Chapter 61 Vascular Trauma
Treatment of vascular trauma in the civilian and military venues has had a symbiotic relationship. Although German surgeons accomplished a limited number of arterial repairs in the early part of World War I, ligation of major arterial injuries was not abandoned until the Korean Conflict in the 1950s. Until that time, arterial ligation was used preferentially, and some experts of the day felt there was little place for definitive arterial repair of the combat wound. Review of the arterial injuries during World War II revealed that arterial ligation was followed by gangrene and amputation in half the cases.1 After World War II, significant advances made in civilian hospitals included atraumatic vascular clamps, monofilament sutures, arteriography, and prosthetic grafting. These advances made it possible to repair arterial injuries in the battlefield, such that during the Korean conflict, the overall amputation rate was reduced to 13%.2 The latest innovation in vascular surgery, the “endovascular revolution,” is rapidly changing our approach to managing some components of vascular trauma in the civilian setting.3 It is unclear to what extent these techniques will facilitate care in the battlefield.
Trauma remains the leading cause of death in the 15- to 44-year-old age group in the United States. The prevalence of iatrogenic vascular injury has increased in tandem with the number of catheter-based invasive procedures.4 Overall, arteries in the lower extremities are injured more than other anatomical areas. In this chapter, the basic definitions and treatment strategies for vascular trauma are described.
Basic Concepts and Definitions
Signs of Vascular Trauma
The hard signs of vascular trauma warrant urgent or immediate operative exploration and intervention (Box 61-1). In many instances, these patients present with systemic shock, and resuscitative efforts are best coordinated in the operating room. The soft signs of vascular injury (see Box 61-1) require further diagnostic evaluation to plan intervention if needed. Depending on the hemodynamic status of patients with soft signs of vascular injury, subsequent evaluation may include invasive or noninvasive imaging techniques.
Hemorrhagic Shock
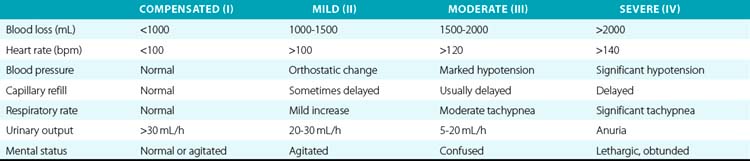
Hemorrhagic shock is a condition of reduced tissue perfusion due to inadequate delivery of oxygenated blood, resulting in anaerobic metabolism, acidosis, and deleterious alterations to cell function. Because bleeding may be a major complication associated with vascular trauma, it is important to provide information on the kinds of shock that may be associated with these injuries. There are four types of hemorrhagic shock, ranging in severity based on the amount of blood lost. Clinical characteristics associated with the degree of hemorrhagic shock are described in Table 61-1.
Ischemia/Reperfusion Injury
Ischemia/reperfusion is a complex pathological process involving intracellular and extracellular processes that result in metabolic, thrombotic, and inflammatory changes in brain, intestine, heart, kidney, and skeletal muscle. A devastating component of ischemia/reperfusion injury is the paradoxical increase in tissue injury associated with restitution of blood flow to ischemic tissues. The myonephropathic-metabolic syndrome was described by Haimovici in a few patients who underwent lower-extremity revascularization following acute ischemia in the late 1950s, providing one of the first published clinical observations of limb ischemia and reperfusion.5,6 These patients experienced ongoing lower-extremity necrosis and myoglobin-induced renal failure in the presence of palpable pulses.
One of the most severe components of ischemia/reperfusion injury, the paradoxical decrease in blood flow following restoration of perfusion, was described initially as a no-reflow phenomenon in the brain,7 and May et al. described the same phenomenon 10 years later in skin flaps.8 These investigators described cellular swelling, intravascular aggregation of cellular components of blood (platelets, neutrophils), and leakage of intravascular fluid into the interstitial space as basic mechanisms whereby tissue flow is decreased during reperfusion, providing the first definitive histological evidence to support the existence of the no-reflow phenomenon. From a biochemical perspective, it is useful to discuss this process in terms of its components: ischemic injury and reperfusion injury.
Compartment Syndrome
Acute compartment syndrome is a surgical emergency. Compartment syndrome may be defined as increased tissue pressure in a closed myofascial space, resulting in disturbed microcirculation that leads to irreversible neuromuscular ischemic damage.9 Acute compartment syndrome most commonly occurs following lower-limb trauma. Emergency decompression through open and extensive fasciotomies is the treatment of choice. The cardinal clinical feature is severe pain, greater than would be expected from the original insult. The pain may be exacerbated by passive extension of the tendons crossing the symptomatic compartment or arising from the muscles within it. Among other clinical features, the first is usually hypoesthesia, followed by compartment distension and muscle weakness in the later stages. The need for prompt intervention and the benefits of timely surgical decompression require a high index of suspicion and effective clinical assessment to make the diagnosis. Clinical assessment may be supported by compartment pressure measurements, in which a needle is inserted into the compartment and pressure monitored using a pressure transducer. The pressure that indicates a need for fasciotomy has not been universally established, although a pressure greater than 30 mmHg is widely accepted as abnormal. Other authors advocate a threshold that relates to the diastolic blood pressure, with compartment pressures within 30 mmHg of the diastolic pressure indicating a need for fasciotomy.
A recent 10-year review of the incidence of compartment syndrome in a mature level I trauma center indicated that after lower-extremity trauma, 2.8% of patients will require fasciotomy. A stepwise logistic regression identified the presence of vascular injury (arterial, venous, or combined), need for packed red blood cell (RBC) transfusion, male gender, open fracture, elbow or knee dislocation, and age younger than 55 as independent predictors for the need for fasciotomy after extremity trauma. Combined arterial and venous injury resulted in fasciotomy in 42% of patients.10
Thoracic Vascular Injury
With the heart, great vessels, brachiocephalic vessels, and descending aorta housed within the confines of the thorax, mortality following vascular injury is associated with exsanguinating hemorrhage at the scene. Hemothorax associated with penetrating trauma can be managed with a chest tube, but if a major vascular structure is involved, patient survival is largely dependent on whether there is free hemorrhage or a contained hematoma. It is estimated that over 80% of patients suffering blunt trauma to the aorta will die at the scene of the accident.11
Thoracic/Cardiac Box
Thoracic aorta
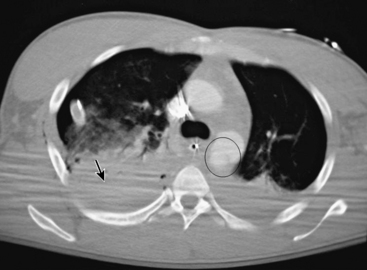
Traumatic aortic rupture is a devastating clinical problem that is difficult to manage owing to the need to approach aortic repair in concert with management of complex associated injuries to nonvascular organ systems (Fig. 61-1). The natural history of aortic transection is relatively self-selective, with a significant majority of patients exsanguinating at the scene. Of those who make it to the hospital alive, upwards of 38% die largely as a result of associated injuries.12 Fabian et al. estimate the incidence of traumatic aortic disruptions in the United States to be between 7500 and 8000 patients annually; about 1000 to 1500 of these patients arrive at hospitals alive.13 It took 50 centers 2.5 years to generate 274 patients for this prospective report. This averages just over two patients per year per center. Despite the low volume, contemporary analysis reveals that of the 9% to 19% who reach the hospital alive,14 approximately 30% will die within the next 6 hours, and a total of 50% within 24 hours.15
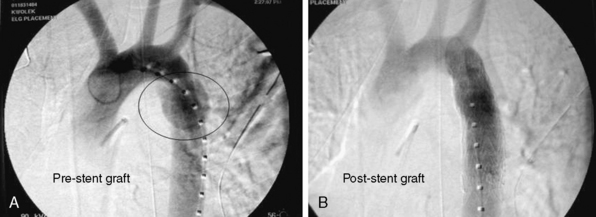
Until recently, open operative intervention for these traumatic injuries required thoracotomy, anticoagulation, and application of an aortic cross-clamp. These operative maneuvers conceded the potential of exacerbating associated injuries and spinal cord injury.13,16,17 Proximal-to-distal aortic bypass using a Gott Shunt, left heart bypass with heparin-bonded circuits, and cardiopulmonary bypass (CPB) may all significantly reduce the likelihood of paraplegia.18,19 Thus, the current status of traumatic aortic disruption and traditional repair is not ideal. Advances in endovascular repair techniques for traumatic rupture have resulted in a major change in the approach to treatment of this devastating clinical entity.20–22 This change in approach is based on and supported by improved mortality associated with repair by endovascular vs. open techniques in patients with multiple trauma23 (Fig. 61-2).
Open Surgical Approach
Open repair may be accomplished either with direct cross-clamping alone or with circulatory assistance (left-sided heart bypass with heparin-coated conduits, cardiopulmonary bypass, or femoral-femoral bypass).19,24,25 Optimal exposure of the thoracic aorta is gained through a posterolateral thoracotomy with an incision in the fourth intercostal space. The aortic arch may be controlled either with clamping between the left common carotid and left subclavian, or just distal to the left subclavian artery. The descending thoracic aorta is controlled distally immediately after the traumatic injury to avert sacrifice of the intercostal arteries. The aorta can then be repaired with direct suturing or graft interposition.
Endovascular Approach
Trauma centers equipped with radiographic fixed or mobile C-arms and angiographic equipment in the operating rooms would be ideal. This affords the opportunity for patients to be treated for their vascular injury(s) via open cutdown or percutaneously in a setting where surgical interventions for associated injuries may also be treated if indicated at that time.26 Though not specifically designed to treat thoracic aortic transections, the use of endoluminal abdominal aortic extension cuffs has been shown to be technically feasible in several small series.26–28 This has been particularly evident in young individuals with normal-sized aortas. Broad prepping and draping of the patient should be undertaken so that emergent intervention for associated abdominal or thoracic injuries can be pursued while the patient is under anesthesia. Patients should be placed supine on the appropriate x-ray table, with slight rotation to a decubitus position. This orientation of the patient provides access to the femoral and iliac vessels and the abdominal aorta if needed.
