Chapter 11 Vascular Emergencies
ARTERIAL EMERGENCIES
Improved diagnostic methods and increasing specialization have enhanced our ability to treat vascular emergencies. The treatment of these patients has changed radically in recent years, becoming increasingly complex and involving a team approach, of which diagnostic and interventional radiologists are essential elements. In the evaluation of vascular emergencies the role of catheter angiography as a diagnostic tool is being progressively replaced by computed tomographic angiography (CTA) and sometimes magnetic resonance angiography (MRA). With technological advances these modalities, and particularly multidetector CTA (MD-CTA), have become an integral part in the initial assessment of acute vascular insults, as they are minimally invasive techniques, currently available in most emergency departments and trauma centers, and permit a prompt and accurate diagnosis within a short period of time. These are the methods of choice for the diagnosis of patients who do not have an indication for immediate surgical exploration. In addition, patients with direct signs (Box 11-1) of vascular injury on CTA can be taken to surgery without diagnostic angiography given its high sensitivity for the detection of vascular injuries. Furthermore, CT/CTA can be safely performed in patients with metallic fragments from bullets or other foreign objects, as opposed to MRI/MRA. MD-CTA has been increasingly used to diagnose arterial injuries from blunt and penetrating trauma not only to the chest and abdomen but also to the neck and extremities.
Arterial Emergencies of the Neck
Trauma of the Extracranial Carotid and Vertebral Arteries
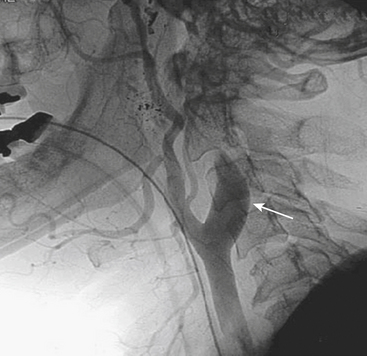
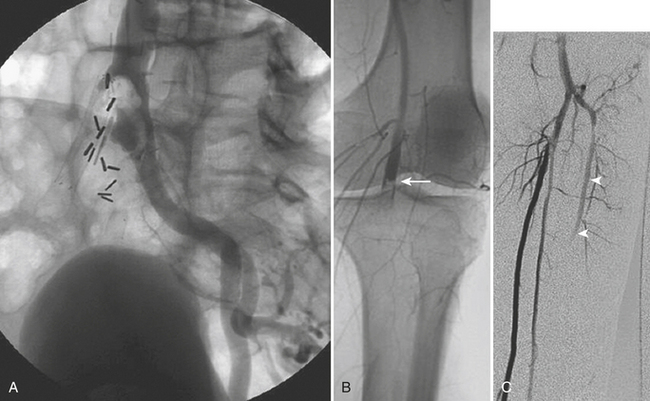
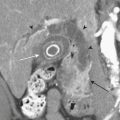
Classically, the neck is divided into three zones for injury classification and management purposes. Zone 1 is from the clavicles to the cricoid cartilage, zone 2 is from the cricoid to the angle of the mandible, and zone 3 is from the angle of the mandible to the skull base. Zones 1 and 3 are extremely difficult to approach surgically. Injuries to zone 2 are the most common (60% to 70%), and this represents a clinical advantage since they are readily accessible for physical examination and surgery. The management of unstable patients who have suffered a penetrating neck injury is emergency surgical exploration. For the hemodynamically stable patients who present with a wound that penetrates the platysma there is still some controversy, as the local standards of care and resources may vary (Fig. 11-1). In general, surgeons opt for a conservative approach starting with CT/CTA and use additional diagnostic studies or take the patient to surgery if medically indicated. CTA has high sensitivity and specificity with reported values for the diagnosis of cervical vascular injuries ranging between 90% and 100%, respectively. The routine use of conventional angiography has been discouraged because of the high number of examinations with negative findings and the potential risk for complications due to its invasive nature. Nevertheless, the presence of any indirect sign (Box 11-2) around the carotid artery should warrant a correlation with angiography given the potential catastrophic consequences of missing a lesion in this location.
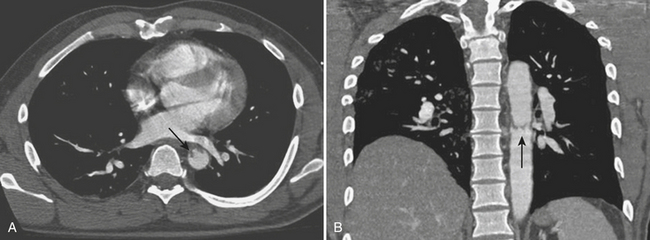
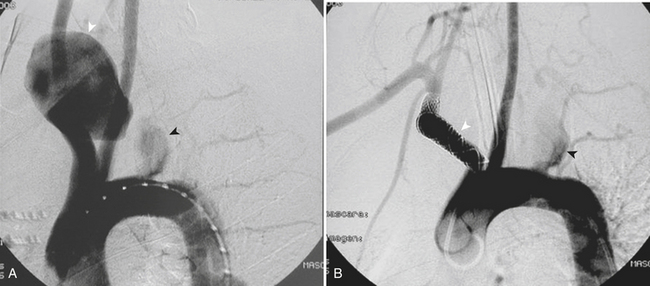
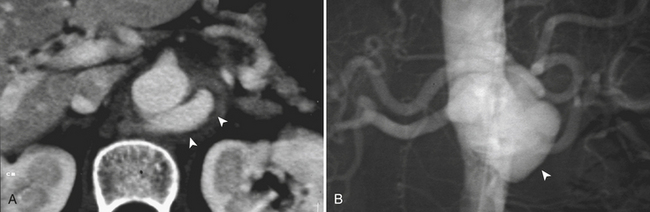
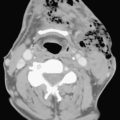
Blunt traumatic injuries of the extracranial carotid and vertebral arteries may have potential devastating consequences with an incidence that oscillates between 0.33% and 2.7% according to reports from centers performing aggressive angiographic screening. Although CTA and MRA have a great potential as screening tools in patients with blunt cerebrovascular injury, catheter angiography is still considered the gold standard. Intimal dissection and occlusion are more common with blunt trauma than with penetrating injuries (Fig. 11-2). Approximately 10% of patients have focal neurologic findings on initial presentation, and two thirds of the patients develop symptoms within 24 hours. The remainder may present with neurological findings weeks to months later.
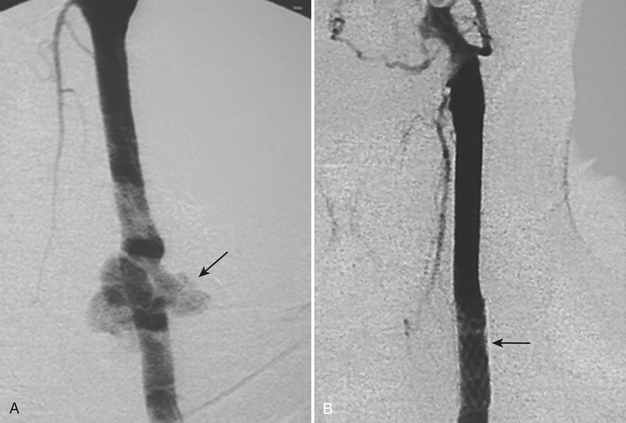
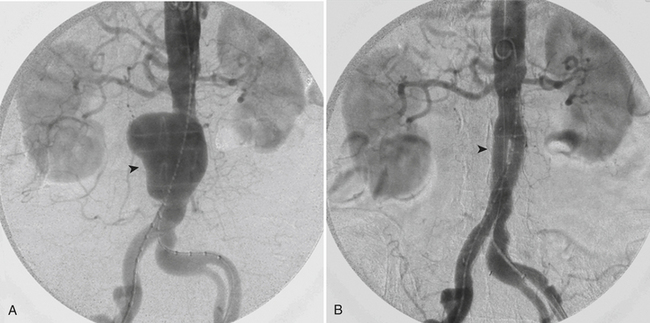
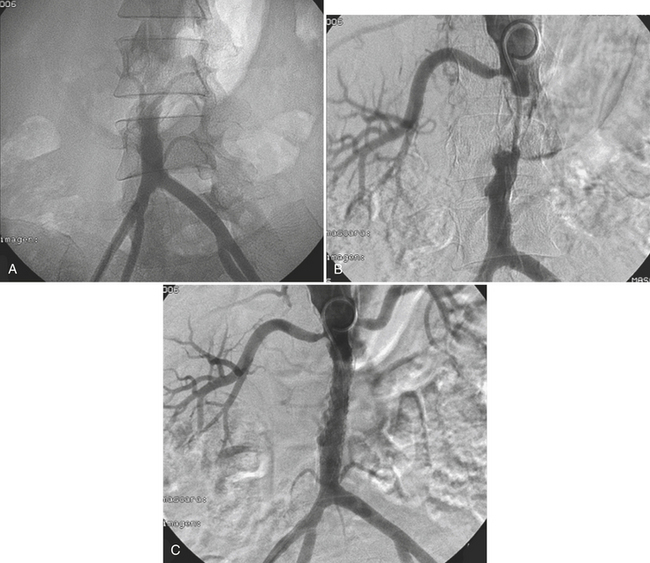
The type of intervention performed for carotid and vertebral injuries is determined by the nature of the lesion, the symptoms and clinical condition of the patient, and the feasibility of accessing the injured segment either with open surgery or with an endovascular approach. In general, intimal flaps and nonocclusive dissections are managed with anticoagulation provided there are no contraindications. Stent placement is reserved for flow-limiting dissections, while covered stents (stent grafts) have been successfully used to treat pseudoaneurysms and arteriovenous arteriovenous (AV) fistulas with good immediate results and patency rates (see Fig. 11-1). Embolization is another alternative for cases of vascular transection where surgical or endovascular vessel wall reconstruction cannot be achieved and adequate collateral pathway is demonstrated.
Nontraumatic Emergencies of the Carotid and Vertebral Arteries
Carotid and Vertebral Artery Dissection
Spontaneous dissection of the carotid or vertebral arteries in the absence of trauma is rare. It usually occurs in patients with underlying history of hypertension, fibromuscular dysplasia, or an underlying collagen disorder such as Marfan or Ehlers-Danlos syndrome. It can occur at any age, and clinical manifestations are variable, including neck pain or ipsilateral headache, Horner syndrome (ptosis, myosis, and unilateral anhydrosis), and acute or chronic focal neurologic events either in the form of transient ischemic attack or as a permanent neurological deficit. All imaging modalities including ultrasound, CT, and MRI can be used to demonstrate the intimal flap, a compressed true lumen, slow flow in the false lumen, or even thrombus formation. Catheter angiography is generally indicated to better assess flow patterns or the absence of flow (see Fig. 11-2). The majority of patients are successfully treated with anticoagulation, but stent placement in the true lumen may be necessary to maintain or restore cerebral perfusion.
Aortic Emergencies
Acute aortic syndromes encompass a spectrum of aortic emergencies that include traumatic aortic injury (TAI), aortic dissection, penetrating atherosclerotic ulcer of the aorta, intramural hematoma, and aortic aneurysm rupture. Chapter 2 covers the topics of aortic dissection, intramural hematoma, and penetrating ulcer. This section focuses on traumatic aortic injuries.
