CHAPTER 1 Traumatic and Nontraumatic Emergencies of the Brain, Head, and Neck
INTRACRANIAL HEMORRHAGE AND TRAUMATIC BRAIN INJURY
General Imaging Characteristics of Hemorrhage
MR imaging has greatly revolutionized the evaluation of intracranial hemorrhage. The evolution of hemorrhage from the hyperacute to the chronic stage will have corresponding signal changes on T1-weighted images (T1WI), T2-weighted images (T2WI), fluid attenuated inversion recovery (FLAIR) images, and gradient-echo sequences. These properties can assist in detection and understanding of the time course of the injury. While beyond the scope of this chapter, description of the physics of the signal characteristics of blood products on MR is generally based on the paramagnetic effects of iron and the diamagnetic effects of protein in the hemoglobin molecule. The usual signal characteristics of hemorrhage and the general time course over which they evolve is summarized in Table 1-1.
EXTRA-AXIAL HEMORRHAGE
Epidural Hemorrhage
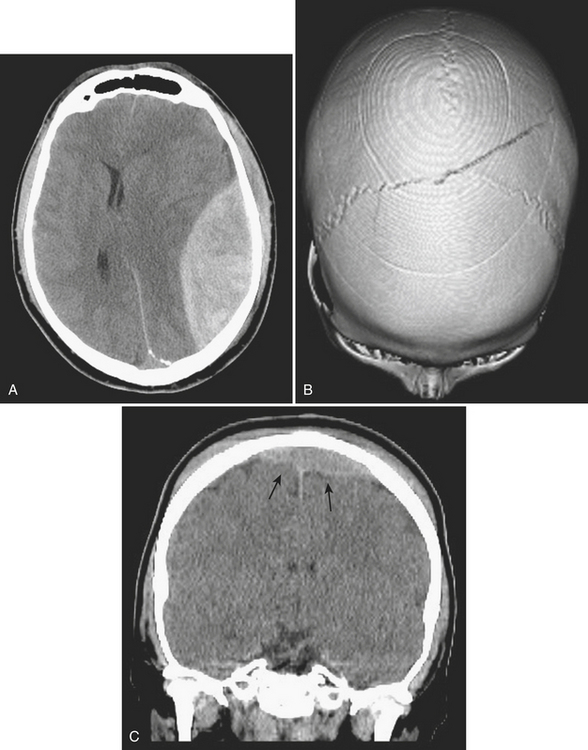
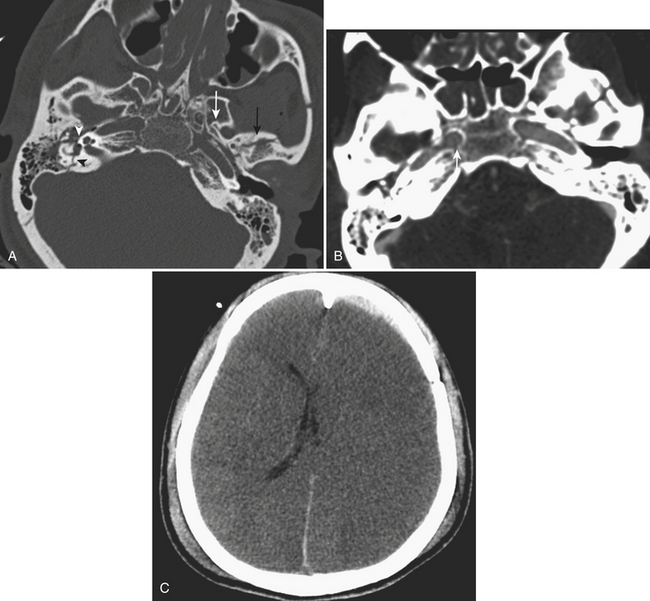
Epidural hematoma is the term generally applied to hemorrhage that forms between the inner table of the calvarium and the outer layer of the dura due to its masslike behavior. More than 90% are associated with fractures in the temporoparietal, frontal, and parieto-occipital regions. CT is usually the most efficient method for evaluation of this type of hemorrhage. Epidural hematoma typically has a hyperdense, biconvex, or lens-shaped appearance. It may cross the midline but does not cross sutures (since the dura has its attachment at the sutures). This might not hold true if a fracture disrupts the suture. There is usually an arterial source, commonly due to tear of the middle meningeal artery and much less commonly (less than 10%) due to tear of the middle meningeal vein, diploic vein, or venous sinus (Figs. 1-1 and 1-2). The classic clinical presentation describes a patient with a “lucid” interval, although the incidence of this finding varies from 5% to 50% in the literature. Prompt identification of an epidural hematoma is critical, as evacuation or early reevaluation may be required. Management is based on clinical status, and therefore alert and oriented patients with small hematomas may be safely observed. The timing of follow-up CT depends on patient condition, but, generally, the first CT scan may be obtained in 6 to 8 hours and, if the patient is stable, follow-up may be extended to 24 hours or more afterward.
