Vascular Lesions
Vascular lesions of the spine are uncommon in the pediatric population. The most common vascular lesions include fast-flow lesions such as arteriovenous malformations (AVMs) and arteriovenous fistulas (AVFs). Less common are venous (formerly “cavernous”) malformations and spinal cord infarcts. Several syndromes are associated with spinal cord AVMs and AVFs, including hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu), neurofibromatosis, and Parkes-Weber, Cobb, and CLOVES (congenital, lipomatous, overgrowth, vascular malformations, epidermal nevi, and spinal/skeletal anomalies or scoliosis) syndromes.1,2 Pediatric spinal AVMs and AVFs presenting at less than 2 years of age are especially likely to occur in association with syndromes such as hereditary hemorrhagic telangiectasia.1,3 However, recent reports have indicated that the association between Klippel-Trénaunay syndrome and AVMs may be erroneous. In a review of 208 patients with Klippel-Trénaunay syndrome, none was found to have spinal AVMs, as has been reported previously.4 Further review revealed that many patients previously reported in the literature as having Klippel-Trénaunay syndrome and spinal AVMs likely had CLOVES syndrome.5 This finding underscores the issues surrounding variable and inconsistent classification of pediatric vascular lesions.
Classification
Vascular lesions of the spine have undergone various classifications,1,6–10 in part because of the complexity of their diagnosis and features. The term “spinal arteriovenous shunt” is used to encompass both types of high-flow lesions (AVMs and AVFs). Because AVMs and AVFs are found in various locations (i.e., intramedullary, intradural extramedullary, and extradural sites) with variable feeding vessels, structural features, drainage, and hemodynamic changes, additional subclassifications have been proposed.6,8,9 On the basis of histopathologic discoveries, pediatric vascular anomalies increasingly have been classified using the International Society for the Study of Vascular Anomalies (ISSVA) system first published by Mulliken and Glowacki.3,11,12 This system, which allows for correlation between lesion histology, clinical presentation, and treatment, is now used widely by pediatric subspecialists including dermatologists, interventional radiologists, plastic surgeons, and neurosurgeons.10 The ISSVA classification system divides lesions into two broad categories neoplasms and vascular anomalies.10–12 Vascular anomalies are further divided into slow/low (venous and/or lymphatic components) and fast/high (arterial components) flow lesions.3 This chapter discusses pediatric vascular anomalies, including fast-flow vascular anomalies such as AVMs and AVFs, and slow-flow venous (formerly “cavernous”) malformations. Spinal cord infarcts are also discussed in this chapter although as neither neoplasms nor vascular malformations, and they fall outside the ISSVA system.
Arteriovenous Malformations
Overview and Etiology: AVMs are high-flow congenital communications between arteries and veins that result in arteriovenous shunting, causing the involved vessels to become enlarged and tortuous.12 AVMs contain a nidus (a Latin word meaning “nest”), or vascular network, that communicates between the arteries and veins. The high-flow dynamics within the AVM predisposes the involved arteries to aneurysm and veins to variceal formation.10 AVMs can be characterized further by the location of the nidus (within the spinal cord or on the cord surface), as well as by morphology of the nidus (the central, tightly formed “glomus” type or the diffuse, typically extensive, extramedullary “juvenile” or “metameric” type). In the pediatric population, the glomus spinal cord fast-flow AVM is the most common. In one large pediatric series, twice as many children had AVMs as had AVFs.6 AVMs may occur anywhere along the spine in approximate proportion with each segment, including 30% to 40% cervical and 60% to 70% thoracolumbar.