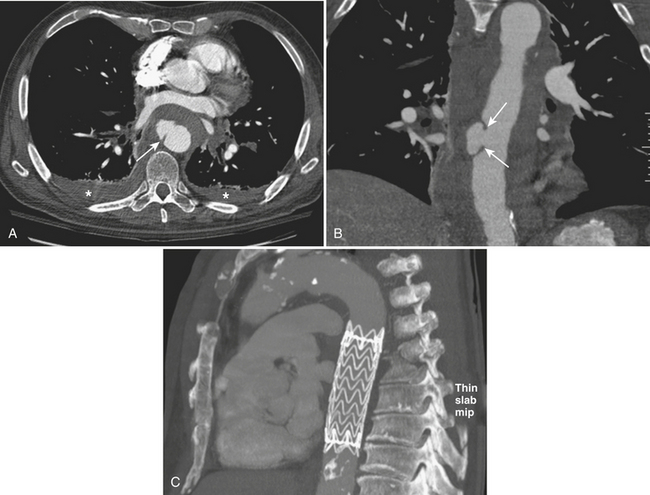
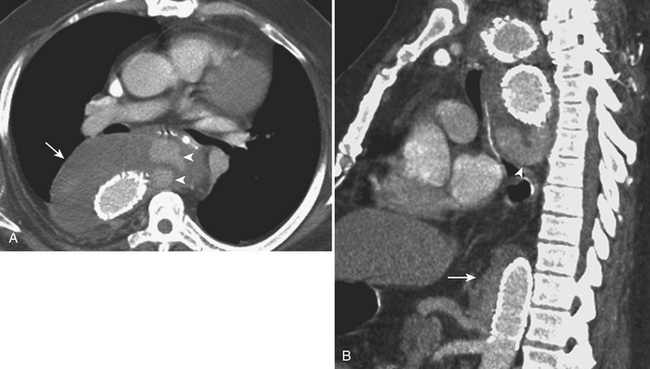
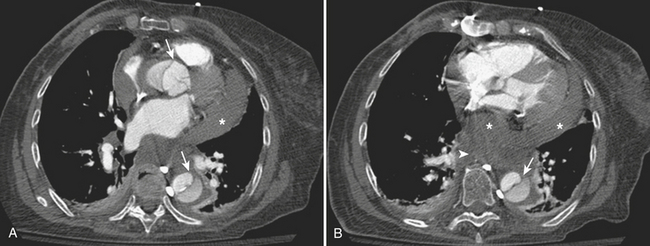
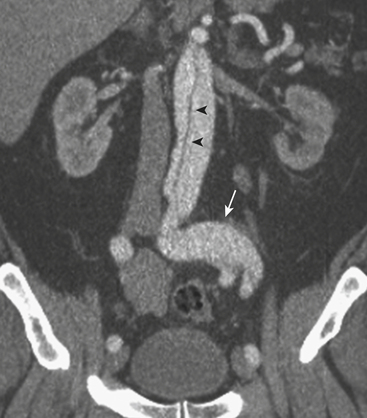
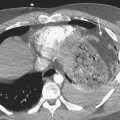
TAI is a novel term that encompasses a spectrum of injuries characterized by a variable degree of aortic wall laceration and that include intimal tear, intramural hematoma, traumatic dissection, traumatic pseudoaneurysm, and, in the most severe form, aortic transection. TAI may result from rapid decelerations, crush injuries, penetrating wounds, and surgical or angiographic instrumentation. Blunt trauma is the most frequent cause of TAI, usually as the result of shearing, hydrostatic forces, and/or torsion forces applied along the aortic arch during a motor vehicle accident and falls from heights. Less common causes include displaced clavicular and thoracic vertebral fractures with entrapment of the aorta between the anterior chest wall and the spine. The overall mortality at the scene of the accident has been reported to be as high as 80% in autopsy series, with only 10% to 20% of the victims surviving the initial trauma. Bleeding from a laceration or rupture can be controlled by the aortic adventia or the periaortic tissues, which can help the patient survive the initial injury; however, the end result is pseudoaneurysm formation, which, due to its instability, requires prompt diagnosis and treatment (Fig. 11-3). The region of the aorta most susceptible to blunt injury is the isthmus, where the relatively mobile proximal thoracic aorta and arch join the fixed distal arch at the insertion of the ligamentum arteriosum, just distal to the left subclavian artery origin (Fig. 11-4). This area is involved in as many as 90% to 95% of the cases. Other common areas of injury are the aortic root and the diaphragmatic hiatus. Injuries to the ascending aorta are uncommon (5% to 9%) and usually lethal due to the lack of surrounding connective tissue, resulting in rapid death due to exsanguination or cardiac tamponade. Clinical suspicion as well as a prompt diagnosis and treatment remain of crucial importance in TAI, given that 30% of the victims will die within 6 hours and 40% to 50% will die within 24 hours.
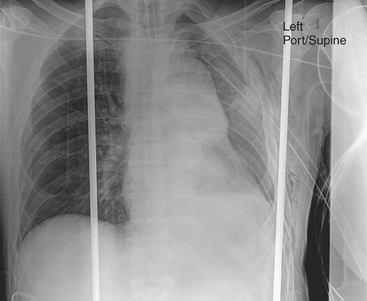
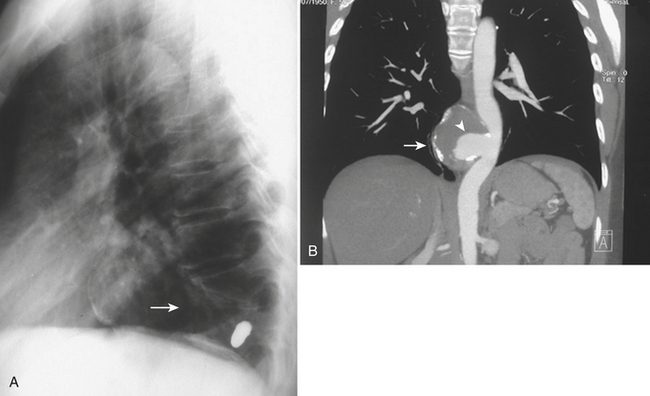
The initial imaging screening of trauma patients is obtained with plain radiographs due to the availability and capacity to quickly assess injuries such as pneumothorax, hemothorax, mediastinal hematoma (Fig. 11-5), and fractures. Clinical signs and symptoms found in patients with TAI include chest pain, back pain, dyspnea, cough, hoarseness, hypotension, pulse discrepancy, shock, and coma; as many as 30% to 50% of these patients may not show external signs of trauma.
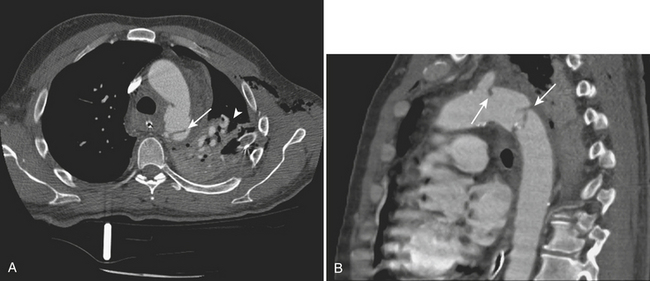
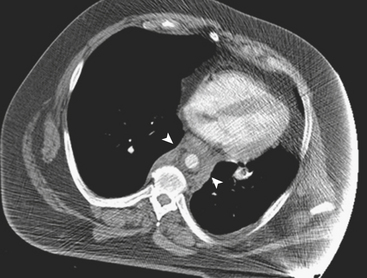
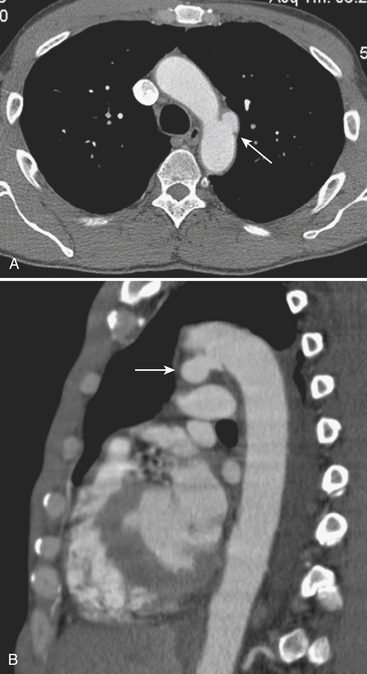
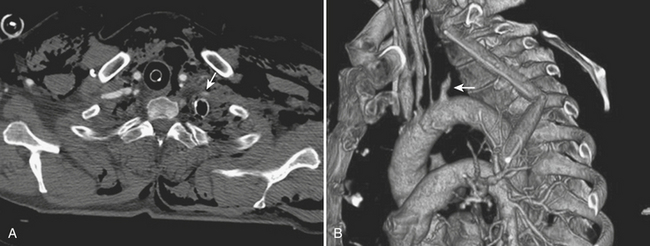
Catheter aortogram has long been considered the gold standard for the diagnosis of TAI with reported sensitivities and specificities approaching 100%. However, MD-CTA has become the preferred method for screening major traumatic and nontraumatic aortic emergencies, thanks to its improved spatial and contrast resolution and supplemental postprocessing techniques (thin sections, multiplanar reconstructions [MPRs], three-dimensional volume-rendered images), with a performance that rivals that of catheter angiography. MD-CTA has helped to better characterize the location and extent of TAI and other vascular injuries, allowing for faster diagnosis and treatment planning. In addition, MD-CTA can also recognize a greater number of normal variants of the vascular anatomy and subtle vascular injuries, which were less likely to be seen with conventional CT scans (Fig. 11-6). Furthermore, the need to perform an aortography to confirm the diagnosis of TAI is eliminated when direct signs of injury are present on CTA. Direct signs of TAI in CT include active extravasation of contrast, pseudoaneurysm formation, irregularity of the aortic wall, abrupt change in caliber of the aorta, aortic dissection, intimal flaps, and filling defects. Indirect signs of possible aortic injury include periaortic hematoma and mediastinal hematoma (Fig. 11-7).
Treatment
Alternative management of TAI consists of delayed operative intervention or no operation while the patient is kept under close monitoring and blood pressure (BP) control to reduce the risk of free rupture. The mainstay of treatment is to maintain systolic BP below 120 mm Hg (mean BP below 80 mm Hg) and allow patients who have suffered associated severe trauma to stabilize before surgical repair. This approach is indicated in hemodynamically stable patients with small aortic tears or those with associated injuries such as significant head, cardiac, or pulmonary trauma, large body surface burns, contaminated wounds, large retroperitoneal hematomas, or other high-risk medical comorbidities. Some of these aortic injuries might develop into a chronic pseudoaneurysm (Fig. 11-8), and others might even resolve during the period of observation. Close clinical and imaging follow-up is imperative to detect injury progression or aortic rupture.
More recently, endovascular treatment of TAI using stent grafts has been introduced as an alternative in the management of hemodynamically stable patients and patients with contraindications to cardiopulmonary bypass, such as severe coagulopathy, extensive additional injuries, and severe underlying cardiac or pulmonary disease. Mortality and morbidity appear to be lower than those of surgery, most likely because of the less invasive nature of the procedure, shorter operation time, and lack of the surgery-related comorbidities. The stent grafts are delivered via a common femoral artery cut-down access and positioned under angiographic and fluoroscopic guidance. The left subclavian artery origin often has to be covered with the endograft to provide adequate proximal support without significant clinical consequence in many patients. A significant number of patients with TAI are young, and therefore their aortic size is small; most stent grafts available today are made for the treatment of larger aortas with degenerative aortic aneurysms. Even though it is usually recommended to oversize the stent graft by 10% to 20% to ensure an adequate seal, excessive oversizing has been associated with type I endoleaks (Table 11-1) and stent graft collapse. Complications related to the endovascular repair include groin hematoma, iliofemoral dissection, endoleak, graft migration, and arterial rupture. Overall morbidity rates have been found to be around 12% with mortality rates of 4%. Clinical and imaging follow-up in both surgical and endovascular treatment modalities is required for early identification of post-treatment complications.
| Endoleak type I | Inadequate seal around the proximal or distal end of the stent graft |
| Endoleak type II | Retrograde flow from branch vessels (intercostal, lumbar, inferior mesenteric, gonadal arteries) resulting in persistent filling of aneurysm |
| Endoleak type III | Stent graft laceration or fabric tears, dislodgment of modular graft devices |
| Endoleak type IV | Porosity of the graft fabric, diffuse leakage through interstices |
| Endotension | Excluded sac continues to enlarge without apparent endoleak formation |
Penetrating Aortic Injury
Penetrating injuries to the intrathoracic great vessels are uncommon, with an incidence of 1%, and a high mortality rate that ranges between 50% and 85% despite advances in trauma care and prehospital resuscitation. These types of injuries can be caused by gunshot wounds (GSWs) or stab wounds (SWs) that traverse the chest or the base of the neck (Fig. 11-9). Overall, GSWs are more common and more lethal than SWs. Penetrating injuries to the thoracic aorta are more common along the ascending aorta and the arch branches, with a documented low incidence of descending thoracic injuries. These types of lesions are commonly associated with coexisting lethal intrathoracic injuries, which worsen the patient’s prognosis. Although the aorta is protected by osseous structures, a laceration in this large-caliber vessel with high intraluminal pressure can cause rapid exsanguination. Thoracic aortic injuries have a worse prognosis than injuries to the abdominal aorta, probably due to the retroperitoneal location of the abdominal aorta, which can slow down exsanguination. They usually manifest as hemorrhage into the mediastinum or pleural cavity presenting as a hemothorax, cardiac tamponade, or mediastinal hematoma.
Nontraumatic Aneurysms of the Thoracic and Abdominal Aorta
Thoracic Aortic Aneurysms
Thoracic aortic aneurysms (TAAs) are often the result of atherosclerotic disease or cystic medial degeneration with subsequent weakening and dilatation of the aortic wall. It is usually a silent process, less commonly seen than abdominal aortic aneurysm, and generally occurs in older men in their sixth and seventh decades. Based on their morphology, aneurysms are classified as either fusiform aneurysms, which involve the full circumference of the vessel wall, or saccular aneurysms involving only a focal portion. Thoracic aneurysms occur in the ascending aorta in 40% to 60% of the cases, descending aorta in 30% to 40% of the cases, and aortic arch and thoracoabdominal aorta in 10% of the cases. The incidence and prevalence of thoracic aortic disease including TAA are steadily increasing due to the aging population and improved diagnostic techniques. The reported incidence of TAA ranges from 6 to 10 cases per 100,000 patients per year. The natural history of untreated TAA is progressive expansion of the aneurysm and ultimately rupture (Fig. 11-10).
Given that most patients are asymptomatic, TAAs are commonly diagnosed as an incidental finding on imaging studies. However, they can also present with signs and symptoms related to compression of adjacent structures. Ascending aortic aneurysms can compress the coronary arteries or the origin of the great vessels causing myocardial or cerebral ischemia; together with arch aneurysms, they can erode into the mediastinum and compress the left recurrent laryngeal nerve causing hoarseness, or the phrenic nerve and lead to hemidiaphragmatic paralysis. They can also press on the tracheobronchial tree causing wheezing, dyspnea, cough, hemoptysis, and pneumonitis; on the esophagus and cause dysphagia; and on the superior vena cava (SVC) causing SVC syndrome. Mural thrombus from the aneurysms can be the source of emboli and cause strokes, as well as renal, mesenteric, or limb ischemia. Heart failure can result from aortic root aneurysms leading to aortic regurgitation, or from the rupture of a sinus of Valsalva aneurysm into the right side of the heart. Chest and back pain are rare symptoms associated with compression of intrathoracic structures or erosion into adjacent bones (Fig. 11-11).