The diameter of stent grafts should be oversized at least 20% based on sizing obtained from computed tomographic angiography (CTA). Unlike treatment of patients with degenerative aneurysms, the aortic diameter proximal to the injured aorta is most often in the 18- to 26-mm range. Generous oversizing can lead to collapse of a stent graft.29
Thoracic venae cavae
Simple isolated injuries to the thoracic venae cavae can be managed with lateral venorrhaphy. Partial-occluding clamps or temporary inflow occlusion can be used in these circumstances to facilitate repair.30 Complex injuries to the venae cavae or associated injuries to the heart may require CPB and/or interposition grafts for exposure and repair.
Pulmonary vessels
Trauma to the main right or left pulmonary arteries is extremely rare and almost exclusively found after penetrating traumatic injury. Some case reports have described blunt traumatic injury to the main pulmonary arteries, but it remains exceedingly rare.31 As with many of the great vessel injuries, cardiac tamponade or hemopericardium is the common presenting finding. Usually the diagnosis is made in the operating room during an empirical thoracotomy for hemopericardium. Distal pulmonary vascular injuries beyond the mediastinum can be seen following both blunt and penetrating trauma. Extensive vascular injury or significant injury to the hilar region may necessitate a pneumonectomy, which bears a significantly high mortality rate in trauma situations.32
Carotid and Vertebral Vascular Trauma
Penetrating Carotid Artery Trauma
Stab and low-velocity missile wounds account for the vast majority of civilian penetrating carotid artery injuries. Demographics of the injured generally include young, healthy males and associated alcohol and/or illegal drug use precipitating the injury. Studies have shown that carotid injuries occur in roughly 17% of all penetrating neck trauma.33 Common carotid artery (CCA) injury is more frequent than internal carotid injuries. Iatrogenic injury to the carotid artery is most commonly due to attempted central venous catheter insertion.
Anatomical considerations
The neck is divided anatomically into three zones of injury (Box 61-2). Zone I comprises the area from the thoracic outlet (the level of jugular notch) to the cricoid cartilage, zone II begins at level of the cricoid cartilage and terminates at the angle of the mandible, and zone III extends from the angle of the mandible to the skull base. The zone divisions were based on the anatomical relationships of the neurovascular and aerodigestive tract structures, as well as the surgical approach for exposure.
Zone II Injuries
In stable patients, the management of zone II injuries of the neck is the source of some debate among trauma centers. Historically, all zone II injuries were operatively explored because of improved mortality rates with immediate operative intervention, as opposed to delayed or expectant intervention.34 The relative ease of operative exposure in zone II and low morbidity rate with operation, coupled with the many vital structures passing through this region, made it the mainstay of treatment for many years, despite the high negative exploration rate. With advancements in diagnostic imaging, selective exploration has become more popular in stable patients. Thus, the decision whether operative exploration is necessary can be predicated on further diagnostic evaluation and imaging to confirm or rule out vascular injury. Usually this evaluation includes serial physical examinations, esophagoscopy, bronchoscopy, and imaging studies (i.e., catheter-based angiography, CTA, magnetic resonance angiography [MRA], or duplex ultrasound scanning) when vascular injury is suspected.33,35–37 Centers that have used angiography to evaluate penetrating neck injuries in hemodynamically stable patients have found that only 13% to 17% of these patients have major vascular (carotid and vertebral) injuries requiring repair.33,37 Because of this, some have begun to advocate angiography only in cases of suspected vascular injury and report very few missed injuries with this approach. More recent studies have also advocated CT scanning as an adjunct to the physical examination in zone II neck injuries, but angiography remains the gold standard.
Operative approach/open surgical management
The surgical approaches for zone I and III injuries can be significantly more complicated and challenging. To obtain adequate proximal vascular control and exposure, vascular injuries within zone I frequently require a median sternotomy or supraclavicular “trap-door” incision in addition to the standard cervical oblique incision. Zone III injuries may necessitate cephalad extension of the exposure to attain distal vascular control. Maneuvers such as dislocation or partial resection of the mandible are not infrequent, especially in high zone III injuries. Temporary control of bleeding at or near the skull base can be accomplished through insertion and inflation of a Fogarty catheter into the injured vessel.38
Endovascular management
Though endovascular management is less prevalent in blunt carotid injury, it has provided some promising results in the management of penetrating carotid trauma, especially in zone I and high zone III (internal carotid artery [ICA]) injuries.39,40 Traditionally, ICA injuries (pseudoaneurysm and dissections), albeit rare, warrant anatomical reconstruction to prevent devastating ischemic neurological insults. Operating high in the neck is technically demanding and can be associated with cranial nerve injury; thus, endovascular stenting and coiling has the potential to limit iatrogenic damage associated with open exploration. Recent reviews have demonstrated that stent graft placement for traumatic ICA pseudoaneurysms have been successful in neurologically symptomatic patients following trauma.41,42 Consideration must be given to thromboembolic events associated with stent graft placement, the potential for intimal hyperplasia formation, and restenosis. The anatomical tortuosity of the vessel may prevent safe crossing of the traumatic lesion with a guidewire. A recent retrospective review of 113 patients with blunt or penetrating carotid injury demonstrated a promising short-term patency (up to 2 years) of carotid stent grafts (80%).43
Blunt Carotid/Vertebral Artery Injury
Blunt carotid and vertebral artery injury (BCVI) is diagnosed roughly once in every 1000 patients.44 Though BCVI is considered a rare occurrence, without prompt and appropriate care, cerebral ischemia rates range from 40% to 80%, and mortality rates from 25% to 60%.44,45 BCVI lesions vary in mechanism and location; skull base fractures, for example, can contuse the intrapetrous/cavernous portion of the internal carotid artery. Blunt trauma to the carotid artery can be induced via a stretching, twisting, or shearing effect to the neck. BCVI after strangulation and choking has been described as well.45 Several authors have proposed signs, symptoms, or injury patterns that should raise the suspicion for BCVI.44,46,47 Skull base, midface, mandible, and cervical spine fractures are associated with BCVI and should raise a healthy concern. Significant blunt trauma to the chest and neck in a patient with a Glasgow Coma Scale score of 8 or less represents another clinical scenario that warrants further investigation. Note that these injuries can also be insidious in their presentation, so in a patient with a Glasgow Coma Scale score above 8 but a history of a high-risk mechanism (motor vehicle accident/deceleration injury) and/or trauma to the face and neck, suspicion for BCVI should remain.
Diagnostic evaluation
In most emergency situations, multidetector CTA remains the initial tool for evaluation of carotid injuries because it can often be performed expeditiously near or in the emergency room. Adequacy of the older single-slice helical CTA in diagnosing BCVI was limited by sensitivities and specificities of 68% and 67%, respectively.48 However, several studies have shown the effectiveness of 16-channel multislice CTA in diagnosis of BCVI,44,49 with sensitivities and specificities each greater than 94%. The somewhat subtle nature of blunt carotid injury makes digital subtraction angiography (DSA) the gold standard for the diagnosis of blunt carotid injury. Imaging by CTA may be compromised in situations where metallic artifact is present (shrapnel, plates, prosthesis, etc.). Although DSA is an invasive method, it not only depicts the extent and severity of vessel injury, it can also provide information about the integrity of cerebral circulation. Magnetic resonance angiography is another safe, noninvasive technique that can provide data concerning vessel morphology and blood flow. Its accuracy in diagnosing BCVI may rival that of CTA, but time required for the examination, difficulty monitoring the patient during the study, and high cost limit its use.45 Duplex ultrasonography is used more in penetrating carotid trauma but is not sensitive enough to be the screening modality of choice in BCVI.44
Classification of blunt carotid injury: the Denver scale
The Denver Scale (Table 61-2) has been widely accepted and used to classify blunt carotid injury.50 It was employed to standardize and direct care for the different grades of vessel injury based on multiplanar CTA or DSA findings. Grade I to IV injuries typically warrant anticoagulation as the mainstay of treatment, with consideration of surgical intervention if contraindication of anticoagulation or further neurological deterioration exists. Grade V injury, which represents complete transection with extravasation, is most appropriately treated through surgical intervention, whether it be an endovascular or open approach.
| Type/Grade | Radiological/Angiographic Findings and Criteria |
|---|---|
| I | Vessel wall irregularity or dissection/intramural hematoma with <25% luminal stenosis |
| II | Presence of intraluminal thrombus, raised intimal flap, or dissection/intramural hematoma with ≥25% luminal stenosis |
| III | Presence of pseudoaneurysm |
| IV | Vessel occlusion |
| V | Complete transection with extravasation |
From Biffl WL, Moore EE, Offner PJ, et al: Blunt carotid arterial injuries: Implications of a new grading scale. J Trauma 47:845–853, 1999.
Clinical management
Despite increased awareness of blunt carotid injury, there is no agreement on the best therapeutic approach. Accumulated data suggest that conservative therapy using antithrombotic therapy with heparin prevents cerebral infarction. Similarly, antiplatelet therapy and anticoagulation with warfarin (international normalized ratio [INR] 2-3) were equally effective in reducing risk of stroke following BCVI.45,51 In a recent analysis of the national trauma database, a comparison of functional independence following BCVI in patients treated conservatively (anticoagulation and/or antiplatelet agents) vs. operatively (open and endovascular treatment) was performed.52 There was no difference in functional outcome in these patients, regardless of conservative or operative intervention. The only demographic difference among the groups was greater injury severity score in patients undergoing endovascular repair. In most studies, BCVI in surgically accessible areas are treated operatively; however, the vast majority of BCVI lesions occur in surgically challenging areas high within the carotid canal or foramen transversarium. Such locations make standard operative approaches for thrombectomy or reconstruction difficult.53
Abdominal Vascular Injuries
Abdominal vascular injury represents 5% to 25% of all abdominal traumatic injuries54 and carries a mortality rate of 31% to 87%.55 With penetrating trauma overwhelmingly the most common etiology of traumatic vascular injury (>90% of cases), any penetrating injury to the torso from the upper thigh to the level of the nipples should generate high suspicion for vascular injury.56 Most patients with major abdominal vascular injury present with a contained or partially contained retroperitoneal hematoma. Patients who suffer major free intraperitoneal hemorrhage frequently die at the scene of injury. Patients with free retroperitoneal or intraabdominal hemorrhage who make it to the hospital usually present with profound hypotension in class IV hemorrhagic shock.56 The incidence of arterial and venous injury is similar and depends on the location, force, and mechanism of the insult. The association of abdominal vascular trauma with assault and aggressive behavior accounts for a shocking 90% to 95% of cases.55
When vascular injury is present in the abdomen, the aorta and IVC are most commonly injured—25% and 33%, respectively.57 The overall mortality rate from penetrating abdominal vascular injuries is 45%, but associated injuries to the abdominal aorta, hepatic veins, retrohepatic vena cava, and/or the portal vein can elevate it to as high as 90%.35,54 Despite our advances in technology and surgical/medical techniques, no significant changes have occurred with regard to mortality associated with abdominal vascular trauma over the last 20 to 30 years. This reflects the lethal potential of these injuries and the fact that patients presenting in shock with vascular injury continue to have a high mortality rate.58
Clinical Presentation and Evaluation
Of those patients with abdominal vascular injury who make it to the hospital, about 14% will lose vital signs en route; thus, minimizing the time from injury to delivery at a medical care facility can result in a significant improvement in outcomes.59 In addition to prehospital time, the clinical presentation will depend on the mechanism of injury, vessel injured, severity of the injury, and presence of other associated injuries. Blunt trauma causes vascular injury through rapid deceleration, anterior/posterior crushing, or laceration from sharp bony fractures. These patients often initially present with stable vital signs, but may rapidly decompensate because of the insidious evolution of blunt abdominal trauma. In contrast, penetrating vascular trauma to the abdomen presents in a less subtle manner.
