CHAPTER 111 Vascular Imaging of Hepatic Transplantation
With more than 6000 surgeries performed in 2008,1 liver transplantation is the current treatment of choice for many causes of progressive acute and chronic end-stage liver disease. In the United States, liver transplantation typically involves harvesting of an organ from a deceased donor (orthotopic liver transplantation). However, demand for liver transplantation is increasingly outstripping the supply of cadaveric livers, and transplantation of a portion of the liver from a live donor (i.e., living related donor) is increasing in popularity. Knowledge of recipient and live donor hepatic vascular anatomy is critical for proper preoperative planning for transplantation.
Although malignant disease is a contraindication to transplantation in most other solid organs, liver transplantation offers the only potential cure for primary, low- and moderate-volume hepatocellular carcinoma. The overall 5-year survival in this setting is expected to be 70%, with a recurrence rate below 15%. Compared with hepatocellular carcinoma, transplantation for cholangiocarcinoma is controversial because 5-year survival rates are much lower (20% to 30%) and recurrence rates are much higher. Transplantation for metastatic liver lesions is generally not performed; rare exception is made for metastatic carcinoid and other neuroendocrine tumors.2
PRETRANSPLANTATION WORK-UP
Imaging of Liver Transplant Recipients
The radiologic evaluation of a potential candidate for orthotopic liver transplantation primarily focuses on an assessment of the degree of cirrhosis (liver size and morphology, presence of ascites) and patency and caliber of the portal vein and associated feeding veins (splenic vein and superior mesenteric vein) to determine whether a suitable site for venovenous anastomosis between the donor liver and recipient portal vein or feeding branches exists. Because of the greatly increased risk for development of hepatocellular carcinoma in cirrhotic livers, most centers perform routine sonographic surveillance for early detection of hepatocellular carcinoma. Whereas ultrasonography is sensitive in the evaluation of portal vein caliber, patency, and direction of flow, its operator-dependent nature limits its sensitivity for detection of hepatocellular carcinoma (20% to 40% for lesions smaller than 3 cm).3
Dynamic contrast-enhanced (multiphase) multidetector computed tomography (CT) and magnetic resonance imaging (MRI) enable rapid, whole-liver evaluation during arterial and portal venous phases of intravenous contrast enhancement, allowing a comprehensive assessment of the hepatic artery and the portal, splenic, and superior mesenteric veins as well as major collateral veins. In general, both contrast-enhanced CT and MRI are more sensitive than sonography for detection of hepatocellular carcinoma lesions larger than 2 cm (sensitivity, 74% to 96%). The 2-cm size is important because by the Model for End-Stage Liver Disease scoring system, cirrhotic patients with 2-cm or larger lesions are assigned priority for liver transplantation. Although both multiphase CT and MRI are less sensitive for detection of hepatocellular carcinoma lesions smaller than 2 cm, they are better than ultrasonography, which has sensitivities as low as 20% for these lesions.4–9 MRI benefits from its ability to provide additional lesion characterization for further differentiation of the myriad types of liver nodules in cirrhotic livers based on signal intensity (e.g., T1 and T2) and contrast enhancement characteristics. Multiphase CT and MR examinations can determine vascular patency and are superior to ultrasound examination in the characterization of the recipient vascular anatomy.
Imaging of Potential Living Donors for Hepatic Transplantation
As an alternative to cadaveric liver transplantation, which is not an option in many European and Asian countries and usually entails a long wait time in many regions of the United States, living related and living donor hepatic transplantations have been advocated to expand the pool of available hepatic allografts. In adult-to-adult living donor transplantation, the right lobe (segments V, VI, VII, and VIII) is resected and transplanted. In adult-to-pediatric liver donation, the left lateral segment (segments II and III) is transplanted into the pediatric recipient. However, because of concerns of donor safety in the United States, fewer than 250 adult living donor hepatic transplantations were performed in 2007.10
Approximately 55% of patients have a conventional right and left hepatic artery arising from a common hepatic artery (Michel type I, Fig. 111-1).11,12 However, in the remaining 45% of patients, there can be a wide range of hepatic artery variations (Fig. 111-2) that can be described by the Michel classification (Table 111-1). Proper identification of hepatic artery anatomy in the donor is critical for surgical planning and minimization of postoperative complications in both the donor and recipient. A donor may be rejected when aberrant arterial anatomy is also associated with other biliary and venous variants, which in combination result in an increased complexity of the operation.
| Type 1 Normal anatomy: proper hepatic artery dividing into sole right and left hepatic arteries |
| Type 2 Left hepatic artery replaced to the left gastric artery |
| Type 3 Right hepatic artery replaced to the superior mesenteric artery |
| Type 4 Both right and left hepatic arteries replaced |
| Type 5 Accessory left hepatic artery |
| Type 6 Accessory right hepatic artery |
| Type 7 Accessory right and left hepatic arteries |
| Type 8 Proper hepatic artery originating from the superior mesenteric artery combined with an accessory left hepatic artery |
| Type 9 Proper hepatic artery arising from the superior mesenteric artery |
| Type 10 Proper hepatic artery arising from the left gastric artery |
From Artioli D, Tagliabue M, Aseni P, et al: Detection of biliary and vascular anatomy in living liver doners: value of gadobenate dimeglumine enhanced MR and MDCT angiography. Eur J Radiol. In press.
Ideal portal venous anatomy has the right portal vein branching from the main portal vein (Fig. 111-3) and not from the left portal vein. Cases in which portal venous branches to segment IV arise from the right anterior portal vein may be a contraindication to transplantation.