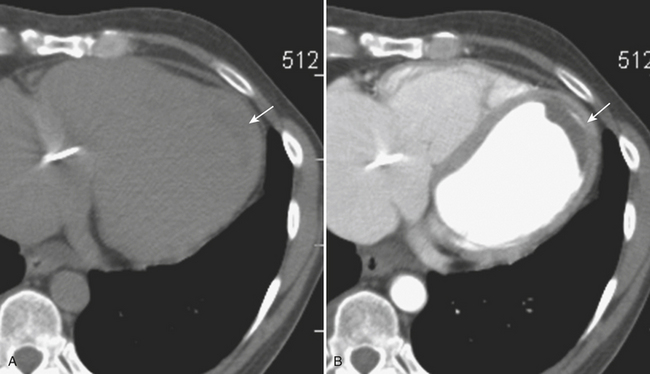
The most feared complications of TAA are aneurysm dissection and rupture. Dissection can lead to arterial occlusion and end-organ ischemia, while rupture can cause massive hemorrhage that usually cannot be contained by adjacent structures and therefore is considered a surgical emergency. Patients present with hypotension and acute onset of chest pain, abdominal pain, back pain, or neck pain. Rupture occurs more commonly into the left pleural space, but it can also occur into the pericardium causing pericardial tamponade (Fig. 11-12), and into the esophagus resulting in aortoesophageal fistula and dramatic upper gastrointestinal bleeding.
Multiple etiologies have been associated with thoracic aortic aneurysms, and they differ depending on their location within the thoracic aorta. Aneurysms of the ascending aorta and aortic arch are more commonly seen in elderly patients secondary to atherosclerosis or aortic valve stenosis leading to poststenotic dilatation. The younger patient population presenting with thoracic aneurysms in the ascending aorta and aortic arch (Tables 11-2 and 11-3) usually have a connective tissue disorder such as Marfan syndrome (mutation of fibrillin-1 gene), Ehlers-Danlos syndrome (defect in type III collagen), Loeys-Dietz syndrome, or Turner syndrome (associated to bicuspid aortic valves). Other infrequent etiologies include mycotic and syphilitic aneurysms. Aneurysms of the sinus of Valsalva can be congenital, infectious, or postsurgical in origin, and they are seen as dilatations in connection with the aortic root. Vasculitis can affect all portions of the thoracic aorta. Takayasu arteritis is one of the most striking entities, characterized by a chronic inflammatory process of unknown etiology that affects the aorta, its major branches, and the pulmonary arteries. It affects the vessel wall causing obliterative luminal changes, occlusion, or dilatation. Aneurysm formation can be a fatal complication of this disease since it may lead to heart failure secondary to aortic valve regurgitation or aortic rupture.
| Degenerative | Associated with atherosclerosis |
| Inheritable/connective tissue | Marfan syndrome |
| Ehlers-Danlos syndrome | |
| Loeys-Dietz syndrome | |
| Turner syndrome | |
| Osteogenesis imperfecta | |
| Rheumatoid arthritis | |
| Bicuspid aortic valve | |
| Aneurysm of sinus of Valsalva | |
| Arteritis | Giant cell arteritis |
| Takayasu arteritis | |
| Behcet’s disease | |
| Relapsing polychondritis | |
| Infectious | Syphilis |
| Mycotic aneurysms |
Table 11-3 Aortic Arch Aneurysms
| Degenerative | Secondary to atherosclerosis |
| Arteritis | Giant cell |
| Takayasu | |
| Behcet’s disease | |
| Infectious | Syphilis |
Thoracic aneurysms of the descending aorta are also most commonly degenerative in origin caused by atherosclerosis and usually associated with hypertension, hypercholesterolemia, and smoking. These aneurysms are generally fusiform with significant intimal calcifications and demonstrate associated tortuous aorta and mural thrombus. Other causes include chronic aortic dissection (Stanford type A or B), which can dilate over time as the wall of the false lumen weakens, creating a dissecting aneurysm with a high risk of rupture. Injuries to the descending thoracic aorta associated with blunt trauma can result in pseudoaneurysm formation, characteristically located near the aortic isthmus distal to the subclavian artery, and with a high risk of rupture (these are discussed in the TAI section). As with ascending thoracic aneurysms, younger patients with descending thoracic aortic aneurysms usually have an underlying connective tissue disorder (Table 11-4) or vasculitis. Mycotic aneurysms can also affect this segment.
Table 11-4 Descending Thoracic Aortic Aneurysm
| Degenerative | Secondary to atherosclerosis |
| Focal pseudoaneurysm secondary to penetrating aortic ulcer | |
| Arteritis | Giant cell |
| Takayasu | |
| Behcet’s disease | |
| Inherited/connective tissue | Marfan syndrome |
| Ductus aneurysm | |
| Loeys-Dietz syndrome | |
| Infectious | Mycotic aneurysms |
| Traumatic | Post-traumatic pseudoaneurysms |
| Chronic aortic transection |
Aneurysms located in the thoracoabdominal aorta are less frequent than abdominal or thoracic aneurysms, which is fortunate because these are difficult-to-treat lesions due to the numerous visceral branches that arise along this segment. They are divided into four types according to the Crawford classification (Table 11-5). Types II and III are the more complex ones, with type II having the highest risk of treatment-induced spinal cord infarction and renal failure.
Table 11-5 Crawford Classification of Thoracoabdominal Aneurysms
| I | Descending thoracic aorta to suprarenal aorta |
| II | Proximal descending thoracic aorta to infrarenal aorta (below the diaphragm) |
| III | Mid-descending thoracic aorta to infrarenal aorta |
| IV | Supravisceral aorta to infrarenal aorta |
Diagnosis
CTA provides sufficient information to diagnose and follow up progression of TAA; angiographic sequences allow for precise delineation of the extension of the aneurysm, involvement of the great vessels, and other associated thoracic pathology (see Fig. 11-11). Unenhanced CT is used to identify areas of aortic calcification, mural thrombus, acute wall hematoma (circumferential or crescentic), and recent hemorrhage. Contrast administration helps to differentiate the patent lumen from mural thrombus and to demonstrate an intimal flap and a false lumen in areas of aortic dissection. CT also shows the relationship of the aneurysm to the adjacent structures and helps correlate associated patient symptomatology with imaging findings. Mycotic aneurysms can affect any portion of the aorta and are seen as saccular dilatations with multilobulated contour (Fig. 11-13). Cross-sectional CT imaging features include a perianeurysmal soft tissue mass, fluid collection, and occasionally gas-forming inflammation. Syphilitic aortic aneurysms are rarely seen nowadays, but if encountered they usually demonstrate extensive aortic calcification or linear calcifications with longitudinal wrinkling of the wall causing a “shaggy tree-bark” pattern; approximately 75% of the cases exhibit a saccular morphology.
Treatment
TAA repair depends on its location. Aneurysms located in the ascending aorta are usually treated surgically via a median sternotomy with aneurysm resection and prosthetic tube graft placement. Surgical treatment is recommended for patients who are symptomatic, present with large aneurysms, have a high aneurysmal growth rate, or have associated complications. The best time to treat patients with TAA is still uncertain given the limited understanding of its natural history. Nonetheless, for most ascending thoracic aneurysms, repair is considered in patients with aneurysms that are 5.5 cm in diameter or larger. The decision to intervene is made depending on the patient’s operative risks versus the risk of developing an aneurysm-related complication such as dissection or rupture (e.g., patients with Marfan syndrome have their aneurysms repaired earlier due to the high risk of dissection and valvular insufficiency). Mortality of elective surgical repair in ascending aortic aneurysms ranges from 3% to 5%, and it carries a high risk of postsurgical morbidity including paraplegia, stroke, and bleeding; however, successful repair practically eliminates the risk of rupture. Lesions that are not treated by a surgical approach can have a mortality rate as high as 74%. Repair of arch aneurysms is even more challenging due to the high risk of stroke during replacement of the abnormal arch and reimplantation of the brachiocephalic vessels. Elective surgical repair for descending thoracic and thoracoabdominal aortic aneurysms has an associated mortality rate of 5% to 14% and a significant risk of paraplegia due to occlusion of the spinal cord blood supply; the risk has decreased with the implementation of protective techniques such as regional epidural hypothermic protection and cerebrospinal fluid drainage. Indications for surgical repair of descending aortic aneurysms include diameter equal to or larger than 6.5 cm, a patent primary entry site, expanding false lumen of either a dissection or aneurysm, symptomatic patients, or signs of impending rupture (Box 11-3).
Endovascular treatment of aortic arch aneurysms requires surgical transposition of supra-aortic vessels and is currently used in selected cases to help reduce the risk inherent in surgical repair. Patients with descending thoracic aortic aneurysms can also be offered endograft repair as an alternative to surgical treatment, in particular those patients with high operative risk. Endografts successfully exclude the aneurysm sac in most patients, with an apparent lower rate of persistent neurologic deficits in the range of 2% to 3% (versus 5% to 6% for surgically treated patients). Nevertheless, stent grafts are also associated with complications such as stroke, paraplegia, and device-related complications such as endoleaks and graft migration. Long-term studies are on their way to assess the durability of endograft aneurysm repair (see Fig. 11-10).
Abdominal Aortic Aneurysms
Diagnosis
MD-CTA has emerged as the new gold standard for the diagnosis of aortic abdominal aneurysms, replacing catheter arteriography. MD-CTA offers high-resolution imaging and shorter scan times, allowing the detection and characterization of aneurysms and related complications including impending rupture and contained or complete rupture. Several signs of impending rupture have been identified (see Box 11-3) on CT including increased aneurysm size, a low thrombus-to-lumen ratio, and mural thrombus hemorrhage that is usually identified as high-attenuation crescents in the wall of the aortic aneurysm in unenhanced CT images (Fig. 11-14). There are various signs that suggest a contained AAA rupture, including loss of definition of the posterior aortic wall; presence of an organized hematoma with contour abnormalities of the vessel wall; interruption of a continuous ring of aortic wall calcification; and a posterior wall of the aorta following the contour of the vertebra with or without associated vertebral erosion (sign known as a “draped” aorta). A ruptured AAA is determined by the presence of hemorrhage contiguous to the aorta, almost always involving the retroperitoneal space and rarely the iliopsoas compartment. Periaortic blood might be seen in the pararenal space, perirenal space, or both; a hematocrit sign (cellular-fluid level) and rectus sheath bleeds are more rare but helpful if present, and tend to be associated with coagulopathic hemorrhage (Fig. 11-15).
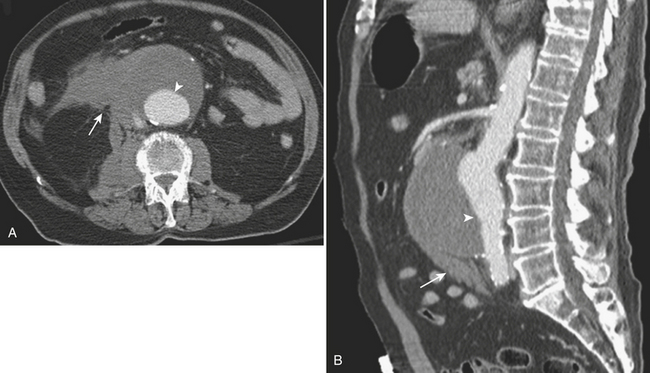
Figure 11-15 A and B, CTA images demonstrating a large infrarenal aortic aneurysm (arrowheads) with rupture (arrows).
(Courtesy of Dr. Daniel J.A. Margolis, UCLA, David Geffen School of Medicine.)
CT is also useful in differentiating between different types of aortic aneurysms. Mycotic aneurysms are usually seen as saccular-shaped collections with a lobulated contour; other features may include a periaortic soft tissue mass with stranding, retroperitoneal para-aortic fluid collection, vertebral erosion, gas-forming inflammation around the aneurysm, intra-aortic air pockets, and thrombus formation within a false lumen after aneurysmal rupture (see Fig. 11-13). In inflammatory aneurysms, CT and MRI can detect the cuff of soft tissue inflammation surrounding the aneurysm, thickening of the aneurysm wall, perianeurysmal and retroperitoneal fibrosis, and adherence of the anterior aneurysm wall to adjacent structures (Fig. 11-16). On precontrast CT images the thickened aortic wall has soft tissue attenuation that enhances after intravenous contrast administration. The arterial wall may become undistinguishable from periaortic fibrosis. Periaortic fibrotic tissue can adhere to the ureters, small bowel, duodenum, and inferior vena cava causing entrapment of these structures and further complicating surgical repair. Preoperative imaging is of great importance in the planning of surgical and endovascular treatment of patients with AAA. Important preoperative features and measurements done on CTA include the maximum transverse aneurysm diameter, relation of the aneurysm to the renal arteries, presence of a proximal neck (renals to aneurysm distance), presence of a distal neck (aneurysm to aortic bifurcation distance), extension of the aneurysm into the iliac arteries, identification of concomitant aneurysms, and appearance of the access pathway including femoral and iliac arteries. Some conditions that limit the delivery of an endograft include very large AAA, markedly tortuous aorta, pelvic arteries with abrupt angles, and extensive calcification causing luminal narrowing. Any of those factors can represent an exclusion criterion depending on the specific anatomy, and the degrees of angulation and stenosis. They determine the suitability or unsuitability for endovascular treatment, and cautious analysis of the images in conjunction with the surgical/interventional team is crucial to decrease the risk of endoleaks, attachment failures, graft migration, and conversion to open repairs. MRA has shown similar properties as CTA for the diagnosis of and for preoperative planning for AAA, providing precise information about the location and extent of the aneurysm. Conventional arteriography is currently used as an adjunct imaging modality for evaluating patients with complex vascular anatomy, for aneurysms that could not be fully characterized with CTA and MRA, and for intraprocedural graft positioning.