Clinical Presentation: The initial clinical presentation varies from acute paraparesis to slowly progressive myelopathy with weakness, sensory loss, and bowel and bladder dysfunction. Hemorrhage is more commonly seen in children (78%) compared with adults, especially in cervical cord AVMs. Children present acutely because of an arteriovenous malformation hemorrhage in 56% of cases.6
Imaging: Magnetic resonance imaging (MRI) is the best noninvasive modality for initial diagnosis and follow-up of a spinal arteriovenous shunt.6,13 Imaging findings of spinal cord AVMs include tortuous dilated vessels that can be within or on the surface of the cord. Hemorrhage has a variable appearance on T1-weighted and T2-weighted images depending on chronicity. T2-weighted images may demonstrate spinal cord edema and expansion.3 MRI also documents the presence of cord cavitation, atrophy, and venous thrombosis. Images obtained after administration of gadolinium may reveal cord enhancement, which is attributed to venous stasis, ischemia, or spinal cord infarction1 (Fig. 46-1). Distinguishing normal physiologic cerebrospinal fluid (CSF) pulsation and flow (particularly in the dorsal extramedullary midthoracic region) from vascular abnormalities requires optimization of spine imaging, which may include switching the phase and frequency encoding directions to decrease the effect of physiologic CSF pulsation. Computed tomography has a limited role in imaging AVMs but may document bony changes often seen in large, diffuse metameric-type lesions. Although MRI is excellent for the initial diagnosis, spinal angiography remains the gold standard for diagnosis and delineation of AVMs.
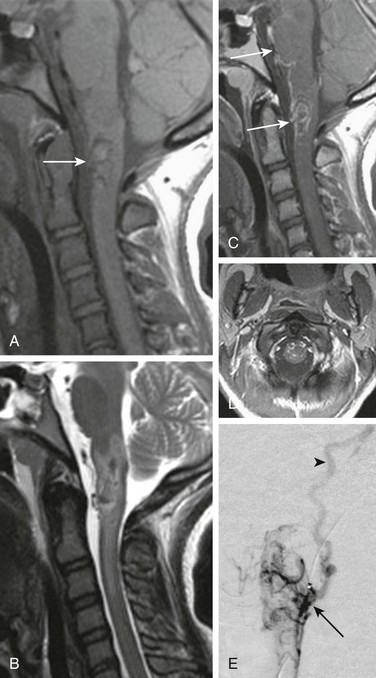
Figure 46-1 Arteriovenous malformation in a 14-year-old girl with sudden onset of severe headache, left-sided weakness, and loss of consciousness.
Head computed tomography (not shown) revealed intraventricular hemorrhage. A, A sagittal T1-weighted image shows a hypointense lesion with subtle foci of high signal hemorrhage (arrow). B, A sagittal T2-weighted image confirms low-signal central hemorrhage with hyperintense surrounding edema. C and D, Postcontrast T1-weighted sagittal and axial images demonstrate enhancing superficial serpiginous vessels and peripheral intramedullary enhancement (arrows). E, A catheter angiogram injection of the vertebral artery reveals an intramedullary nidus (arrow) with arterial supply from anterior and lateral spinal artery contributors. Venous drainage is toward the jugular bulb via a recurrent perimedullary vein (arrowhead).
Treatment: Endovascular embolization is the primary line of treatment for fast-flow vascular anomalies, with the exception of lesions at the conus, which are managed surgically.1,3,6 In cases in which complete embolization or resection are not possible because of an unacceptable risk of morbidity or mortality, the goal of treatment is to reach a hemodynamic equilibrium between the lesion and the spinal cord that will reduce the risk of ischemia and hemorrhage.14,15
Arteriovenous Fistula
Overview and Etiology: The term AVF is used to describe a fast-flow lesion with a direct artery to vein fistula without a focal nidus, which is seen with AVMs. It is thought that AVFs likely are an acquired deformity resulting from microvascular injury and reparative healing, leading to an abnormal communication between an artery and vein.8,16 In a large pediatric and adult series, a higher percentage of multiple spinal cord fistulas was present in pediatric patients (46%) compared with adults (27%).6 AVFs are divided by location into two groups, spinal cord and spinal dural. Spinal cord AVFs are perimedullary in location and one third as common as AVMs in children. Spinal dural AVFs are extremely rare in the pediatric population.6
Clinical Presentation: The clinical presentation of AVFs is similar to AVMs, including slowly progressive weakness, sensory loss, and bowel and bladder dysfunction due to venous congestion, venous hypertension, and resultant myelopathy. However, AVFs hemorrhage less than AVMs, and acute presentations are uncommon.6
Imaging: Imaging findings of spinal cord AVFs are virtually identical to spinal cord AVMs in the pediatric population.1 Dural AVFs, which are common in adults, are uncommon in children.6