Subdural Collections
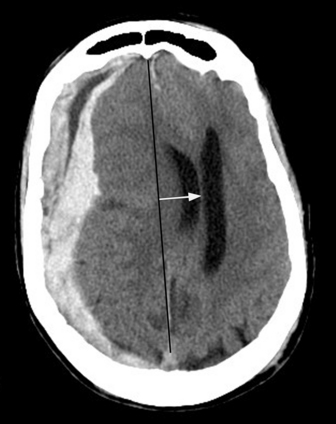
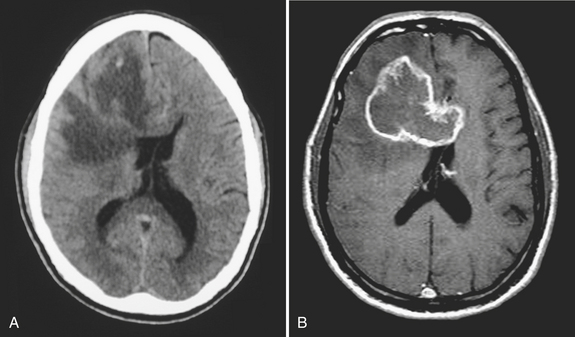
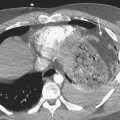
Subdural hematoma (SDH) is the term generally applied to hemorrhage that collects in the potential space between the inner layer of the dura and the arachnoid membrane. It is typically the result of trauma (motor vehicle collision, assaults, and falls, the latter especially in the elderly population), causing tear of the bridging vein(s), and has a hyperattenuating, crescentic appearance overlying the cerebral hemisphere (Fig. 1-3). These hemorrhages can cross sutures and may track along the falx and tentorium but do not cross the midline. Inward displacement of the cortical vessels may be noted on a contrast-enhanced scan. There is a high association with subarachnoid hemorrhage. Acute SDHs thicker than 2 cm, seen with other parenchymal injuries, are associated with greater than 50% mortality. As the SDH evolves to the subacute (5 days to 3 weeks) and then chronic (more than 3 weeks) stage, it decreases in attenuation, becoming isodense to the brain and finally to CSF. Subacute SDH can have a layered appearance due to separation of formed elements from serum. Subacute hemorrhages may be relatively inconspicuous when isodense, and therefore it is especially important to recognize signs of mass effect, such as sulcal effacement, asymmetry of lateral ventricles, and shift of midline structures, as well as sulci that do not extend to the skull (Fig. 1-4). Bilateral isoattenuating SDHs can be especially challenging since findings are symmetric. Beware of this, particularly in the elderly patient who does not have generous sulci and ventricles. At this stage, the SDH should be conspicuous on MR imaging, especially on FLAIR sequences. A subacute SDH may also be very conspicuous on T1-weighted images due to the hyperintensity of methemoglobin.