Treatment
Endovascular aortic aneurysm repair (EVR) offers a less invasive approach to reduce the operative morbidity and mortality. Blood losses are significantly reduced since the graft is placed intravascularly via femoral access, and, likewise, the risk for lower limb and visceral ischemia is lower since aortic clamping is not performed. EVR is offered to patients who are undergoing repair for asymptomatic AAA or symptomatic nonruptured AAA or those who are at high risk surgically and have significant comorbidities. The role of the endograft is to exclude the aneurysmal sac from the arterial circulation and decrease the mechanical stress over the vessel wall (Fig. 11-17). Studies have shown benefits in the perioperative mortality and a 30-day morbidity and mortality rate of less than 3% with EVR. Despite its short-term advantages, long-term survival and quality-adjusted life expectancy do not seem to vary significantly when compared with open surgery repair.
A common complication related to stent graft placement is the development of endoleaks (Fig. 11-18). An endoleak is defined as an incomplete exclusion of the aneurysmal sac. The estimated incidence oscillates in the 10% to 45% range, and currently lifelong imaging follow-up is recommended in order to detect endoleaks and other graft-related complications. Delayed rupture is rare (0.1% to 1% per year) and has been associated with type I and type III endo-leaks, graft migration, and endograft kinking. When type I or type III endoleaks are recognized, immediate treatment is indicated through graft extension, reintervention, or conversion to open repair. Other serious complications include graft infection and occlusion (Fig. 11-19). Occlusion is usually due to distortion of one of the graft limbs and can cause lower extremity ischemia if not promptly treated. Continuous aortic expansion at the “neck” can cause endograft migration and delayed type I endoleak, but this is rare since endografts are oversized at the time of placement by up to 20% to ensure adequate seal.
Traumatic Abdominal Aortic Injury
Traumatic abdominal aortic injuries are relatively rare, accounting for only 4% to 6% of all aortic injuries. Despite medical advances, it remains one of the most lethal causes of early death in trauma with a mortality rate that ranges between 50% and 80%. Penetrating injuries to the abdominal aorta that cause complete transection are uncommonly seen in the hospital setting due to rapid exsanguination. The most common causes of traumatic penetrating injuries to the aorta include gunshot wounds and stab wounds. Besides the obvious risk that a direct aortic injury implies, the high mortality is also linked to the high incidence of associated injuries to other organs. Abdominal aortic injuries due to penetrating trauma have lower mortality rates when compared with thoracic aortic injuries due to the compartmental retroperitoneal location of the abdominal aorta. However, when the aortic rupture extends beyond the retroperitoneum into the suprarenal segment or intraperitoneally, the protective effect of retroperitoneal tamponade is lost and the risk of death increases. Common complications seen in patients who survive penetrating injuries include arteriovenous fistula and pseudoaneurysm formation (see Fig. 11-11).
Nontraumatic Aortic Dissection
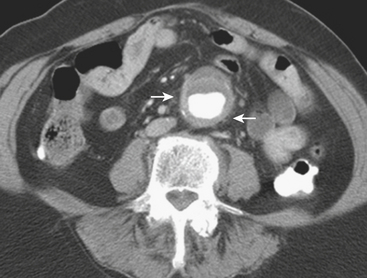
Spontaneous isolated dissection of the abdominal aorta is a rare event with an estimated incidence of 2% to 4% and is more frequently the extension of a thoracic aortic dissection. It is caused by a tear in the intima, usually associated with degeneration of the media or cystic medial necrosis. Blood separates the intima from its surrounding media creating a false lumen filled with blood, which can propagate distally or proximally to the initial tear. Isolated abdominal aorta dissections more frequently originate below the renal arteries with some originating even lower, near the inferior mesenteric artery. Propagation of the dissection can involve branch vessels including the renals and the celiac and mesenteric arteries or can extend into the iliac arteries (Fig. 11-20). The renal arteries are involved most frequently. The most common predisposing factors to abdominal aortic dissection are hypertension and atherosclerosis. Other etiologies include penetrating atherosclerotic ulcer, aortic aneurysms, trauma, Marfan syndrome, fibromuscular dysplasia, and iatrogenic causes secondary to surgical or angiographic procedures. Clinical presentation is usually characterized by sudden onset abdominal or back pain, but it may vary from completely asymptomatic to frank visceral or limb ischemia. MD-CTA allows a fast, accurate, and precise diagnosis. CTA findings of aortic dissection include visualization of an intimal flap separating the true and false lumen, inhomogeneous enhancement of the aortic lumen, presence of a double channel in the aorta, and asymmetric vessel wall thickening. Unenhanced images can help identify internal displacement of the intimal calcifications. The imaging findings that help distinguish the false lumen from the true lumen include the “beak” sign, which represents a wedge-shaped area of the false lumen at the edge of the dissection; the “cobweb” sign, manifested as low-attenuation linear densities that represent residual strands of medial tissue that did not separate completely from the intima during the dissection and are floating in the false lumen; and a relatively larger cross-sectional area of the false lumen with respect to the true lumen. CT can also assess the extent of the dissection and possible involvement of visceral and iliac vessels (see Fig. 11-20). MRA is also a highly accurate diagnostic tool that has the advantage of showing different sequences and can even demonstrate flow dynamics with the implementation of flow-enhanced sequences and cine images. In addition, some of the noncontrast MRA sequences offer an alternative to those patients in whom the use of contrast is limited because of severe impairment of the renal function or iodine allergy. Unfortunately, MRI/MRA has restricted availability in the emergency setting, is usually more time-consuming, and has limited applicability in emergency patients who may have noncompatible implants, metallic fragments, and numerous monitoring devices attached. MRI is then usually reserved for stable patients to confirm the diagnosis after equivocal imaging findings in previous exams.
Acute Abdominal Aortic Occlusion
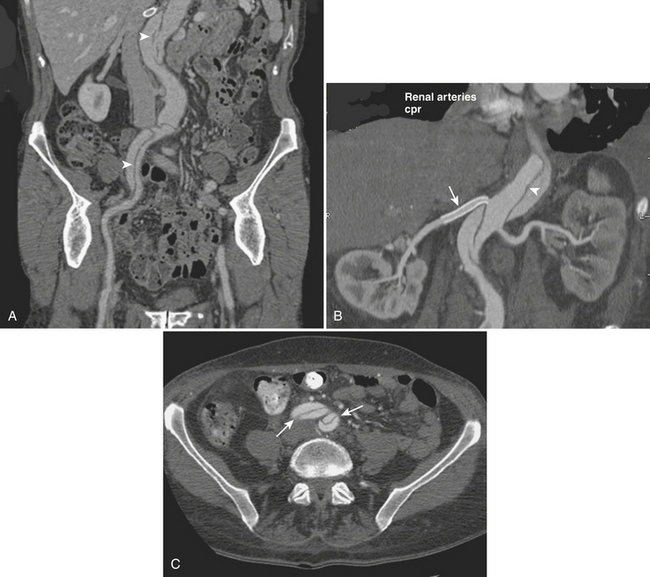
Acute thrombosis of an AAA causing AAO is rare. Aortic dissection can also cause AAO by propagation of the dissection to the aortic bifurcation and compression of the true lumen by the false lumen. This is also a rare event and is considered an emergency requiring urgent surgical repair or fenestration of the dissection. Less common causes of acute aortic thrombosis include trauma, iatrogenic injuries, and hypercoagulable states (antithrombin III, protein C/protein S deficiencies, lupus, among others). AAO is usually preceded by a progressive stenosis with associated signs and symptoms of chronic ischemia, such as lower extremity claudication and rest pain. A partial occlusion allows for collateral circulation to build over time and maintain supply even in the setting of a superimposed acute event; these patients present with worsening claudication or pain at rest. However, presentation may be more severe, as symptoms typically get aggravated with development of cyanosis below the level of the umbilicus, pallor and coldness of the lower extremities, absent femoral pulses, and neurologic symptoms ranging from numbness and weakness to complete anesthesia and paralysis. The diagnosis of AAO can be made with ultrasound, CTA, and catheter angiography. The use of MRA is limited for the various reasons cited previously but can also be used if readily available. All cross-sectional modalities can also detect branch vessel involvement and determine the extension of the thrombus; however, ultrasound is operator dependent and can be limited by the patient’s habitus and bowel gas interposition. MD/CTA scanning can evaluate the complete thoracoabdominal aorta and the cardiac chambers when looking for a cardiac source of emboli (Fig. 11-21). The treatment of AAO requires immediate systemic anticoagulation to prevent thrombus propagation, hydration, and optimization of cardiac and renal function. As mentioned above, surgery is the first treatment option and should be performed promptly. A delay in treatment can predispose to the development of limb ischemia, compartment syndrome, thrombus propagation to renal and mesenteric arteries, reperfusion syndrome, limb loss, and even death. Surgery usually involves aortic repair attempt, aortobifemoral bypass, or, in high surgical risk patients, an axillobifemoral bypass. Despite surgical intervention, acute abdominal aortic occlusion has a high mortality rate of approximately 50%. In select patients, catheter angiography may provide a therapeutic alternative if there is realistic probability of reestablishing patency and signs of irreversible ischemia are not yet present (Fig. 11-22).
Abdominal Compartment Syndrome
Compartment syndrome (CS) is defined as an increased pressure in a body compartment that causes deficient tissue perfusion and risks tissue and organ viability. Abdominal CS (ACS) is a critical condition characterized by a continuous elevation of the intra-abdominal pressure (IAP, normal 0 to 5 mm Hg) associated with abdominal distention and leading to respiratory insufficiency with decreased lung capacity, increased airway pressure, hypoxia, hypercarbia, reduced cardiac output, oliguria, and eventually multiorgan failure. Increased intracranial pressure and impairment of the portosystemic circulation can also result from the rise in central venous pressure. Abdominal trauma with hepatic, vascular, and/or splenic injury is the most common cause of acute ACS. Other causes are massive fluid resuscitation and packing for uncontrolled hemorrhage. Ruptured aortic aneurysms can cause ACS by increasing abdominal fluid volume (Box 11-4).
Vascular Emergencies of the Mesenteric-Visceral Arteries
Nontraumatic Emergencies of the Mesenteric and Visceral Vasculature
Acute Gastrointestinal Bleeding
Acute gastrointestinal bleeding is classified into upper and lower causes based on its origin proximal or distal to the ligament of Trietz. Upper gastrointestinal (GI) bleeds are approximately five times more common than lower gastrointestinal causes, and lower gastrointestinal bleeding originates in the colon in approximately 80% of the cases. Localization of the bleed is critical to determine management. Upper bleeds occur secondary to gastritis and gastric or duodenal ulcers, while the most common cause of lower GI bleed is colonic diverticulosis followed by angiodysplasia. Depending on the site of origin (proximal or distal) and the severity of the hemorrhage, a GI bleed will manifest as hematemesis, melena, and/or hematochezia (Fig. 11-23). A positive nasogastric aspirate and lavage is an effective way to determine if there is an upper GI bleed. In these patients, the initial evaluation is performed with endoscopy, which not only confirms the diagnosis and identifies the source in more than 95% of the cases, but also can offer therapy with sclerotherapy, clipping, cauterization, or banding. With lower GI bleeds, identification of the bleeding site with colonoscopy is more difficult, with an overall reported success rate of 70%. More than 85% of lower GI bleeds resolve spontaneously with supportive therapy alone (Fig. 11-24). It is crucial that all GI-bleeding patients be stabilized, with large bore venous accesses placed for fluid resuscitation and transfusion as needed. If the source of bleeding is still unknown despite endoscopy and colonoscopy, there are various diagnostic imaging alternatives available. One option is to perform a tagged red-blood cell nuclear scan, which can detect bleeding rates as low as 0.1 mL/min; however, its ability to localize the bleeding to a particular segment of bowel is limited. Catheter angiography is another option, which detects a bleeding rate of approximately 0.5 to 1 mL/min. Given the often intermittent nature of GI bleed, and the time required to stabilize and transfer the patient, angiograms are positive in only about 50% of the cases. However, if performed immediately after a positive tagged red-blood cell scan, or when obtained during an episode of active bleed, the likelihood of finding the source increases. MD-CTA is a promising noninvasive first-line diagnostic modality that offers fast scanning times that vary between 6 and 20 seconds and allows for accurate diagnosis or exclusion of active gastrointestinal hemorrhage by comparing pre– and post–intravenous contrast images and identifying abnormal hyperattenuating areas of extravasated contrast material within the bowel lumen.