Ideal hepatic venous anatomy has the single dominant right hepatic vein available for anastomosis without significant (>5 mm diameter branch veins) hepatic venous branches draining into the now-missing middle hepatic vein. However, many variants in hepatic venous drainage exist. Hepatic venous branches larger than 5 mm that drain the right posterior lobe (VII and VI) and insert into the inferior vena cava (IVC) may require separate venous anastomoses in the recipient to prevent hepatic segmental congestion or bleeding and subsequent liver dysfunction. Hepatic venous branches larger than 5 mm that drain the right anterior lobe branches (VIII and V) into the middle hepatic vein will invariably transect the surgical resection plane and probably require separate venous anastomoses to also prevent complications such as hepatic venous congestion in the recipient.13,14
SURGICAL ANATOMY (CADAVERIC LIVER TRANSPLANTATION)
The hepatic artery anastomosis is typically a branch patch anastomosis formed at the origin of the gastroduodenal artery from the common hepatic artery in the recipient and at the origin of the splenic artery from the celiac trunk in the donor (Fig. 111-4). In some instances, a donor anastomosis is made at the celiac axis with use of an aortic patch (Carrel patch).15,16 On occasion, an aortic jump graft will be formed from the donor common and external iliac arteries joined to the recipient abdominal aorta in an end-to-side anastomosis and to the hepatic artery of the donor by a branch patch anastomosis. This technique can be used in cases of small native hepatic artery or celiac artery stenosis in which an adequate inflow cannot be ensured.
When there is variant donor hepatic artery anatomy, modification of normal technique is required. For example, in Michel type III variant (a replaced right hepatic artery from the superior mesenteric artery, the most common arterial variant; see Fig. 111-2),17 a primary anastomosis between the donor celiac artery with an aortic patch and the recipient branch patch at the gastroduodenal artery takeoff and a secondary anastomosis between the replaced right hepatic artery and the proximal stump of the donor splenic artery can be performed.15 In situations of variant hepatic arterial anatomy in the recipient, such as a replaced right hepatic artery, the larger of the two inflow vessels is used.16
The portal vein anastomosis is typically an end-to-end anastomosis between the donor and recipient portal veins.18 A venous jump graft between the donor portal vein to the recipient superior mesenteric vein may be needed in cases of portal vein thrombosis or prior portal venous surgery.16 Rarely, arterialization of the portal vein, in which the donor portal vein is anastomosed to the arterial vessels of the recipient, has been performed when a suitable visceral venous anastomosis is impossible because of extensive thrombus.19
For the caval anastomosis, the IVC is transected above and below the intrahepatic segment during cadaveric hepatectomy, and an end-to-end anastomosis is made between the upper and lower margins of the IVC in the recipient.16 In the “piggyback” technique, a single end-to-end anastomosis is made between the donor hepatic vein–IVC stump and the recipient’s common hepatic vein stump off the IVC (Fig. 111-5).18 This technique is preferred at some institutions because IVC flow is not interrupted during most of the operation, thus reducing inherent operative risk as well as obviating the need for venovenous bypass.20
COMPLICATIONS AFTER LIVER TRANSPLANTATION
Orthotopic liver transplantation is a technically complex procedure with inferior vena caval, portal venous, and hepatic arterial vascular anastomoses. A variety of short- and long-term complications may arise. Of particular relevance to interpreting physicians are the vascular complications, which are among the most frequent causes of early acute graft failure.21 The transplant team is particularly sensitive to the patency of the allograft vessels in the immediate postoperative period in patients with signs of bile leak or sepsis or more commonly a persistent elevation in serum liver enzymes. When any of these are suspected, ultrasonography with color, power, and spectral Doppler imaging is immediately performed. If sonography is inconclusive or suggests arterial stenosis or occlusion, MR angiography (MRA) or CT angiography (CTA) is typically performed to determine the extent of these potentially morbid vascular complications to enable revascularization with endovascular interventional procedures.
Both CTA and MRA are performed for definitive evaluation of vascular complications after liver transplantation, with a sensitivity reported to be 100%, specificity between 74% and 96%, and negative predictive value of 100%.22–27 Although no studies directly comparing their performance exist in the post-transplantation setting, the latest generation of CT and MR equipment with optimized imaging protocols is generally regarded as equivalent in terms of their ability to evaluate vascular complications. CTA is usually more widely available with regard to equipment and expertise, and it can be a more reliable examination in uncooperative patients with fewer limitations on motion of the patient. Its major limitation is in those patients for whom iodinated contrast material cannot be administered for concern of nephrotoxicity or severe contrast allergy. In a minimally cooperative patient, contrast-enhanced three-dimensional MRA is a robust examination that allows assessment of hepatic vessels and hepatic parenchyma. The biliary tree is also easily assessed with the use of MR cholangiopancreatography sequences incorporated into a single examination. Its major limitations are an increased susceptibility to erratic respiratory motion, the possible overestimation of vascular stenosis due to slow flow or surgical clips–related susceptibility artifact,28 and a small chance of inducing potentially progressive and debilitating intravenous gadolinium–associated nephrogenic systemic fibrosis in post-transplantation patients with severe acute renal failure (glomerular filtration rate <30 mL/min/1.73 m2).29
Hepatic Artery
With an occurrence between 5% and 12% of adult patients and up to 42% of pediatric patients, presumably owing to small size of the transplant vessel, hepatic artery thrombosis is the most frequent and most serious vascular complication after liver transplantation.21,27,30 Although the liver parenchyma has dual arterial and portal venous blood supply, the hepatic artery alone perfuses the biliary tree of the transplanted liver.
