Chapter 18
Vascular Laboratory
Venous Duplex Scanning
Luis R. León Jr.,, Nicos Labropoulos
Based on a chapter in the seventh edition by Luis R. Leon, Jr. and Nicos Labropoulos
Duplex ultrasonography (DUS) is the best screening, perioperative, and follow-up tool available for the evaluation of vascular disease. In the assessment of venous disease, DUS is used to detect acute deep venous thrombosis (DVT) and venous reflux and to evaluate chronic venous obstruction. It is also a great method to provide differential diagnosis and thus improve management of the patient. The progress achieved by DUS during the last 20 years has led to its current central role in the diagnosis of venous disease.
Basic Principles
DUS is a sensitive and specific tool for the assessment of most venous diseases. However, like many other modalities, it is operator and equipment dependent. There are considerable variations and training levels among investigators who perform this test. Reliable information can be obtained from DUS only by experienced operators who have extensive knowledge of venous anatomy, physiology, and pathology. Technologists, not always certified or registered, perform these examinations in the United States. Technologists, vascular scientists, and physicians perform these tests in many other countries. It is also common for surgeons, angiologists, and phlebologists to perform their own testing. It would be ideal if the personnel performing the investigations undergo systematic training. Proof of continuing sonographic education, at least yearly, should be also required.
Of all outpatient DUS examinations for suspected acute lower extremity DVT, only 12% to 25% detect an abnormality; an increasing prevalence of abnormal findings is seen in patients with pertinent symptoms or thrombotic risk factors.1–6 Until recently, there was no systematic consensus on how DUS is best performed for chronic venous disease. The American College of Radiology published practice guidelines for the performance of peripheral venous DUS in 1993 (last revision in 2006). The clinical aspects of this document (indications, specifications of the examination and equipment) were developed collaboratively by the American College of Radiology and the American Institute of Ultrasound in Medicine. In 2006, a consensus document on DUS investigation of the veins in chronic venous disease of the lower limbs, released in two parts by the Union Internationale de Phlébologie,7,8 summarized the current standards for assessment of the lower extremity veins with DUS.
All maneuvers and techniques that will be used during the examination should be explained to the patient. Certain conditions regarding equipment and examination technique must be met for optimal results to be achieved. Enough lighting should be provided to enable a thorough evaluation of the lower limbs and to establish the distribution of varicosities. The lower extremities should be inspected for varicosities and scars from surgery to help predict the source of reflux and to facilitate the examination. The room should also be warm and comfortable to ensure that there is no vein spasm, which would hinder accurate determination of vein size. The importance of these seemingly trivial suggestions was pointed out by van Bemmelen and associates.9 By modifying room conditions at the time of DUS, they showed that vein diameters are significantly larger after warm-water submersion in a sitting position without a tourniquet than after other conditions.
Choosing the right transducer for a certain patient, illness, or vein is important. Axial resolution and tissue penetration are inversely related. A 4- to 7-MHz linear array transducer is optimal for assessing most veins, which normally lie between 1 and 3 cm below the skin. Deep veins, such as those in the abdomen or pelvis, or veins in obese or edematous patients are better assessed by using lower frequency curvilinear transducers for deeper penetration.
Low blood flow settings are used most often. The pulse repetition frequency (the number of pulses transmitted per second) is set at 1500 Hz or lower. When vein stenosis or arteriovenous fistula is suspected, pulse repetition frequency should be increased because blood flow velocity is considerably elevated. The imaging focus should be set at the far wall (in relation to the skin) to achieve better lateral resolution. The lumen of the vein should be set to appear dark in the absence of stasis and thrombosis. Time-gain compensation (increasing amplification of ultrasound echoes with depth to compensate for their progressive attenuation) is set according to the echogenicity and location of the examined tissues to improve imaging. The weaker the signal seen with increasing depth, the higher the gain that will be required. When velocity waveforms are obtained, gain should be set so that the background is dark to avoid overestimation. The insonation angle is often set at 0 degrees. However, because most veins run parallel to the skin, this angle has to be set to parallel the vein flow channel. An angle of insonation of 45 to 60 degrees between the transducer and the vein should be used to achieve the optimum Doppler waveform. This is done only when the velocity of reflux is estimated.
Thrombosis
Prompt and accurate diagnosis of DVT has the potential to prevent major morbidity. Clinical diagnosis of DVT is misleading in roughly 50% of cases because of its low sensitivity and specificity.10–13 As other methods were developed, ascending contrast-enhanced venography became the “gold standard.” DUS has replaced contrast-enhanced venography as the method of choice because of its high accuracy, portability, availability, noninvasive nature, and ability to perform a differential diagnosis.14–18
Venous obstruction can be caused by extrinsic and intrinsic conditions. Tumors, hematomas, cysts, aneurysms,19,20 and musculoskeletal structures can cause extrinsic vein compression. Such pathologic processes are not usually associated with signs and symptoms of chronic venous disease unless concomitant reflux or obstruction is present. Diagnosis of these conditions is important because it can alter management.
However, the most common cause of obstruction is venous thrombosis. DVT is a significant cause of morbidity and mortality in hospitalized patients. In the acute phase, pulmonary embolism and pulmonary hypertension can develop. Venous thromboembolism is the leading cause of preventable in-hospital mortality in the United States. On the other hand, postthrombotic syndrome describes the sequelae of chronic venous disease. Typical signs and symptoms are lower extremity pain, aching, cramps, tiredness, or heaviness; restless limbs; burning sensation or itching; chronic limb swelling; spider, reticular, or varicose veins; skin changes; atrophie blanche; lipodermatosclerosis; and ulceration.
DUS evaluation of the veins can determine the presence of anatomic obstruction with a sensitivity and specificity of more than 90%.21 Functional evaluation of obstruction cannot be achieved with DUS because it assesses a single vein segment at a time. Unlike the case of vein reflux, no adequate tests exist for the accurate measurement of functional obstruction.
Examination
For performance of the examination, the patient is placed in a reverse Trendelenburg position with the knee bent and in external rotation. The examination begins below the inguinal ligament at the common femoral vein (CFV) and the saphenofemoral junction (SFJ). The transducer is placed in a transverse orientation to the vein and compression is applied. The transducer is then turned longitudinally to evaluate for flow and augmentation. The veins are examined in 3- to 5-cm intervals. In a similar manner, all the deep veins of the extremity, including the femoral, deep femoral, popliteal, peroneal, soleal, gastrocnemius, and posterior tibial veins, are examined. It is important to bear in mind the possibility of anatomic variation in venous anatomy. For instance, duplications of the femoral vein were seen in 26% and triplications in 1.2% of consecutive patients referred for abdominal or lower limb imaging. These figures were 37% and 2.6% for the popliteal vein, respectively.22 Unless local signs or symptoms are present, the anterior tibial veins are not routinely examined because of their low incidence of DVT.23 The saphenous vein trunks are then evaluated.
It is crucial to assess the iliac veins and the inferior vena cava (IVC) when disease is suspected at that level. In this case, however, flow is evaluated chiefly because compression can be difficult and uncomfortable. Asymmetry of flow velocity, waveform, and pattern at rest and during flow augmentation in the CFV indicates proximal obstruction. However, the absence of asymmetry cannot exclude obstruction. Accordingly, when iliocaval obstruction is suspected, the full extent of these veins must be imaged. The presence of stenosis, usually from extrinsic compression, is recognized by the mosaic color (which denotes post-stenotic turbulence), abnormal Doppler waveform at the stenotic area, slow flow, spontaneous contrast, and vein dilatation before the stenosis.24 The reduction in vein diameter can be measured by planimetry to compare the smallest lumen with the normal lumen and by the peak vein velocity ratio (post-stenotic/prestenotic). The four components that should be examined are visualization, compressibility, flow, and augmentation.
Signs and symptoms are present in both lower extremities in patients with bilateral vein obstruction or when the IVC is involved. The iliac veins and IVC may exhibit extrinsic compression from masses. Such compression can lead to signs and symptoms of chronic venous disease, and in affected patients, thrombosis is a common event. Veins are either totally or partially obstructed and completely or partially recanalized. Timely recanalization may help maintain valve competence. Otherwise, the valve leaflets are damaged or destroyed.
Deep Venous Thrombosis
It is important not only to diagnose DVT accurately but also to determine whether the thrombotic event is acute or chronic because of considerable differences in management. DUS allows such differentiation,10,25 but no reliable methods are currently available to calculate thrombus age precisely. Different methods have been tested, including triplex ultrasound,26 magnetic resonance imaging of the thrombus (signal changes over time),27 and DUS (quantification of thrombus echogenicity)—all with mixed results.28
Acute Thrombosis
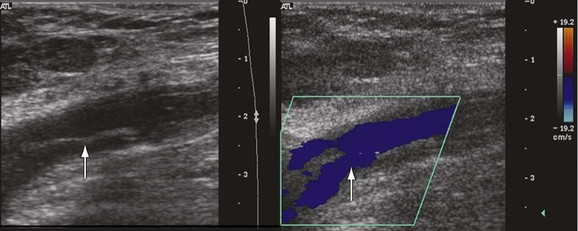
A fresh thrombus is mostly hypoechoic, homogeneous, partially compressible, seen in a dilated vein, and sometimes floating.10 Veins with acute thrombosis are echolucent and distended with smooth walls. Acute thrombus is spongy on compression, but it will still keep the walls of the vein from coapting. On color-flow examination, acute thrombus will have confluent flow channels (Fig. 18-1).
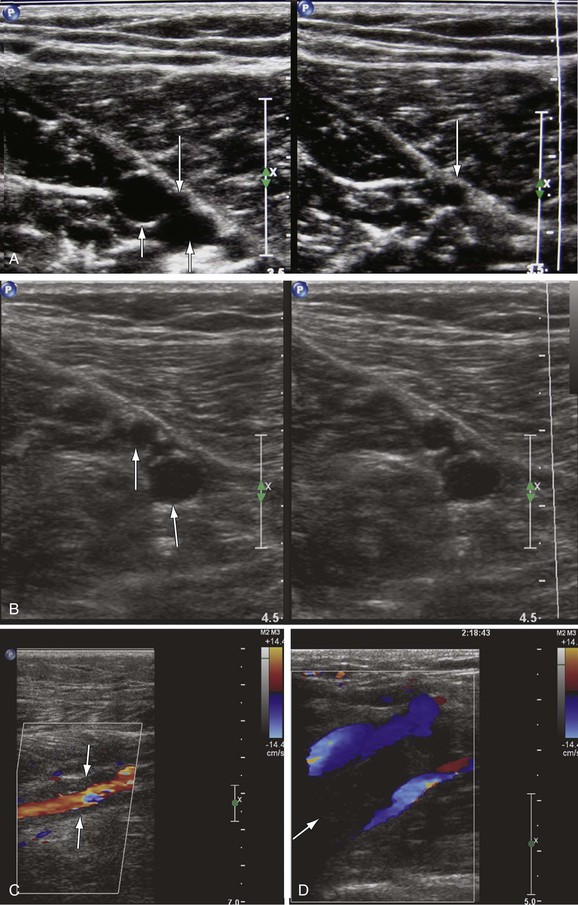
Figure 18-1 Diagnosis of acute deep venous thrombosis with duplex ultrasound. A, Cross-sectional view of the posterior tibial veins at the midcalf. Two veins (upward arrows) and the artery (downward arrow) in the center are seen below the soleus muscle (left panel). Both veins were collapsed normally with pressure applied by the transducer, so that only the artery is seen (right panel), indicating absence of thrombus within the veins. B, Cross-sectional view at the midcalf in a patient with acute calf pain. The posterior tibial veins (arrows) are distended and not compressible. C, Thrombosis of both posterior tibial veins in a high-risk asymptomatic patient. The artery is patent (red), and the accompanying veins (arrows) demonstrate absence of flow. D, Longitudinal view of the left common femoral vein from a patient with acute swelling and pain. There is a free-floating thrombus (arrows) surrounded by flowing blood at its proximal end.