Aneurysms of the Visceral Arteries
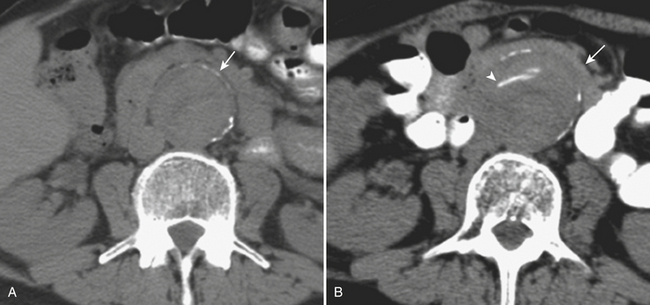
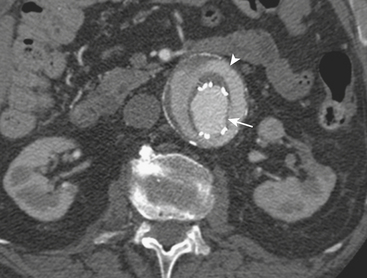
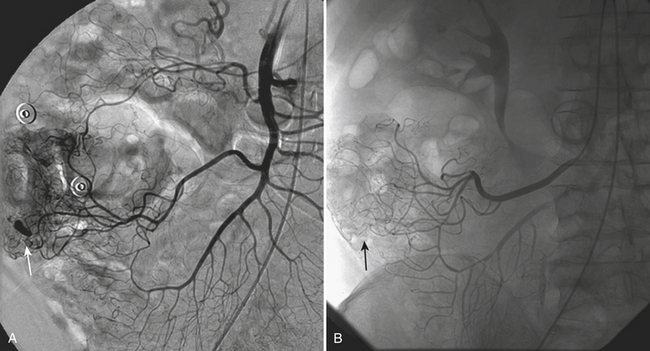
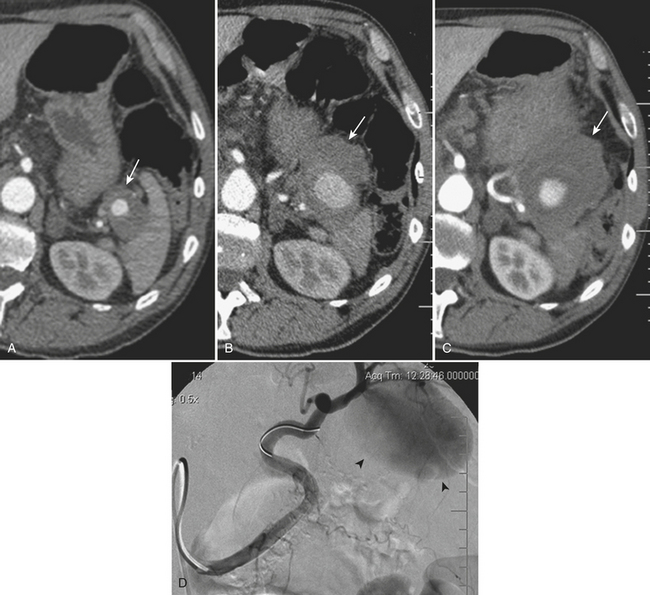
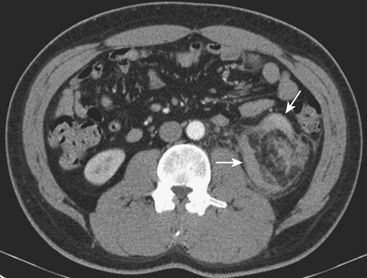
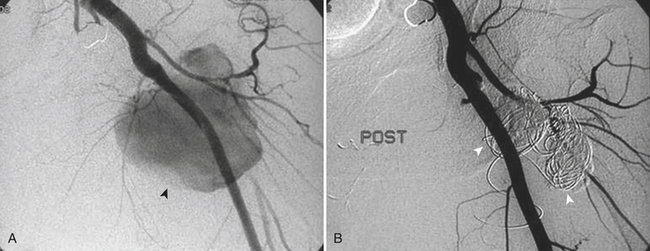
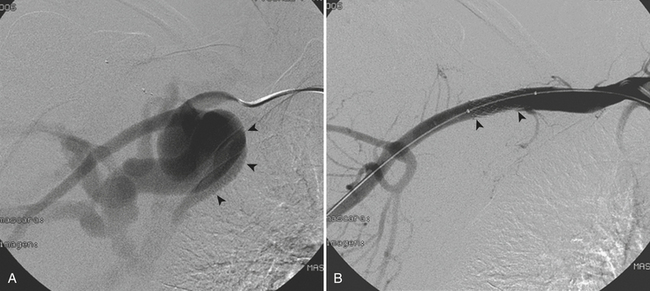
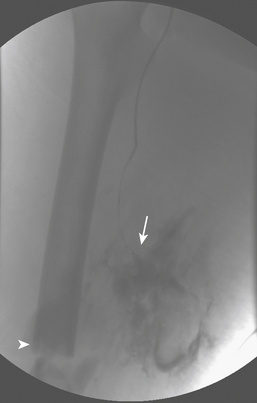
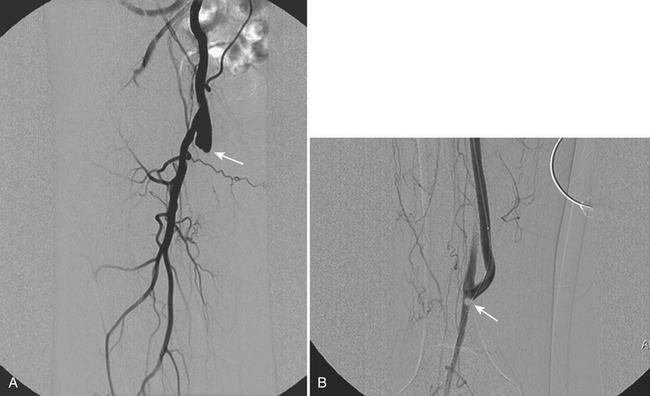
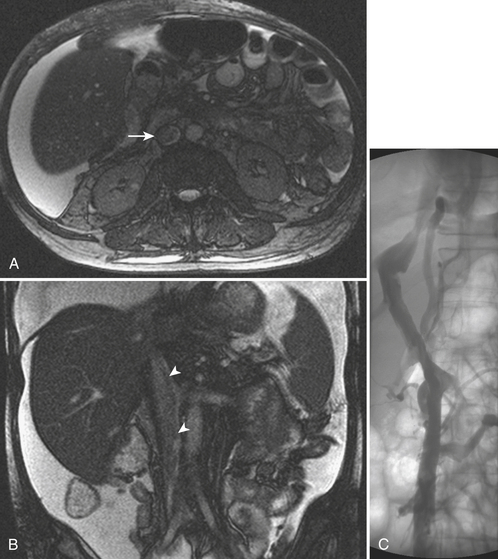
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after aortic and iliac aneurysms, and account for 60% of the cases of visceral aneurysms (Fig. 11-25). Most are saccular, single, and less than 3 cm in diameter. As with other intra-abdominal aneurysms, common causes include pancreatitis, atherosclerosis, trauma, and fibrodysplastic disease. Interestingly, these aneurysms are four times more prevalent in females than males, with higher incidence among pregnant and multiparous women. The catastrophic complication of rupture in pregnancy results in high mortality rates in the order of 70% to 90% for both the mother and fetus. Treatment options include surgical resection and embolization. Endovascular stent graft placement can be another option in select cases.
Involvement of the celiac, ileocolic, and gastroduodenal arteries has also been described. Aneurysms of the gastroduodenal artery are often associated with pancreatic pathology and can cause bleeding in the peritoneal or retroperitoneal space and more rarely into the portal vein or into a pancreatic pseudocyst (Fig. 11-26). In general, elective repair is recommended for splachnic aneurysms when they reach a size greater than 2 to 2.5 cm. Pseudoaneurysms are always considered to carry a high risk of rupture, and so repair is recommended on detection regardless of their size.
Traumatic Injury of the Renal Arteries
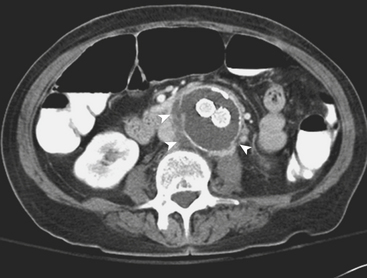
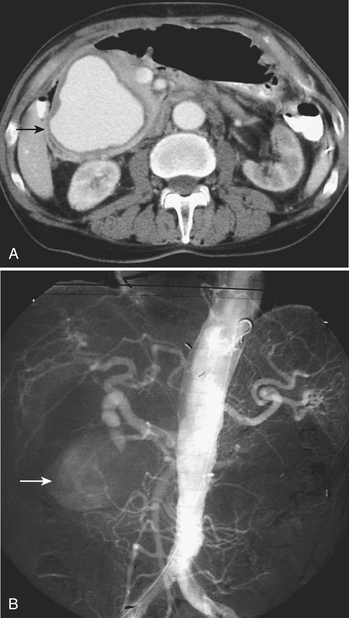
Iatrogenic renal vascular injuries can also be included in this category, as biopsies, ablations, and partial nephrectomies can lead to bleeding or pseudoaneurysms and AV fistula formation. These might result in active extravasation into the perirenal space or the renal collecting system (Fig. 11-27).
Nontraumatic Renal Arterial Emergencies
Acute Renal Ischemia
Normal kidneys have no significant collateral blood supply, and acute occlusion will lead to rapid loss of kidney function if not effectively revascularized within 1 to 2 hours. The most common cause of acute occlusion in middle-aged and elderly patients is embolic disease of a cardiac source, while in the young patient population it is trauma. Other etiologies include aortic or renal artery dissection, thrombosis of a focal stenosis, thromboembolism from renal artery aneurysm, and procedural complications (Fig. 11-28). Patients with acute renal artery ischemia classically present with flank pain and hematuria. Occlusion in the setting of progressive renal artery stenosis is less likely to end in acute ischemia as collateral flow usually via capsular, adrenal, or gonadal arteries will have developed. Both CT and MRI are useful to demonstrate the areas of asymmetric renal perfusion with generalized lack of enhancement seen with complete arterial occlusion or areas of segmental infarction due to smaller emboli. CTA and MRA can demonstrate renal artery stenosis, arterial dissection, and other renal artery anomalies as well as accessory arteries or collateral flow. Revascularization of acute renal artery occlusion is difficult due to the narrow time window before irreversible changes develop. If an occlusive dissection develops during renal artery angioplasty, stenting is indicated; if the occlusion is secondary to thrombosis, then treatment is done with thrombolysis, and thrombectomy or surgical revascularization is indicated.
Renal Neoplasm–Related Vascular Emergencies
Some benign and malignant renal neoplasms can be the source of acute clinical symptoms including pain, hemorrhage, and gross hematuria. Angiomyolipomas are benign renal neoplasms that contain fat, smooth muscle, and blood vessels. They are well known for their association with tuberous sclerosis and their tendency to bleed, particularly when larger than 4 cm. Ultrasound or CT evaluation is usually performed in the emergency setting due to acute flank pain that may be associated with hemodynamic changes. Images will demonstrate the acute renal and perirenal hemorrhage with or without contrast extravasation. The mass itself as well as the characteristic intralesional fat can usually be identified, although it can be difficult in the setting of acute hemorrhage (Fig. 11-29). The presence of other angiomyolipomas can help the diagnosis. These lesions are usually treated with percutaneous embolization using a combination of particles, coils, and sometimes alcohol. Follow-up scans are performed to document resolution of the hematoma and shrinkage of the lesion.