Hepatic artery thrombosis generally develops within the first 3 months after transplantation.30 Risk factors for development of hepatic artery thrombosis include small native hepatic arteries, use of a Roux-en-Y biliary reconstruction, length of cold ischemia and operative times, volume of blood and plasma products used intraoperatively, and use of aortic conduits in arterial reconstruction.15,31
Clinical presentation of hepatic artery thrombosis can be variable, depending on the time and severity of hepatic injury related to hepatic artery thrombosis. The most devastating is fulminant hepatic necrosis, which occurs in up to 33% of patients with hepatic artery thrombosis. Patients have rapid decompensation of liver synthetic function, fever, sepsis, hypotension, and coagulopathy, which can necessitate retransplantation. Somewhat more variable in nature are the biliary tract complications resulting from hepatic arterial ischemia or infarction. These include intrahepatic biliary strictures, intrahepatic bile leaks or bilomas, and biliary necrosis in up to 60% of cases of anastomotic hepatic artery stenosis and in up to 80% of cases of hepatic artery thrombosis.32 The clinical presentation of patients is variable. Some patients may present with intermittent episodes of sepsis without a clear source, presumably caused by focal abscesses in areas of infarcted liver or biliary necrosis. Others may remain asymptomatic or mildly symptomatic, with a diagnosis of hepatic artery thrombosis made incidentally.
For suspected postoperative hepatic artery thrombosis, ultrasonography is usually the first imaging modality performed because of its speed and portability. In addition to gray-scale two-dimensional imaging, color, power, and spectral Doppler ultrasound examinations may be performed to maximize chances of evaluating arterial flow. In experienced hands, ultrasonography has achieved up to 92% sensitivity for detection of hepatic artery thrombosis. Spectral Doppler findings include progressively decreased to absent diastolic flow, decreased slope and amplitude (tardus et parvus) of the peak systolic velocity, and finally absence of the hepatic waveform altogether. If this process is prolonged, the development of intrahepatic arterial collateral vessels can lead to a false-negative examination.33 However, these collateral intrahepatic arterial vessels are often not completely normal, also often demonstrating a tardus-parvus waveform. CTA (Fig. 111-6) or MRA studies are usually relied on to confirm ultrasound findings and to determine the extent of the problems. False-positive diagnoses by Doppler study can be due to severe hepatic edema, hypotension, high-grade hepatic arterial stenosis, or technical limitations.
By CTA or MRA, arterial thrombosis of the hepatic artery (Fig. 111-7) or of other vessels (celiac artery, Fig. 111-8) is readily diagnosed. Hepatic arterial thrombosis can be diagnosed by the lack of contrast media opacification of the hepatic artery or graft. Hepatic artery thrombosis is often associated with hepatic and biliary complications (Figs. 111-9 to 111-12). Intrahepatic collateral vessels can be seen if progression toward hepatic artery thrombosis is advanced.
Because hepatic artery thrombosis may be clinically manifested with a spectrum of mild to severe clinical symptoms, its treatment varies correspondingly. In fulminant hepatic failure, the patient is resuscitated and listed for retransplantation. If thrombosis is acute and patients are symptomatic or minimally symptomatic, operative exploration with arterial reconstruction is performed. If the celiac artery inflow is adequate, thrombectomy with revision of the thrombosed segment may be performed without arterial reconstruction. If the inflow is inadequate, revascularization with an end-to-side anastomosis with the aorta or an interposition arterial graft is performed (Figs. 111-13 and 111-14). In some centers, percutaneous catheter–mediated thrombolysis, with or without angioplasty and stenting, is performed in those patients who are asymptomatic or minimally symptomatic. This technique works best in acute or subacute thromboses. Percutaneous management of biliary complications may be sufficient, but retransplantation may be required when diffuse biliary injury and breakdown are accompanied by infection. If the thrombosis is late and the patient is asymptomatic, the patient may be expectantly observed with no intervention performed because hepatic arterial collateral vessels may be sufficient.34–36
Hepatic artery stenosis is a common vascular complication of orthotopic liver transplantation, with a prevalence between 5% and 11%.25,30,37 It can be difficult to diagnose because many cases progress to hepatic artery thrombosis by the time clinical symptoms develop. Hepatic artery stenosis most often occurs at the vascular anastomosis. Early clinical findings may be manifested by biliary dysfunction or graft failure progressing to cholangitis or areas of ischemic infarcts.
Doppler ultrasound study is frequently performed in the evaluation of hepatic artery stenosis. Elevation of blood flow velocities by twofold to threefold at the hepatic artery anastomosis, increased downstream diastolic flow, and downstream tardus-parvus arterial waveforms within intrahepatic arteries are diagnostic criteria. Mild degrees of stenosis, however, may have normal Doppler findings. CTA and MRA are more direct methods for anatomic assessment of the anastomosis and are sensitive in the evaluation for stenosis. Both CTA and MRA can overestimate the degree of narrowing.22,25,26,38 Stenosis at the celiac axis should be assessed in all cases during evaluation of the hepatic arterial inflow (Figs. 111-15 to 111-17).
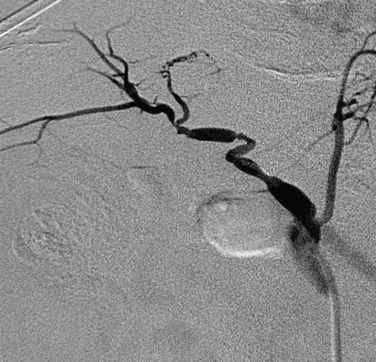
 FIGURE 111-17 Conventional x-ray hepatic arteriogram demonstrates hepatic artery stenosis in an orthotopic liver transplantation patient.
FIGURE 111-17 Conventional x-ray hepatic arteriogram demonstrates hepatic artery stenosis in an orthotopic liver transplantation patient.
In patients who are symptomatic or who have a long-segment stenosis, operative revision may be performed. Otherwise, hepatic artery stenosis can be successfully treated with angioplasty, which can improve graft survival by preventing progression to arterial thrombosis.39–41
Hepatic artery pseudoaneurysm (Fig. 111-18) is a rare complication of liver transplantation. Often at the vascular anastomosis, and frequently mycotic in nature, it is at increased risk for rupture. A pseudoaneurysm can be intrahepatic or peripheral after biopsy, instrumentation, or infection.16,30 It can be treated surgically or by transarterial embolization.
