (i.e., the ascending branch of the lateral femoral circumflex artery and the two veins). Attention is initially directed toward the venous anastomosis. We have continued to enjoy success with our venous anastomoses with the use of a coupling device (Microvascular Anastomotic Coupler system, Medical Companies Alliance, Homewood, AL). The device comes in sizes that range from 1.0 mm to 3.5 mm in 0.5-mm increments; however we use either the 2.5 -mm or 3.0 -mm coupler for the majority of cases. After this, the microscope is brought into the surgical field, a blue microsurgical suction mat (Micromat, PMT, Chanhasen, MN) is placed as a backdrop, and the arterial anastomosis is completed with the use of an 8-0 or 9-0 black nylon monofilament suture with a 100-μm needle (Sharpoint, Pearsalls Limited, Taunton, Somerset TAI, IRY, UK) (Figure 31-13). After the anastomosis, the repair site is observed for any leaks. We will often place a small local fat graft over a minor leak, whereas larger leaks require additional suturing. Next, the exposed end of the fibular graft is observed for endosteal bleeding. Bleeding from the endosteal vessels is seen within 5 minutes of completing the anastomosis in more than 90% of cases. For those cases in which flow is absent after 5 minutes, we recommend several steps. First, we check the patient’s blood pressure and core body temperature. If either or both are low, elevation will often produce adequate flow. In addition, we irrigate the medullary canal of the exposed fibula with papavarin and heparin. If this does not improve the flow, we recheck the anastomosis site for leaks and perform a patency test on both sides of the anastomosis. If flow is sluggish or absent on the femoral side of the anastomosis, we trace the artery back to its origin from the lateral femoral circumflex artery. The ascending branch can be either kinked or tethered anywhere along this course. If this is the case, the removal of the aggravating tissue (most often the vastus intermedius) will often allow for adequate flow. If poor flow is localized to the fibular pedicle side, we check the vein that was not used for the venous anastomosis, because often this vein can have a small leak that induces spasm throughout the vessels or that causes a vascular steal. Any leaks are repaired with either 8-0 suture or micro hemoclips. Rarely if ever does the graft have to be removed entirely for size adjustment if accurate measurements have been made during the preparation of the core and of the fibular graft. If all else fails, the anastomosis is redone, and an interpositional vein graft is used if there is tension.
Technical Pearls
Postoperative rehabilitation
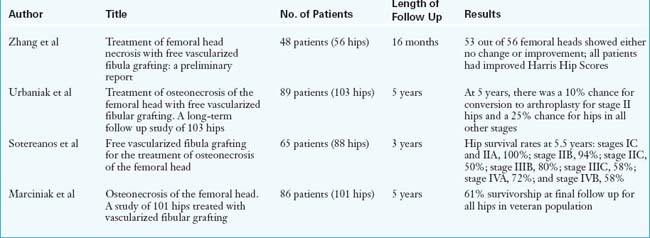
Postoperatively, all patients are placed on an intravenous infusion of dextran for 3 days and then transitioned to aspirin and Persantine daily, which is continued for 6 weeks. The operative drains, the Foley catheter, and the epidural catheter are removed on the second postoperative day, and physical therapy is also initiated on that day. The average hospital stay is 3 days. Patients do not bear weight on the operative side for 6 weeks, after which time progressive weight bearing is permitted. Full weight bearing is achieved by 5 to 6 months. We recommend aquatherapy and stationary bicycling at the 6-week postoperative mark. Patients are encouraged to begin early active and passive motion of the toes and ankle, with special emphasis on the passive stretching of the great toe. The great toe is susceptible to a flexion contracture as a result of the scarring of the flexor hallucis longus muscle. Follow up radiographic and clinical examinations are performed at 3 months, 6 months, and yearly thereafter. Unrestricted activity is allowed at 1 year after surgery (Tables 31-1 and 31-2).
Table 31–1 Results and Outcomes for Patients with Avascular Necrosis of the Femoral Head Treated with Free Vascularized Fibular Grafting
| Type | No. of Cases | No. of Revisions |
|---|---|---|
| Idiopathic | 492 | 107 (22%) |
| Steroids | 726 | 123 (17%) |
| Alcohol | 380 | 77 (20%) |
| Trauma | 274 | 53 (17%) |
| Perthes | 25 | 2 (8%) |
| Other | 37 | 4 (11%) |
| SCFE | 32 | 1 (3%) |
| Pregnancy | 34 | 2 (6%) |
SCFE, Slipped capital femoral epiphysis.
Annotated references and suggested readings
Aldridge J.M.3rd, Berend K.R., Gunneson E.E., Urbaniak J.R. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. Surgical technique. J Bone Joint Surg Am.. 2004;86-A(suppl 1):87-101.
Aldridge J.M., Urbaniak J.R. Avascular necrosis of the femoral head: role of vascularized bone grafts. Orthop Clin North Am.. 2007;38(1):13-22.
Kawate K., Yajima H., Sugimoto K., et al. Indications for free vascularized fibular grafting for the treatment of osteonecrosis of the femoral head. BMC Musculoskelet Disord.. 2007;8:78.
Kim S.Y., Kim Y.G., Kim P.T., Ihn J.C., Cho B.C., Koo K.H. Vascularized compared with nonvascularized fibular grafts for large osteonecrotic lesions of the femoral head. J Bone Joint Surg Am.. 2005;87(9):2012-2018.
Marciniak D., Furey C., Shaffer J.W. Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am.. 2005 Ap;Volume 87(4):742-747.
Plakseychuk A.Y., Kim S.Y., Park B.C., Varitimidis S.E., Rubash H.E., Sotereanos D.G. Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am.. 2003;85-A(4):589-596.
Sotereanos D.G., Plakseychuk A.Y., Rubash H.E. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clinical Orthopaedics & Related Research (344); 1997 No:243-256.
Urbaniak J.R., Coogan P.F., Gunneson E.B., Nunley J.A. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting: a long-term follow-up study of one hundred and three hips. J Bone Joint Surg.. 1995;77A:681-694.
Urbaniak J.R., Jones J.P. Osteonecrosis: etiology, diagnosis, and treatment. AAOS publication, 1997.
Vail T.P., Urbaniak J.R. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am. 1996 Fe;Volume 78(2):204-211.
Zhang C., Zeng B., Xu Z., et al. Treatment of femoral head necrosis with free vascularized fibula grafting: a preliminary report. Microsurgery. 2005;25(4):305-309.