Chronic Thrombosis
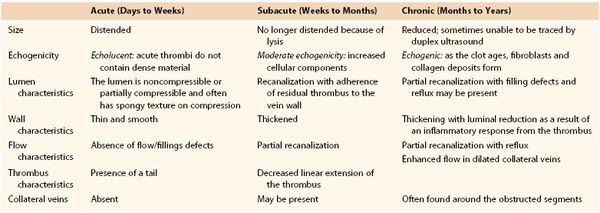
Chronic thrombosis is characterized by the presence of an organized, hyperechoic, heterogeneous, noncompressible thrombus firmly adherent to the vein wall on DUS.10 Chronic thrombus is firm. Chronic thrombi are echogenic and contracted with thick and irregular walls (Fig. 18-2). Chronic thrombus may have multiple channels or collateralization. Intraluminal webs and wall thickening, with or without reflux, indicate previous thrombosis in the absence of a visible thrombus. The presence of dilated collateral veins is a sign of obstruction, but their absence cannot exclude it. It is also possible for the veins to be fully recanalized without any evidence of anatomic obstruction. However, the thickening and increased stiffness of the vein wall can cause functional obstruction. Criteria for differentiation of acute from subacute and chronic DVT are depicted in Table 18-1.
Recurrent Thrombosis
Recurrent thrombosis is defined as a repeated thrombotic event. It may occur in the contralateral or ipsilateral limb. New thrombus may be formed in new vein segments that were not involved in the previous episode or in segments that previously had thrombosis (Fig. 18-3). After monitoring 738 patients for 3.7 to 8.8 years, Hansson and colleagues found a 5-year incidence of recurrent DVT of 21% after the first episode of DVT and 27% after a second episode.29 The cumulative incidence of pulmonary embolism was 2.6%. Proximal DVT, cancer, and a previous history of venous thromboembolism were identified as risk factors for recurrence. Patients with postoperative DVT and a longer duration of anticoagulation were at a lower risk for the development of DVT.29 Others have confirmed that patients with iliofemoral DVT have a twofold higher risk for development of recurrent DVT than do patients without iliofemoral involvement.30 Three DUS criteria can be used to diagnose recurrent DVT: extension of the thrombus more than 9 cm,31 noncompressibility of a vein segment that had previously been compressible or had previously recanalized, and increase in thrombus thickness by 4 mm.32 Patients with a single DVT episode must be monitored closely for recurrence. Patients who are at lower risk should undergo repeated DUS at 6 months before cessation of anticoagulation. Those at higher risk for recurrence may require a longer duration of anticoagulation, and a second DUS examination should be performed at a 1-year interval. Further studies are needed to determine the optimal duration of anticoagulation for patients who are at higher risk for recurrent DVT. DVT often recurs in new vein segments, and on such occasions the diagnosis is straightforward. When it occurs in previously thrombosed segments, the criteria of thrombus extension and thrombus thickness are used. In these instances, accurate recording of the baseline event and a subsequent follow-up scan are important for the diagnosis of recurrent events.
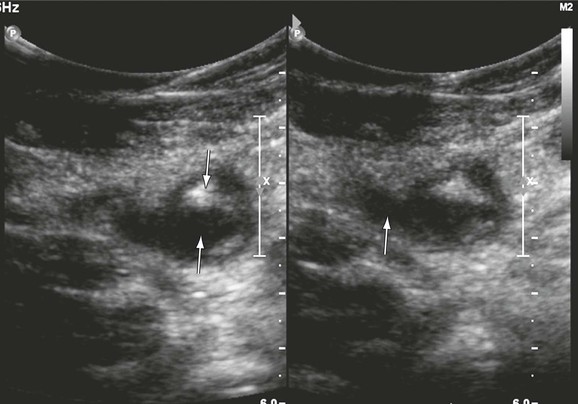
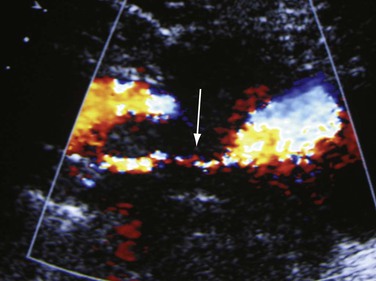
Figure 18-3 Recurrent thrombosis in the common femoral vein in a patient with limb swelling and chest pain. The patient previously had a femoropopliteal thrombosis documented in our hospital. The vein contains both echogenic (downward arrow) and echolucent (upward arrow) material (left panel). It is dilated (significantly larger than the common femoral artery, arrow, right panel) and noncompressible.
Central Vein Stenosis
Criteria have been developed to detect central vein stenosis by DUS in patients with signs and symptoms of venous outflow obstruction.24 Patients with limb swelling or pain (or both) and vein stenosis (41 stenoses in 37 patients) detected by DUS subsequently underwent contrast-enhanced venography with measurement of the pressure gradient. The gradient had to be 3 mm Hg or greater for patients to be included in this analysis. The best sonographic criterion for detecting significant vein stenosis was found to be a V2/V1 ratio of greater than 2.5 (with only two false-positive and false-negative test results reported) (Fig. 18-4). However, this was only a pilot study, and further studies need to be done to evaluate the best DUS parameters for hemodynamically or clinically significant vein stenosis. Planimetric evaluation by calculating directly the percentage diameter stenosis, vein dilation before stenosis, mosaic color from the turbulent flow at the exit of the stenosis, reduced flow augmentation, and presence of collateral veins are indicative of the diagnosis. The percentage stenosis may vary throughout the cardiac and respiratory cycles as the vein diameter can change. This is important in evaluating iliac vein compression by the iliac arteries. In patients with nutcracker syndrome (renal vein compression by superior mesenteric artery), we have used a vein velocity ratio of 4 to 5 as an indicator of significant stenosis, often combined with B-mode–measured diameter reduction.
Superficial Vein Thrombosis
Also known as superficial thrombophlebitis, superficial vein thrombosis is most often found in the extremity veins, but it can occur in many other locations. Although its diagnosis was based solely on clinical assessment, use of DUS has enabled accurate detection of its extent and progress (Fig. 18-5).
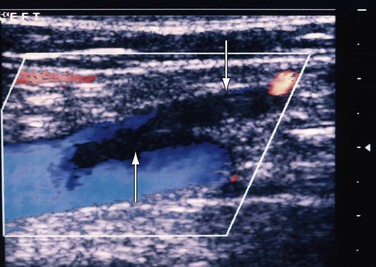
Figure 18-5 Extension of thrombosis from the thigh segment of the great saphenous vein (downward arrow) to the common femoral vein (upward arrow). The patient had pain in the lower part of the thigh, and within a week the thrombus extended proximally.
Thrombosis occurs most commonly in the saphenous veins and their tributaries, followed in frequency by the cephalic and basilic veins. Propagation of thrombus can occur in a contiguous or noncontiguous fashion. Contiguous extension of the thrombotic process from the superficial to the deep veins can occur in three ways, most commonly from the great saphenous vein (GSV) to the femoral vein. Less often, the thrombus extends from the small saphenous vein (SSV) to the popliteal vein. Extension through perforator veins to deep veins can also occur. In addition, it is possible that thrombosis can extend from deep veins to superficial ones, but this has not been documented. Most patients with superficial thrombophlebitis should have their deep veins evaluated even if the lowest prevalence of DVT ever reported (5.6%) is accepted.33 The diagnosis of superficial thrombophlebitis can be made on the basis of clinical or DUS data. Superficial thrombophlebitis is often easy to diagnose because of its superficial location, but the correlation between clinical examination and surgical findings is poor. Clinical examination does not reveal the true extent of superficial thrombophlebitis; surgical exploration often shows extension of the thrombotic process 5 to 10 cm higher than the level that was clinically diagnosed. DUS is recommended for confirmation of the diagnosis, for estimation of the extent of thrombosis, and for follow-up.33
Unusual Sites of Venous Thrombosis
The precise anatomic distribution of venous thrombosis in “unusual locations” has rarely been addressed. Among 15,850 DUS examinations performed in a 10-year period to rule out DVT, our group studied patients with DVT in all thigh veins except the femoral vein.34 The deep femoral, femoropopliteal, lateral thigh, sciatic, and muscular thigh veins were examined. We identified 14 cases (7 men, 0.54% of patients with DVT, and 0.088% of the entire population) involving thrombosis in unusual locations (Fig. 18-6). Ten cases involved the left lower extremity and 4 the right. The veins involved were the deep femoral (8); the femoropopliteal (2), which connects the popliteal with the deep femoral vein; and the deep external pudendal (1). Of the three patients with venous malformation, one had involvement of the muscular thigh veins, and in two patients the lateral thigh vein and the sciatic vein were thrombosed. Propagation of thrombi with extension to the CFV was seen in 4 of the 14 patients: 2 from the deep femoral vein, 1 from the femoropopliteal vein, and 1 from the deep external pudendal vein. Pulmonary embolism occurred in two patients, one of which was fatal. At final follow-up, recurrent DVT developed in two patients, and nine had signs and symptoms of chronic venous disease.34
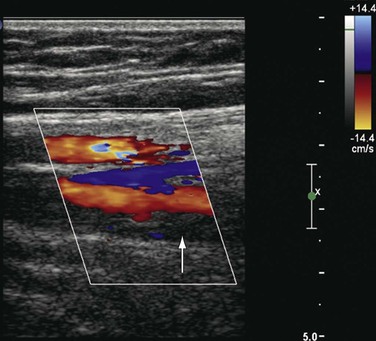
Figure 18-6 Acute thrombosis in the deep femoral vein in a high-risk asymptomatic patient. There is color filling (top to bottom) in the femoral artery and vein and in the deep femoral artery but not in the deep femoral vein (arrow).
The incidence of DVT of the thigh veins studied was extremely low. The signs and symptoms in our patients, both initially and at follow-up, spanned the whole spectrum of the natural history of DVT as reported in the literature for the more usual sites of thrombosis. Cases of thrombus propagation, pulmonary embolism, recurrent DVT, and postthrombotic syndrome were seen. The association of these veins with all the complications of thromboembolic disease suggests that routine imaging of these veins would be beneficial.34
Differential Diagnosis and Incidental Findings
DUS can be helpful not only when it yields a positive result for the diagnosis of a lower extremity ulcer, such as severe reflux of the posterior accessory vein of the calf, but also when the results show a completely normal venous system by extending the evaluation to rule out other causes. A multi-institutional study of 799 limbs with leg ulcers at a vein clinic reported 17 patients with 21 ulcers of nonvenous, nonarterial etiology.35 Fourteen of these patients had completely normal arterial and venous findings on DUS. The normal DUS results in these 14 patients prompted a search for alternative causes. Chronic inflammation, vasculitis, Kaposi’s sarcoma, chronic inflammation, basal cell carcinoma, pyoderma gangrenosum, hydroxyurea administration, and sickle cell disease were identified as the most prevalent causes for most of those ulcers.35 Venous and arterial DUS is recommended before any other test because of the overwhelming prevalence of venous and arterial disease in limbs with ulcers. In addition, many ulcers may have a mixed etiology, and venous and arterial testing can demonstrate the specific vascular disease that may contribute to ulcer formation.35
Several other conditions that mimic the symptoms and signs of venous disease can be diagnosed with DUS. Among them, Baker’s cysts, enlarged hematomas, lymph nodes, musculoskeletal injuries, aneurysms, and tumors may be identified. This is particularly important because some of these diagnoses may have a crucial impact on the therapy chosen (Fig. 18-7). For instance, when DUS is ordered to exclude DVT in a patient with the initial symptom of leg swelling and a hematoma or Baker’s cyst is found, anticoagulation becomes ill advised.36 Large ruptured Baker’s cysts can rarely compress the adjacent neurovascular bundle, leading to neurogenic symptoms.37 Patients with muscle rupture, hematoma, and ruptured Baker’s cyst are symptomatic and D-dimer levels are positive. Anticoagulation may be given inappropriately in these cases before objective testing, particularly if the patients present at hours when DUS may not be available.