Treatment: Because each spinal AVF is unique, treatment must be tailored to the specific lesion, taking into account location, size, number of fistula formation sites, and associated symptoms.3 Therapies are the same as for other fast-flow anomalies, including endovascular embolization, microsurgical resection, radiation therapy (used rarely), or a combination of these therapies.1,3,17 Endovascular embolization has the advantage of being less invasive than the other therapies, but the feeding arteries may not be accessible, and thus open surgery may be needed in some cases.18
Prognosis: Early diagnosis of AVFs is essential to preserve neurological function. Patient outcome is proportional to the severity of symptoms at the time of diagnosis. Patients who are nonambulatory and those with poor bladder or bowel function before treatment are unlikely to regain neurological functions after therapy.17,18
Venous (Cavernous) Malformations
Overview: Slow-flow venous (cavernous) malformations (VMs), also previously termed cavernous angiomas and cavernomas, are congenital vascular malformations made up of dysmorphic venous vascular spaces lined by a single layer of endothelial cells with no intervening neural tissue.19 According to the ISSVA classification system, VMs of the spinal cord have been reclassified as slow-flow vascular anomalies. Similarly, venous vertebral anomalies were previously misclassified as hemangiomas, although they are neoplastic. Pathologically, the cells of this lesion are anomalous vessels that do not divide and undergo mitosis because they are not neoplasms. Unfortunately, some classification systems still refer to this malformation as a neoplasm, which may lead to confusion.8 The term venous (cavernous) VM, rather than the misnomer cavernoma, should be used to avoid confusion with a true neoplasm. Spinal cord VMs are less common than similar lesions found in the brain, with one study showing that 5% of pediatric VMs are located within the spine.20
Etiology: The specific etiology of spinal VMs is unclear. However, the presence of multiple brain and/or spinal VMs in any pediatric patient at presentation should prompt a careful history and genetic analysis. Associated mutations predisposing patients to multiple VMs have been found on chromosomes 3 and 7, with an autosomal-dominant variable penetrance pattern of inheritance.20 Deep venous anomalies are associated with sporadic VMs in up to 40% of cases. Patients who previously have undergone radiation therapy, typically of the craniospinal axis, are predisposed to the development of a VM. Approximately 12% to 16% of pediatric patients have multiple lesions.20,21
Clinical Presentation: The clinical presentation of VMs is a result of hemorrhage and ranges from nearly asymptomatic to severe neurologic symptoms. Pediatric patients are more likely to have a sudden clinical onset compared with adults, with up to 75% of children presenting acutely with hemorrhage.20
Imaging: MRI findings of spinal cord VMs are similar to their intracranial counterparts, with T1-weighted signal heterogeneity within the lesion caused by blood products of varying age. On T2-weighted imaging, a hypointense rim representing hemosiderin is typical, with an internal heterogeneous T2 signal. Cord edema may be seen if recent hemorrhage has occurred (Fig. 46-2). On T2* gradient recalled echo imaging and more recently reported susceptibility weighted imaging, a prominent blooming effect is noted because of the susceptibility effect of hemorrhage degradation products.22 Susceptibility imaging has been shown to be significantly better than T2* gradient imaging at detecting hemorrhage.22 Distinguishing a spinal cord VM from other pathologies can be difficult in some cases but usually is based on identification of the classic rim of hemosiderin along the lesion margins and the absence of other morphologic features that would suggest a neoplasm, such as an enhancing mass.19 Because VMs are very slow-flow lesions, they typically are not seen on catheter angiography.
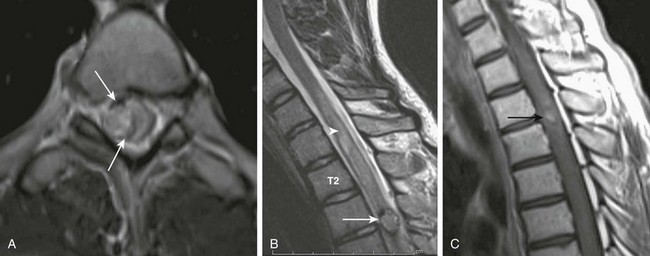
Figure 46-2 Venous (cavernous) malformation in a 16-year-old boy with arm weakness.