Vascular Emergencies of the Pelvis
Trauma of the Pelvic Arteries
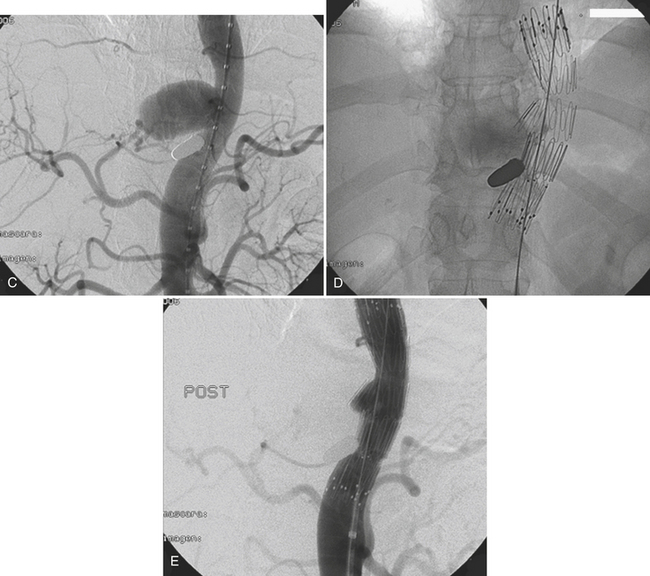
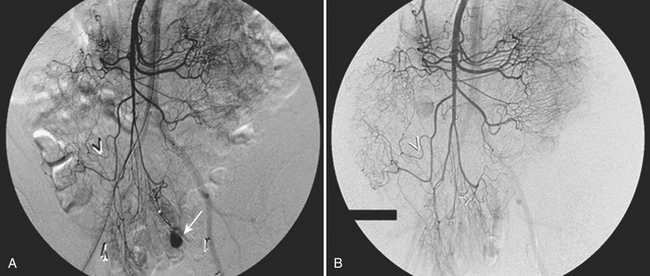
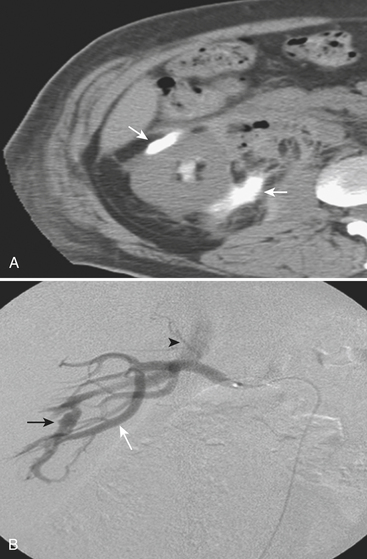
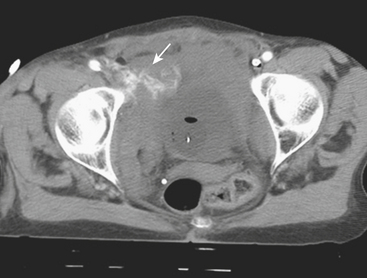
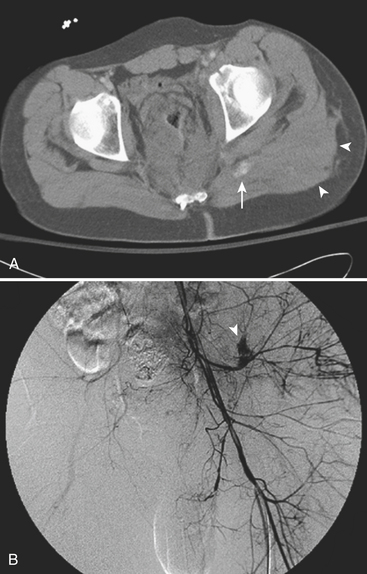
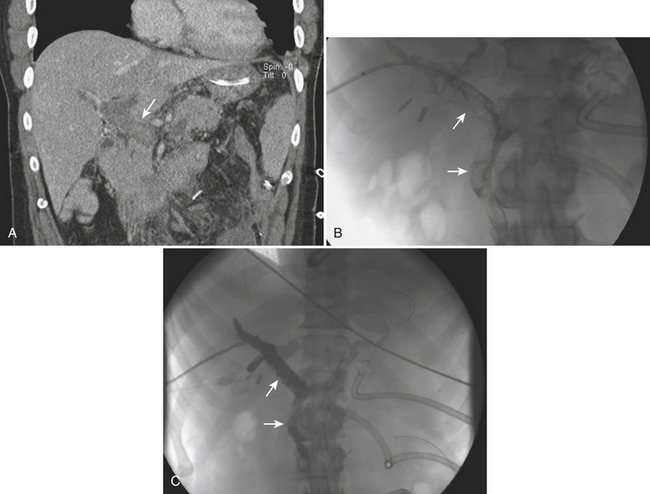
Traumatic injury to the vascular structures of the pelvis is more frequently the result of pelvic fractures in combination with violent shear and traction forces. Pelvic vascular injuries can lead to rapid hypovolemic shock and death from exanguination if not treated promptly. Most pelvic fractures are the consequence of lateral compression, and the majority of those are successfully stabilized with pelvic fixation. There are also unstable pelvic fractures secondary to more complex forces that do not respond well to pelvic fixation and can be aggravated by associated coagulopathy and cancellous bone bleeding. If the patients are hemodynamically stable, an expedited MD-CTA can be quite helpful in characterizing the fracture and assessing the location and degree of vascular involvement. When both intra-abdominal hemorrhage and pelvic fractures are present, the patients are taken directly to the operating room for repair of their abdominal injuries and pelvic fixation. Endovascular intervention is usually postponed until the more emergent conditions are treated, unless CT/CTA determines that the more ominous finding can be best approached with endovascular techniques (Fig. 11-30). Angiography in hemodynamically unstable patients should be directed first to a rapid evaluation of the source of bleeding and then expeditiously to embolization. A previous CT/CTA helps to direct the angiogram and possibly obviate the need for an initial nonselective pelvic angiogram, since the absence of abnormal findings in nonselective angiography does not exclude an active bleed and selective internal and external iliac angiograms are still mandated. It is important to note, however, that an initial pelvic angiogram can be useful to identify massive extravasation and depict the patient’s specific anatomy. Also, one should not confuse the normal cavernosal blush at the penoscrotal junction with an area of extravasation. The primary goal of embolization in patients with pelvic fractures is to promptly stop the hemorrhage and decrease the arterial flow to the injured vessel. Gelfoam pieces are mixed with contrast, and vessel embolization is performed until no further extravasation is identified (Fig. 11-31). In stable patients, subselective embolization can be performed, and other embolic agents such as coils and glue can be used. The use of covered stents has also been described with great success, but it is recommended that a specialist familiarized in their use be the one performing the procedure. Both coil embolization and stent graft placement have been used successfully to treat traumatic pseudoaneurysms (Fig. 11-32). After embolization, completion of the angiography is important to exclude other sources of bleeding.
Nontraumatic Emergencies of the Iliac Arteries
Iliac Occlusive Disease
Atherosclerosis is the most common cause of iliac occlusive disease, and it frequently involves the distal aorta. Patients tend to present with unilateral or bilateral leg claudication or ischemia depending on the level of obstruction and the presence of collateral circulation; bilateral symptoms suggest aortic involvement. Aortoiliac occlusive disease is part of the lower extremity peripheral vascular disease spectrum (see section covering the lower extremities), and as such it can lead to loss of the extremity or even the loss of life when it becomes acute. Causes for acute presentation include embolism, thrombosis, dissection, trauma, low cardiac output states, and hypotension. Emboli are the most common cause of sudden lower extremity ischemia, with 80% originating in the heart due to atrial fibrillation, valvular disease, or recent myocardial infarction. Emboli can also originate in the peripheral circulation proximal to the occlusion as a consequence of irregular or ulcerated plaque, aneurysms, and previous interventions such as stent grafts. Emboli tend to get wedged at bifurcation points or in areas where vessels narrow abruptly. The iliac arteries are involved in 18% of the cases of acute ischemia of the lower extremity, the aorta in 15%. Emboli tend to have a more acute and severe presentation owing to the lack of collateral circulation. Occlusive thrombosis is caused by the disruption of an atheromatous plaque that leads to exposure of its core products with subsequent activation of the coagulation cascade (Fig. 11-33). In patients who present with acute ischemic symptoms, a history of thigh or buttock claudication is suggestive of underlying iliac atherosclerotic disease. Leriche syndrome is described as the combination of intermittent claudication, impotence, and significantly decreased or absent femoral pulses. This syndrome can be identified in approximately one third of male patients with aortoiliac occlusive disease and indicates chronic peripheral arterial insufficiency due to narrowing of the distal aorta. Iliac occlusive disease can also present as “blue toe” syndrome when it causes distal emboli.
Catheter angiography is usually reserved for cases where intervention is anticipated or to answer specific inquiries regarding pressure gradient measurements and information on flow dynamics. Definitive treatment of hemodynamically significant aortoiliac disease is usually done by aortobifemoral bypass, with a 5-year patency rate of approximately 90%. Patients in whom aortoiliac disease becomes symptomatic but who have comorbidities that increase the operative risk may benefit from a less invasive approach such as endovascular repair with angioplasty and stenting, axillary-femoral bypass, or femoro-femoral bypass. Focal concentric iliac artery stenosis usually responds well to angioplasty with more than 60% primary patency rate at 4 years; however, since stents are approved for iliac use, most iliac lesions are treated nowadays with primary stenting, with a 4-year patency rate of almost 80% for stenotic lesions and 60% for recanalized occlusions. Minimally invasive treatment of bilateral common iliac artery occlusive disease will require placement of bilateral stents, also known as “kissing” stents when they touch each other at the aortoiliac confluence. When dealing with patients complaining of acute or recent onset of symptoms and a fresh thrombus is suspected, thrombolysis is an excellent recanalization alternative (see Fig. 11-33).
Iliac Aneurysms
Degenerative aneurysms of the iliac arteries tend to involve the common iliac artery (CIA) and are frequently seen in association with abdominal aortic aneurysms or dissection (Fig. 11-34). External and internal iliac artery aneurysms are rare. As with AAA, the most feared complication is aneurysm rupture, which can be free, contained, or into a venous structure. Findings of rupture can be subtle with only mild perivascular stranding noticed, or dramatically florid with retroperitoneal and/or intraperitoneal hemorrhage. Rupture into venous structures causes high output congestive heart failure. Chronic ruptures usually contain thrombus associated with saccular dilatation and disruption of intimal calcification. Isolated CIA aneurysms warrant repair when they reach 2.5 to 3.0 cm in diameter, and they can be managed with stent graft placement provided there is an adequate access pathway and sufficient landing zones to allow appropriate attachment of the graft.
Iliac Artery Dissection
Dissection of the iliac arteries is usually an extension of thoracoabdominal aortic dissection. Primary iliac artery dissection is relatively rare and has been described in association with fibromuscular dysplasia and vigorous training in professional athletes. Both CTA and MRA are excellent for the evaluation of thoracoabdominal and iliac dissection (see Fig. 11-20).
Vascular Emergencies of the Extremities
Arterial Injury of the Upper Extremities
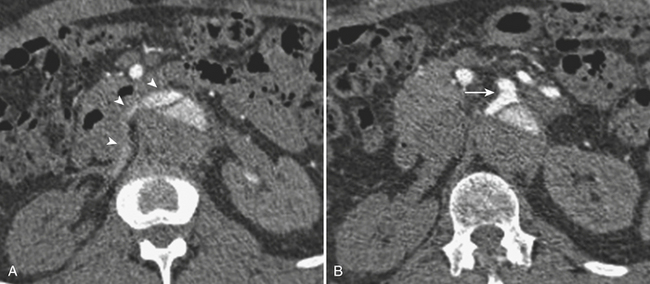
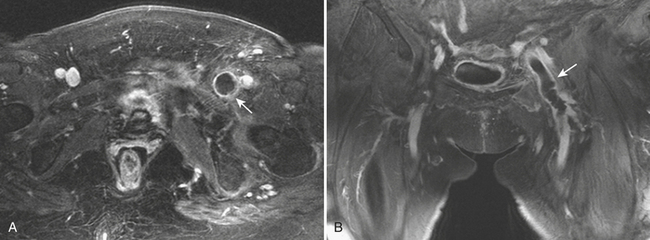
Traumatic vascular lesions to the upper extremities can occur in as many as 40% to 50% of penetrating trauma patients when “hard” (or direct) clinical signs are present. Hard clinical signs include pulsating hemorrhage, expanding hematoma, presence of a thrill or bruit, pulse deficit, and extremity ischemia (Box 11-5). Indirect (or “soft”) signs include stable hematoma, extensive soft tissue injury, adjacent fracture or adjacent nerve injury, nonpulsatile bleeding, and delayed capillary refill (Box 11-6). In the absence of hard clinical signs the patients can be treated conservatively, and noninvasive imaging modalities play a major role in their evaluation and follow-up. Besides the conventional penetrating and blunt traumatic injuries that any vessel is subject to, the arteries of the upper extremity are also at risk for unique injuries due to the major functional role they play in our lives. Among them are stretch injuries that occur during extreme traction when attempting to stop a fall. The subclavian-axillary segment is particularly at risk, and associated brachial plexus injuries are common. Intimal tears and disruption of the media can then lead to thrombosis and distal embolization (Fig. 11-35). Other injuries include blunt trauma from incorrect use of crutches, affecting the axillary-brachial segment, iatrogenic injuries during central venous access procedures, and injuries related to self-administered drugs.
CTA is the preferred imaging modality to emergently assess the arteries of the proximal upper extremities, reserving catheter angiography for patients with equivocal diagnosis or when therapeutic intervention is required. CTA offers the advantage of simultaneously assessing for additional nonvascular injuries, while conventional angiography offers the advantage of diagnosing and treating at the same time. Both modalities can identify any of the vascular injuries including intimal tear, occlusion, pseudoaneurysm, active extravasation, complete transection, and arteriovenous fistulas. Branch vessel extravasation and pseudoaneurysms can be treated with coil embolization. Main arterial pseudoaneurysms and fistulas can also be treated with embolization or covered stents (Fig. 11-36).
Arterial Trauma of the Lower Extremities
Vascular injuries of the lower extremities are a serious complication of both penetrating and blunt trauma. The rate of limb loss with major injuries is approximately 15%, and 25% of the patients eventually develop some degree of limb dysfunction due to related osseous or nerve injury. As mentioned previously, clinical signs of arterial injury are divided into “hard” and “soft” signs (see Boxes 11-5 and 11-6). Patients with a profoundly ischemic limb or active hemorrhage from the wound are taken directly to surgery, while stable patients with viable limbs should undergo a complete limb and pulse examination. A fast determination of the ABI can be helpful, as the incidence of vascular injury in patients with a normal ABI and normal examination is less than 10%. This number can increase to 20% when there are “soft” signs present and the ABI is less than 1, and increases to 40% when the ABI is less than 1 and the mechanism is a gunshot wound or there is associated pulse deficit or neurologic deficit.
In the setting of a normal physical exam, a penetrating wound in proximity to vascular structures is considered an indication for angiography only in cases in which the bullet tract follows the course of a major artery over a long segment. In the past, some practices called for angiography to be performed on every patient with a penetrating injury in proximity to a runoff vessel and when the overall identification of clinically relevant vascular injuries was less than 10%. CTA has become a useful minimally invasive tool in this scenario, with the ability to accurately diagnose the type of injury as well as its extent and location. CTA can depict injuries that do not routinely require surgical intervention, such as intimal flaps, branch vessel occlusions, and partial luminal narrowing with preservation of distal flow. Catheter angiography remains as a useful examination for patients with nonconclusive CTA findings and whenever endovascular transcatheter therapy is indicated. In general, partial injuries (such as intimal flaps) are managed conservatively, branch vessel extravasation may require embolization with coils (Fig. 11-37), and pseudoaneurysms are treated with embolization or stent grafts. Major injuries demand open surgical repair when extensive reconstruction or bypass is required.