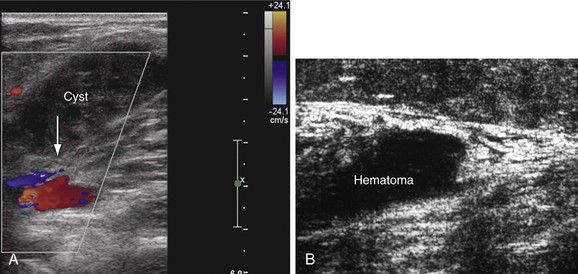
Figure 18-7 Incidental findings in patients who underwent ultrasound examination to rule out deep venous thrombosis. A, Hemorrhagic, ruptured popliteal cyst partially compressing the popliteal vein (arrow). An acute onset of pain and swelling developed the day before the examination. B, Rupture of the gastrocnemius muscle at its distal end near the origin of the Achilles tendon. The architecture of the muscle fibers is destroyed, and hematoma is seen over the area of the rupture.
Upper Extremity Veins
Upper extremity DVT has not been as extensively studied as lower extremity DVT. They have similar clinical findings: limb swelling or discoloration (or both) and pain and tenderness to palpation. However, these symptoms are nonspecific, and less than 50% of all symptomatic limbs will have imaging proof of DVT.
Compared with contrast-enhanced venography, the sensitivity and specificity of DUS have been reported to be 78% to 100% and 82% to 100%, respectively. However, except in highly specialized centers, contrast-enhanced venography remains the standard in more thorough evaluations of upper extremity DVT. Whereas false-positive results are rare, false-negative results may occur with small nonobstructive thrombi that cannot be directly assessed with compression techniques because of bones overlying centrally situated veins, including the medial subclavian vein segment, the brachiocephalic vein, and their confluence with the superior vena cava. Furthermore, differentiation between a normal vein and large collaterals in patients with chronic venous thrombosis may be difficult. Therefore, contrast-enhanced venography or magnetic resonance venography (MRV) is used in select cases in which DUS findings are equivocal or when clinical suspicion for upper extremity DVT is high despite negative DUS results.
Technique and Normal Anatomy
The patient should be placed in the supine position with the arm comfortably extended to approximately 60 degrees from the chest. Hyperextension should be avoided because it may inhibit normal flow and affect waveform shape and amplitude. Routine DUS examination includes assessment of the internal jugular vein, the brachiocephalic vein, the confluence of the cephalic and subclavian veins, the subclavian vein, the axillary vein, the brachial vein, the basilic vein, and the veins of the forearm. Evaluation of the brachiocephalic veins and superior vena cava requires a supraclavicular or suprasternal approach, and a sector or phased array transducer with a small footprint may be needed to facilitate imaging in areas surrounded by bone. The jugular vein is then scanned with the patient’s head rotated away from the side of examination. This testing is best performed with 5- to 7-MHz linear array transducers. Curved array transducers may be useful in the proximal axillary vein in larger patients to achieve better penetration and to obtain a larger field of view so that longer deep vein segments can be visualized. The superior vena cava and brachiocephalic veins are best imaged with small footprint transducers superior to the clavicle and through the sternal notch. The subclavian vein is imaged with the transducer placed above and below the clavicle. When it is viewed from beneath the clavicle, the vein lies superficial and caudal to the adjacent artery. The proximal axillary vein is seen below the clavicle under the pectoralis minor. The rest of the vein is imaged through the axillary area. Next, by placement of the transducer over the midportion of the inner aspect of the upper part of the arm in a transverse orientation, the brachial artery can be seen in the midsection of the upper part of the arm and used as a landmark. The paired brachial veins are closely applied to the artery.38 It is important to be aware of the multitude of anatomic variations of the arterial system in the upper extremity because these paired veins will change accordingly.39
The basilic vein lies more superficial. Although it is technically a superficial vein, the basilic vein is often larger than the brachial veins and may have a considerable thrombus burden. It unites the brachial vein at the midarm region or more proximally. Once the arm veins are interrogated, they can be followed centrally into the axillary vein. Waveforms should be obtained along the longitudinal vein axis at a Doppler angle of 60 degrees or less.
Knowledge of the normal spectral waveform in the upper extremity veins is essential for examination of these veins. The spectral Doppler signals are characterized by two phasic variations in amplitude. Cardiac pulsatility is manifested as a choppy and sometimes biphasic flow pattern that is more prominent in the central veins. Peak forward flow occurs during mid-diastole, whereas flow slows or reverses as the tricuspid valve closes. Respiratory variation may also be pronounced in the upper extremity veins, with increased flow during inspiration and decreased flow during expiration. With end-inspiration or end-expiration, little flow may be seen in healthy subjects. This should not be mistaken for blockage or an anechoic thrombus. Bilateral DUS is recommended for detecting waveform asymmetry, which may indicate more central obstruction that is difficult to directly visualize. As in the case of lower extremity examination, noncompressibility is the most sensitive and specific sign of venous thrombosis. In this area, compression is preferably done in the transverse plane because the transducer may slide off the vessel in the longitudinal axis and potentially result in false-negative results. Color Doppler imaging becomes useful when overlying bones prevent direct compression (Fig. 18-8).38
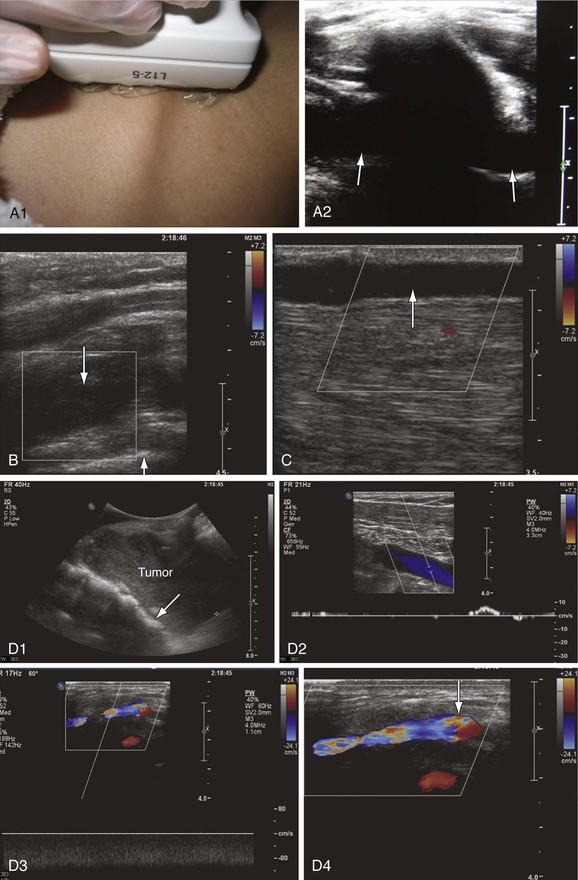
Figure 18-8 Upper extremity venous examination. A, Imaging of the subclavian vein through the clavicle (A1). This is an area where imaging has been difficult. However, use of the clavicle and pleura as landmarks makes imaging of this segment easier (A2). The subclavian vein is seen on both sides of the clavicle (arrows). B, Acute thrombosis of the proximal subclavian vein in a patient with acute swelling and pain in the left upper extremity. The vein is distended with absence of flow (down arrow). The white line crossing the right bottom corner of the color box is the pleura (up arrow). C, Spontaneous thrombosis of the cephalic vein in a patient with pain along the length of the vein for at least 2 days before the examination. The cephalic vein is dilated, with echolucent material in the lumen and absence of flow (arrow). D, Compression of the proximal left subclavian vein (arrow) by a tumor (D1). There is very low flow with poor augmentation in the axillary vein distal to the site of compression (D2). The venous flow is shifted from left to right through the jugular arch vein, which is dilated with high nonphasic flow (D3). The jugular arch vein diameter measures 5.6 mm in its largest segment (arrow) (D4). This vein is usually very small and is seen only in cases of proximal obstruction.
Diagnostic Criteria
Criteria for diagnosis of acute DVT in the veins of the upper extremity are identical to those described for their lower extremity counterparts, with some peculiarities. The difficulty of directly visualizing central vein thrombus makes examination of the upper extremities more reliant on indirect findings. Collaterals are very often visualized as prominent venous structures in the soft tissues surrounding the thrombosed main vein. Spectral analysis may also be useful in the evaluation of central thromboses in which the thrombus may be difficult to identify. Conversion of the normal biphasic pattern to a nonpulsatile signal similar to portal venous flow is strongly suggestive of central venous impairment, such as thrombosis, stenosis, or extrinsic compression by an adjacent mass (see Fig. 18-8). In cases in which a diagnosis of central thrombosis is considered on the basis of dampening of the spectral waveform, comparison should be made with the opposite side to confirm that the pattern is present only on the symptomatic side. Absent or reduced cardiac pulsatility was found to be a sensitive parameter. Respiratory phasicity is also a helpful finding. It is often asymmetrical in patients with unilateral venous occlusion. However, in cases of bilateral subclavian vein or superior vena cava occlusion, subtle dampening of pulsatility or phasicity may be difficult to appreciate. In situations with equivocal DUS results, especially when the clinical signs are highly suggestive of DVT, MRV or contrast-enhanced venography is advised for further evaluation.38
Not uncommonly, the only manifestation of chronic venous thrombosis is an inability to show a specific vessel in the expected anatomic location because the vein is simply collapsed and fibrosed. Collateral veins may become so prominent in patients with chronic disease that they may be mistaken for the main vein, which is essentially absent. In patients thought to have chronic thrombosis, any deviation from the expected normal anatomy or flow direction should be viewed with suspicion. Identification of the vein and its adjacent artery is also an important clue to differentiating main veins from enlarged collaterals. In addition, the loss of respiratory phasicity and in particular cardiac pulsatility by spectral analysis may be the only positive finding in patients with central venous obstruction who have otherwise normal findings. A caveat of using this criterion is that sometimes these collateral vessels are large and extensive enough to provide adequate drainage, and pulsatility and respiratory variations may be preserved.
Reflux
Although venous thrombosis and obstruction must be ruled out, most patients are examined with DUS for detection of vein reflux. This study also aids in determining the best therapy for each particular situation. DUS allows the examiner to quantify venous reflux in individual veins.39–49 Several studies have attested to its superiority over contrast-enhanced venography, and it is now regarded as the test of choice to diagnose vein reflux.
Venous reflux is synonymous with reversal of vein flow, which can be seen in physiologic and pathologic conditions. Physiologic flow reversal accounts for the fraction of the second that it takes for the valve leaflets to appose. A prospective study has demonstrated that the acceptable physiologic flow reversal is different for different veins.41 The theory supporting this concept is that larger veins have fewer valves. Therefore, the expected time for the valve leaflets to come together is longer than that for smaller, shorter veins. Consequently, in the CFV, femoral vein, and popliteal vein, the accepted physiologic time is less than 1000 milliseconds. For the superficial, deep femoral, deep calf axial, and muscular veins, the value is less than 500 milliseconds; and in the perforating veins, it is less than 350 milliseconds.