Regardless of the mechanism, operative exploration is paramount and must be done expeditiously, foregoing any diagnostic imaging in a hemodynamically unstable patient with suspected abdominal vascular injury. Cautious resuscitation is prudent in hemodynamically unstable patients. Excessive fluid resuscitation of an actively hemorrhaging patient can potentiate ongoing blood loss and coagulopathy.60–62 To maintain end-organ perfusion until definitive control of hemorrhaging is obtained, cessation of bleeding and permissive hypotension should be the initial goals of resuscitation.
Retroperitoneal hematomas
Retroperitoneal hematomas occur in more than 90% of abdominal vascular injuries.59 The retroperitoneum is divided into three main zones of injury: zone I is the central/midline retroperitoneum, zone II encompasses the perinephric space, and zone III comprises the pelvic retroperitoneum. Treatment of retroperitoneal hematomas varies depending on the anatomical location and mechanism of injury.
Diagnostic Imaging
Ultrasonography is an easy and readily accessible means of acutely assessing for hemoperitoneum following trauma to the abdomen.63 The FAST exam, which is an acronym for Focused Assessment with Sonography in Trauma, is performed immediately after the primary survey per the Advanced Trauma Life Support (ATLS) protocol. The confirmation of fluid in the abdomen during the FAST exam, coupled with hemodynamic instability, affirms the plan of immediate laparotomy. Ultrasonography is limited in its ability to delineate the presence of retroperitoneal hematomas and the morphology of visceral vasculature injury. Therefore, it is not the modality of choice to assess the extent of vascular injury in the abdomen. However, its use is significantly valuable in assessing morphology of vascular injury elsewhere in the body.
Operative Management and Damage Control
Different maneuvers can be undertaken to gain the needed exposure for particular regions and structures in the abdomen. To expose the entire length of the abdominal aorta and most of its branches, a left-sided medial visceral rotation was described by Mattox et al. in 1974.64 The only artery not accessible via this approach is the right renal artery. The Mattox maneuver is implemented by taking down the lateral peritoneal attachments of the sigmoid and left colon. The left colon, left kidney, and spleen are then swept/mobilized medially toward the midline, exposing the retroperitoneum and aorta. A right-sided medial visceral rotation can also be done, providing exposure to the infrarenal vena cava and iliac vessels. This is called an extended Kocher maneuver. The right colon along with the third and fourth portion of duodenum may be released from their lateral attachments, then reflected medially and superiorly using the Cattell-Braasch maneuver.65 This maneuver provides additional exposure to the suprarenal IVC and makes the portal venous system accessible. This exposure is extensive and provides access to most of the structures in the retroperitoneum.
Appropriate operative management for the different zones of the retroperitoneum and for specific vascular injuries are covered elsewhere in the chapter. However, once the extent of injury has been identified, the American Association for the Surgery of Trauma Organ Injury Scale (AAST-OIS) for abdominal vascular injury should be used to determine the severity of injury and overall prognosis (Table 61-3). This grading scale has demonstrated excellent correlation between mortality and extent of vascular damage following trauma.66 Understanding the extent of injury and risk of mortality should be factored into the treatment plan that ensues.
Table 61-3 American Association for the Surgery of Trauma Organ Injury Scale for Vascular Injury*
| Grade | Extent of Vascular Injury |
|---|---|
| I | Injury to an unnamed branch of the superior mesenteric artery or vein Injury to an unnamed branch of the inferior mesenteric artery or vein Phrenic artery or vein injury Lumbar artery or vein injury Gonadal artery or vein injury Ovarian artery or vein injury Unnamed small artery or vein requiring ligation |
| II | Right hepatic, left hepatic or common hepatic artery injury Splenic artery or vein injury Right or left gastric artery injury Gastroduodenal artery injury Inferior mesenteric artery, trunk, or vein injury Primary named braches of mesenteric artery or vein injury Other named vessels requiring ligation of repair |
| III | Superior mesenteric vein, trunk injury Renal artery or vein injury Iliac artery or vein injury Hypogastric artery or vein injury Vena caval, infrarenal injury |
| IV | Superior mesenteric artery, trunk injury Celiac axis proper injury Vena caval, suprarenal, and infrahepatic injury Aortic, infrarenal injury |
| V | Portal vein injury Extraparenchymal hepatic vein injury Vena caval, retrohepatic, or suprahepatic injury Aortic, suprarenal, and subdiaphragmatic injury |
* Classification system applicable for extraparenchymal vascular injury. Increase one grade for multiple grade III or grade IV injuries involving >50% of the vessel circumference. Downgrade one grade if the laceration is <25% of the vessel circumference for grade IV or V injuries.
Many patients with abdominal vascular injury who warrant immediate surgery will also face the “trauma triad of death”: acidosis, hypothermia, and coagulopathy. Under these conditions, a definitive repair or reconstruction of the injured vessel(s) may place an insurmountable burden on the patient. In these circumstances, a damage-control philosophy is the most appropriate therapeutic management.67 In a damage-control scenario, major venous injuries are ligated, retroperitoneal bleeding/oozing is packed off tightly, and arterial injuries are temporarily shunted. The abdomen is temporarily closed with a prosthetic material, and the patient is transferred to the intensive care unit (ICU) for resuscitation. Definitive vascular repair and abdominal closure are deferred until the patient has been adequately resuscitated and his/her condition has stabilized.
Specific Abdominal Vascular Injuries
Abdominal aorta
The aorta is the largest artery in the body, and its abdominal portion spans from where it traverses the diaphragm at the level of T12 to where it bifurcates into the iliac arteries at the L4/L5 level. It is the most commonly injured artery in the abdomen following trauma; many times, aortic injury presents as a lethal condition. Blunt injury to the abdominal aorta is most often associated with motor vehicle accidents, followed by falls and direct blows to the abdomen. The mortality associated with blunt aortic injury is estimated to be around 30%.56,68 Penetrating trauma remains the most common method of injury to the abdominal aorta and carries a significantly higher mortality than blunt injury. Mortality following penetrating injury (e.g., fire arm blasts, knife stab wounds, etc.), has been estimated to be between 67% and 85%.69
Many patients with significant aortic injury never make it to the hospital. The fortunate few who make it to a medical care facility present with injuries such as intimal disruptions, thrombosis, and/or contained retroperitoneal hematomas.54,55,59,63,70 The clinical presentation will depend on the mechanism of trauma, type of aortic injury, prehospital time, and associated traumatic vascular and nonvascular injuries. Penetrating injuries typically present in a dramatic fashion with profound hypotension, shock, and hemodynamic collapse. Under certain conditions, an emergency resuscitative thoracotomy can temporize cardiovascular collapse in order to get the patient to the operating room for definitive surgical intention.
Ultrasonography during the FAST exam can immediately inform the trauma team of the presence of free intraperitoneal fluid and, in the setting of hemodynamic instability, the affirmative need for operative intervention. Blunt injuries tend to have a less dramatic presentation with normotensive patients but can still have significant injury leading to visceral, renal, or lower-extremity ischemia. Multidetector CT scans provide a fast, accurate method of evaluating aortic injuries in hemodynamically stable patients. The diagnosis in unstable patients should be made during laparotomy. Depending on the findings from clinical examination and imaging studies, endovascular techniques can be implemented to treat both penetrating and blunt acute traumatic injuries.3,71–74
The significantly high incidence of associated injuries following penetrating abdominal trauma (>90%)75 make open surgical intervention the gold standard for repair, in part because assessing and treating bowel and solid-organ injuries are critical. When the abdomen is explored, all four quadrants should be packed off, then examination of the quadrants should be done meticulously to identify all injuries, vascular and nonvascular alike. For supramesocolic zone I injuries, proximal control is obtained by clamping or compressing the aorta as it traverses the diaphragm. If the injury to the aorta resides high on the abdominal aorta, proximal control can be gained above the diaphragm through a left thoracotomy. The injured portion of the aorta or associated injured vessels can be exposed by means of a left-sided medial visceral rotation (Mattox maneuver). Infra-mesocolic zone I hematomas result from injury to either the infrarenal aorta or IVC. In this scenario, proximal control is gained at the supraceliac aorta, and exposure is provided by opening the posterior peritoneum in the midline.
Endovascular management is becoming far more frequently used with aortic trauma, and is more common following blunt injury than penetrating injury.3,73,74 Much of the impetus for its use in trauma has been drawn from the success of stent grafting in the treatment of nontraumatic aortic disease (e.g., aneurysms, ruptures, dissections, fistula formations, thrombosis). The extension of endovascular techniques into the setting of trauma is logical and has provided more treatment options for patients presenting with contained retroperitoneal hematomas.73 If a patient has a perforated viscous, small-bowel anastomosis, partial colectomy, or ostomy, endoluminal treatment of associated arterial injuries avoids opening the retroperitoneum and placing a prosthetic conduit in a contaminated field.