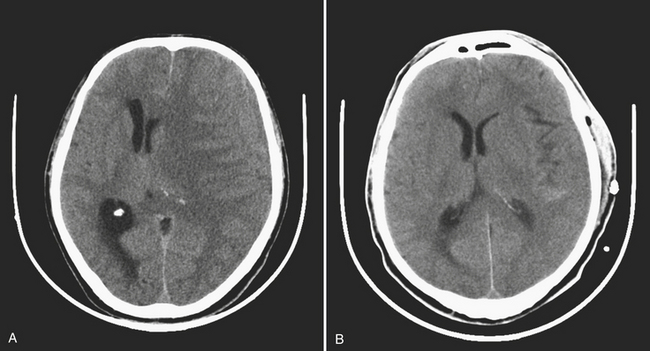
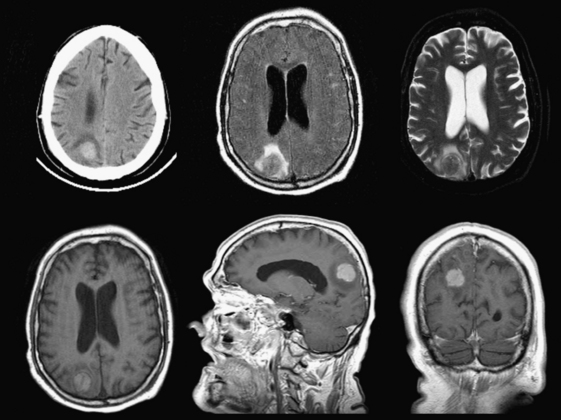
Chronic subdural hematomas are collections that have been present for more than 3 weeks. Even a chronic hematoma may present in the emergency setting; for example, in a patient prone to repeated falls, brought in because of a change in mental status. On both CT and MR, these collections typically have a crescentic shape and may demonstrate enhancing septations and membranes surrounding the collection after contrast administration. Calcification of chronic SDH can occur and be quite extensive (Fig. 1-5). Areas of hyperdensity within a larger hypodense SDH may indicate an acute component due to recurrent bleeding, an “acute on chronic subdural hemorrhage.” Mixed density collections may also be acute, due to active bleeding or CSF accumulation due to tear of the arachnoid membrane. Chronic SDH is usually isointense to CSF on both T1WI and T2WI, but the appearance can be variable depending on any recurrent bleeding within the collection. The FLAIR sequence is typically very sensitive for detection of chronic SDH due to hyperintensity based on protein content. Hemosiderin within the hematoma will cause a signal void due to susceptibility effect, and “blooming” (appears to be larger than its true size) will be noted on gradient-echo sequence.
Subarachnoid Hemorrhage
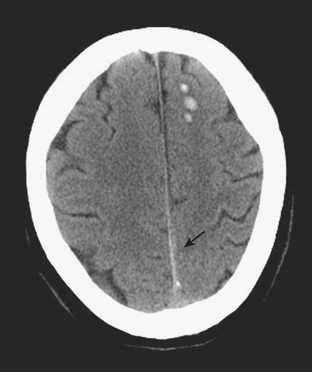
Subarachnoid hemorrhage (SAH) fills the space between the pia and the arachnoid membrane, outlining the sulci and basilar cisterns. This can be due to a variety of causes, including trauma, ruptured aneurysm, hypertension, arteriovenous malformation, occult spinal vascular malformation, and hemorrhagic transformation of ischemic infarction. SAH is often associated with overlying traumatic subdural hematoma. Subarachnoid hemorrhages generally do not cause mass effect or edema. On CT, hyperdensity is seen within the sulci and/or basilar cisterns (Figs. 1-6 and 1-7).
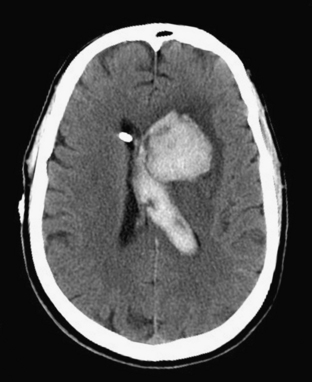
Intraventricular Hemorrhage
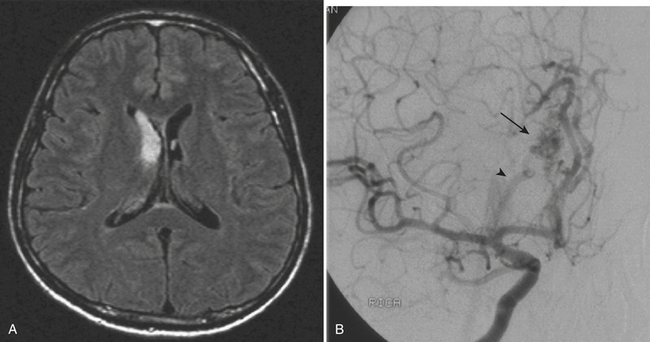
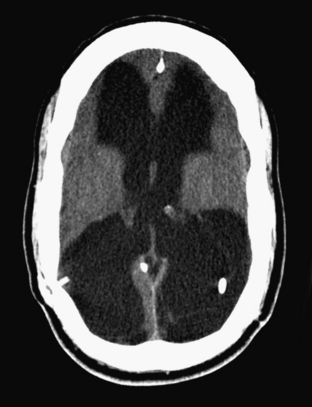
Intraventricular hemorrhage (IVH) is typically caused by trauma in the adult population. It can result from extension of parenchymal hemorrhage into the ventricles or redistribution of subarachnoid hemorrhage. Primary intraventricular hemorrhage is uncommon and usually caused by a ruptured aneurysm, intraventricular tumor, vascular malformation, or coagulopathy (Fig. 1-8). Large IVHs are quite conspicuous on CT or MR. They may occupy a majority of the ventricle(s) and may result in hydrocephalus and increased intracranial pressure. Small amounts of IVH may be difficult to detect; one must check carefully for layering hemorrhage within the atria and occipital horns of the lateral ventricles. Normal choroid plexus calcifications in the atria of lateral ventricles, the fourth ventricle, and extending through the foramina of Luschka should not be mistaken for acute IVH.