Numerous recommendations regarding the precise criteria for defining reflux have been published ![]() (Table e18-2). However, most studies have used more than 500 milliseconds as the ideal cutoff value for detecting reflux in the superficial and perforator veins. The veins have been tested in both supine and standing positions; the standing position is ideal because the supine position may have both false-positive and false-negative findings. The Valsalva maneuver is used only in the groin area because its effectiveness in inducing reflux diminishes below that level. Attempts have been made to use a critical peak velocity for reflux and also to calculate the reflux volume in a vein segment or even to add all the values from all the refluxing segments. Both have not been established as they lack reproducibility and clinical relevance. There are many factors that affect measurements, such as vein diameter, wall compliance, vein location, presence of edema or lipodermatosclerosis, degree of recanalization, and size and number of the refluxing veins connecting to the examined vein. DUS is very good in determining reflux in individual vein segments and creating a map for the extent and distribution of the disease, but it cannot provide an accurate account of disease severity in the entire extremity.
(Table e18-2). However, most studies have used more than 500 milliseconds as the ideal cutoff value for detecting reflux in the superficial and perforator veins. The veins have been tested in both supine and standing positions; the standing position is ideal because the supine position may have both false-positive and false-negative findings. The Valsalva maneuver is used only in the groin area because its effectiveness in inducing reflux diminishes below that level. Attempts have been made to use a critical peak velocity for reflux and also to calculate the reflux volume in a vein segment or even to add all the values from all the refluxing segments. Both have not been established as they lack reproducibility and clinical relevance. There are many factors that affect measurements, such as vein diameter, wall compliance, vein location, presence of edema or lipodermatosclerosis, degree of recanalization, and size and number of the refluxing veins connecting to the examined vein. DUS is very good in determining reflux in individual vein segments and creating a map for the extent and distribution of the disease, but it cannot provide an accurate account of disease severity in the entire extremity.
Table e18-2
Published Criteria for Diagnosis of Reflux

* Common femoral, superficial femoral, deep femoral, and great saphenous veins.
BMI, Body mass index; CFV, common femoral vein; CVI, chronic venous insufficiency; FV, femoral vein; GSV, great saphenous vein; RT-15, 15-degree reverse Trendelenburg position; SV, standardized Valsalva maneuver; tr, reflux time; VD%, relative change in vein diameter; VDmax, vein diameter during standardized Valsalva maneuver; VDmin, vein diameter during normal breathing.
In addition, DUS adds valuable information on the pathogenesis of chronic venous disease. On the basis of the pathophysiologic mechanism of the reflux, it can be classified as primary, secondary, or congenital.50,51 The most common type (64% to 79%) is primary, without a demonstrated cause. Secondary chronic venous disease is most frequently the result of DVT and accounts for 18% to 28% of cases. Congenital reflux exists since birth but is rarely recognized early because there is a lag in the initial signs and symptoms. It accounts for less than 5% of cases. The differentiation between primary and secondary venous reflux is important, and DUS is excellent for this. It allows a clear visualization of the wall and luminal changes and the pattern of reflux. Many patients have asymptomatic thrombosis that is discovered only during the DUS examination.
Examination
The examination to assess for reflux should start with the patient standing, facing toward the examiner, and the patient’s weight on the contralateral limb. The examined limb should be in slight flexion and external rotation. If a patient is unable to stand, the veins from the midthigh and below can be assessed in the sitting position. If the test is performed on a bed, the torso should be elevated above 45 degrees. A horizontal position is inappropriate for detection of reflux and measurement of vein diameters.
Compression with release distal to the point of examination on the limb is a reliable method of evaluating reflux and is referred to as augmentation. Venous reflux is elicited by imaging the vein under investigation while applying compression to the limb with one of the following methods: release after a calf squeeze for proximal veins or a foot squeeze for calf veins, manual compression of vein clusters, pneumatic calf cuff deflation, active foot dorsiflexion and relaxation, or the Valsalva maneuver (which may be the preferred technique to demonstrate SFJ incompetence). The compression is abruptly removed, and the presence and duration of reflux are observed. With compression, vein flow is initially increased as the blood is pushed in the normal direction of flow in a distal to proximal direction. Once the pressure is released, blood flow reverses momentarily. If valves are competent, there is minimal or no backflow. However, with incompetent valves, blood continues to flow in the reverse direction (Fig. 18-9). For precision and standardization of the measurements to be obtained, automated pneumatic cuffs with rapid inflation and deflation are used.52 However, some find it technically more difficult. The cuffs are placed around the leg at different levels. One is placed 5 cm below the probe site. A 24-cm cuff is used around the thigh, a 12-cm cuff around the calf, and a 7-cm cuff around the foot. The inflation lasts for 3 seconds, followed by rapid deflation in 0.3 second. To ensure complete venous emptying and to overcome the hydrostatic pressure from above, 80 mm Hg is used in the thigh, 100 mm Hg in the calf, and 120 mm Hg in the foot.
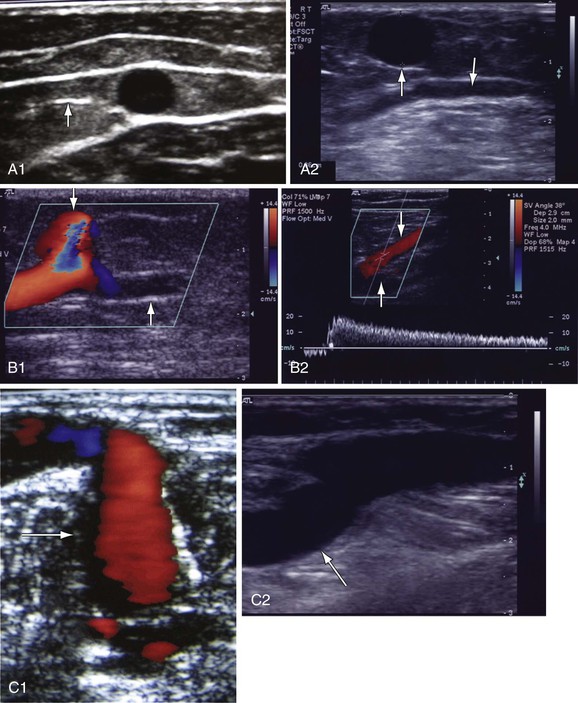
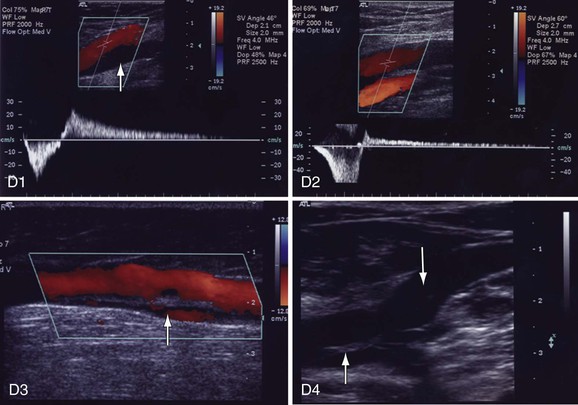
Figure 18-9 Identification of reflux in patients with chronic venous disease. A, Cross-sectional view of the great saphenous vein (GSV) in the thigh (A1). The saphenous vein is found in the saphenous canal, which is bounded by the superficial and deep fascia. The vein is anchored to the canal by the saphenous ligament (white line on the left side of the vein [arrow]). GSV aplasia is present at the knee because no vein is seen in the saphenous canal (down arrow, A2). The vein has been replaced by an accessory saphenous vein that is dilated (8.6 mm, up arrow) and incompetent. B, GSV reflux at the midthigh draining into a tributary (B1, down arrow). The GSV below the tributary has no reflux (up arrow). Prolonged reflux is noted in the small saphenous vein (SSV) (down arrow) at the saphenopopliteal junction (B2). The popliteal vein is normal (up arrow). C, Reflux in a dilated (6.8 mm) midcalf medial perforator vein (arrow) that is connected to incompetent GSV tributaries (C1). The deep veins are normal. The medial gastrocnemius vein that connects with a large midcalf posteromedial perforator vein (8.2 mm, arrow) is dilated. Both veins were incompetent (C2). D, Reflux in the popliteal vein because of prolonged reflux in the SSV (D1). The popliteal vein below the SSV has no reflux (arrow). Reflux is occurring in a fully recanalized popliteal vein that had a documented episode of thrombosis 3 years ago (D2). Unlike the segmental popliteal vein reflux induced by the SSV in D1, the whole popliteal vein was involved in this one. Reflux is seen in a partially recanalized GSV at the lower part of the thigh (D3). Two refluxing channels are seen separated by echogenic material (chronic luminal changes, arrow). Thrombus in the common femoral vein (CFV) extended into the GSV (D4). The patient had CFV thrombosis that on subsequent Duplex ultrasonography was shown to extend to the GSV. Two years later, the patient had significant postthrombotic syndrome with reflux and obstruction in the CFV, femoral vein, popliteal vein, and GSV. Echogenic material is seen in the saphenofemoral junction (down arrow) and CFV (up arrow).
On occasion, in patients with significant edema, the aforementioned techniques are inadequate, and dorsiflexion/plantar flexion is also used. A second technique is use of the Valsalva maneuver to increase intra-abdominal pressure, which can lead to reversal of vein flow if valve incompetence is present. This method is mainly useful for evaluation of valves in the groin because competent valves proximally will limit its usefulness in the distal part of the lower extremity. This method is used in the groin only when the distal augmentation does not elicit reflux.
Examination starts with the deep veins. The probe is placed at the CFV to the union of the femoral and deep femoral veins. The SFJ at the terminal and preterminal valves and the associated tributaries are examined next, followed by the popliteal vein and deep calf veins.
Next, the superficial vein system is examined. The GSV, the SSV, their tributaries, and the nonsaphenous veins are the most common sites for identification of reflux. The GSV can be identified and differentiated from other veins because it is surrounded by two layers of fascia in the saphenous eye.53,54 The SSV is found in the triangular fascia, surrounded by the crural fascia and the gastrocnemius muscle heads.55 Superficial vein tributaries that are often incompetent are, in the thigh, the anterior and medial accessory veins, and, in the calf, the anterior and posterior accessory veins, and they should be routinely included in the examination.
Anatomic variations of the lower extremity veins are common. The most frequent anatomic variations in the saphenous veins are segmental hypoplasia and aplasia.56 The segments with aplasia and hypoplasia are replaced through the accessory veins.57 The saphenous veins are duplicated when both veins are found inside the saphenous canal. A second vein that is parallel to the saphenous but outside the saphenous canal is termed an accessory vein. Duplication of the saphenous veins is rare and found in less than 3% of patients with chronic venous disease.58
The perforator veins are the last to be examined. They extend perpendicular to the superficial and deep veins traversing the deep fascia. The deep fascia is dense and echogenic because of its collagen content and is thus easy to visualize on DUS. There are approximately 150 perforator veins in the lower extremity, only 20 of which are of importance in terms of clinically significant reflux.59 The normal direction of flow is from superficial to the deep veins through the perforator veins. These veins are examined by transverse and oblique scanning because their long axis is seen in these planes. They are found by following the course of the GSV, the SSV, and their tributaries. Outward flow in these veins is seen only in conjunction with superficial and deep vein reflux.