A and B, Axial and sagittal T2-weighted images demonstrate a centrally hyperintense intramedullary lesion with a hypointense rim (arrows). Central bright signal cord edema is noted proximally as well (arrowhead). C, A postcontrast T1-weighted image shows no enhancement. Central hyperintense hemorrhage within the lesion (arrow) also was seen on precontrast T1-weighted images (not shown).
Treatment: Asymptomatic VMs and those located in delicate regions are treated conservatively in many cases rather than with endovascular techniques. Although symptomatic venous malformations located elsewhere in the body often are treated initially with sclerotherapy, this treatment is not an option for intramedullary spinal cord lesions. Instead, patients with symptomatic spinal cord lesions may undergo surgical resection or, rarely, radiotherapy when the lesions are found in hard-to-reach locations.19,21
Prognosis: The immediate postoperative outcome after VM resection in one study showed that 11% of patients had worse symptoms, 83% were the same, and 6% were improved. At 5-year follow-up, the percentages of patients who were the same and improved were 68% and 23%, respectively,23 showing a moderate response to treatment.
Spinal Cord Infarction
Overview: Infarction of the spinal cord is uncommon, particularly in children. The most vulnerable region of the spinal cord is the anterior half, which is supplied by the single anterior spinal artery. The posterior spinal cord is supplied by paired paramedian posterior spinal arteries, allowing more extensive collateral circulation.
Etiology: Trauma and infection (e.g., meningitis) are the most common causes of cord infarction in children.24 Other causes of spinal cord infarction include thrombotic and/or embolic phenomena from cardiac disease, hypercoagulable states, umbilical arterial lines, or fibrocartilaginous embolization, as well as hypoperfusion and cardiac arrest.24,25 Fibrocartilaginous embolization is well described in dogs in the veterinary literature, and rarely occurs in humans. Fibrocartilaginous embolization is believed to result from vertical disc herniation and embolization of the nucleus pulposus. The mechanism by which disc fragments enter the spinal artery is unclear, and some authors do not subscribe to this causality.25,26
Clinical Presentation: Spinal cord infarction most often presents with acute paraparesis or quadriparesis depending on the level of cord involvement.26
Imaging: Because the single anterior artery supplies the anterior two thirds of the spinal cord, infarction often shows anterior cord involvement of both gray and white matter, seen as T1-weighted hypointensity and T2-weighted hyperintensity (Fig. 46-3). T2-weighted hyperintensity may not be seen initially if MRI is performed in a hyperacute setting. Cord edema and swelling can be seen acutely, and contrast enhancement can occur in the subacute (>5 days) phase.27 Current MRI techniques allow diffusion imaging of the spinal cord as a supplement to standard sequences to confirm ischemia27,28 (Fig. 46-4). In the differential diagnosis of cord infarction is transverse myelitis, which is more common than cord infarction in children (see Chapter 44).29 Isolated involvement of the spinal cord gray matter suggests an infectious or postinfectious autoimmune process rather than infarction. Fibrocartilaginous embolization may be suspected when the infarct is located in the lumbar spine in association with adjacent disc disease (Fig. 46-5). Careful review of the patient’s history, spinal fluid analysis, and the anatomic distribution of signal abnormality in spinal gray and/or white matter may be helpful to distinguish cord infarct from transverse myelitis.24,29
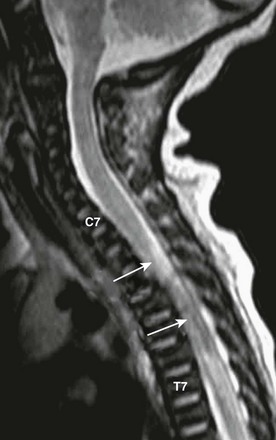
Figure 46-3 A thoracic spinal cord infarct in a newborn boy with paraplegia as a result of a traumatic delivery.
Sagittal T2-weighted magnetic resonance image reveals a large region of heterogeneous signal intensity (arrows) due to cord transection. Proximal hyperintense edema also is seen. Labeled are the seventh cervical (C7) and thoracic (T7) vertebrae.