INTRA-AXIAL HEMORRHAGE
Contusion
Parenchymal contusions result from blunt trauma and can occur in the cortex or white matter. Their locations are typically at the site of greatest impact of brain on bone, including the anterior/inferior frontal lobes and the lobes. They can be considered coup (occurring at the site of impact) or contrecoup (opposite the site of impact) types. On CT, a contusion typically appears as an area of hyperdensity with a surrounding rim of hypodense edema. It can initially appear as a focal area of subtle hypodensity and may blossom on follow-up exam at 12 to 24 hours with development of an obvious central area of hyperdensity and a larger surrounding zone of hypodense edema (Fig. 1-9). On MR, signal characteristics reflect the hemorrhagic and edematous components. Over time, the density and signal characteristics of the hemorrhage will evolve in a fashion similar to a spontaneous hemorrhage. Parenchymal hemorrhage due to penetrating trauma, such as from gunshot wound or impalement, will follow the same general pattern of evolution.
Diffuse Axonal Injury
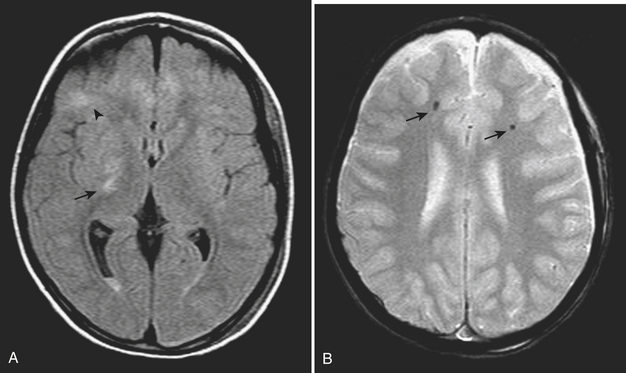
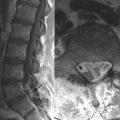
Diffuse axonal injury (DAI) is another type of traumatic brain injury that may present with parenchymal hemorrhages and is distinct from parenchymal contusion. DAI is an injury to the axons caused by acceleration/deceleration injury with a rotational component (usually from motor vehicle collision or other blunt trauma to the head). There may be complete transection of axons with injury to the associated capillaries, or partial disruption of the axons. The lesions of DAI typically occur at the inter-faces of gray and white matter in the cerebral hemispheres, the body and splenium of the corpus callosum, midbrain, and upper pons. Lesions may also be seen in the basal ganglia. Patients sustaining DAI typically lose consciousness at the moment of impact. DAI may be suspected when the clinical exam is worse than expected based on the findings on initial CT scan. Usually, the greater the number of lesions, the worse the prognosis. Individuals who recover usually demonstrate lingering effects such as headaches and cognitive deficits. Initial CT scans in more than half of patients with DAI may be negative. CT findings include hypodense foci due to edema in areas of incomplete axonal disruption and hyperdense foci due to petechial hemorrhage where there is complete transection of the axons and associated capillaries (Fig. 1-10). MR is more sensitive than CT for detection of DAI. Approximately 30% of those negative by CT will demonstrate abnormal findings on MR. These findings include FLAIR and T2 hyperintensities (edema) and gradient-echo hypointensities (hemorrhages) (Fig. 1-11). Lesions may appear hyperintense on diffusion-weighted images. It is estimated that more than 80% of the lesions of DAI are nonhemorrhagic. Generally, if imaging is repeated within 3 to 5 days, more lesions will become apparent as the process evolves. A staging system for DAI based on locations of lesions on histopathology may be applied to MR findings. Stage 1 is based on subcortical lesions in the frontal and temporal lobes. Stage 2 will also show lesions in the corpus callosum and lobar white matter, and stage 3 will have lesions in the midbrain and pons. Diffusion tensor imaging, particularly helpful in evaluation of white matter tracts, has been shown to be more sensitive than conventional MR for detection of diffuse axonal injury and correlates more closely with clinical outcomes.