Patients with C1 and C2 disease have reflux confined to the superficial system. As clinical severity worsens (C3 to C6), the prevalence of incompetence in the perforator and deep veins increases. In limbs with chronic venous disease, reflux alone exists in 80% of patients, reflux and obstruction are present in 17% of patients, and obstruction alone is found in only 2% of patients. In addition, the combination of the two has a worse prognosis for the development of skin lesions.60
Superficial Veins
Great Saphenous and Accessory Saphenous Veins
The scan should begin in the groin of the first limb to be examined. A transverse view is used to identify the GSV and CFV, both of which lie medial to the common femoral artery, by looking for the “Mickey Mouse” sign. If the junction is not present after surgery to remove the GSV, “Mickey’s” medial ear is missing. Several veins can be visualized in the region of the SFJ, and two GSV valves (terminal and preterminal) can be imaged near the SFJ. It is important to assess these tributaries and GSV valves because several hemodynamic patterns can be seen. Possible sources of reflux or proximal points of insufficiency are sought, such as SFJ incompetence, veins from the lower abdomen or pelvis, thigh or calf perforator veins, or the vein of Giacomini. The destination of the reflux is determined on the transverse view: (1) reflux into the GSV within the saphenous compartment; (2) reflux into the accessory anterior saphenous vein, which is slightly external to the GSV and aligned with the femoral vessels below; or (3) reflux into major thigh tributaries superficial to the saphenous fascia. A connection between the GSV and pelvic sources of venous reflux is suspected if there is a sudden increase in GSV diameter, whereas the diameter may decrease distal to a major incompetent tributary. It is also recommended to scan within the inguinal lymph node area distal to the SFJ, where normal and varicose veins may lie. The full length of the GSV or of tributaries to the ankle needs to be followed. This vein lies within a fascial compartment, which can easily be identified on the B-mode ultrasound image. This appearance is widely known as the saphenous eye. Scanning for compressibility and reflux should be performed every few centimeters. If reflux is diagnosed, diameters at the junction and along the GSV need to be measured. Many authors measure GSV diameter 3 cm below the SFJ. Further useful sites of measurement are at the midthigh and at the knee. Measurement should be made of the saphenous trunk vein and not of any varix or dilated segment with an incompetent valve. Measurement of the diameter can be used to help decide among different types of treatment, for example, among DUS-guided sclerotherapy, radiofrequency ablation, endovenous laser therapy, and surgery. The depth of the saphenous trunk beneath the skin may also be important in patients in whom radiofrequency closure or endovenous laser therapy is being considered. These measurements can be used as a baseline for follow-up after endovenous procedures.
Small Saphenous Vein and Its Thigh Extension
The patient should be standing and facing away with the knee slightly bent, heel on the ground, and weight on the opposite limb. The study starts at the back of the knee. A transverse view is used to identify the major veins of the popliteal fossa. It needs to be determined whether the saphenopopliteal junction (SPJ) is present. If so, the junction is shown in a longitudinal view. The popliteal vein is tested proximal and distal to the SPJ, the gastrocnemius vein insertion, and the SPJ for reflux or thrombosis. It is important to rule out SPJ incompetence with SSV reflux. SSV reflux may occur during calf muscle contraction or manual calf compression (systolic phase) in some patients and suggests possible popliteal or femoral vein obstruction (or both), but reflux is typically most obvious during calf release (diastolic phase). If reflux is present, the diameter of the SSV 3 cm distal to the SPJ (or at the popliteal crease) and at the midcalf is documented while any varix in the vein is avoided. The level of the SPJ in relation to the popliteal skin crease also needs to be recorded. The SSV may join the popliteal vein medially, posteriorly, or laterally, so it is advisable to record its position in relation to the popliteal vein circumference. The presence or absence of an artery accompanying the SSV or the gastrocnemius vein is of importance, for instance, when DUS-guided sclerotherapy is to be undertaken. Alternative sources of reflux need to be assessed, including communication of the SSV with a popliteal fossa perforator vein, GSV tributaries, pelvic veins traced to the buttock or perineum, or SSV thigh extensions. Alternative destinations for GSV reflux are also investigated, including tributaries and SSV thigh extensions. The SSV thigh extension and its connections with deep thigh veins or pelvic veins are scanned. The vein of Giacomini is deep to the fascia in most of its course. Its distal SSV connection and proximal connection into the GSV need to be seen. The direction of reflux from the saphenofemoral incompetence to the SSV or from the saphenopopliteal incompetence to the GSV is also recorded.
Nonsaphenous Veins
Nonsaphenous superficial veins are those that are not part of the GSV or SSV system. On occasion, tributaries from these veins do connect with the saphenous veins. Of a total of 835 limbs in patients with chronic venous disease, 84 lower extremities (72 patients) were identified as having nonsaphenous venous reflux, for a prevalence of about 10%. Most of these patients had clinically evident varicose veins. Such reflux was significantly more prevalent in women (67 versus 5, P < .0001), probably because of a gender-specific etiologic mechanism. Signs from at least one of the clinical chronic venous disease classes were present in all limbs. Four of five of these patients were symptomatic. Veins involved included the gluteal, posterolateral thigh perforator, vulvar, lower posterior thigh, popliteal fossa (a direct tributary of the popliteal vein), knee perforator, and sciatic nerve veins. Assessment of these veins requires slight modification of the standard recommended technique. These veins are imaged best in the standing position, and patients frequently have to rotate so that these veins can be traced in all aspects of the limb. Low-frequency probes (3 MHz) are often needed to trace the deep connections of nonsaphenous veins to the deep vein system. Knowledge of these veins and their importance in chronic venous disease is key because distinguishing the true anatomic site of reflux is necessary to facilitate appropriate and effective treatment.61
The presence of gluteal and vulvar varices deserves special comment. Their presence should raise suspicion for pelvic congestion syndrome and ovarian vein reflux. DUS is not ideal for imaging of the ovarian and internal iliac vein tributaries. In these instances, alternative methods of imaging to be considered include MRV, endovaginal ultrasound, and varicography through the vulvar veins. If ovarian vein reflux is suspected, MRV or descending contrast-enhanced venography through the IVC or left renal vein could be considered.61
Perforator Veins
Thigh Perforators
It is recommended that the examiner look for perforator veins on the medial aspect of the thigh during examination of the GSV and deep veins. Not all thigh perforator veins, competent or incompetent, will be detected. They are usually found in the middle and lower thirds of the thigh but can also occur in the proximal part of the thigh near the SFJ. It is necessary to look for lateral and posterior thigh perforator veins if clinical assessment shows varices in these regions. Spectral or color Doppler (or both) examination is used to test for inward and outward flow in the perforator veins during calf or thigh compression. If an incompetent thigh perforator vein is found, it may be useful to record its diameter measured at the muscle fascia and its location in relation to the knee joint to help decide the best management for this vein.
Calf Perforators
Perforator veins pass through the deep fascia, which is a distinct band on the B-mode image. Perforator veins around the circumference of the calf are examined. Not all calf perforator veins, competent or incompetent, will be detected. If outward flow is detected, their diameters at the deep fascia are measured, and their location is described according to the topographic anatomy (i.e., lower calf medial perforator vein). The larger the perforator vein diameter, the greater the chance of reflux. It has been shown in at least two studies that perforator vein diameters larger than 3 mm have a higher association with reflux (>90%).62,63 However, the sensitivity of these criteria is not ideal because as many as a third of all incompetent perforators have a measured diameter of less than 3 mm. Therefore, size alone cannot distinguish competent from incompetent perforator veins. Bidirectional flow can be determined by color Doppler or spectral analysis after a distal muscle squeeze or isometric calf muscle contraction. However, no consensus has been reached on the pathologic significance of bidirectional flow. Bidirectional flow in a perforator vein indicates incompetence of the vein, but some authors argue that true pathologic incompetence is present only if reflux is elicited during the diastolic phase of calf muscle relaxation or release of compression. Accordingly, some authors suggest testing for inward and outward flow separately during calf muscle contraction or compression and calf muscle relaxation or release to help distinguish pathologic from reentry perforator veins. Assessing the approximate duration of inward and outward flow may provide an estimate of net flow.
Deep Veins
Deep Thigh Veins
The CFV should be tested in the longitudinal view for phasic flow with normal respiration, cessation of flow with deep inspiration, possible reflux with the Valsalva maneuver, and flow during manual compression of the thigh or calf. This may be demonstrated better with the patient in the supine position. If continuous flow is detected in the CFV, which can indicate a proximal obstruction, it is recommended that scanning be extended to the iliac veins and IVC. The CFV should be examined above and below the SFJ because retrograde flow in the CFV is seen at the SFJ level or higher in the presence of SFJ reflux, whereas retrograde flow distal to this level represents true deep venous reflux. It is then necessary to follow the full length of the femoral vein to the popliteal vein. If necessary, the femoral vein may be seen better by moving the probe to an anterior window through the vastus medialis at the adductor hiatus.
Popliteal Vein
The popliteal fossa is a complex site to investigate, both from an anatomic point of view and for assessment of venous hemodynamics. Multiple longitudinal and transverse views are required. The popliteal vein is properly scanned with the patient lying in the prone position when phasicity of flow with respiration has to be elicited, although some patients may not have this phasicity in the absence of any abnormality; it is usual to assess flow augmentation with calf squeezing because the Valsalva maneuver is of limited utility at this level. The popliteal vein should be examined above and below the SPJ when it is present because retrograde popliteal vein flow is present above the SPJ when the SPJ terminal valve is incompetent, and only retrograde flow distal to this level represents true deep venous reflux. The anatomic and hemodynamic relationship of the SPJ and the popliteal and gastrocnemius veins should be established.
Deep Calf Veins
The patient should be standing (preferable for examination of superficial veins) or sitting with the foot hanging down and resting on the examiner’s knee or on a step. With experience, the examiner will be able to identify all deep crural veins. Reflux in the posterior tibial veins best reflects the clinical features. The posterior tibial veins need to be examined on a medial or posteromedial view and the peroneal veins on a posteromedial or posterior view. These veins should be examined in patients with past or present DVT and in those with incompetent perforating veins in the calf. The peroneal veins are the most frequently affected calf veins after previous venous thrombosis. Examination of the soleal and gastrocnemius veins deep in their muscle groups completes the basic investigation of deep veins in the leg.
Endovenous Procedures
Treatment of superficial and perforator veins by ablation is now performed under DUS guidance. It is important to document vein diameter, proximity to the skin, tortuosity, obstruction, and areas with anatomic variations to formulate a good treatment plan.64 During the procedure, DUS is used to obtain percutaneous vein access and for guidance of wires and catheters. Accurate positioning for the treatment area of interest is achieved easily because the tip of the catheter is placed in the correct location safely. Before the ablation takes place, tumescence fluid is injected around the vein for the thermal methods (pharmacomechanical and chemical methods do not require tumescence). The goal is to create a halo sign over the entire length of the treated segment with the vein being collapsed around the catheter. Enough fluid is injected around the vein to protect the surrounding structures and skin from thermal damage. During catheter pullback, the immediate effect on the vein can be observed. The vein is reexamined at the end of the procedure to ensure complete ablation and that the saphenous junctions and deep veins are free of thrombus.65 The standard for follow-up of endovenous therapies is DUS, which is important for monitoring success, identifying complications, and treating recurrent or new sites when it is deemed appropriate by the patient and the specialist.
Foam sclerotherapy has become an effective method for the treatment of vein varicosities and is as successful as traditional surgical ligation and stripping. However, treatment may need to be repeated to achieve secondary success.66 It is used as stand-alone therapy or frequently as an adjuvant to laser or radiofrequency vein ablation.67,68 Under DUS guidance, it can be used to treat varicosities of all sizes, saphenous main trunks, perforator veins, and venous stasis ulcers.