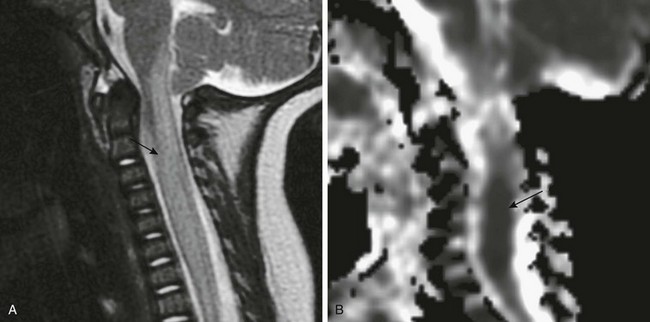
Figure 46-4 Cord ischemia as a result of infection from herpes simplex virus type 6.
A, A sagittal fast spin echo T2-weighted magnetic resonance image shows T2 hyperintensity and cord swelling as a result of edema (arrow). B, An apparent diffusion coefficient map of a sagittal echo planar diffusion-weighted image shows hypointensity representing cytotoxic cord edema (arrow). The findings are consistent with necrotizing myelitis.
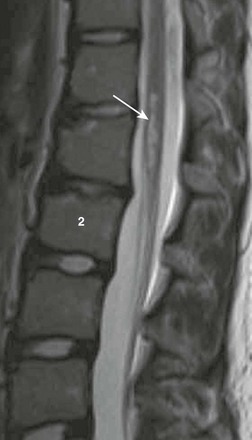
Figure 46-5 A lumbar spinal cord infarct in a 13-year-old girl with pain and leg weakness for 2 weeks, possibly fibrocartilaginous in etiology.
Sagittal T2-weighted magnetic resonance image reveals a well-defined region of oblong hyperintensity (arrow). Irregularity at the anterior superior margin of L1 and L2 are consistent with Schmorl nodes.
Bemporad, JA, Sze, G. Magnetic resonance imaging of spinal cord vascular malformations with an emphasis on the cervical spine. Neuroimaging Clin N Am. 2001;11:111–129.
Boo, S, Hartel, J, Hogg, JP. Vascular abnormalities of the spine: an imaging review. Curr Probl Diagn Radiol. 2010;39(3):110–117.
Mulliken, JB, Glowacki, J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast reconstr Surg. 1982;69:412–422.
Nagib, MG, O’Fallon, MT. Intramedullary cavernous angiomas of the spinal cord in the pediatric age group: a pediatric series. Pediatr Neurosurg. 2002;36:57–63.
Nozaki, T, Nosaka, S, Miyazaki, O. Syndromes associated with vascular tumors and malformations: a pictorial review. Radiographics. 2013;33(1):175–195.
References
1. Cullen, S, Krings, T, Ozanne, A, et al. Diagnosis and endovascular treatment of pediatric spinal arteriovenous shunts. Neuroimaging Clin N Am. 2007;17(2):207–221.
2. Krings, T, Chng, SM, Ozanne, A, et al. Hereditary hemorrhagic telangiectasia in children: endovascular treatment of neurovascular malformations: results in 31 patients. Neuroradiology. 2005;47(12):946–954.
3. Cahill, AM, Nijs, EL. Pediatric vascular malformations: pathophysiology, diagnosis, and the role of interventional radiology. Cardiovasc Intervent Radiol. 2011;34(4):691–704.
4. Alomari, AI, Orbach, DB, Mulliken, JB, et al. Klippel-Trenaunay syndrome and spinal arteriovenous malformations: an erroneous association. AJNR Am J Neuroradiol. 2010;31(9):1608–1612.
5. Alomari, AI, Chaudry, G, Rodesch, G, et al. Complex spinal-paraspinal fast-flow lesions in CLOVES syndrome: analysis of clinical and imaging findings in 6 patients. AJNR Am J Neuroradiol. 2011;32(10):1812–1817.
6. Niimi, Y, Berenstein, A, Song, J. Spinal vascular malformations. In: Albright AL, Pollack IF, Adelson PD, et al, eds. Principles and practice of pediatric neurosurgery. New York: Thieme Medical Publishers, 2008.