Portal Vein
Portal vein thrombosis (Figs. 111-19 and 111-20) is an uncommon complication after orthotopic liver transplantation. Seen in 1% to 3% of cases, it can develop as late as 5 years after transplantation.18,21–23,25,30 Causes include surgical technique, misalignment or excessive vessel length, hypercoagulable states, previous portal vein surgery, and thrombus formation from portal venous bypass cannula used at transplantation. By ultrasound examination, echogenic thrombus within the portal vein is demonstrated. In some acute settings, the thrombus can be anechoic. In all instances, color Doppler and spectral analysis should be performed. On CT and MR, portal vein thrombosis is evident with lack of contrast media enhancement, either complete or partial, within the portal vein. MR has the added ability to detect thrombus without the need for contrast media by bright blood pulse sequences such as steady-state free precession. Postoperative venous aneurysms can also be seen (Fig. 111-21).
Portal vein stenosis (Fig. 111-22) can be an incidental finding or can be manifested by symptoms of portal hypertension.30 In interpreting studies, however, it is important to note that size discrepancy between the donor and recipient portal vein can cause an apparent anastomotic stenosis. By Doppler ultrasound examination, an elevation in the velocity at the anastomosis by three or four times suggests a hemodynamically significant stenosis. Both CTA and MRA can demonstrate anastomotic narrowing. If it is not associated with other stigmata of portal hypertension, it is nonspecific in terms of hemodynamic significance. In evaluating the functional significance of an anastomotic narrowing, portal venography should be performed. A pressure gradient of 5 mm Hg or higher is compatible with a significant stenosis. Symptomatic stenosis can be treated by segmental portal vein resection or percutaneously by angioplasty with or without stent placement (Figs. 111-23 and 111-24).39,42
Hepatic Veins and Inferior Vena Cava
IVC stenosis is rare, occurring in less than 1% of transplants.23,30 It is caused by technical problems related to surgery or compression by fluid collections.42 The clinical findings in significant IVC stenosis include hepatomegaly, pleural effusions, ascites, and extremity edema.16 A hemodynamically significant stenosis at the anastomosis may be manifested as a threefold to fourfold increase in velocity by spectral ultrasound examination. There may be dampened or reversed flow within the hepatic veins in a significant supracaval stenosis. With CTA or MRA, lack of or partial opacification of portions of the IVC due to thrombus or narrowing of the caval anastomosis can be seen (Figs. 111-25 to 111-28). As in portal vein stenosis, care must be taken not to mistake size discrepancy at the anastomosis between donor and recipient vessels for a hemodynamically significant stenosis (see Fig. 111-26). If a hemodynamically significant stenosis is suspected, venography should be performed to determine the presence of a significant pressure gradient. If one is found, angioplasty and stent placement can be successful.39
In cases of a piggyback caval anastomosis, there is an increased risk for venous outflow obstruction. Presumptive causes include direct compression of the vein by a graft that is too large, twisting of the venous anastomosis by a graft that is too small, surgical factors such as tight sutures, and, in late cases, intimal hyperplasia and fibrosis. Endovascular treatment with balloon-expandable stents can be an effective treatment in these cases.43
CONCLUSION
KEY POINTS
 Liver transplantation is the primary treatment of many fulminant acute and chronic liver diseases as well as of certain hepatic malignant neoplasms.
Liver transplantation is the primary treatment of many fulminant acute and chronic liver diseases as well as of certain hepatic malignant neoplasms. In the pretransplantation setting, multidetector CT or CTA and dynamic contrast-enhanced MR or MRA are used primarily for surgical anatomic assessment, evaluation of vascular patency, and detection of focal or diffuse liver lesions.
In the pretransplantation setting, multidetector CT or CTA and dynamic contrast-enhanced MR or MRA are used primarily for surgical anatomic assessment, evaluation of vascular patency, and detection of focal or diffuse liver lesions. In the limited instances of living related liver donor transplantation, radiologic evaluation of the donor liver vascular anatomy by CT or MR angiography is mandatory.
In the limited instances of living related liver donor transplantation, radiologic evaluation of the donor liver vascular anatomy by CT or MR angiography is mandatory. Spectral and color Doppler ultrasound studies are often performed as an initial screening examination in the evaluation for complications in the post-transplantation setting, predominantly because these are rapid, portable examinations that can provide useful although limited information.
Spectral and color Doppler ultrasound studies are often performed as an initial screening examination in the evaluation for complications in the post-transplantation setting, predominantly because these are rapid, portable examinations that can provide useful although limited information. Multidetector CT or CTA and dynamic contrast-enhanced MR or MRA are more reliable whole-liver examinations. They provide a complete assessment of each surgical vascular anastomosis in the evaluation for post-transplantation vascular complications.
Multidetector CT or CTA and dynamic contrast-enhanced MR or MRA are more reliable whole-liver examinations. They provide a complete assessment of each surgical vascular anastomosis in the evaluation for post-transplantation vascular complications.Alonso-Torres A, Fernandez-Cuadrado J, Pinilla I, et al. Multidetector CT in the evaluation of potential living donors for liver transplantation. Radiographics. 2005;25:1017-1030.
Limanoud P, Raman SS, Ghobial RM, et al. Preoperative imaging of adult-to-adult living related liver transplant donors. J Comput Assit Tomogr. 2004;28:149-157.
Onofrio AC, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359-378.
Pannu HK, Maley WR, Fishman EK. Liver transplantation: preoperative CT evaluation. Radiographics. 2001;21:S133-S146.
Saad WE, Orloff MC, Davies MG, et al. Postliver transplantation: vascular and biliary surgical anatomy. Tech Vasc Interv Radiol. 2007;10:172-190.