DUS has been applied in several other scenarios. Venous outflow obstruction has been related to venous hypertension, a key factor in the pathophysiologic process of chronic venous disease. Endovascular vein angioplasty plus stenting has replaced surgery as the therapy of choice for iliocaval outflow obstruction. DUS is used preoperatively to support the diagnosis of outflow obstruction, intraoperatively to guide access to the femoral vein, and postoperatively to evaluate stent patency. Intravascular ultrasound (IVUS) plays a greater perioperative role in this setting than DUS does.69 Although contrast-enhanced venography is considered the gold standard for imaging before insertion of an IVC filter, bedside transabdominal DUS has also been found to be a safe and effective alternative. When IVC visualization was adequate, contrast-enhanced venography and DUS had high success and low complication rates. Technical success was significantly higher with the use of contrast-enhanced venography. However, this must be weighed against the advantage of bedside application offered by DUS, which may be especially important in an immobilized or critically ill patient.70 All these interventions are monitored by subsequent DUS at time intervals appropriate to each particular procedure and when symptoms require.
Patients with Recurrent Varicose Veins After Surgery
Residual and recurrent varicose veins are a common problem after interventions to correct reflux in patients with chronic venous disease.71,72 Uniform identification of the causes and patterns of recurrence has not been reported, mainly because of the inconsistency in defining recurrence, initial therapy, and method and length of follow-up. In 1998, a consensus group developed a classification for patients with recurrent varices after surgery (REVAS)71 to be used as a complement to the CEAP classification.73 The CEAP classification can be used in two ways. In the basic CEAP classification, only the single highest class of C is used, and only the first descriptor is used for E (etiology), A (anatomy), and P (pathophysiology). In the advanced CEAP classification, all the signs described in the clinical classes are provided, and for A or P (or both), the 18 named venous segments are used to locate venous disease. Use of the advanced CEAP classification is more appropriate for REVAS patients because it allows a more defined description and better comparison among different patient groups.
REVAS is a clinical definition that includes true recurrences, residual refluxing veins, and varicose veins caused by progression of the disease. It includes six items: T is for topographic sites of REVAS, S is for sources of reflux, R is for the degree of reflux, N is for nature of the sources, P is for contribution from a persistent incompetent saphenous trunk, and F is for possible contributory factors.71 Until the CEAP and REVAS classifications, it was difficult to report these occurrences. The frequency of REVAS has been reported to be between 20% and 80%, depending on the definition of the condition and the duration of follow-up.71
By use of the REVAS scheme, new insights into the pathophysiologic mechanism of REVAS have been described.74 About 75% of REVAS patients are symptomatic. The sources of reflux feeding the recurrence are of multiple origins and occur at the SFJ in almost 50% of patients. Persistent or recurrent SFJ reflux has been attributed to neovascularization, to failure in ligating the SFJ, or to overlooked junctional tributaries (Fig. 18-10). No apparent source of reflux is seen in 10% of patients, and it is of pelvic or abdominal origin in 17%. About 75% of limbs have incompetent perforator veins. The number of REVAS patients with skin changes is higher than the number of patients with varices that had not been operated on, probably because of the significantly older mean age of REVAS patients than those before surgical intervention. These age differences are expected because patients who seek treatment after their first surgical intervention are likely to be older as a result of significant time elapsing before disease recurrence. The below-knee saphenous trunks had a higher prevalence of reflux than the above-knee trunks because the GSV is most often stripped to the knee level and the SSV is often ligated without stripping.
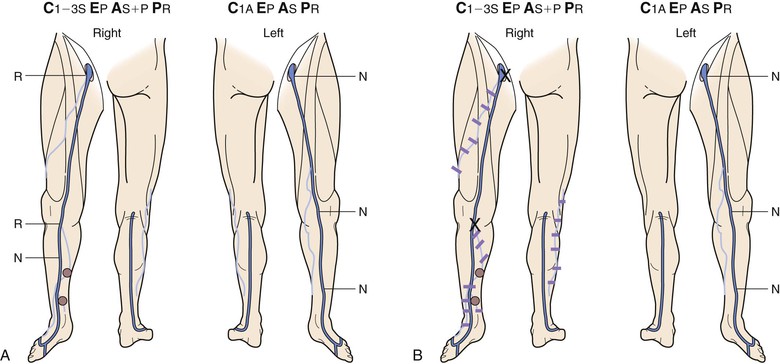
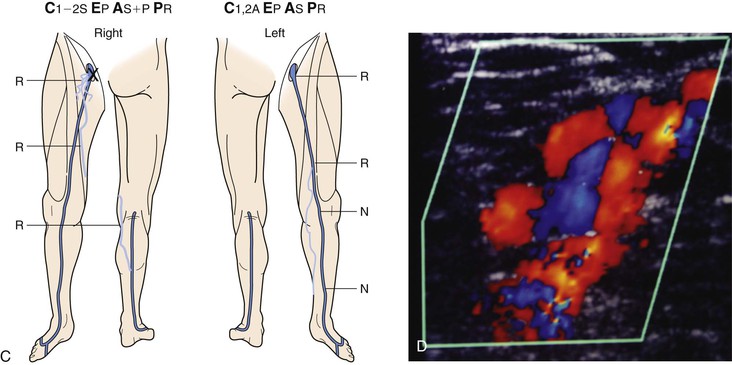
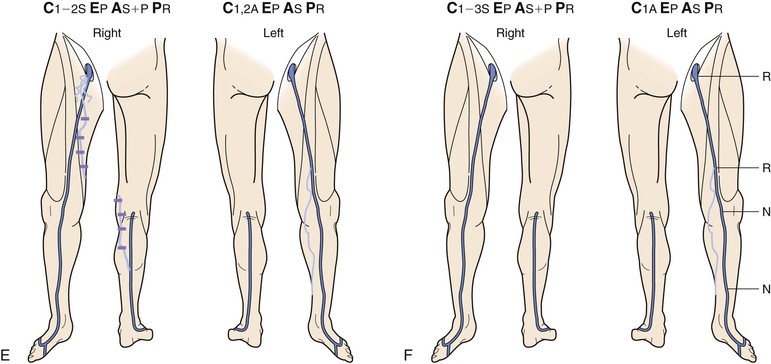
Figure 18-10 Serial duplex ultrasound (DUS) examination and treatment of a 57-year-old woman with pain, swelling, and itching in the right lower extremity. She had two pregnancies and a positive family history for reflux from both parents. C, Clinical manifestations; E, etiologic factors; A, anatomic distribution of disease; P, pathophysiologic findings. A, Baseline examination before treatment showing mapping of the vein for reflux. B, Two months later, she underwent stripping of the great saphenous vein (GSV) from the saphenofemoral junction (SFJ) to the upper portion of the calf and phlebectomies in multiple tributaries. C, She came back years later with symptoms in the same limb. D, DUS revealed neovascularization at the SFJ. These vessels were connected to a posteromedial thigh tributary that extended to the calf. She underwent DUS-guided injection sclerotherapy for the area in the groin and phlebectomies for the tributary in the thigh and calf. E, Thirteen months later, she was free of vein reflux in this limb. Interestingly, the left lower extremity had reflux in an accessory vein of the GSV (shown in blue). F, In her subsequent examinations, ascending reflux was noted in the GSV up to the SFJ. However, she was asymptomatic even at her last examination and did not want treatment. N, Normal; R, reflux.
Ultrasound-Directed Inferior Vena Cava Filter Placement
The gold standard and most widespread method for IVC filter placement is through the use of fluoroscopy-guided, contrast-enhanced venography. The disadvantages of this procedure include the need for radiation, the administration of iodinated contrast material, and the necessity of transporting the patient to the interventional suite. This proves cumbersome in many patients in the intensive care setting who require filter placement. Advances in DUS technology have improved the diagnostic evaluation of the IVC. As a logical consequence, bedside placement of IVC filters by transabdominal DUS was proposed and later proved to be feasible in most patients. Direct comparison of both imaging modalities has shown IVC filter placement technical success rates with transabdominal DUS of more than 97%, compared with almost 100% with contrast venography. The overall complication rates were 0.6% for venography and 1.8% for transabdominal DUS.75 The IVC and right renal vein are imaged first. The IVC is measured to ensure that the maximal size limitation of 2.8 cm (for most available filters) is not exceeded. With use of the Seldinger technique, a wire is advanced by either a transjugular or transfemoral approach, under DUS visualization, until it reaches the desired location. Next also under DUS guidance, the introducing system is directed into position, just caudal to the confluence of the IVC and the right renal vein. The filter is deployed and the position verified with DUS.
Patients with inadequate transabdominal DUS visualization can be considered for IVUS imaging. Proper placement has been reported in more than 90% of IVUS-directed procedures. Filter malposition and tilting of more than 15 degrees have been reported in up to 10% of bedside IVUS-guided procedures, which has been shown to be lower than the prevalence reported for filter placement under fluoroscopic guidance. IVUS accurately measures the IVC diameter and precisely localizes the renal veins.76 A single- or double-puncture access technique can be used. Double-puncture technique has the advantage of continuous real-time ultrasound imaging. However, concern of CFV thrombosis is high if the ipsilateral CFV is used for double-puncture access. We favor the single-puncture method. A 0.035-inch guide wire is introduced into the IVC and an introducer sheath is placed over the wire. An IVUS probe is passed to the level of the right atrium. Venous anatomy is interrogated with a pullback technique to visualize the hepatic veins, renal artery, and renal veins. The transverse IVC diameter is then measured. Markers are placed in the IVUS catheter at the site of entry into the sheath in two places, one when the more inferior renal vein is identified and one at the site of the IVC bifurcation. Next, the length from the sheath hub to the wished deployment site between the more distal renal vein and IVC bifurcation is determined. The IVC filter sheath is introduced at the ideal distance and then deployed. Abdominal x-ray films were routinely obtained to evaluate the IVC filter location, but now this is left to the operator’s discretion.
Ultrasound Mapping of the Extremity Veins
The GSV is the most commonly used conduit for lower extremity bypass surgery. The morbidity associated with vein harvesting has been reported to be as high as 40%, including wound infection, leg pain, reduced mobility of the patient, prolonged in-hospital stay, and increase in readmission rates and increased cost.77,78 The routine use of DUS vein mapping decreases leg wound complications by reducing unnecessary leg incisions due to poor-quality veins or difficulty in delineating the venous anatomy, particularly in obese patients, with ensuing reduced lengths of incision, harvest time, morbidity, and hospital stay.77,78
The adequacy of the ipsilateral GSV is assessed first. The vein is examined from the SFJ to the ankle with the patient in a reverse Trendelenburg position. Venous measurement diameters are taken. Next, the degree of compressibility, the thickness of the vein wall, and the presence of intraluminal echoes suggestive of previous thrombosis are assessed. Often the GSV has segmental hypoplasia and aplasia. In this situation, it is usually replaced by accessory veins. DUS easily identifies such anatomic variations, aiding in selection of the best conduit. If the ipsilateral GSV is inadequate, an identical process can be undertaken for the contralateral GSV. The course of the vein in the lower extremity is then marked with indelible ink if the vein is believed to be adequate for use as a bypass. Veins are thought to be adequate when the diameter is more than 2.5 mm and they are easily and completely compressible, nonsclerotic, and free of intraluminal echoes (Fig. 18-11). If the vein is of good quality but of inadequate size, the study is repeated after the patient has been standing for 5 minutes. In cases in which the GSV is inadequate, both cephalic veins are studied from the wrist to the shoulder. Cephalic veins are similarly judged adequate when the previously stated criteria are fulfilled.77,78 The SSV is imaged from the lateral malleolus to its thigh extension when the other veins are not suitable. DUS is very helpful to map the saphenous veins in patients with varicose veins. It has been shown that the saphenous veins themselves are rarely varicose and can often be used after DUS imaging identifies them and excludes significant varicosities.79
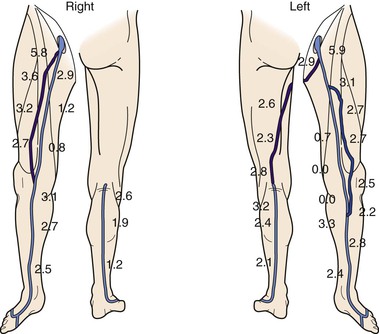
Figure 18-11 Bilateral lower extremity vein mapping in a 64-year-old man who presented with left lower extremity rest pain. The great saphenous vein (GSV) and small saphenous vein (SSV) were mapped throughout their length. There is variable anatomy in both extremities with GSV hypoplasia and aplasia and SSV thigh extension in one extremity only. The hypoplastic and aplastic GSV segments have been “replaced” by an accessory vein in both limbs. (Numbers indicate vein diameter.)