7. Anson, JA, Spetzler, RF. Interventional neuroradiology for spinal pathology. Clin Neurosurg. 1992;39:388–417.
8. Spetzler, RF, Detwiler, PW, Riina, HA, et al. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2 suppl):145–156.
9. Zozulya, YP, Slin’ko, EI, Al, Q, II. Spinal arteriovenous malformations: new classification and surgical treatment. Neurosurg Focus. 2006;20(5):E7.
10. Dubois, J, Garel, L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol. 1999;29(12):879–893.
11. Van Aalst, JA, Bhuller, A, Sadove, AM. Pediatric vascular lesions. J Craniofac Surg. 2003;14(4):566–583.
12. Mulliken, JB, Glowacki, J. Classification of pediatric vascular lesions. Plast Reconstr Surg. 1982;70(1):120–121.
13. Dormont, D, Gelbert, F, Assouline, E, et al. MR imaging of spinal cord arteriovenous malformations at 0.5 T: study of 34 cases. AJNR Am J Neuroradiol. 1988;9(5):833–838.
14. Veznedaroglu, E, Nelson, PK, Jabbour, PM, et al. Endovascular treatment of spinal cord arteriovenous malformations. Neurosurgery. 2006;59(5 suppl 3):S202–S209. [discussion S203-S213].
15. Juszkat, R, Zabicki, B, Checinski, P, et al. Endovascular treatment of arteriovenous malformation. Aesthetic Plast Surg. 2009;33(4):639–642.
16. Spetzler, RF, Zabramski, JM. Surgical management of large AVMs. Acta Neurochir Suppl (Wien). 1988;42:93–97.
17. Patsalides, A, Knopman, J, Santillan, A, et al. Endovascular treatment of spinal arteriovenous lesions: beyond the dural fistula. AJNR Am J Neuroradiol. 2011;32(5):798–808.
18. Song, D, Garton, HJ, Fahim, DK, et al. Spinal cord vascular malformations in children. Neurosurg Clin N Am. 2010;21(3):503–510.
19. Mottolese, C, Hermier, M, Stan, H, et al. Central nervous system cavernomas in the pediatric age group. Neurosurg Rev. 2001;24(2-3):55–71. [discussion 72-73].
20. Acciarri, N, Galassi, E, Giulioni, M, et al. Cavernous malformations of the central nervous system in the pediatric age group. Pediatr Neurosurg. 2009;45(2):81–104.
21. Deutsch, H, Shrivistava, R, Epstein, F, et al. Pediatric intramedullary spinal cavernous malformations. Spine (Phila Pa 1976). 2001;26(18):E427–E431.
22. Wang, M, Dai, Y, Han, Y, et al. Susceptibility weighted imaging in detecting hemorrhage in acute cervical spinal cord injury. Magn Reson Imaging. 2011;29(3):365–373.
23. Mitha, AP, Turner, JD, Abla, AA, et al. Outcomes following resection of intramedullary spinal cord cavernous malformations: a 25-year experience. J Neurosurg Spine. 2011;14(5):605–611.
24. Nance, JR, Golomb, MR. Ischemic spinal cord infarction in children without vertebral fracture. Pediatr Neurol. 2007;36(4):209–216.
25. Tosi, L, Rigoli, G, Beltramello, A. Fibrocartilaginous embolism of the spinal cord: a clinical and pathogenetic reconsideration. J Neurol Neurosurg Psychiatry. 1996;60(1):55–60.
26. Raghavan, A, Onikul, E, Ryan, MM, et al. Anterior spinal cord infarction owing to possible fibrocartilaginous embolism. Pediatr Radiol. 2004;34(6):503–506.
27. Krings, T, Lasjaunias, PL, Hans, FJ, et al. Imaging in spinal vascular disease. Neuroimaging Clin N Am. 2007;17(1):57–72.
28. Manara, R, Calderone, M, Severino, MS, et al. Spinal cord infarction due to fibrocartilaginous embolization: the role of diffusion weighted imaging and short-tau inversion recovery sequences. J Child Neurol. 2010;25(8):1024–1028.
29. Han, JJ, Massagli, TL, Jaffe, KM. Fibrocartilaginous embolism—an uncommon cause of spinal cord infarction: a case report and review of the literature. Arch Phys Med Rehabil. 2004;85(1):153–157.