Sahani D, Mehta A, Blake M, et al. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367-1380.
Schroeder T, Malago M, Debatin JF, et al. “All-in-one” imaging protocols for the evaluation of potential living liver donors: comparison of magnetic resonance imaging and multidetector computed tomography. Liver Transpl. 2005;11:776-787.
1 Organ Procurement and Transplantation Network Available at http://optn.transplant.hrsa.gov/
2 Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Tranplantation. 1998;66:1307-1312.
3 Bennett GL, Krinsky GA, Abitbol RJ, et al. Ultrasound detection of hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: correlation of pretransplant ultrasound findings and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179:175-180.
4 Taouli B, Krinsky GA. Diagnostic imaging of hepatocellular carcinoma in patients with cirrhosis before liver transplantation. Liver Transpl. 2006;12:S1-S7.
5 Krinsky GA, Lee VS, Theise ND, et al. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156-1164.
6 Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445-454.
7 Hanna RF, Kased N, Kwan SW, et al. Double-contrast MRI for accurate staging of hepatocellular carcinoma in patients with cirrhosis. AJR Am J Roentgenol. 2008;190:47-57.
8 Ronzoni A, Artioli D, Scardina R, et al. Role of MDCT in the diagnosis of hepatocellular carcinoma in patients with cirrhosis undergoing orthotopic liver transplantation. AJR Am J Roentgenol. 2007;189:792-798.
9 Brancatelli G, Baron RL, Peterson MS, Wallis M. Helical CT screening for hepatocellular carcinoma in patients with cirrhosis: frequency and causes of false-positive interpretation. AJR Am J Roentgenol. 2003;180:1007-1014.
10 Anselmo DM, Baquerizo A, Geevarghese S, et al. Liver transplantation at Dumont-UCLA Transplant Center: an experience with over 3,000 cases. Clin Transpl. 2001:179-186.
11 Sahani D, Mehta A, Blake M, et al. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367-1380.
12 Onofrio AC, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359-378.
13 Limanond P, Raman SS, Ghobrial RM, et al. Preoperative imaging in adult-to-adult living related liver transplant donors: what surgeons want to know. J Comput Assist Tomagr. 2004;28:149-157.
14 Alonso-Torres A, Fernandez-Cuadrado J, Pinilla I, et al. Multidetector CT in the evaluation of potential living donors for liver transplantation. Radiographics. 2005;25:1017-1030.
15 Ishigami K, Zhang Y, Rayhill S, et al. Does variant hepatic artery anatomy in a liver transplant recipient increase the risk of hepatic artery complications after transplantation? AJR Am J Roentgenol. 2004;183:1577-1584.
16 Nghiem HV, Tran K, Winter TCIII, et al. Imaging of complications in liver transplantation. Radiographics. 1996;16:825-840.
17 Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50-52.
18 Quiroga A, Sebastia C, Margarit C, et al. Complications of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics. 2001;21:1085-1102.
19 Stange BJ, Glanemann M, Nussler NC, et al. Indication, technique and outcome of portal vein arterialization in orthotopic liver transplantation. Transplant Proc. 2001;33:1414-1415.
20 Khan S, Silva MA, Tan YM, et al. Conventional versus piggyback technique of caval implantation; without extra-corporeal veno-venous bypass. A comparative study. Transpl Int. 2006;19:795-801.
21 Varotti G, Grazi GL, Vetrone G, et al. Causes of early acute graft failure after liver transplantation: analysis of a 17-year single centre experience. Clin Transplant. 2005;19:492-500.
22 Brancatelli G, Katyal S, Federle MP, Fontes P. Three-dimensional multislice helical computed tomography with the volume rendering technique in the detection of vascular complications after liver transplantation. Transplantation. 2002;73:237-242.
23 Stafford-Johnson DB, Hamilton BH, Dong Q, et al. Vascular complications of liver transplantation: evaluation with gadolinium enhanced MR angiography. Radiology. 1998;207:153-160.
24 Finn JP, Edelman RR, Jenkins RL, et al. Liver transplantation: MR angiography with surgical validation. Radiology. 1991;179:265-269.
25 Glockner JF, Forauer AR, Solomon H, et al. Three-dimensional gadolinium enhanced MR angiography of vascular complications after liver transplantation. AJR Am J Roentgenol. 2000;174:1447-1453.
26 Kim BS, Kim TK, Jung DJ, et al. Vascular complications after living related liver transplantation: evaluation with gadolinium-enhanced three-dimensional MR angiography. AJR Am J Roentgenol. 2003;181:467-474.
27 Legmann P, Costes V, Tudoret L, et al. Hepatic artery thrombosis after liver transplantation: diagnosis with spiral CT. AJR Am J Roentgenol. 1995;164:97-101.
28 Pandaripande PV, Lee VS, Morgan GR, et al. Vascular and extravascular complications of liver transplantation: comprehensive evaluation with three-dimensional contrast-enhanced volumetric MR imaging and MR cholangiopancreatography. AJR Am J Roentgenol. 2001;177:1101-1107.
29 Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148-157.
30 Wozney P, Zajko AM, Bron KM, et al. Vascular complications after liver transplantation: a 5 year experience. AJR Am J Roentgenol. 1986;147:657-663.
31 Silva MA, Jambulingham PS, Gunson BK, et al. Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl. 2006;12:146-151.
32 Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. AJR Am J Roentgenol. 1995;165:1145-1149.
33 Hall TR, McDiarmid SV, Grant EG, et al. False-negative duplex Doppler studies in children with hepatic artery thrombosis after liver transplantation. AJR Am J Roentgenol. 1990;154:573-575.
34 Sheiner PA, Varma CV, Guarrera JV, et al. Selective revascularization of hepatic artery thromboses after liver transplantation improves patient and graft survival. Transplantation. 1997;64:1295-1299.