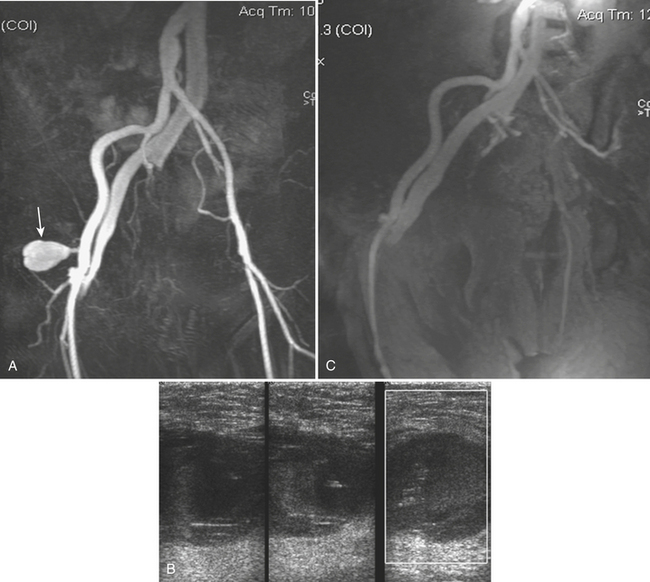
Femoral Artery Pseudoaneurysm
Pseudoaneurysm of the femoral artery is more frequently the result of an arterial puncture to perform cardiac or peripheral angiography, occurring in 1% to 5% of the cases. Patients may present with a large inguinal hematoma or a pulsatile mass. Most pseudoaneurysms thrombose spontaneously, but up to 3% to 5% may persist, increase in size, or rupture. Because of their location these aneurysms are generally diagnosed with duplex ultrasound. Occasionally MRA or CTA is required when they cannot be adequately characterized with ultrasound due to their large size or multilobulated characteristics, ultrasound’s inability to visualize the pedicle, or when they persist despite an initial attempt to treat them. Most femoral pseudoaneurysms can be treated with ultrasound-guided compression of the pedicle (or neck) to interrupt the flow and induce thrombosis; when this fails, thrombin injection can be performed under direct ultrasound visualization to control the aneurysm flow as the thrombin is being administered (Fig. 11-38).
Nontraumatic Acute Lower Extremity Limb Ischemia
Acute lower limb ischemia is most frequently the result of atherosclerosis that gradually progresses to the point of complete occlusion; it manifests with insufficient limb perfusion and worsening pain that may acutely exacerbate. The level of arterial compromise correlates closely with the location of ischemic symptoms; aortoiliac disease manifests as pain in the thigh and buttock, whereas femoral-popliteal disease manifests as pain in the calf. Symptoms of chronic peripheral vascular disease are precipitated by walking a predictable distance and are relieved by rest (claudication). Ischemic rest pain is more worrisome; it can be partially relieved by placing the extremity in a dependent position, so that perfusion is facilitated by the effect of gravity. An acute, profoundly ischemic leg is a surgical emergency. Among the causes for acute limb ischemia are emboli, plaque disruption and thrombosis, dissection, trauma, and low cardiac output states. Among the various causes of acute limb ischemia, the major diagnostic goal is to differentiate embolic from thrombotic occlusion. Emboli are the most common cause of sudden lower extremity ischemia, and approximately 80% originate in the heart due to atrial fibrillation, valvular disease, or recent myocardial infarction. Emboli can also originate in the peripheral circulation (proximal to the level of occlusion) as a consequence of irregular or ulcerated plaques, aneurysms, dissection flaps, previous interventions, or stent grafts (Fig. 11-39). Emboli tend to get wedged at bifurcation points or stenotic areas; the femoral artery bifurcation is the most common site (40%), followed by the iliac arteries (20%), the aorta (15%), and the popliteal arteries (15%). Acute thrombosis occurs more often in the setting of established atherosclerotic disease, where the disruption of an existing atheromatous plaque leads to exposure of its core products and subsequent activation of the coagulation cascade.
Catheter angiography is indicated when emergent surgical revascularization is not required to salvage a viable extremity and is generally reserved for cases where endovascular intervention is anticipated. The evaluation should include the aortic bifurcation and runoff vessels if CTA or MRA has not been performed. If indicated, thrombolysis can be performed (see Fig. 11-39). Thrombolytic therapy is contraindicated in the presence of intracranial tumor, recent surgery, intracranial bleed, severe hypertension, bleeding at a noncompressible site, and gastrointestinal hemorrhage. Contraindications for thrombolysis have been further subdivided into absolute and relative, understanding that clinical judgment for each patient and careful evaluation of the risks and benefits are crucial in the decision-making process (Boxes 11-7 and 11-8). Follow-up angiograms are obtained in patients treated with thrombolytic infusion to reassess patency and determine the underlying cause for the occlusion.
VENOUS EMERGENCIES
Venous Emergencies of the Abdomen
Inferior Vena Cava Obstruction
The clinical presentation of IVC obstruction varies. A slow, progressive occlusion allows for the development of collateral flow, while a more acute occlusion causes edema of the lower extremities and even hypotension from decreased blood return. Ultrasonography, CT, and MRI can be useful in identifying IVC occlusion, thrombosis, and extrinsic compression by surrounding structures or tumor (Fig. 11-40). Acute thrombus may appear hyperdense on CT. Direct IVC venography is rarely required for diagnosis and is usually obtained only if a filter is being considered or if thrombolysis is to be performed.
Portal Vein Thrombosis
Portal vein thrombosis is more frequently chronic in nature, and results from a focal occlusion that leads to the development of collateral pathways, known as cavernous transformation of the portal vein. Eventually, portal hypertension and portosystemic collaterals develop, which may cause variceal gastrointestinal bleed. Acute thrombosis of the portal vein is rare and usually occurs in patients with underlying hypercoagulable states or severe trauma. It may induce thrombosis of the superior mesenteric vein and cause intestinal ischemia. Endovascular recanalization with thrombolysis and thrombectomy can be attempted from a transjugular or transhepatic approach in selected cases (Fig. 11-41).
Venous Emergencies of the Pelvis and Lower Extremities
The widespread use of CT to identify pulmonary emboli has led researchers to look for DVT during the same exam. Results demonstrate that thrombus can be identified in up to 10% of the lower extremity and pelvis scans obtained during the venous phase of enhancement of pulmonary CTA. MR venography has a sensitivity and specificity greater than 95% for the detection of DVT; however, its current use in the acute setting remains limited (Fig. 11-42). Conventional catheter venography has been practically abandoned for diagnosis of DVT. Today, catheter venography is used only in patients who represent a diagnostic dilemma or in those who require a therapeutic intervention. Thrombi are seen as filling defects outlined by contrast material. Differentiation of acute and chronic DVT can be sometimes challenging but the presence of collateral veins is a helpful sign of chronicity. The traditional treatment of acute DVT is anticoagulation to prevent extension of the thrombus while the endogenous mechanisms progressively lyse the clot and reestablish patency. With this approach, complete resolution of the thrombosis is achieved in up to 50% of the cases. Thrombolysis and mechanical thrombectomy can be performed as well. The principle is to macerate the thrombus to expose greater portions of the thrombus to the lytic agent and at the same time facilitate its removal or breakdown by endogenous mechanisms. The great majority of patients have prompt relief of their symptoms, and venous patency at 1 year is maintained in more than 70% of the patients. There are many contraindications to thrombolytic therapy, mostly involving factors that increase the risk of serious bleeding, such as recent surgery, upper gastrointestinal bleeding, recent CVA, and central nervous system tumor or trauma. Bleeding requiring transfusion was reported in 11% of patients in the national venous thrombolysis registry, and the rate of intracranial bleeding was only 0.2%. Venous stent placement after venous thrombolysis is usually reserved for patients with underlying stenosis of a large vein.
AbuRahma A.F. Fate of endoleaks detected by CT angiography and missed by color duplex ultrasound in endovascular grafts for abdominal aortic aneurysms. J Endovasc Ther. 2006;13:90-95.
Ahrar K., Smith D.C., Bansal R.C., et al. Angiography in blunt thoracic aortic injury. J Trauma. 1997;42:665-669.
Al-Bahrani A.Z., Abid G.H., Sahgal E., et al. A prospective evaluation of CT features predictive of intra-abdominal hypertension and abdominal compartment syndrome in critically ill surgical patients. Clin Radiol. 2007;26:676-682.
Asensio J.A., Chahwan S., Hanpeter D., et al. Operative management and outcome of 302 abdominal vascular injuries. Am J Surg. 2000;180:528-534.
Atar E., Belenky A., Hadad M., et al. MR Angiography for abdominal and thoracic aortic aneurysms: Assessment before endovascular repair in patients with impaired renal function. AJR Am J Roentgenol. 2006;186:386-393.
Attia C., Villard J., Boussel L., et al. Endovascular repair of localized pathological lesions of the descending thoracic aorta: Midterm results. Cardiovasc Intervent Radiol. 2007:30:628-637.
Berthet J.P., Marty-Ane C.H., Veerapen R., et al. Dissection of the abdominal aorta in blunt trauma: Endovascular or conventional surgical management? J Vasc Surg. 2003;38:997-1004.
Bhalla S., Menias C.O., Heiken J.P. CT of acute abdominal aortic disorders. Radiol Clin North Am. 2003;41:153-169.
Biffi W.L., Ray C.E.Jr., Moore E.E., et al. Noninvasive diagnosis of blunt cerebrovascular injuries: A preliminary report. J Trauma. 2002;53:850-856.
Bolognesi R., Manca C., Tsialtas D., et al. Aortic intramural hematoma: An increasingly recognized aortic disease. Cardiology. 1998;89:178-183.
Branchereau A., Jacobs M., editors. Vascular Emergencies. Elmsford, NY: Wiley-Blackwell, 2003.
Brandt M., Walluscheck K.P., Jahnke T., et al. Endovascular repair of ruptured abdominal aortic aneurysm: Feasibility and impact on early outcome. Vasc Interv Radiol. 2005;16:1309-1312.
Castaner E., Andreu M., Gallardo X., et al. CT in nontraumatic acute thoracic aortic disease: Typical and atypical features and complications. RadioGraphics. 2003;23(Suppl):S93-S110.
Chan F.Y., Crawford E.S., Coselli J.S., et al. In situ prosthetic graft replacement of mycotic aneurysm of the aorta. Ann Thorac Surg. 1989;47:193-203.
Demers P., Miller D.G., Mitchell R.S., et al. Stent-graft repair of penetrating atherosclerotic ulcers in the descending thoracic aorta: Mid-term results. Ann Thorac Surg. 2004;77:81-86.
Deree J., Shenvi E., Fortlage D., et al. Patient factors and operating room resuscitation predict mortality in traumatic abdominal aortic injury: A 20-year analysis. J Vasc Surg. 2007;3:493-497.
Dogra VS, Bhatt S (eds): Emergency Cross-Sectional Imaging. Radiol Clin of North Am 45, 2007.
Dyer S., Moore E.E., Ilke D.N., et al. Thoracic aortic injury: How predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1,561 patients. J Trauma. 2000;48:673-683.
Evangelista A., Mukherjee D., Mehta R.H., et al. Acute intramural hematoma of the aorta: A mystery in evolution. Circulation. 2005;111:1063-1070.
Faggioli G., Stella A., Freyrie A., et al. Early and long-term results in the surgical treatment of juxtarenal and pararenal aortic aneurysms. Eur J Vasc Endovasc Surg. 1998;15:205-211.
Fang J.F., Wong Y.C., Lin B.C., et al. Usefulness of multidetector computed tomography for the initial assessment of blunt abdominal trauma patients. World J Surg. 2006;30:176-182.
Farber A., Wagner W.H., Cossman D.V., et al. Isolated dissection of the abdominal aorta: Clinical presentation and therapeutic options. J Vasc Surg. 2002;36:205-210.
Fattori R., Napoli G., Lovato L., et al. Descending thoracic aortic disease: Stent-graft repair. Radiology. 2003;229:176-183.
Federle M.P., Pan K.T., Pealer K.M. CT criteria for differentiating abdominal hemorrhage: Anticoagulation or aortic aneurysm rupture? AJR Am J Roentgenol. 2007;188:1324-1330.
Fishman J.E. Imaging of blunt aortic and great vessel trauma. J Thorac Imaging. 2000;15:97-103.
Fishman J.E., Nunez D.Jr., Kane A., et al. Direct versus indirect signs of traumatic aortic injury revealed by helical CT: Performed characteristics and interobserver agreement. AJR Am J Roentgenol. 1999;172:1027-1031.
Garzon G., Fernandez-Velilla M., Marti M., et al. Endovascular stent-graft treatment of thoracic aortic disease. Radiographics. 2005;25(Suppl):S229-S244.
Gavant M.L. Helical CT grading of traumatic aortic injuries: Impact on clinical guidelines for medical and surgical management. Radiol Clin North Am. 1999;37:553-573.
Görich J., Asquan Y., Seifarth H., et al. Initial experience with intentional stent-graft coverage of the subclavian artery during endovascular thoracic aortic repairs. J Endovasc Ther. 2002;9(Suppl 2):II-39-II-43.
Gunn M., Campbell M., Hoffer E.K. Traumatic abdominal aortic injury treated by endovascular stent placement. Emerg Radiol. 2007;13:329-331.
Hallet J.W. Splenic artery aneurysms. Semin Vasc Surg. 1995;8:321-326.
Halliday K.E., al-Kutoubi A. Draped aorta: CT sign of contained leak of aortic aneurysms. Radiology. 1996;199:1-3.
Hellmann D.B., Grand D.J., Freischlag J.A. Inflammatory abdominal aortic aneurysm. JAMA. 2007;297:395-400.