Celiac axis injury
Celiac artery injuries are rare and almost always a result of penetrating trauma. In a review of 302 patients with abdominal vascular injuries, the celiac artery was injured in only 3.3% of cases.59 The celiac axis gives rise to the left gastric, splenic, and common hepatic arteries. Injuries amenable to primary repair should be carried out, but ligation of the artery can be done without ischemic sequelae because of the robust collateral circulation of the proximal gastrointestinal tract. That being said, endovascular coiling and embolization is a frequently used technique for controlling hemorrhage from the hepatic and splenic vasculature following blunt trauma.76 The mortality rate for celiac axis injuries has been reported to range between 38% and 75%.56 However, many of these patients have associated vascular injuries that contribute to the high mortality rate; isolated celiac injury likely carries a much lower mortality rate.
Superior mesenteric artery and vein injury
Superior mesenteric artery injury presents as either free intraperitoneal hemorrhage, a central (zone I) supramesocolic retroperitoneal hematoma, ischemic proximal small bowel, or any combination of the three. Superior mesenteric artery injuries account for roughly 10% of all abdominal vascular injuries59 and are diagnosed in less than 0.1% of total trauma admissions.77 It is the second most commonly injured abdominal vascular structure following blunt trauma, but penetrating trauma accounts for the significant majority of SMA injuries.77 The mortality rate associated with SMA injury is estimated to be as high as 47% to 67%.54,56,59,77
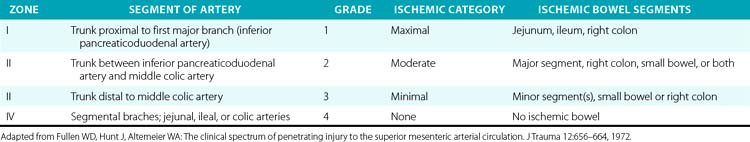
The SMA is divided into four anatomical zones of injury, which were first described by Fullen et al.,78 who also correlated grade of ischemia with injury location (Table 61-4). The affected zone, ischemic grade, and AAST-OIS for abdominal vascular trauma correlate well with mortality.77,79 The surgical approach is driven by the location of the injury. Injuries to the first two zones should undergo primary repair whenever possible. Ligation of the artery at these levels would result in significant small-bowel ischemia and a poor prognosis without revascularization. Revascularization with autogenous or prosthetic conduits is another option and sometimes necessitates extra-anatomical routes. Zone III and IV injuries should be primarily repaired as well. Should ligation at these levels or of the segmental branches prove necessary for hemostasis, only local ischemia to the small bowel will follow, which segmental bowel resection can remedy. A second-look laparotomy is mandatory to asses the viability and integrity of the small bowel following any surgical manipulation of the SMA. Endovascular techniques have been implemented in limited cases but have demonstrated promise.80
Table 61-4 Fullen’s Anatomical Classification of Superior Mesenteric Artery Injury by Zone and Grade
Superior mesenteric vein (SMV) injuries are infrequent but incur high mortality rates due to the difficulty in obtaining prompt exposure and hemorrhage control, combined with the high incidence of concomitant portal vein injury.81 Lateral venorrhaphy is the preferred surgical means of repair for an isolated SMV injury. Graft conduits can restore flow, but thrombosis can be a devastating complication. Ligation of the vein is a plausible surgical option, especially in hemodynamically unstable patients,81 but carries a risk of venous mesenteric ischemia secondary to splanchnic sequestration. Aggressive resuscitation is vital following venous ligation, and a second-look laparotomy is again standard, as with SMA injuries.
Inferior mesenteric artery and vein injury
Injury to the inferior mesenteric artery (IMA) is typically managed by ligation.56 In rare cases, collateral circulation to the descending and sigmoid colons and the upper rectum may be inadequate and result in ischemia to these regions of bowel. Again, this is a very rare occurrence because of the rich collateral flow via the marginal artery and its arcade. Ligation of the inferior mesenteric vein is tolerated much better than that of the SMV and can be done with impunity.
Renovascular injuries
Renovascular injury is relatively uncommon following trauma. The kidney itself is injured in only 1.2% of all trauma cases, and vascular injury only accounts for 2.5% to 4% of those cases.82 It does, however, represent roughly 16% of all abdominal vascular trauma.59 Renovascular injury is more common following blunt as opposed to penetrating trauma, with blunt trauma making up almost 80% of cases.
The clinical presentation is most often subtle with regard to the vascular injury itself, but associated injuries can cause a more concerning clinical picture, frequently taking precedence over the renovascular injuries. Unlike many other vascular injuries, exsanguination from the renal vasculature is uncommon and is usually contained in the retroperitoneum. Flank pain, proteinuria, and hematuria (gross and microscopic) are findings that indicate the presence of renal vascular injury, but are neither always present nor specific for vascular injury itself. Computed tomography is the best first-line imaging modality for renovascular injuries.83 It can illustrate the extent of parenchymal damage and perfusion, along with the morphology of the retroperitoneal hematoma. Angiography coupled with endovascular techniques is frequently needed to confirm and sometimes treat the vascular injuries.
Renal function is diminished significantly following just 3 to 6 hours of ischemic insult. If revascularization is to be done, it should be accomplished within this window, despite a success rate of 28% for renal function preservation.84 Revascularization can be done via primary repair, vein patch angioplasty, interposition grafting, or segmental resection with reanastomosis. This is usually performed if the injury is found during operative exploration or when there is bilateral vascular injury in an attempt to preserve some renal function. A nephrectomy is an accepted surgical treatment/option for devastating unilateral renovascular injuries. Studies support that nephrectomy in the setting of major unilateral vascular injury has comparable mortality, posttreatment renal function, transfusion requirements, and level of service as that of repair.82,85 Renal vein injury can be repaired similarly to the artery. If devastating injury occurs to the left renal vein, ligation is acceptable. Ligation of the left renal vein near its confluence with the IVC can be tolerated because of accessory venous drainage through the left gonadal, left adrenal, and lumbar veins. Attempts to repair the right renal vein should be made, since the absence of adequate venous collateral flow on the right side will lead to a right nephrectomy. Endovascular approaches to manage renovascular trauma have been useful in selected instances of pseudoaneurysm formation, intimal tears, or AVFs.85–87
Mortality is hard to estimate because of the significant occurrence of major associated injuries that drive the data. Rates of posttraumatic renal failure and hypertension are low and in two studies were estimated to be 6.4% and 4.5%, respectively.85,88
Iliac vessel injury
Exsanguination from iliac vessel injuries is common and associated with high mortality resulting from refractory hemorrhage and associated injuries.89–91 Mortality rates range from 25% to 40%, but the incidence of iliac vessel injury represents 10% of all abdominal vascular injuries and less than 2% of all vascular trauma.91 Gaining surgical control of the bleeding can be challenging, and many of these patients present acidotic, coagulopathic, and hypothermic from extensive blood loss.90,91 The close proximity and shared course of the iliac veins make combined arteriovenous injuries a frequent occurrence. The small bowel, colon, urinary bladder, and ureters intimately cohabitate the microenvironment of the iliac vessels, and concomitant injury to these structures are the rule rather than the exception. Extent of injury to the surrounding structures and degree of enteric contamination are principal factors that can increase the complexity of management with regard to repair and/or revascularization.
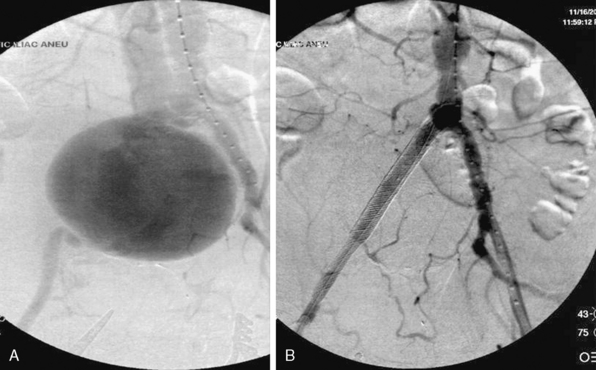
Injury to the iliac artery or suspicion of it is a clear indication for surgery in zone III retroperitoneal injuries. Proximal control is gained at the infra-mesocolic aorta, with distal control gained at the external iliac at the level of the inguinal ligament. The ascending colon is reflected medially via a Kocher or Cattell-Braasch maneuver, exposing the pelvic retroperitoneum. Primary arteriorrhaphy is the preferred method of repair for a simple injury. Reconstruction can also be accomplished with end-to-end anastomosis of autogenous saphenous vein or polytetrafluoroethylene (PTFE) grafts.90 It is important to be cognizant of the fact that prosthetic grafts may be problematic in an environment contaminated by associated small-bowel or colon injury. Iliac injuries are amenable to bailout or damage-control procedures in the circumstance of a patient critically ill due to other traumatic injuries, where an extensive operation is too high risk. Temporary shunt insertion, arterial ligation with delayed extra-anatomical reconstruction, or balloon tamponade of venous injury may be employed in this situation. Iliac vein injury can be even more complex with regard to gaining access and control of the vessel. Occasionally, the iliac artery must be divided to allow adequate access to the venous structures, and then reconstructed following venous repair. Concerns of edema and compartment syndrome following prolonged ischemic time or vein ligation merit a low threshold for lower-extremity fasciotomies. Endovascular techniques have shown utility in selected cases of isolated iliac artery injury. Patients with AVFs, pseudoaneurysms, or major intimal tears may benefit from endovascular stenting and/or coiling rather than open exploration92,93 (Fig. 61-3).
Inferior vena cava
The IVC is the most commonly injured vessel in the abdomen, accounting for one fourth of abdominal vascular injuries.59 About 90% of injuries to the IVC are a result of penetrating trauma, and approximately 18% have associated aortic injury.94,95 More than half of patients with IVC injuries will die before reaching the hospital. Of those who make it to a medical care facility, more than half are in class III hemorrhagic shock.95 The most important prognostic factors following IVC injury are the grade of hemorrhagic shock, anatomical level of IVC injury, and associated vascular injuries.58,94–96 Supra- and retrohepatic lacerations have the worst prognosis, and their management can be challenging.