Preoperative Mapping for Arteriovenous Fistulae
As in the case of lower extremity bypasses, preoperative DUS mapping results in a marked increase in placement of arteriovenous fistulae80–82 and a reduction in the use of tunneled hemodialysis catheters83 in the management of patients with chronic kidney disease in whom creation of an arteriovenous fistula is considered. This is true because of its noninvasive nature, ease of performance, safety, and success. Both the National Kidney Foundation Dialysis Outcomes Quality Initiative for vascular access and the National Vascular Access Improvement Initiative (Fistula First) now mandate vascular mapping in all patients approaching chronic dialysis. For optimal results, both arterial and venous evaluation is required. In general, three means are available to perform such mapping: physical examination, DUS, and venography. An adequate vein assessed by clinical inspection alone was present in only 47% of consecutive chronic kidney disease patients (poor clinically visible or clinically absent veins were found in 54%). Patients with poor clinically visible or clinically absent veins then underwent DUS. About 75% of these patients showed adequate veins on DUS for successful creation of an arteriovenous fistula. We believe that the evaluation should be performed by the individual who will be constructing the arteriovenous fistula and that it should become a routine tool of all vascular access surgeons. Upper extremity DUS should also be routinely used to determine which arteries have optimal arterial inflow for successful creation of an arteriovenous fistula. Arterial narrowing and calcification are relatively common in patients with chronic kidney disease, especially those who are diabetic and hypertensive. The presence of a stenotic or calcified artery may jeopardize the surgeon’s attempt to create an arteriovenous fistula. It is for these reasons that arterial evaluation should be performed (Fig. 18-12).
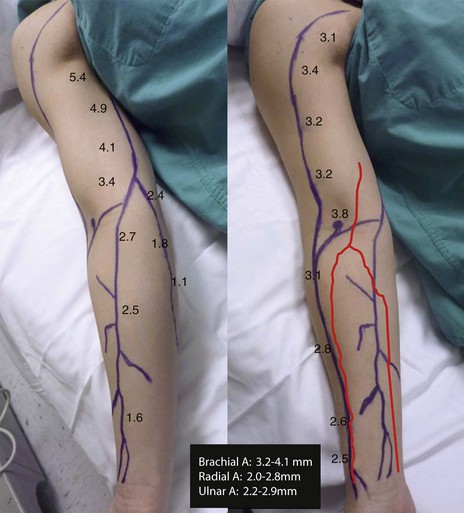
Figure 18-12 Mapping of the basilic and cephalic veins and brachial, radial, and ulnar arteries. The diameter of the veins and arteries in millimeters is depicted throughout the length of the vessels. A, The upper extremity is slightly rotated to demonstrate the basilic vein. B, The cephalic vein and the arteries are drawn.
Preoperative DUS has also been used to investigate the subsequent function of the arteriovenous fistula. Malovrh84 used DUS to examine forearm arteries and veins before creating arteriovenous fistulae in 116 consecutive patients. It was shown that DUS helps identify the optimal location for successful creation of vascular access and the time necessary for its development on the basis of parameters such as feeding artery internal diameter, resistance index, blood flow before and after reactive hyperemia, and internal diameter of the vein before and after proximal vein compression. This approach is particularly helpful in patients with diabetes and in the elderly. In general, preoperative arterial diameter of 1.6 mm or more and venous diameter of 2.5 mm are required for arteriovenous fistula creation (Fig. 18-13).80,84
Figure 18-13 Mapping for creation of dialysis access fistula. Examples of normal basilic vein (A) and cephalic vein (B) are shown. Both veins have a good diameter without wall thickening or any intraluminal material. C, Basilic vein wall thickening due to previous asymptomatic thrombosis (arrow) from needle injury. D, Chronic thrombosis of the cephalic vein and one of its tributaries. The lumen in both veins is contracted, having echogenic material from the chronic effects of thrombosis (arrows).
Furthermore, adequate veins should be less than 0.5 cm deep from the skin and possess an 8- to 10-cm straight segment for successful cannulation; otherwise, vein superficialization is often required. Environmental conditions seem to be of high importance when vein diameters are measured. van Bemmelen and colleagues9 studied the effect on cephalic and basilic vein diameters of room conditions (i.e., room temperature), a tourniquet inflated to 65 mm Hg, and a warm-water bath with a mixture of warm and cold tap water to a temperature of 43° C to 44° C. Warm-water immersion yielded larger measurements compared with multiple other conditions, such as the sitting and supine positions. Measurements obtained without warm-water immersion represented only about a third of the veins’ fully distended size. The authors concluded that warm-water immersion measurements were more likely to resemble venous diameters after distention with arterial pressure. They suggested that this maneuver be added when inadequate vein diameters are seen on examination without warm-water immersion, before concluding that these patients do not have adequate veins for fistula construction.9
Whereas DUS provides an accurate assessment of peripheral vasculature, it may not always detect central vein stenosis, a weakness that could potentially result in development of swelling associated with central obstruction after arteriovenous fistula placement.
Limitations of Duplex Ultrasonography
DUS is extremely operator dependent, and when the technique is not standardized, results become difficult to interpret. Individual laboratories sometimes determine the type of venous ultrasonography to use and the segments of the venous system to be examined on the basis of local technologist experience and technical expertise. Unfortunately, venous DUS examinations are not yet uniformly standardized and range from compression of as few as two deep veins to complete duplex and color Doppler evaluation of the entire lower extremity. Limited examinations as stand-alone studies are obviously incompatible with the recommendations of the American College of Chest Physicians. Complete color-flow venous DUS has become the standard of care for assessment of lower extremity DVT. It is recommended that whenever possible, venous DUS for exclusion of the presence of DVT consist of evaluation of both the proximal and calf veins, as has been described in the preceding paragraphs.
There are also anatomic limitations that influence which venous segments can be evaluated in an individual examination, such as interrogation of veins in the abdomen (bowel gas overlying the iliac veins), pelvis, or Hunter’s canal. Visualization and compression of the distal femoral vein within the adductor canal can also be difficult, although isolated segmental thrombus is rare. Examination of veins in a limb that recently underwent surgical intervention or examination of patients with lower limb edema, renal failure, or congestive heart failure may prove difficult. Finally, anatomic variations, such as duplicated or aplastic segments, may make this technique difficult in that, for instance, thrombosis in a duplicated segment may be overlooked.
Some DUS examinations are constrained by practical matters involved in the clinical care of patients. Morbid obesity, inability of the patient to fully cooperate with positioning for the examination, intolerance of the pressure of the ultrasound scan head on the skin, or inability of the examiner to perform a complete examination because of the presence of bandages, casts, or extremity wounds may, in some cases, lead to a need for serial examinations or performance of an alternative diagnostic procedure, such as catheter-based contrast-enhanced venography, computed tomography, or MRV, to exclude DVT. These techniques have challenged DUS as the main method of studying venous disease. MRV uses flow dynamics with black blood imaging or gadolinium enhancement of the vein lumen in looking for a thrombus, and computed tomography involves the use of intravenous contrast material with multidetector scanners. However, because of its simplicity, low relative cost, and noninvasive nature, at this time DUS remains the gold standard for the diagnosis of venous disease.
Selected Key References
Cavezzi A, Labropoulos N, Partsch H, Ricci S, Caggiati A, Myers K, Nicolaides A, Smith PC. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part II. Anatomy. Eur J Vasc Endovasc Surg. 2006;31:288–299.
This consensus document systematically agrees about the anatomy of the superficial and perforator venous system of the lower limbs in health and disease as shown by ultrasound. This document was agreed on by a group of clinicians who regularly use this technology in their daily practice. It also defines best practices for venous duplex ultrasound imaging and interpretation of images.
Coleridge-Smith P, Labropoulos N, Partsch H, Myers K, Nicolaides A, Cavezzi A. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part I. Basic principles. Eur J Vasc Endovasc Surg. 2006;31:83–92.
This consensus document summarizes best practices for venous duplex ultrasound examination of the lower limbs agreed on by a group of clinicians who regularly use this technology in their daily practice. No systematic consensus agreement from phlebology or vascular societies on how vascular ultrasound for chronic venous disease is best performed existed before this publication.
Karakitsos D, Labropoulos N. Venous imaging for reflux using duplex ultrasonography. AbuRhama AF, Bandyk DF. Noninvasive vascular diagnosis: a practical guide to therapy,. ed 3. Springer: London; 2013:509–518.
This chapter provides an excellent summary of the details of imaging for reflux in all lower extremity and pelvic veins. The distribution and extent of reflux of the lower extremity are described.
Labropoulos N, Patel PJ, Tiongson JE, Pryor L, Leon LR, Tassiopoulos AK. Patterns of venous reflux and obstruction in patients with skin damage due to chronic venous disease. Vasc Endovascular Surg. 2007;41:33–40.
This study was carried out to identify the distribution, etiology, and pathophysiology of venous disease unique to patients who have skin damage as a result of chronic venous disease and to investigate the role of previous venous thrombosis in the same group of patients. The findings of this study should significantly influence the treatment of chronic venous disease with skin damage.
Labropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, Ashraf Mansour M, Baker WH. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003;38:793–798.
This is the only study that evaluated most veins in the lower extremity and had the largest sample size; it provided cutoff values to define venous reflux in the superficial veins, femoropopliteal veins, perforator veins, deep femoral veins, and deep calf veins.
Perrin M, Guex JJ, Ruckley CV, dePalma RG, Royle JP, Elkof B, Nicolini P, Jantet G. Recurrent varices after surgery (REVAS): a consensus document. Cardiovasc Surg. 2000;8:233–245.
This consensus document was written by an international group that met in Paris and developed for the first time a classification for patients with recurrent varices after surgery. Before this work, it was very difficult to systematically report true occurrences, residual refluxing veins, and varicose veins caused by progression of disease.
Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–645.
This consensus document represents the most accepted classification of venous disease and is intended to be used by both clinicians and researchers. It was produced by an ad hoc committee of the American Venous Forum based on clinical status (C), etiology (E), anatomic pattern of the problem (A), and pathophysiologic underlying mechanism (P) in patients with chronic venous disease.
Prandoni P, Bernardi E, Marchiori A, Lensing AW, Prins MH, Villalta S, Bogatella P, Sartor D, Piccioli A, Simioni P, Pagnan A, Girolami A. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484–485.