35 Bhattacharjya S, Gunson BK, Mirza DF, et al. Delayed hepatic artery thrombosis in adult orthotopic liver transplantation—a 12-year experience. Transplantation. 2001;71:1592-1596.
36 Langnas AN, Marujo W, Stratta RJ, et al. Hepatic allograft rescue following arterial thrombosis. Role of urgent revascularization. Transplantation. 1991;51:86-90.
37 Glockner JF, Forauer AR. Vascular or ischemic complications after liver transplantation. AJR Am J Roeentgenol. 1999;173:1055-1059.
38 Vignali C, Bargellini I, Cioni R, et al. Diagnosis and treatment of hepatic artery stenosis after orthotopic liver transplant. Transplant Proc. 2004;36:2771-2773.
39 Raby N, Kirani J, Thomas S, et al. Stenoses of vascular anastomosis after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol. 1991;157:167-171.
40 Abbasoglu O, Levy MF, Vodapally MS, et al. Hepatic artery stenosis after liver transplantation—incidence, presentation, treatment, and long term outcome. Transplantation. 1997;63:250-255.
41 Saad WE, Davies MG, Sahler L, et al. Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol. 2005;16:795-805.
42 Ito K, Siegelman ES, Stolpen AH, Mitchell DG. MR imaging of complications after liver transplantation. AJR Am J Roentgenol. 2000;175:1145-1149.
43 Wang SL, Sze DY, Busque S, et al. Treatment of hepatic venous outflow obstruction after piggyback liver transplantation. Radiology. 2005;236:352-359.

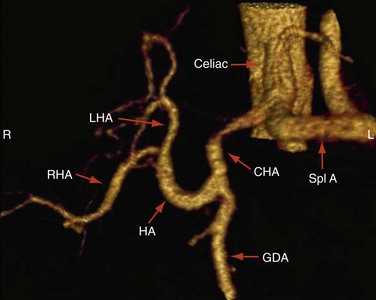
 FIGURE 111-1
FIGURE 111-1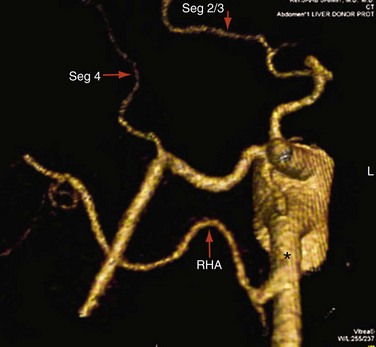
 FIGURE 111-2
FIGURE 111-2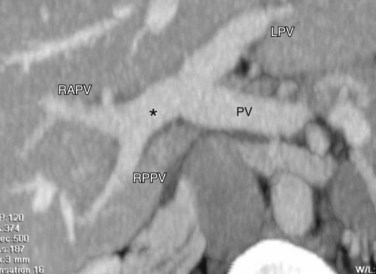
 FIGURE 111-3
FIGURE 111-3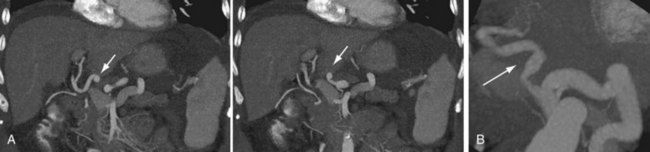
 FIGURE 111-4
FIGURE 111-4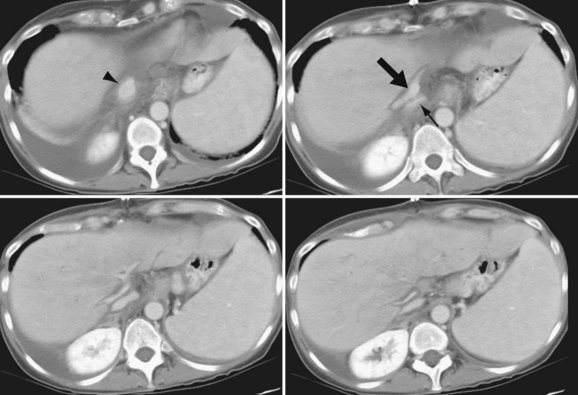
 FIGURE 111-5
FIGURE 111-5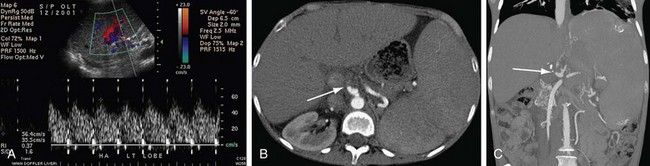
 FIGURE 111-6
FIGURE 111-6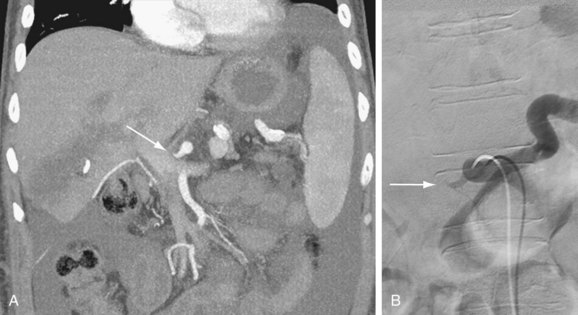
 FIGURE 111-7
FIGURE 111-7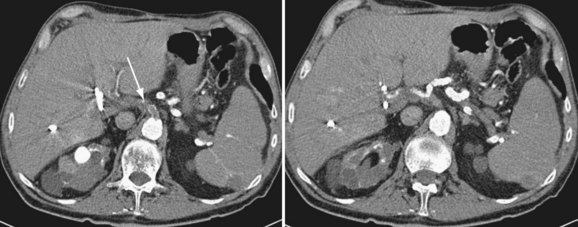
 FIGURE 111-8
FIGURE 111-8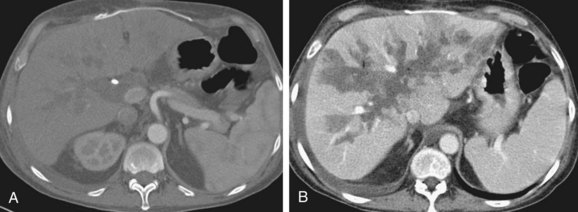
 FIGURE 111-9
FIGURE 111-9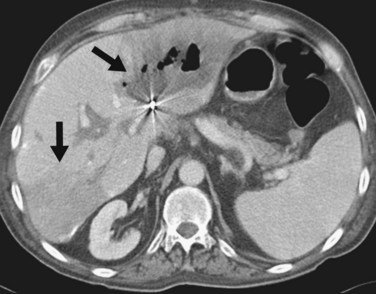
 FIGURE 111-10
FIGURE 111-10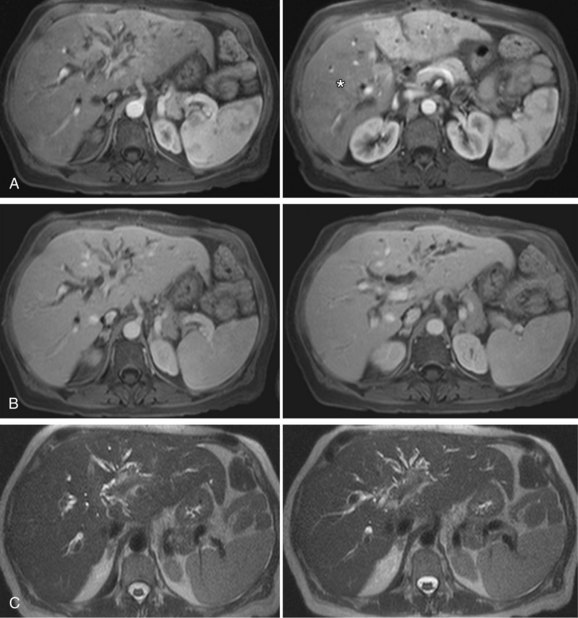
 FIGURE 111-11
FIGURE 111-11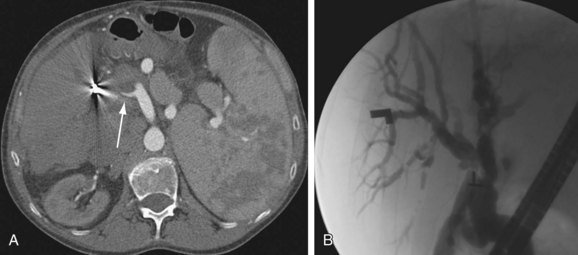