Some IVC injuries present with a contained zone I retroperitoneal hematoma, and the patient will remain hemodynamically stable. In this circumstance, a decision for expectant management for lesions in the retro- or suprahepatic vena cava would be reasonable, despite the dogma of zone I retroperitoneal hematoma treatment. There is a considerable degree of difficulty and mortality associated with surgical exploration.95,96 Surgical exploration for lesions behind the liver may involve combined entry into the chest and abdomen and require extensive mobilization of the liver, atriocaval shunting, total vascular occlusion, or hepatic vascular isolation. In extreme circumstances, direct exposure of the IVC can also be accomplished through division of the liver along the Cantlie line, though it is rarely employed and only if there is already significant injury to the liver.
Infrarenal caval ligation can be an acceptable treatment in a hemodynamically unstable patient with significant associated traumatic injuries. Ligation above the level of the renal veins would lead to fulminant renal failure and is not an appropriate option. Reconstruction with autogenous vein or prosthetic conduits are the most appropriate treatments despite the low success rate due to the extreme condition of the patient. If ligation of the infrarenal IVC is done, the patient’s bilateral lower extremities should be wrapped and elevated to prevent morbid lower-extremity edema. Patients can experience transient edema in the lower extremities following primary repair of the IVC that causes stenosis. Older patients with IVC stenosis following repair are at increased risk for a pulmonary embolus, so some have advocated vena cava filter placement superior to the repair.95
Portal vein injury
The portal vein is made up by the confluence of the superior mesenteric and splenic veins behind the neck of the pancreas. It constitutes 80% of the hepatic blood flow and provides 50% of the hepatic oxygen demand. Its injury accounts for 5% of all abdominal vascular injuries.59 The vast majority are a result of penetrating trauma (90%) and associated with other vascular injuries 70% to 90% of the time.97 Following penetrating injury, patients usually present with hemorrhagic shock and require an emergent laparotomy. Blunt trauma causing a direct blow to the abdomen or severe deceleration forces can injure the portal vein, usually in combination with the SMV.
The operative exposure for these injuries can be extensive, using an extensive right-sided medial visceral rotation of the ascending colon and duodenum. In some circumstances, especially with combined SMV injuries, this exposure may not be adequate, warranting division of the neck of the pancreas to increase exposure. These patients present in such poor condition that complex reconstructions are rarely feasible or advisable. Primary repair, if acquiescent, is the best surgical treatment. Complex reconstructions should only be done in patients with associated hepatic artery injury not amenable to repair. The combined absence of blood flow through the portal vein and hepatic artery is not compatible with life. These situations merit revascularization with autogenous saphenous vein graft.97 Ligation is another option that should be considered for devastating retropancreatic injuries. Similar to ligation of the SMV, splanchnic bed sequestration can lead to patchy bowel-wall necrosis. Massive fluid resuscitation should be expected postoperatively. Historical data indicate survival rates between 55% and 85% following ligation.97–99 The abdomen should be left open and vacuumed packed, with a planned return visit to the operating room in roughly 48 hours. The mortality rate associated with portal vein injuries can be as high as 50% to 72%.59,98,99
Extremity Vascular Injury
Evaluation of patients with suspected peripheral vascular injury should be performed promptly and thoroughly. Special attention should be paid to the hard and soft signs of vascular injury, as well as the viability of the extremity. Despite the fact that over 80% of patients with vascular injury will present with absent pulses, shock, or neurological deficits, some will have arterial injuries yet also have distal pulses in the affected extremity and be hemodynamically stable.100
Mangled Extremity Score
Assessing the viability of an extremity following trauma can be difficult. Some patients require a primary amputation because of the unsalvageable nature of their limb after trauma. The Mangled Extremity Severity Score (Table 61-5) is an objective set of criteria used to predict the need for amputation in the upper and lower extremities following trauma. It takes into account the extent of soft-tissue/skeletal damage, degree of ischemia, level of systemic hypotension, and age. A score of 7 or higher is the threshold for performing an amputation.
| Factor | Score |
|---|---|
| Skeletal/Soft-Tissue Injury | |
| Low energy (stab, simple fracture, low-energy GSW) | 1 |
| Medium energy (open or multiple fractures, dislocation) | 2 |
| High energy (close-range shotgun or “military” GSW, crush) | 3 |
| Very high energy (above conditions plus contamination, avulsion) | 4 |
| Limb Ischemia (Score Double For >6 Hours of Ischemia) | |
| Pulse reduced but perfusion normal | 1 |
| Pulseless, paresthesias, decreased capillary refill | 2 |
| Cool, paralyzed, insensate, numbness | 3 |
| Hypotension | |
| Systolic BP >90 mmHg | 0 |
| Transient hypotension | 1 |
| Persistent hypotension | 2 |
| Age (Years) | |
| <30 | 0 |
| 30-50 | 1 |
| >50 | 2 |
BP, blood pressure; GSW, gunshot wound. Adapted from Johansen K, Daines M, Howey T, et al: Objective criteria accurately predict amputation following lower extremity trauma. J Trauma 30: 568–572, 1990.
Noninvasive Studies
Doppler ultrasonography has been a valuable resource in the acute assessment of extremity vascular injury. Its accuracy in detecting vascular injury is high, but limited in its ability to detect intimal defects and small false aneurysms. B-mode ultrasonography is also an easily accessible and inexpensive study that provides good resolution and the ability to diagnose small vascular defects that would otherwise be missed by Doppler or physical examination.100
Angiography
In hemodynamically stable patients, catheter-based contrast angiography is an excellent modality for evaluation and provides the opportunity for therapeutic measures as indicated. In general, angiography is commonly used following blunt extremity injury but may prove useful in many circumstances. For example, it can be extremely useful in cases with extensive global damage to the limb, multiple complex fractures, gunshot wounds, and injuries resulting in extensive soft-tissue defects.100 Its use intraoperatively and even for postoperative evolution makes it the gold standard of most vascular evaluation and treatment modalities.
Specific Peripheral Vascular Injuries
Subclavian-axillary injury
Much of the course of the subclavian/axillary artery and vein are covered by the clavicle and overlying pectoralis musculature, making injury to these structures uncommon. Penetrating trauma accounts for the overwhelming majority of cases.101 Concomitant injury of artery and vein occurs in roughly 20% of cases.102 Blunt injury to these structures are rare, but clavicle and first rib fractures/dislocations have been associated with blunt vascular injury in multiple studies.102–104
Minimizing prehospital time is key to successful management of these injuries. Upon presentation, critical ischemia of the upper extremity is uncommon, and a palpable pulse may be present in the extremity owing to the rich collateral circulation around the axillary artery. Uncontained hemorrhage from these injuries can be devastating and result in mortality at the scene. One study demonstrated an overall mortality rate of 39% in a series of 54 consecutive subclavian artery injuries.101 Another review of 79 patients illustrated that more than 20% of patients with subclavian/axillary injuries arrived at a medical care facility with no vital signs or imminent cardiac arrest due to massive hemorrhage.102
Similar to other types of vascular injury, multidetector CT scanners are a useful modality, especially with penetrating missile injuries. One study demonstrated that multidetector CT scans were helpful in avoiding unnecessary angiography in 85% of cases involving transmediastinal gunshot wounds.105 Angiography is less used in the diagnostic setting because of the utility and efficacy of color-flow Doppler and CT, but it continues to have a vital role in the therapeutic arena for managing subclavian and axillary injuries.3,73,105–107
Endovascular therapy has been used with high success in both blunt and penetrating trauma in selected patients. Those who are hemodynamically stable and found to have traumatic AVFs, false aneurysms, and focal dissections are ideal candidates. It is important to note that lesions in close proximity to the origins of the vertebral and/or right CCAs may preclude safe deployment of an endovascular stent graft without covering the origin. One series reviewed 23 patients who underwent intervention for traumatic subclavian/axillary artery injuries. Patients who met the criteria for and underwent endovascular stenting had shorter operative time, less blood loss, and similar patency rates.107
Brachial and forearm vessel injury
The incidence of upper-extremity vascular trauma historically represents 30% of vascular trauma. The upper-arm arterial supply is made up by the brachial and deep brachial arteries. The forearm blood supply consists of the radial, ulnar, and interosseous arteries. Penetrating trauma is the most common etiology of injury, and the brachial artery is damaged more than half the time in upper-extremity vascular injuries.108 The vascular component of upper-extremity trauma per se does not play a large role in overall mortality, compared to associated traumatic injuries. Nerve injury is widely accepted as the most important prognostic factor/indicator of function.108 Concomitant bone, nerve, or venous injury has been observed to be as high as 73% in some studies.109
Angiography remains the gold standard for evaluation/diagnosis of upper-extremity injuries, but noninvasive studies provide good utility in the acute setting. Physical examination and duplex ultrasound studies can diagnose most significant injuries but may miss smaller or more subtle injuries. Compared to conventional angiography, small series have shown CTA to approach 95% sensitivity and 98% specificity for upper-extremity vascular lesions.108 That accuracy, combined with the easy accessibility of CTA, makes it one of the most common modalities used in evaluating these injuries.
Expedient repair of all brachial or forearm arterial injuries is vital. Critical limb ischemia may develop in as short as 4 hours following injury if not repaired.108 Furthermore, studies of arterial injuries of the upper extremity repaired more than 12 hours after the traumatic insult suggest that only 25% return to normal arm function.110 Isolated injury to the radial or ulnar artery can usually tolerate ligation without subsequent ischemia because of the rich collateral circulation of the forearm and hand. However, it is important to remember that only 80% to 85% of the population has an intact palmer arch. Under these circumstances, the patient will need repair to the ulnar or radial artery to prevent hand ischemia. Although compartment syndrome is less commonly seen in the upper extremity than the lower extremity, a fasciotomy should be considered following any arterial repair in the arm.
Femoral vessels
Femoral vessel injury is among the most common vascular injuries, constituting approximately 70% of all peripheral vascular injuries.111 More than 90% of femoral artery injuries result from penetrating trauma, primarily gunshot wounds.100 Exsanguination does occur following these injuries, but the superficial course of the vessel allows for prehospital control of hemorrhage through direct pressure.