Whereas the diagnosis and treatment of acute symptomatic DVT of the arm were extensively documented before this study, its long-term clinical course was poorly defined. It demonstrated a low risk of recurrent thromboembolism (7.7% within the first 5 years) and a 27.3% risk for recurrent thromboembolism within the first 2 years, both risks being lower than those previously reported in patients with venous thrombosis of the leg.
Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E. The long-term clinical course of acute deep vein thrombosis. Ann Intern Med. 1996;125:1–7.
This prospective cohort study documented for the first time the long-term risk for recurrent venous thromboembolism and the incidence and severity of postthrombotic syndrome in patients who had symptomatic DVT. A high risk of recurrent thromboembolism was demonstrated as long as 8 years after the diagnosis of DVT, and the results challenged the widely adopted use of short-course anticoagulation therapy in these patients.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Markel A, et al. Pattern and distribution of thrombi in acute deep venous thrombosis. Arch Surg. 1992;127:305–309.
2. Criado E, et al. Predictive value of clinical criteria for the diagnosis of deep vein thrombosis. Surgery. 1997;122:578–583.
3. Glover JL, et al. Appropriate indications for venous duplex ultrasonic examinations. Surgery. 1996;120:725–730.
4. Hill S, et al. Selective use of the duplex scan in diagnosis of deep venous thrombosis. Am J Surg. 1995;170:201–205.
5. Naidich JB, et al. Suspected deep venous thrombosis: is US of both legs necessary? Radiology. 1996;200:429–431.
6. Vogel P, et al. Deep venous thrombosis of the lower extremity: US evaluation. Radiology. 1987;163:747–751.
7. Coleridge-Smith P, et al. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part I. Basic principles. Eur J Vasc Endovasc Surg. 2006;31:83–92.
8. Cavezzi A, et al. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part II. Anatomy. Eur J Vasc Endovasc Surg. 2006;31:288–299.
9. van Bemmelen PS, et al. Improvement in the visualization of superficial arm veins being evaluated for access and bypass. J Vasc Surg. 2005;42:957–962.
10. León LR, et al. Diagnosis of deep venous thrombosis. Davies AH. Vascular surgery highlights 2003-04. Health Press Limited: Oxford; 2004:45–52.
11. Barnes RW, et al. The fallibility of the clinical diagnosis of venous thrombosis. JAMA. 1975;234:605–607.
12. Haeger K. Problems of acute deep venous thrombosis, the interpretation of signs and symptons. Angiology. 1969;20:219–226.
13. Hirsh J, et al. Clinical features and diagnosis of venous thrombosis. J Am Coll Cardiol. 1986;8:114B–127B.
14. Persson AV, et al. Use of the triplex scanner in diagnosis of deep venous thrombosis. Arch Surg. 1989;124:593–596.
15. Schindler JM, et al. Colour coded sonography in suspected deep vein thrombosis of the leg. BMJ. 1990;301:1369–1370.
16. Foley WD, et al. Color Doppler ultrasound imaging of lower extremity venous disease. AJR Am J Roentgenol. 1989;152:371–376.
17. Rose SC, et al. Symptomatic lower extremity deep venous thrombosis: accuracy, limitations, and role of colour duplex flow imaging in diagnosis. Radiology. 1990;175:639–644.
18. Mattos MA, et al. Color-flow duplex scanning for the surveillance and diagnosis of acute deep venous thrombosis. J Vasc Surg. 1992;15:366–376.
19. Lee HY, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771–776.
20. Kim-Gavino CS, et al. Unusual appearance of a popliteal venous aneurysm in a 16-year-old patient: sonographic findings. J Ultrasound Med. 2006;25:1615–1618.
21. Kearon C, et al. Noninvasive diagnosis of deep venous thrombosis. McMaster diagnostic imaging practice guidelines initiative. Ann Intern Med. 1998;128:663–677.
22. Labropoulos N, et al: Clinical significance of the venous anatomic variations of the lower body veins. Abstract book. Paper presented at the 16th annual meeting of the American Venous Forum, Orlando, Fla, 2004.
23. Labropoulos N, et al. Patterns and distribution of isolated calf deep vein thrombosis. J Vasc Surg. 1999;30:787–791.
24. Labropoulos N, et al. Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg. 2007;46:101–107.
25. Labropoulos N, et al. Colour flow duplex scanning in suspected acute deep vein thrombosis; experience with routine use. Eur J Vasc Endovasc Surg. 1995;9:49–52.
26. Emelianov SY, et al. Triplex ultrasound: elasticity imaging to age deep venous thrombosis. Ultrasound Med Biol. 2002;28:757–767.
27. Moody AR, et al. Lower-limb deep venous thrombosis: direct MR imaging of the thrombus. Radiology. 1998;209:349–355.
28. Fowlkes JB, et al. Ultrasound echogenicity in experimental venous thrombosis. Ultrasound Med Biol. 1998;24:1175–1182.
29. Hansson PO, et al. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000;160:769–774.
30. Douketis JD, et al. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110:515–519.
31. Linkins LA, et al. Change in thrombus length on venous ultrasound and recurrent deep vein thrombosis. Arch Intern Med. 2004;164:1793–1796.
32. Linkins LA, et al. Interobserver agreement on ultrasound measurements of residual vein diameter, thrombus echogenicity and Doppler venous flow in patients with previous venous thrombosis. Thromb Res. 2006;117:241–247.
33. León LR, et al. Clinical significance of superficial vein thrombosis. Eur J Vasc Endovasc Surg. 2005;29:10–17.
34. Labropoulos N, et al. Thrombosis in unusual sites of the lower extremity veins. J Vasc Surg. 2008;47:1022–1027.
35. Labropoulos N, et al. Uncommon leg ulcers in the lower extremity. J Vasc Surg. 2007;45:568–573.
36. Labropoulos N, et al. Vascular diagnosis of venous thrombosis. Mansour AM, Labropoulos N. Vascular diagnosis,. WB Saunders: Philadelphia; 2005:429–438.
37. Sanchez JE, et al. Compression syndromes of the popliteal neurovascular bundle due to Baker cyst. J Vasc Surg. 2011;54:1821–1829.
38. Chin EE, et al. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med. 2005;24:829–838.
39. Rodríguez-Niedenführ M, et al. Variations of the arterial pattern in the upper limb revisited: a morphological and statistical study, with a review of the literature. J Anat. 2001;199:547–566.
40. Jeanneret C, et al. Venous reflux and venous distensibility in varicose and healthy veins. Eur J Vasc Endovasc Surg. 2007;34:236–242.
41. Labropoulos N, et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003;38:793–798.
42. Jeanneret C, et al. Physiological reflux and venous diameter change in the proximal lower limb veins during a standardised Valsalva manoeuvre. Eur J Vasc Endovasc Surg. 1999;17:398–403.
43. Lagattolla NR, et al. Retrograde flow in the deep veins of subjects with normal venous function. Br J Surg. 1997;84:36–39.
44. Markel A, et al. A comparison of the cuff deflation method with Valsalva’s maneuver and limb compression in detecting venous valvular reflux. Arch Surg. 1994;129:701–705.
45. Masuda EM, et al. Prospective study of duplex scanning for venous reflux: comparison of Valsalva and pneumatic cuff techniques in the reverse Trendelenburg and standing positions. J Vasc Surg. 1994;20:711–720.
46. Sarin S, et al. Duplex ultrasonography for assessment of venous valvular function of the lower limb. Br J Surg. 1994;81:1591–1595.
47. Araki CT, et al. Refinements in the ultrasonic detection of popliteal vein reflux. J Vasc Surg. 1993;18:742–748.
48. Van Bemmelen PS, et al. The mechanism of venous valve closure. Its relationship to the velocity of reverse flow. Arch Surg. 1990;125:617–619.
49. Van Bemmelen PS, et al. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425–431.
50. Kistner RL, et al. Diagnosis of chronic venous disease of the lower extremities: the “CEAP” classification. Mayo Clin Proc. 1996;7:338–345.
51. Labropoulos N. CEAP in clinical practice. Vasc Surg. 1997;31:224–225.
52. Van Bemmelen PS, et al. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425–431.
53. Caggiati A. Fascial relationships of the long saphenous vein. Circulation. 1999;100:2547–2549.
54. Caggiati A, et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. [International Interdisciplinary Consensus Committee on Venous Anatomical Terminology] J Vasc Surg. 2002;36:416–622.
55. Caggiati A. Fascial relationships of the short saphenous vein. J Vasc Surg. 2001;34:241–246.
57. Caggiati A, et al. Segmental hypoplasia of the great saphenous vein and varicose disease. Eur J Vasc Endovasc Surg. 2004;28:257–261.
58. Labropoulos N, et al. The impact of isolated lesser saphenous vein system incompetence on clinical signs and symptoms of chronic venous disease. J Vasc Surg. 2000;32:954–960.
59. van Limborgh J. L’anatomie du système veineux de l’extremite inférieure en relation avec la pathologie variqueuse. Folia Angiol. 1961;8:240–257.
60. Johnson BF, et al. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995;21:307–312.
61. Labropoulos N, et al. Nonsaphenous superficial vein reflux. J Vasc Surg. 2001;34:872–877.
62. Sandri JL, et al. Diameter-reflux relationship in perforating veins of patients with varicose veins. J Vasc Surg. 1999;30:867–874.
63. Labropoulos N, et al. New insights into perforator vein incompetence. Eur J Vasc Endovasc Surg. 1999;18:228–234.
64. Labropoulos N, et al. Reflux testing and imaging for endovenous ablation. Perspect Vasc Surg Endovasc Ther. 2007;19:67–70.
65. Johnson CM, et al. Endovenous laser ablation of varicose veins: review of current technologies and clinical outcome. Vascular. 2007;15:250–254.
66. Myers KA, et al. Outcome of ultrasound-guided sclerotherapy for varicose veins: medium-term results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33:116–121.
67. Guex JJ. Foam sclerotherapy: an overview of use for primary venous insufficiency. Semin Vasc Surg. 2005;18:25–29.
68. Smith PC. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32:577–583.
69. Neglén P, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990.
70. Corriere MA, et al. Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality? Ann Vasc Surg. 2005;19:229–234.
71. Perrin M, et al. Recurrent varices after surgery (REVAS): a consensus document. Cardiovasc Surg. 2000;8:233–245.
72. van Rij AM, et al. Recurrence after varicose vein surgery: a prospective long-term clinical study with duplex ultrasound scanning and air plethysmography. J Vasc Surg. 2003;38:935–943.
73. Porter JM, et al. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–645.
74. Perrin MR, et al. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43:327–334.
75. Corriere MA, et al. Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality? Ann Vasc Surg. 2005;19:229–234.
76. Garrett JV, et al. Expanding options for bedside placement of inferior vena cava filters with intravascular ultrasound when transabdominal duplex ultrasound imaging is inadequate. Ann Vasc Surg. 2004;18:329–334.
77. Broughton JD, et al. Could routine saphenous vein ultrasound mapping reduce leg wound complications in patients undergoing coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. 2013;16:75–78.
78. Luckraz H, et al. Pre-operative long saphenous vein mapping predicts vein anatomy and quality leading to improved post-operative leg morbidity. Interact Cardiovasc Thorac Surg. 2008;7:188–191.
79. Labropoulos N, et al. The distribution and significance of varicosities in the saphenous trunks. J Vasc Surg. 2010;51:96–103.
80. Silva MB Jr, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27:302–307.
81. Robbin ML, et al. US vascular mapping before hemodialysis access placement. Radiology. 2000;217:83–88.
82. Allon M, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013–2020.
83. Asif A, et al. Conversion of tunneled hemodialysis consigned patients to arteriovenous fistula. Kidney Int. 2005;67:2399–2406.
84. Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis. 2002;39:1218–1225.