BRAIN HERNIATIONS
Subfalcine Herniation
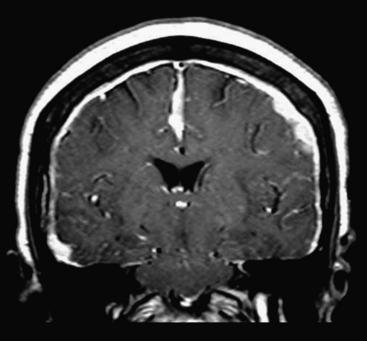
Subfalcine herniation is due to displacement and impingement of the cingulate gyrus underneath the falx. It is usually caused by mass effect on the frontal lobe and is associated with ipsilateral lateral ventricle compression and obstruction of the foramen of Monro with dilatation of the contralateral ventricle (“trapped ventricle”). The degree of midline shift (not synonymous with subfalcine herniation) can be estimated by drawing a line between the anterior and posterior attachments of the falx and measuring the shift of the septum pellucidum relative to this line (see Fig. 1-3). Anterior cerebral artery territory infarct(s) may result from this type of herniation.
Transtentorial Herniation
Transtentorial herniations include two major types, which are the descending transtentorial herniation (DTH) and the ascending transtentorial herniation (ATH). An early DTH is known as uncal herniation, in which the uncus is displaced medially and occupies the ipsilateral suprasellar cistern. A later-stage DTH is caused by continued mass effect with displacement of the medial temporal lobe through the incisura, which completely occupies the suprasellar cistern (along with the uncus) and causes enlargement of the ipsilateral and effacement of the contralateral ambient cisterns. This phenomenon occurs because, as there is marked shifting of brain in the supratentorial compartment, there is also shift of the brainstem in the same direction. There is also compression of the ipsilateral cerebral peduncle. Occasionally, when there is marked mass effect, there can be compression of the contralateral cerebral peduncle against the tentorium, or “Kernohan’s notch,” which leads to ipsilateral motor weakness (this phenomenon may be a false localizing sign). Other imaging findings include a “trapped” temporal horn of the lateral ventricle contralateral to the side of the mass and Duret hemorrhages—hemorrhages of the midbrain and pons caused by stretching and tearing of the arterial perforators. In cases of bilateral mass effect, there can be displacement of both temporal lobes and midbrain through the incisura leading to effacement of the basilar cisterns bilaterally. Complications of this type of herniation include compression of the posterior cerebral artery and penetrating basal arteries with associated infarcts in these vascular distributions (see Fig. 1-2). In addition, there can be compression of the oculomotor nerve (CN III) with an associated palsy. ATH is less common and is caused by superior displacement of the cerebellum and brainstem through the incisura. It is usually due to mass effect in the posterior fossa (as from hemorrhage, tumor, or infarct), and on imaging there is compression on the posterolateral midbrain bilaterally with associated effacement of the ambient and quadrigeminal plate cisterns. There is usually hydrocephalus due to obstruction at the level of the cerebral aqueduct of Sylvius.
Tonsillar Herniation
Tonsillar herniation is caused by downward displacement of the cerebellar tonsils through the foramen magnum into the spinal canal (generally by more than 5 mm). On imaging, there is a peglike configuration to the tonsils with obliteration of the CSF space in the foramen magnum (Fig. 1-12). Complications include obstructive hydrocephalus from compression of the fourth ventricle. Mild tonsillar ectopia, Chiari I malformations, and sagging tonsils due to intracranial hypotension should not be mistaken for acute tonsillar herniation, but should be considered seriously when downward mass effect is expected based on brain edema, mass, or hemorrhage.