Obvious injury to the femoral vessels warrants transfer to a surgical suite for repair. Control of the external iliac vessels may be obtained before accessing the contents in the femoral triangle. Exposure of the femoral vessels can be gained through a linear incision along the medial edge of the sartorius muscle below the inguinal ligament. Combined vein and arterial injury can prove challenging, with venous bleeding occasionally proving more challenging than that of the arterial circuit. Historically, the most commonly injured extremity veins include the superficial femoral vein (42%), the popliteal vein (23%), and common femoral vein (14%).112 The contralateral limb should be prepared in case autogenous vein grafts are needed for repair of the injured artery or vein.
Early diagnosis and aggressive management of femoral vessel injury has led to an amputation rate of less than 9%.100 In contrast to the strategy employed for venous injury in other parts of the body, femoral vein repair is preferred to ligation. If the femoral vein is ligated, early fasciotomy and meticulous monitoring of compartment pressures should be performed.
Calf vessel injuries
The calf vessels include the vascular circuit starting and extending distally from the popliteal fossa. These vessels include the popliteal, anterior and posterior tibial, and peroneal arteries. Popliteal artery injuries represent one of the most challenging of all vascular extremity injuries to manage. The popliteal artery is the second most commonly injured artery in the leg, and more commonly results from penetrating injury. Blunt popliteal injury is most commonly seen following posterior knee dislocations,113 which can have significant orthopedic and neurological consequences.
The outcome from popliteal injury depends importantly on the mode of injury. Amputation rates may reach as high as 20% to 50% following a high-velocity gun or shotgun blast, which may lead to significant soft-tissue injury and septic sequelae. A recent review of 24 published series demonstrated a much lower amputation rate of 11%, indicating the marked improvement in limb salvage in modern civilian series.113,114 The rate of associated injury of local neurovascular structures (popliteal vein, tibial nerve, tibial/peroneal arteries, etc.) involved with penetrating popliteal injury ranges from 20% to 38%.113,115–117
Injury to one of the three infrapopliteal arteries rarely results in limb ischemia in the absence of preexisting occlusive disease. In the setting of isolated hemorrhage from one of these vessels, ligation or embolization is an option. However, when the tibioperoneal truck or two or more of the infrapopliteal arteries are injured, repair or revascularization is vital for limb salvage. Nerve, bone, and soft-tissue damage in this region of the body plays a major role in limb salvage. Historical data demonstrate an amputation rate of 54% when associated orthopedic, nerve, and soft-tissue injuries are present.118
Iatrogenic Vascular Injury
An increased incidence of iatrogenic vascular injury has been associated with the development of catheter-based cardiac and peripheral interventions to treat cardiovascular disease. Most of these injuries fall within the realm of penetrating vascular injuries. In a recent observational series, the commonest cause of vascular trauma (and with the lowest mortality rate) was catheter-based iatrogenic injury. While noniatrogenic injury occurred with the same incidence as penetrating/blunt trauma, it was associated with a fourfold excess mortality.119 Many iatrogenic injuries have therapeutic solutions that can depend on catheter-based intervention, but direct surgical repair is occasionally required. The need for surgical intervention is usually dependent on the hemodynamic status of the patient at the time of injury, or the presence of significant ischemia due to the injury.
Pseudoaneurysms and Arteriovenous Fistulae
False aneurysm
Suspicion of an iatrogenic pseudoaneurysm should occur in the setting of a swollen groin, excessive pain at the insertion site, or soft-tissue hematoma at the puncture site. Presence of a bruit and ongoing pulsatile bleeding are highly suggestive of a pseudoaneurysm. Arterial duplex examination has nearly 100% diagnostic accuracy, and if suspicion is present, no hesitation should delay obtaining the examination (also see Chapter 12). The test is simple, quick, and has excellent sensitivity and specificity. Duplex ultrasonography can be performed at any location in the hospital but may be less efficacious in conditions of morbid obesity or when a very large hematoma is present. The location of certain vessels, including the subclavian, profunda femoris, and visceral arteries can make imaging by duplex ultrasound challenging owing to depth of the vessel, overlying bone, or bowel gas. Duplex ultrasound evaluation in the longitudinal and transverse planes is essential to identify the neck, confirm flow outside the artery, and accurately measure the size of the pseudoaneurysm. Typical characteristics seen on duplex ultrasound imaging include swirling of color flow in a mass distinct from the underlying artery, color flow signal through a tract leading to a sac, and to-and-fro Doppler waveform in the pseudoaneurysm neck. The precise definition of the false aneurysm size and neck is one of the most important parameters for determining treatment options. Larger neck and width often correlates directly with larger arterial defects that are generally more refractory to treatment with minimally invasive techniques. In the setting of a significant drop in blood pressure, decreasing hematocrit, or an obese patient, where duplex ultrasound examination is technically difficult, a CT scan with contrast may be useful to confirm the diagnosis of a pseudoaneurysm and exclude the presence of a large retroperitoneal hematoma.
Arteriovenous fistula
Although physical examination alone is not diagnostic for femoral pseudoaneurysm, it can be highly accurate and specific for detecting the presence of an AVF. A to-and-fro holosystolic/diastolic bruit at the puncture site is both diagnostic and pathognomonic for an AVF. In addition, the intensity of the sounds correlates with the size of the fistula. An ultrasound is unlikely to better characterize the extent of arteriovenous flow in the presence of these clinical findings. In the acute setting, femoral AVFs after groin interventions tend to be small in size and have significantly less flow than AVFs constructed for patients requiring hemodialysis. In certain situations, days, months, or years after trauma or a groin intervention, the insidious onset of heart failure, limb swelling, or claudication can be the initial presentation for an AVF.120 The diagnosis of an AVF can be made by duplex ultrasound when the characteristic physical findings are not clearly present (also see Chapter 12). There are strict duplex ultrasound criteria for establishing a diagnosis of AVF:
Treatment Options
False aneurysms
In the absence of impending cutaneous necrosis, femoral nerve compression symptoms, and continued bleeding despite local compression, most pseudoaneurysms can be managed conservatively with observation. In a study of femoral aneurysms 3 cm or less in diameter, not associated with severe pain, and in the setting of no systemic anticoagulation, there was an 89% spontaneous thrombosis rate with no complications. The mean time for thrombosis was 23 days, with a mean number of 2.6 duplex examinations per patient.121 Loss or compromise of distal circulation, necrosis of overlying skin, uncontrolled bleeding despite compression, and reversal of anticoagulation mandates emergent surgical intervention to repair the femoral pseudoaneurysm.
Endovascular Repair
Traumatic injury to visceral and pelvic vessels can lead to false aneurysms that are best treated with percutaneous methods including microvascular coils, thrombotic agents, and covered stents. In many circumstances, prostheses developed to treat aneurysms can be modified to treat traumatic rupture and pseudoaneurysms of vessels on an individualized basis. To date, these endovascular repairs have been durable, and in the absence of life-threatening bleeding should be considered in light of many significant associated injuries.3,122 Endovascular repair of iatrogenic injury to femoral vessels can be achieved quickly, but could inadvertently occlude the common femoral artery (CFA) because of kinking. The groin is an area of repetitive flexion, which can lead to stent fracture and thrombosis.
Arteriovenous fistulae
Femoral AVFs tend to persist in patients who are on steroids or suffer from chronic renal insufficiency. Implantation of covered stents in the femoral artery has been reported but appears to be contraindicated. As previously mentioned, there is considerable concern regarding complications of this approach, risks for restenosis, stent fracture due to frequent flexion in the groin region, and possible limitations for future femoral intervention.123 Ongoing investigations will provide definitive understanding of the utility of this technique for managing femoral AVFs. In contrast to healthy concern about the durability of covered stent repairs in common femoral AVFs, the use of covered stents to manage iliac vein–iliac artery or aortocaval fistulas have been very effective.124,125 Operative repair of a chronic AVF can be hazardous because of the friable nature of the vessels and the risk for considerable blood loss. Optimal treatment requires obliteration of the fistula and restoration of arterial and venous flow. At times, interposition grafting or patch angioplasty is needed to preserve flow without narrowing vessels. If there is a large defect in the vein, preoperative placement of arterial or venous balloons for intraoperative occlusion may help decrease hemorrhage during repair of the vessels feeding the fistula.
Pediatric Vascular Trauma
Vascular trauma in the pediatric population is uncommon, occurring in only 0.6% of all pediatric trauma patients. Although less frequent than in adults, penetrating trauma is responsible for a slight majority of pediatric vascular injuries.126,127 Vascular trauma in children presents a unique challenge based on the characteristics of small, thin-walled vessels with poor tissue support and pronounced tendency for vasospasm in the setting of small intravascular volumes.127,128 Vessels of the upper extremity are the most commonly injured and are associated with low mortality. Injuries of the thoracic aorta and great vessels are rare. Injury to the carotid artery is exceedingly rare but can have devastating morbidity and mortality if not recognized and managed promptly.129 Decisions regarding operative management in the pediatric population must take into account vessel size and future growth potential, which may require future vascular revision. Amputations are usually reserved for severely mangled extremities; all attempts should be made for limb salvage. Overall, pediatric patients have an improved adjusted mortality when compared to adults.130
1 DeBakey ME, Simeone FA. Battle injuries of the arteries in World War II; an analysis of 2,471 cases. Ann Surg. 1946;123:534–579.
2 Hughes CW. Arterial repair during the Korean war. Ann Surg. 1958;147(4):555–561.
3 Starnes BW, Arthurs ZM. Endovascular management of vascular trauma. Perspect Vasc Surg Endovasc Ther. 2006;18(2):114–129.
4 Giswold ME, Landry GJ, Taylor LM, et al. Iatrogenic arterial injury is an increasingly important cause of arterial trauma. Am J Surg. 2004;187(5):590–592. discussion 592–593
5 Haimovici H. Arterial embolism with acute massive ischemic myopathy and myoglobinuria: evaluation of a hitherto unreported syndrome with report of two cases. Surgery. 1960;47:739–747.
6 Haimovici H. Myopathic-nephrotic-metabolic syndrome and massive acute arterial occlusions. Arch Surg. 1973;106(5):628–629.
7 Ames A3rd, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52(2):437–453.
8 May JWJr, Chait LA, O’Brien BM, et al. The no-reflow phenomenon in experimental free flaps. Plast Reconstr Surg. 1978;61(2):256–267.
9 Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625–632.