Hermsen K., Chong W.K. Ultrasound evaluation of abdominal aortic and iliac aneurysms and mesenteric ischemia. Radiol Clin North Am. 2004;42:365-381.
Hillemanns P., Knitza R., Muller-Hocker J. Rupture of splenic artery aneurysm in a pregnant patient with portal hypertension. Am J Obstet Gynecol. 1996;174:1665-1666.
Hirsch A.T., Haskal Z.J., Hertzer N.R., et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report. Circulation. 2006;113:e463-e654.
Hollerman J.J., Fackler M.L., Coldwell D.M., Ben-Menachem Y. Gunshot wounds: 2. Radiology. AJR Am J Roentgenol. 1990;155:691-702.
Hovsepian D.M., Hein A.N., Pilgram T.K., et al. Endovascular abdominal aortic aneurysm repair in 144 patients: Correlation of aneurysm size, proximal aortic neck length, and procedure-related complications. J Vasc Interv Radiol. 2001;12:1373-1382.
Inaba K., Munera F., McKenney M.G., et al. The nonoperative management of penetrating internal jugular vein injury. J Vasc Surg. 2006;43:77-80.
Ishida M., Kato N., Hirano T., et al. Endovascular stent-graft treatment for thoracic aortic aneurysms: short- to midterm results. J Vasc Interv Radiol. 2004;15:361-367.
Isselbacher E.M. Thoracic and abdominal aortic aneurysms. Circulation. 2005;15(111):16-28.
Iyer V.S., MacKenzie K.S., Tse L.W., et al. Early outcomes after elective and emergent endovascular repair of the thoracic aorta. J Vasc Surg. 2006;43:677-683.
Kaji S., Nishigami K., Akasaka T., et al. Prediction of progression or regression of type A aortic intramural hematoma by computed tomography. Circulation. 1999;100(Suppl 2):II-281-II-286.
Kasirajan K., Heffernan D., Langsfeld M. Acute thoracic aortic trauma: a comparison of endoluminal stent grafts with open repair and nonoperative management. Ann Vasc Surg. 2003;17:589-595.
Kaufman J.A., Lee M.J. Vascular and Interventional Radiology: The Requisites. Philadelphia: Elsevier; 2004.
Kerwin A.J., Bynoe R.P., Murray J., et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma. 2001;51:308-314.
Kieffer E., Bahnini A., Koskas F., et al. In situ allograft replacement of infected infrarenal aortic prosthetic graft: Results in forty-five patients. J Vasc Surg. 1993;17:349-356.
Kim H.S., Fine D.M., Atta M.G. Catheter-directed thrombectomy and thrombolysis for acute renal vein thrombosis. J Vasc Interv Radiol. 2006;17:815-822.
Kim H.S., Patra A., Khan J., et al. Transhepatic catheter-directed thrombectomy and thrombolysis of acute superior mesenteric venous thrombosis. J Vasc Interv Radiol. 2005;16:1685-1691.
Laffargue G., Taourel P., Saguintaah M., et al. CT diagnosis of abdominal compartment syndrome. AJR Am J Roentgenol. 2002;178:771-772.
Laing C.J., Tobias T., Rosenblum D.I., et al. Acute gastrointestinal bleeding: Emerging role of multidetector CT angiography and review of current imaging techniques. Radiographics. 2007;27:1055-1070.
Lamah M., Darke S. Value of routine computed tomography in the preoperative assessment of abdominal aneurysm replacement. World J Surg. 1999;23:1076-1080. discussion 1080–1081
LaRoy L.L., Cormier P.J., Matalon T.A., et al. Imaging of abdominal aortic aneurysms. AJR Am J Roentgenol. 1989;152:85-92.
LeBlang S.D., Dolich M.O. Imaging of penetrating thoracic trauma. J Thorac Imaging. 2000;15:28-35.
Lettinga-van de Poll T., Schurink G.W., De Hann M.W., et al. Endovascular treatment of traumatic rupture of the thoracic aorta. Br J Surg. 2007;94:525-533.
Levy J.R., Heiken J.P., Gutierrez F.R. Imaging of penetrating atherosclerotic ulcers of the aorta. AJR Am J Roentgenol 173. 1999:151-154.
Lohan D.G., Saleh R., Nael K., et al. Contrast-enhanced MRA versus nonenhanced MRA: Pros and cons. Applied Radiol. 2007;Suppl:S3-S15.
Makaroun M.S., Dillavou E.D., Kee S.T., et al. Endovascular treatment of thoracic aortic aneurysms: Results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1-9.
Manghat et al., Manghat NE, Morgan-Hughes GJ, Roobottom CA: Multi-detector row computed tomography: Imaging in acute aortic syndrome. Clin Radiol 60:256–267, 2005.
Mark A., LePage M.A., Quint L.E., Sonnad S.S. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol. 2001;177:207-211.
Mattox K.L. Injury to the thoracic great vessels. In: Moore E.E., Mattox K.L., Feliciano D.V., editors. Trauma. 2nd ed. Norwalk, CT: Appleton & Lange; 1991:393.
May J., White G.H., Waugh R., et al. Improved survival after endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: A 5-year concurrent comparison using life table method. J Vasc Surg. 2001;33(Suppl 2):S21-S26.
McKenney K.L., McKenney M.G., Cohn S.M., et al. Hemoperitoneum score helps determine need for therapeutic laparotomy. J Trauma. 2001;50:650-654.
Miller L.A., Shanmuganathan K. Multidetector CT evaluation of abdominal trauma. Radiol Clin North Am. 2005;43:1079-1095.
Mirvis S.E., Shanmuganathan K., Miller B.H., et al. Traumatic aortic injury: Diagnosis with contrast-enhanced thoracic CT—five-year experience at a major trauma center. Radiology. 1996;200:13-22.
Moizumi Y., Komatsu T., Motoyoshi N., Tabayashi K. Clinical features and long-term outcome of type A and type B intramural hematoma of the aorta. J Thorac Cardiovasc Surg 127. 2004:421-427.
Munera F., Soto J.A., Palacio D., et al. Diagnosis of arterial injuries caused by penetrating trauma to the neck: Comparison of helical CT angiography and conventional angiography. Radiology. 2000;216:356-362.
Munera F., Soto J.A., Palacio D., et al. Penetrating neck injuries: Helical CT angiography for initial evaluation. Radiology. 2002;224:366-372.
Nienaber C.A., Kische S., Ince H. Thoracic aortic stent-graft devices: problems, failure modes, and applicability. Semin Vasc Surg. 2007;20:1-9.
Noel A.A., Gloviczki P., Cherry K.J.Jr., et al. Ruptured abdominal aortic aneurysms: The excessive mortality rate of conventional repair. J Vasc Surg. 2001;34:41-46.
O’Sullivan G.J., Lohan D.G., Gough N., et al. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol. 2007;18:715-724.
Pamela T., Johnson P.T., Chen J.K., Loey B.L. Loeys-Dietz syndrome: MDCT angiography findings. AJR Am J Roentgenol. 2007;189:29-35.
Parellada J.A., Palmer J., Monill J.M., et al. Mycotic aneurysm of the abdominal aorta: CT findings in three patients. Abdom Imaging. 1997;22:321-324.
Patel N.H., Stephens K.E., Mirvis S.E., et al. Imaging of acute thoracic aortic injury due to blunt trauma: A review. Radiology. 1998;209:335-348.
Pauls S., Orend K.H., Sunder-Plassmann L., et al. Endovascular repair of symptomatic penetrating atherosclerotic ulcer of the thoracic aorta. Eur J Vasc Endovasc Surg. 2007;34:66-73.
Peterson A.H., Williams D.M., Rodrigues J.L., Francis I.R. Percutaneous treatment of a traumatic aortic dissection by balloon fenestration and stent placement. AJR Am J Roentgenol. 1995;164:1274-1276.
Picard E., Marty-Ane C.H., Vernhet H., et al. Endovascular management of traumatic infrarenal abdominal aortic dissection. Ann Vas Surg. 1998;12:515-521.
Pickhard P.J., Shimony J.S., Heiken J.P., et al. The abdominal compartment syndrome: CT findings. AJR Am J Roentgenol. 1999;173:575-579.
Quint L.E., Williams D.M., Francis I.R., et al. Ulcerlike lesions of the aorta: Imaging features and natural history. Radiology. 2001;218:719-723.
Rakita D., Newatia A., Hines J.J., et al. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics. 2007;27:497-507.
Rivas L.A., Munera F., Fishman J.E. Multidetector-row computerized tomography of aortic injury. Semin Roentgenol. 2006;41:26-36.
Saletta S., Lederman E., Fein S. Transesophageal echocardiography for the initial evaluation of the widened mediastinum in trauma patients. J Trauma. 1995;39:137-142. discussion 141–142
Sanchez-Ross M., Anis A., Walia J., et al. Aortic rupture: Comparison of three imaging modalities. Emerg Radiol. 2006;13:31-33.
Schoder M., Grabenwoger M., Holzenbein T., et al. Endovascular stent-graft repair of complicated penetrating atherosclerotic ulcers of the descending thoracic aorta. J Vasc Surg. 2002;36:720-726.
Schwartz S.A., Taljanovic M.S., Smyth S., et al. CT findings of rupture, impending rupture, and contained rupture of abdominal aortic aneurysms. AJR Am J Roentgenol. 2007;188:W57-W62.
Sebastià C., Pallisa E., Quiroga S., et al. Aortic dissection: Diagnosis and follow-up with helical CT. Radiographics. 1999;19:45-60.
Shanmuganathan K. Multi-detector row CT imaging of blunt abdominal trauma. Semin Ultrasound CT MR. 2004;25:180-204.
Shanmuganathan K., Mirvis S.E., Sover E.R. Value of contrast-enhanced CT in detecting active hemorrhage in patients with blunt abdominal and pelvic trauma. AJR Am J Roentgenol. 1993;161:5-9.
Shiga T., Wajima Z., Apfel C.C., et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection. Arch Intern Med. 2006;166:1350-1356.
Sicard G.A., Zwolak R.M., Sidawy A.N., et al. Endovascular abdominal aortic aneurysm repair: Long-term outcome measures in patients at high risk for open surgery. J Vasc Surg. 2006;44:229-236.
Sommer T., Fehske W., Holzknecht N., et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996;199:347-452.
Soto J.A., Munera F., Cardoso N., et al. Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr. 1999;23:188-196.
Soto J.A., Munera F., Morales C., et al. Focal arterial injuries of the proximal extremities: Helical CT arteriography as the initial method of diagnosis. Radiology. 2001;218:188-194.
Stone D.H., Brewster D.C., Kwolek C.J. Stent-graft versus open-surgical repair of the thoracic aorta: Mid-term results. J Vasc Surg. 2006;44:188-197.
Suzuki T., Mehta R.H., Ince H., et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the International Registry of Aortic Dissection (IRAD). Circulation). 2003;108(Suppl 2):II-312-II-317.
Symbas P.N., Sherman A.J., Silver J.M., et al. Traumatic rupture of the aorta: immediate or delayed repair? Ann Surg. 2002;235:796-802.
Tespili M., Banfi C., Valsecchi O., et al. Endovascular treatment of thoracic aortic disease: Mid-term follow-up. Catheter Cardiovasc Interv. 2007;70:595-601.
Teufelsbauer H., Polterauer P., Lammer J., et al. Repair of abdominal aortic aneurysms: The benefits of offering both endovascular and open surgical techniques. Perspect Vasc Surg Endovasc Ther. 2006;18:238-246.
Thanila A., Macedo T.A., Stantson A.W., Oderich G.S. Infected aortic aneurysms: Imaging findings. Radiology. 2004;231:250-257.
Thomas T.T., Evangelist A., Nienaber C.A., et al. Long-term survival in patients presenting with type A acute aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114:350-356.
Tsai T.T., Nienaber C.A., Eagle K.A. Acute aortic syndromes. Circulation. 2005;112:3802-3813.
Veith F.J., Baum R.A., Ohki T., et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029-1035.
Vernhet H., Marty-Ane C.H., Lesnik A., et al. Dissection of the abdominal aorta in blunt trauma: Management by percutaneous stent placement. Cardiovasc Intervent Radiol. 1997;20:473-476.
von Kodolitsch Y., Nienaber C.A., Dieckmann C., et al. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. 2004;116:73-77. A-40
Weigel S., Tombach B., Waintz D., et al. Thoracic aortic stent graft: Comparison of contrast-enhanced MR angiography and CT angiography in the follow-up: Initial results. Eur Radiol. 2003;13:1628-1634.
White G.H., Yu W., May J., et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: Classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4:152-168.
Willens H.J., Kessler K.M. Transesophageal echocardiography in the diagnosis of diseases of the thoracic aorta: Part 1. Aortic dissection, aortic intramural hematoma, and penetrating atherosclerotic ulcer of the aorta. Chest. 1999;116:772-779.
Won J.Y., Lee D.Y., Shim W.H., et al. Elective endovascular treatment of descending thoracic aortic aneurysms and chronic dissections with stent-grafts. J Vasc Interv Radiol 12. 2001:575-582.
Yang C.Y., Liu K.L., Lee C.W., et al. Mycotic aortic aneurysm presenting initially as an aortic intramural air pocket. AJR Am J Roentgenol 185. 2005:463-465.
Yeh T.S., Jan Y.Y., Jeng L.B., et al. Massive extra-enteric gastrointestinal hemorrhage secondary to splanchnic artery aneurysms. Hepatogastroenterology. 1997;44:1152-1156.