 FIGURE 111-12
FIGURE 111-12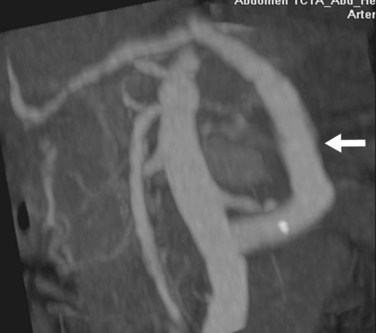
 FIGURE 111-13
FIGURE 111-13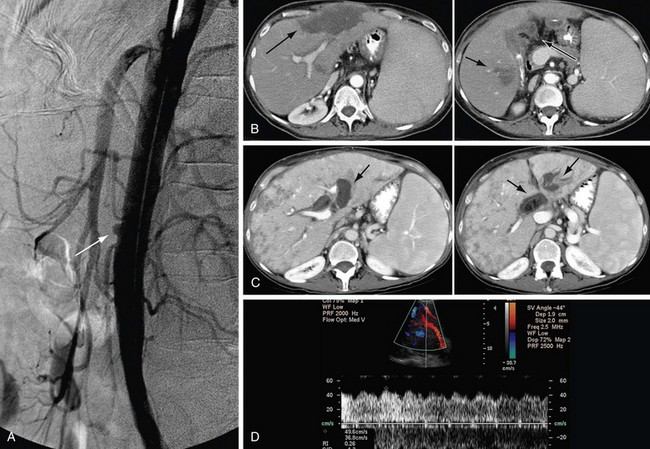
 FIGURE 111-14
FIGURE 111-14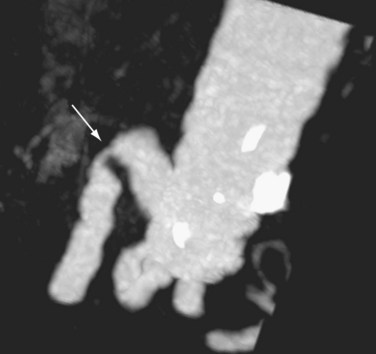
 FIGURE 111-15
FIGURE 111-15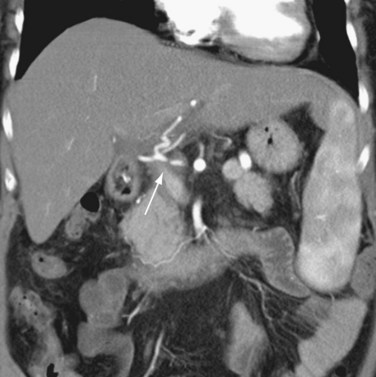
 FIGURE 111-16
FIGURE 111-16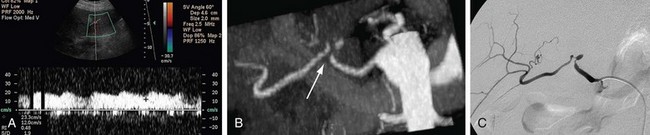
 FIGURE 111-18
FIGURE 111-18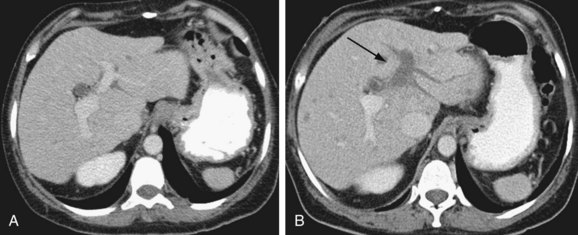
 FIGURE 111-19
FIGURE 111-19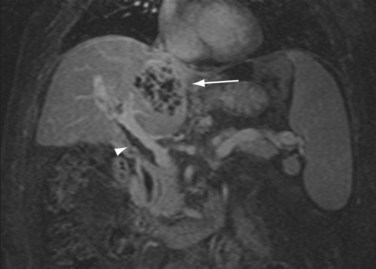
 FIGURE 111-20
FIGURE 111-20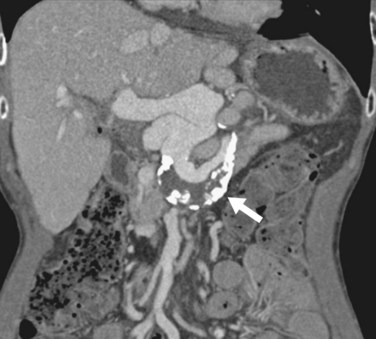
 FIGURE 111-21
FIGURE 111-21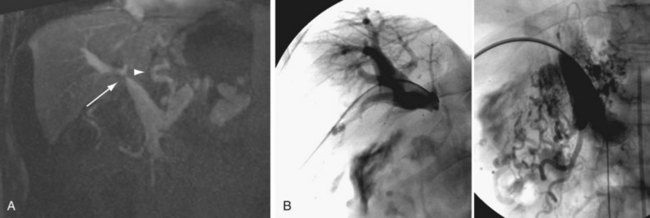
 FIGURE 111-22
FIGURE 111-22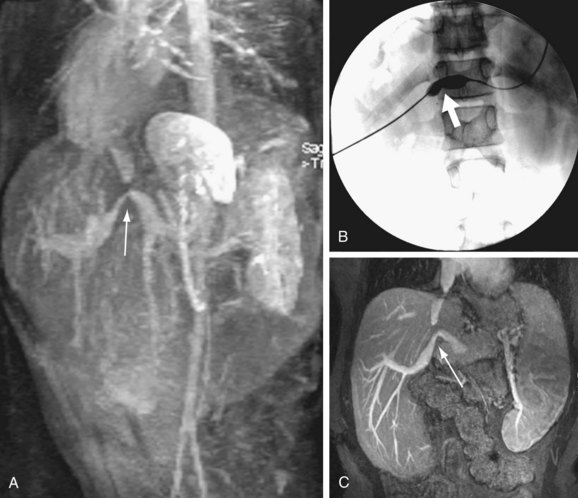
 FIGURE 111-23
FIGURE 111-23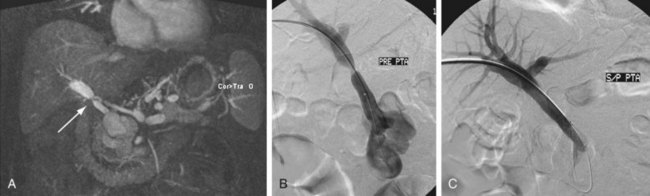
 FIGURE 111-24
FIGURE 111-24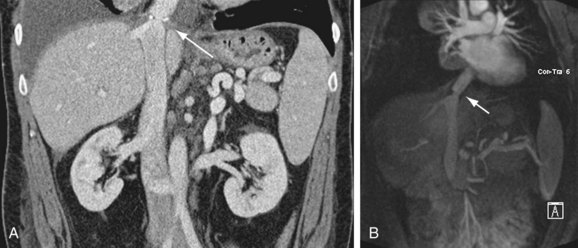
 FIGURE 111-25
FIGURE 111-25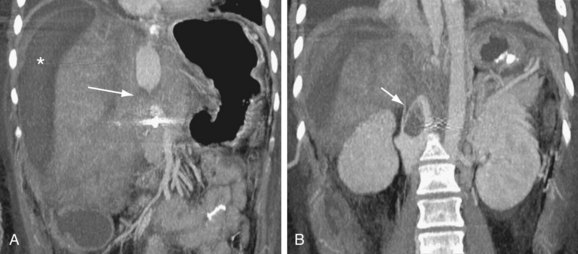
 FIGURE 111-26
FIGURE 111-26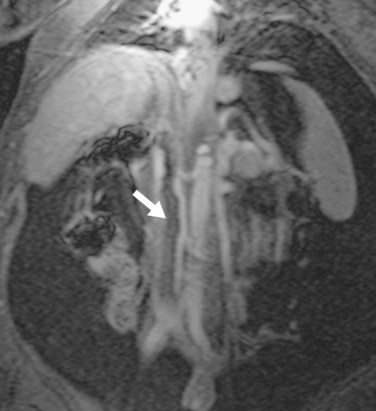
 FIGURE 111-27
FIGURE 111-27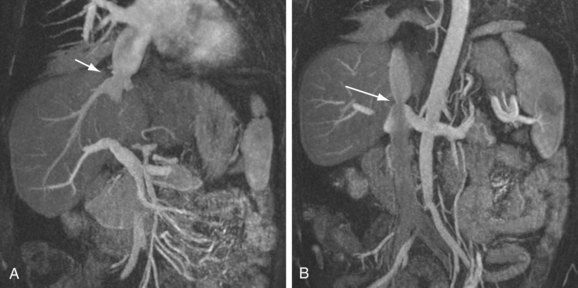
 FIGURE 111-28
FIGURE 111-28