10 Branco BC, Inaba K, Barmparas G, et al: Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre, Injury
11 Yamane BH, Tefera G, Hoch JR, et al. Blunt thoracic aortic injury: open or stent graft repair? Surgery. 2008;144(4):575–580. discussion 580–572
12 Camp PC, Shackford SR. Outcome after blunt traumatic thoracic aortic laceration: identification of a high-risk cohort. Western Trauma Association Multicenter Study Group. J Trauma. 1997;43(3):413–422.
13 Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: multicenter Trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42(3):374–380. discussion 380–373
14 Richens D, Kotidis K, Neale M, et al. Rupture of the aorta following road traffic accidents in the United Kingdom 1992–1999. The results of the co-operative crash injury study. Eur J Cardiothorac Surg. 2003;23(2):143–148.
15 Jamieson WR, Janusz MT, Gudas VM, et al. Traumatic rupture of the thoracic aorta: third decade of experience. Am J Surg. 2002;183(5):571–575.
16 Cowley RA, Turney SZ, Hankins JR, et al. Rupture of thoracic aorta caused by blunt trauma. A fifteen-year experience. J Thorac Cardiovasc Surg. 1990;100(5):652–660. discussion 660–651
17 von Oppell UO, Dunne TT, De Groot MK, et al. Traumatic aortic rupture: twenty-year metaanalysis of mortality and risk of paraplegia. Ann Thorac Surg. 1994;58(2):585–593.
18 Jahromi AS, Kazemi K, Safar HA, et al. Traumatic rupture of the thoracic aorta: cohort study and systematic review. J Vasc Surg. 2001;34(6):1029–1034.
19 Weiman DS, Gurbuz AT, Gursky A, et al. Comparison of spinal cord protection utilizing left atrial-femoral with femoral-femoral bypass in patients with traumatic rupture of the aortic isthmus. World J Surg. 2006;30(9):1638–1641. discussion 1641–1633
20 Kato N, Dake MD, Miller DC, et al. Traumatic thoracic aortic aneurysm: treatment with endovascular stent-grafts. Radiology. 1997;205(3):657–662.
21 Lebl DR, Dicker RA, Spain DA, et al. Dramatic shift in the primary management of traumatic thoracic aortic rupture. Arch Surg. 2006;141(2):177–180.
22 Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331(26):1729–1734.
23 Lettinga-van de Poll T, Schurink GW, De Haan MW, et al. Endovascular treatment of traumatic rupture of the thoracic aorta. Br J Surg. 2007;94(5):525–533.
24 Pate JW, Fabian TC, Walker WA. Acute traumatic rupture of the aortic isthmus: repair with cardiopulmonary bypass. Ann Thorac Surg. 1995;59(1):90–98. discussion 98–99
25 Benckart DH, Magovern GJ, Liebler GA, et al. Traumatic aortic transection: repair using left atrial to femoral bypass. J Card Surg. 1989;4(1):43–49.
26 Peterson BG, Matsumura JS, Morasch MD, et al. Percutaneous endovascular repair of blunt thoracic aortic transection. J Trauma. 2005;59(5):1062–1065.
27 Amabile P, Collart F, Gariboldi V, et al. Surgical versus endovascular treatment of traumatic thoracic aortic rupture. J Vasc Surg. 2004;40(5):873–879.
28 McPhee JT, Asham EH, Rohrer MJ, et al. The midterm results of stent graft treatment of thoracic aortic injuries. J Surg Res. 2007;138(2):181–188.
29 du MM, Reekers JA, Balm R, et al. Collapse of a stent-graft following treatment of a traumatic thoracic aortic rupture. J Endovasc Ther. 2005;12(4):503–507.
30 Bakaeen FG, Wall MJJr, Mattox KL. Successful repair of an avulsion of the superior vena cava from the right atrium inflicted by blunt trauma. J Trauma. 2005;59(6):1486–1488.
31 Ambrose G, Barrett LO, Angus GL, et al. Main pulmonary artery laceration after blunt trauma: accurate preoperative diagnosis. Ann Thorac Surg. 2000;70(3):955–957.
32 Mattox KL, Feliciano DV, Burch J, et al. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg. 1989;209(6):698–705. discussion 706–697
33 Mittal VK, Paulson TJ, Colaiuta E, et al. Carotid artery injuries and their management. J Cardiovasc Surg (Torino). 2000;41(3):423–431.
34 Fogelman MJ, Stewart RD. Penetrating wounds of the neck. Am J Surg. 1956;91(4):581–593. discussion, 593–586
35 Davis TP, Feliciano DV, Rozycki GS, et al. Results with abdominal vascular trauma in the modern era. Am Surg. 2001;67(6):565–570. discussion 570–561
36 McIntyre WB, Ballard JL. Cervicothoracic vascular injuries. Semin Vasc Surg. 11(232), 1998.
37 van As AB, van Deurzen DF, Verleisdonk EJ. Gunshots to the neck: selective angiography as part of conservative management. Injury. 2002;33(5):453–456.
38 Demetriades D, Asensio JA, Velmahos G, et al. Complex problems in penetrating neck trauma. Surg Clin North Am. 1996;76(4):661–683.
39 Duane TM, Parker F, Stokes GK, et al. Endovascular carotid stenting after trauma. J Trauma. 2002;52(1):149–153.
40 McNeil JD, Chiou AC, Gunlock MG, et al. Successful endovascular therapy of a penetrating zone III internal carotid injury. J Vasc Surg. 2002;36(1):187–190.
41 Tsai YH, Wong HF, Weng HH, et al. Stent-graft treatment of traumatic carotid artery dissecting pseudoaneurysm. Neuroradiology. 2010;52(11):1011–1016.
42 Maras D, Lioupis C, Magoufis G, et al. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol. 2006;29(6):958–968.
43 DuBose J, Recinos G, Teixeira PG, et al. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65(6):1561–1566.
44 Bromberg WJ, Collier BC, Diebel LN, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68(2):471–477.
45 Moulakakis KG, Mylonas S, Avgerinos E, et al. An update of the role of endovascular repair in blunt carotid artery trauma. Eur J Vasc Endovasc Surg. 2010;40(3):312–319.
46 Berne JD, Cook A, Rowe SA, et al. A multivariate logistic regression analysis of risk factors for blunt cerebrovascular injury. J Vasc Surg. 2010;51(1):57–64.
47 Ringer AJ, Matern E, Parikh S, et al. Screening for blunt cerebrovascular injury: selection criteria for use of angiography. J Neurosurg. 2010;112(5):1146–1149.
48 Biffl WL, Ray CEJr, Moore EE, et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53(5):850–856.
49 Biffl WL, Cothren CC, Moore EE, et al. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma. 2009;67(6):1150–1153.
50 Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845–853.
51 Edwards NM, Fabian TC, Claridge JA, et al. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg. 2007;204(5):1007–1013. discussion 1014–1005
52 Li W, D’Ayala M, Hirshberg A, et al: Comparison of conservative and operative treatment for blunt carotid injuries: analysis of the National Trauma Data Bank, J Vasc Surg 51(3):593–599, e592.
53 Cothren CC, Biffl WL, Moore EE, et al. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144(7):685–690.
54 Tyburski JG, Wilson RF, Dente C, et al. Factors affecting mortality rates in patients with abdominal vascular injuries. J Trauma. 2001;50(6):1020–1026.
55 Asensio JA, Forno W, Roldan G, et al. Abdominal vascular injuries: injuries to the aorta. Surg Clin North Am. 2001;81(6):1395–1416. xiii–xiv
56 Asensio JA, Forno W, Roldan G, et al. Visceral vascular injuries. Surg Clin North Am. 2002;82(1):1–20. xix
57 Bowley DM, Degiannis E, Goosen J, et al. Penetrating vascular trauma in Johannesburg, South Africa. Surg Clin North Am. 2002;82(1):221–235.
58 Paul JS, Webb TP, Aprahamian C, et al. Intraabdominal vascular injury: are we getting any better? J Trauma. 2010.
59 Asensio JA, Chahwan S, Hanpeter D, et al. Operative management and outcome of 302 abdominal vascular injuries. Am J Surg. 2000;180(6):528–533. discussion 533–524
60 Rhee P, Koustova E, Alam HB. Searching for the optimal resuscitation method: recommendations for the initial fluid resuscitation of combat casualties. J Trauma. 2003;54(5 Suppl):S52–S62.
61 Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33(3):229–241.
62 Stern SA. Low-volume fluid resuscitation for presumed hemorrhagic shock: helpful or harmful? Curr Opin Crit Care. 2001;7(6):422–430.
63 Chapellier X, Sockeel P, Baranger B. Management of penetrating abdominal vessel injuries. J Visc Surg. 2010;147(2):e1–e12.
64 Mattox KL, McCollum WB, Jordan GLJr, et al. Management of upper abdominal vascular trauma. Am J Surg. 1974;128(6):823–828.
65 Cattell RB, Braasch JW. A technique for the exposure of the third and fourth portions of the duodenum. Surg Gynecol Obstet. 1960;111:378–379.
66 Moore EE, Cogbill TH, Jurkovich GJ, et al. Organ injury scaling. III: chest wall, abdominal vascular, ureter, bladder, and urethra. J Trauma. 1992;33(3):337–339.
67 Asensio JA, Petrone P, O’Shanahan G, et al. Managing exsanguination: what we know about damage control/bailout is not enough. Proc (Bayl Univ Med Cent). 2003;16(3):294–296.
68 Aladham F, Sundaram B, Williams DM, et al. Traumatic aortic injury: computerized tomographic findings at presentation and after conservative therapy. J Comput Assist Tomogr. 2009;34(3):388–394.
69 Degiannis E, Levy RD, Florizoone MG, et al. Gunshot injuries of the abdominal aorta: a continuing challenge. Injury. 1997;28(3):195–197.
70 Inaba K, Kirkpatrick AW, Finkelstein J, et al. Blunt abdominal aortic trauma in association with thoracolumbar spine fractures. Injury. 2001;32(3):201–207.
71 Ding X, Jiang J, Su Q, et al. Endovascular stent graft repair of a penetrating aortic injury. Ann Thorac Surg. 2010;90(2):632–634.
72 Yeh MW, Horn JK, Schecter WP, et al. Endovascular repair of an actively hemorrhaging gunshot injury to the abdominal aorta. J Vasc Surg. 2005;42(5):1007–1009.
73 Arthurs ZM, Sohn VY, Starnes BW. Vascular trauma: endovascular management and techniques. Surg Clin North Am. 2007;87(5):1179–1192. x–xi
74 Reuben BC, Whitten MG, Sarfati M, et al. Increasing use of endovascular therapy in acute arterial injuries: analysis of the National Trauma Data Bank. J Vasc Surg. 2007;46(6):1222–1226.
75 Demetriades D, Theodorou D, Murray J, et al. Mortality and prognostic factors in penetrating injuries of the aorta. J Trauma. 1996;40(5):761–763.
76 Durai R, Ng PC. Role of angio-embolisation in trauma–review. Acta Chir Belg. 2010;110(2):169–177.
77 Asensio JA, Berne JD, Chahwan S, et al. Traumatic injury to the superior mesenteric artery. Am J Surg. 1999;178(3):235–239.
78 Fullen WD, Hunt J, Altemeier WA. The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J Trauma. 1972;12(8):656–664.
79 Asensio JA, Britt LD, Borzotta A, et al. Multiinstitutional experience with the management of superior mesenteric artery injuries. J Am Coll Surg. 2001;193(4):354–365. discussion 365–356
80 Patel T, Kuladhipati I, Shah S. Successful percutaneous endovascular management of acute post-traumatic superior mesenteric artery dissection using a transradial approach. J Invasive Cardiol. 2010;22(4):E61–E64.
81 Asensio JA, Petrone P, Garcia-Nunez L, et al. Superior mesenteric venous injuries: to ligate or to repair remains the question. J Trauma. 2007;62(3):668–675. discussion 675
82 Elliott SP, Olweny EO, McAninch JW. Renal arterial injuries: a single center analysis of management strategies and outcomes. J Urol. 2007;178(6):2451–2455.
83 Smith JK, Kenney PJ. Imaging of renal trauma. Radiol Clin North Am. 2003;41(5):1019–1035.
84 Bruce LM, Croce MA, Santaniello JM, et al. Blunt renal artery injury: incidence, diagnosis, and management. Am Surg. 2001;67(6):550–554. discussion 555–556
85 Tillou A, Romero J, Asensio JA, et al. Renal vascular injuries. Surg Clin North Am. 2001;81(6):1417–1430.
86 Lee JT, White RA. Endovascular management of blunt traumatic renal artery dissection. J Endovasc Ther. 2002;9(3):354–358.
87 Weiss VJ, Chaikof EL. Endovascular treatment of vascular injuries. Surg Clin North Am. 1999;79(3):653–665.
88 Knudson MM, Harrison PB, Hoyt DB, et al. Outcome after major renovascular injuries: a Western trauma association multicenter report. J Trauma. 2000;49(6):1116–1122.
89 Asensio JA, McDuffie L, Petrone P, et al. Reliable variables in the exsanguinated patient which indicate damage control and predict outcome. Am J Surg. 2001;182(6):743–751.
90 Asensio JA, Petrone P, Roldan G, et al. Analysis of 185 iliac vessel injuries: risk factors and predictors of outcome. Arch Surg. 2003;138(11):1187–1193. discussion 1193–1184
91 Lee JT, Bongard FS. Iliac vessel injuries. Surg Clin North Am. 2002;82(1):21–48. xix
92 Kiguchi M, O’Rourke HJ, Dasyam A, et al. Endovascular repair of 2 iliac pseudoaneurysms and arteriovenous fistula following spine surgery. Vasc Endovascular Surg. 2010;44(2):126–130.
93 Lyden SP, Srivastava SD, Waldman DL, et al. Common iliac artery dissection after blunt trauma: case report of endovascular repair and literature review. J Trauma. 2001;50(2):339–342.
94 Kuehne J, Frankhouse J, Modrall G, et al. Determinants of survival after inferior vena cava trauma. Am Surg. 1999;65(10):976–981.
95 Buckman RF, Pathak AS, Badellino MM, et al. Injuries of the inferior vena cava. Surg Clin North Am. 2001;81(6):1431–1447.
96 Formisano V, Di Muria A, Muto G, et al. Inferior vena cava gunshot injury: case report and a review of the literature. Ann Ital Chir. 2006;77(2):173–177.
97 Buckman RF, Pathak AS, Badellino MM, et al. Portal vein injuries. Surg Clin North Am. 2001;81(6):1449–1462.
98 Stone HH, Fabian TC, Turkleson ML. Wounds of the portal venous system. World J Surg. 1982;6(3):335–341.
99 Pachter HL, Drager S, Godfrey N, et al. Traumatic injuries of the portal vein. The role of acute ligation. Ann Surg. 1979;189(4):383–385.
100 Carrillo EH, Spain DA, Miller FB, et al. Femoral vessel injuries. Surg Clin North Am. 2002;82(1):49–65.
101 Lin PH, Koffron AJ, Guske PJ, et al. Penetrating injuries of the subclavian artery. Am J Surg. 2003;185(6):580–584.
102 Demetriades D, Asensio JA. Subclavian and axillary vascular injuries. Surg Clin North Am. 2001;81(6):1357–1373. xiii
103 Phillips EH, Rogers WF, Gaspar MR. First rib fractures: incidence of vascular injury and indications for angiography. Surgery. 1981;89(1):42–47.
104 Richardson JD, McElvein RB, Trinkle JK. First rib fracture: a hallmark of severe trauma. Ann Surg. 1975;181(3):251–254.
105 du Toit DF, Strauss DC, Blaszczyk M, et al. Endovascular treatment of penetrating thoracic outlet arterial injuries. Eur J Vasc Endovasc Surg. 2000;19(5):489–495.
106 Janne d’Othee B, Rousseau H, Otal P, et al. Noncovered stent placement in a blunt traumatic injury of the right subclavian artery. Cardiovasc Intervent Radiol. 1999;22(5):424–427.
107 Xenos ES, Freeman M, Stevens S, et al. Covered stents for injuries of subclavian and axillary arteries. J Vasc Surg. 2003;38(3):451–454.
108 Fields CE, Latifi R, Ivatury RR. Brachial and forearm vessel injuries. Surg Clin North Am. 2002;82(1):105–114.
109 Borman KR, Snyder WH3rd, Weigelt JA. Civilian arterial trauma of the upper extremity. An 11 year experience in 267 patients. Am J Surg. 1984;148(6):796–799.
110 Hunt CA, Kingsley JR. Vascular injuries of the upper extremity. South Med J. 2000;93(5):466–468.
111 Asensio JA, Kuncir EJ, Garcia-Nunez LM, et al. Femoral vessel injuries: analysis of factors predictive of outcomes. J Am Coll Surg. 2006;203(4):512–520.
112 Meyer J, Walsh J, Schuler J, et al. The early fate of venous repair after civilian vascular trauma. A clinical, hemodynamic, and venographic assessment. Ann Surg. 1987;206(4):458–464.
113 Mullenix PS, Steele SR, Andersen CA, et al. Limb salvage and outcomes among patients with traumatic popliteal vascular injury: an analysis of the National Trauma Data Bank. J Vasc Surg. 2006;44(1):94–100.
114 Frykberg ER. Popliteal vascular injuries. Surg Clin North Am. 2002;82(1):67–89.
115 Rowe VL, Salim A, Lipham J, et al. Shank vessel injuries. Surg Clin North Am. 2002;82(1):91–104. xx
116 Wagner WH, Calkins ER, Weaver FA, et al. Blunt popliteal artery trauma: one hundred consecutive injuries. J Vasc Surg. 1988;7(5):736–743.
117 Wagner WH, Yellin AE, Weaver FA, et al. Acute treatment of penetrating popliteal artery trauma: the importance of soft tissue injury. Ann Vasc Surg. 1994;8(6):557–565.
118 Shah DM, Corson JD, Karmody AM, et al. Optimal management of tibial arterial trauma. J Trauma. 1988;28(2):228–234.
119 Bains SK, Vlachou PA, Rayt HS, et al. An observational cohort study of the management and outcomes of vascular trauma. Surgeon. 2009;7(6):332–335.
120 Vagefi PA, Kwolek CJ, Wicky S, et al. Congestive heart failure from traumatic arteriovenous fistula. J Am Coll Surg. 2009;209(1):150.
121 Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803–808. discussion 808–809
122 Reed AB, Thompson JK, Crafton CJ, et al. Timing of endovascular repair of blunt traumatic thoracic aortic transections. J Vasc Surg. 2006;43(4):684–688.
123 Thalhammer C, Kirchherr AS, Uhlich F, et al. Postcatheterization pseudoaneurysms and arteriovenous fistulas: repair with percutaneous implantation of endovascular covered stents. Radiology. 2000;214(1):127–131.
124 Gandini R, Ippoliti A, Pampana E, et al. Emergency endograft placement for recurrent aortocaval fistula after conventional AAA repair. J Endovasc Ther. 2002;9(2):208–211.
125 Zhou W, Bush RL, Terramani TT, et al. Treatment options of iatrogenic pelvic vein injuries: conventional operative versus endovascular approach–case reports. Vasc Endovascular Surg. 2004;38(6):569–573.
126 Klinkner DB, Arca MJ, Lewis BD, et al. Pediatric vascular injuries: patterns of injury, morbidity, and mortality. J Pediatr Surg. 2007;42(1):178–182. discussion 182–173
127 Shah SR, Wearden PD, Gaines BA. Pediatric peripheral vascular injuries: a review of our experience. J Surg Res. 2009;153(1):162–166.
128 Mommsen P, Zeckey C, Hildebrand F, et al. Traumatic extremity arterial injury in children: epidemiology, diagnostics, treatment and prognostic value of Mangled Extremity Severity Score. J Orthop Surg Res. 2010;5:25.
129 Chamoun RB, Mawad ME, Whitehead WE, et al. Extracranial traumatic carotid artery dissections in children: a review of current diagnosis and treatment options. J Neurosurg Pediatr. 2008;2(2):101–108.
130 Barmparas G, Inaba K, Talving P, et al. Pediatric vs adult vascular trauma: a National Trauma Databank review. J Pediatr Surg. 2010;45(7):1404–1412.