ACUTE CEREBROVASCULAR DISORDERS
Hemorrhagic Stroke: Spontaneous Parenchymal Hemorrhage
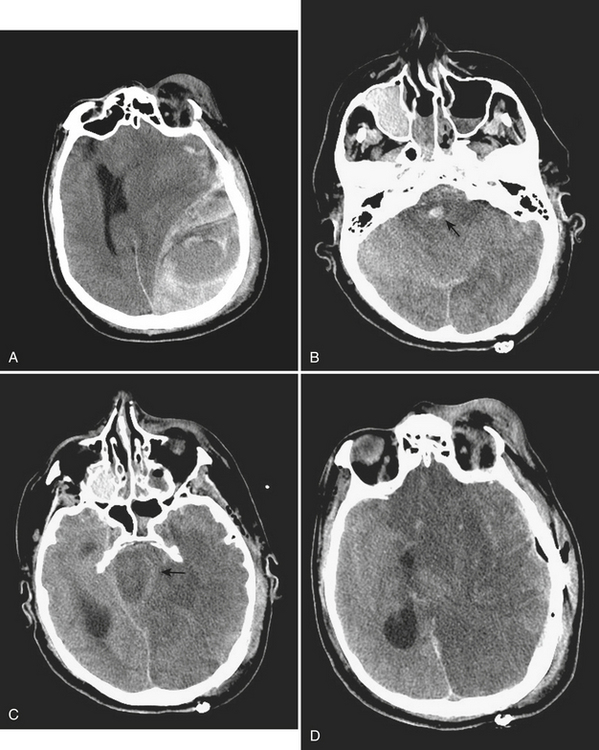
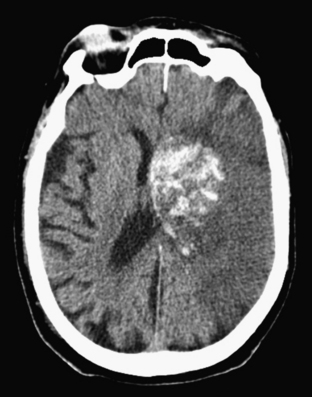
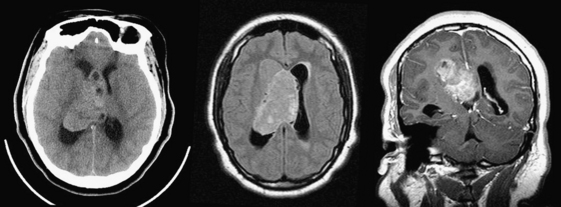
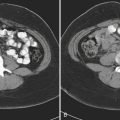
Approximately 10% to 15% of strokes present with an acute parenchymal hemorrhage. The most common cause is hypertension (Fig. 1-13). Coagulopathies, hematologic disorders including hypercoagulable states, amyloid angiopathy, drugs, vascular malformations and aneurysms, vasculitides, and tumors round out the usual list of etiologies. Hemorrhages resulting from illicit drug use and vascular malformations are commonly found in young adults (Fig. 1-14). Sickle cell disease and venous infarcts may also present with parenchymal hemorrhage. A ruptured intracranial aneurysm may occasionally cause a parenchymal hemorrhage in association with subarachnoid hemorrhage. Hypertensive hemorrhages most commonly occur in the basal ganglia and thalami but may also primarily arise within the cerebral hemispheres, brainstem, or cerebellum. Cerebral amyloid angiopathy (CAA) is another common cause of intracranial hemorrhages in patients over 65 years of age. CAA can be found in patients with mild cognitive impairment, dementia of Alzheimer type, and Down syndrome with extracellular deposition of beta amyloid occurring in the cortex and subcortical white matter. CAA can be hereditary (autosomal dominant, Dutch type), sporadic (presence of Apoε4 allele), or acquired (as from hemodialysis). The lobar hemorrhages of CAA typically occur in the frontal and parietal regions. MR is sensitive for the detection of hemosiderin deposition resulting from multiple microhemorrhages over the course of time appearing as small hypointense foci on gradient-echo sequences.
Imaging of Acute Ischemic Stroke
Computed Tomography
The role of imaging in acute stroke diagnosis and management continues to evolve. Since the mid-1970s, unenhanced CT has been the first-line modality to determine the etiology of acute neurologic deficits. CT can offer the chance to detect an ischemic infarct, generally in the middle cerebral artery territory, within 3 hours in up to one third of cases based on findings of subtle parenchymal hypodensity, loss of gray–white matter differentiation (including loss of the insular ribbon or margins of basal ganglia; Fig. 1-15), and effacement of sulci. A hyperdense vessel sign may indicate the presence of an acute thrombus and support the diagnosis. The sensitivity for detection of acute stroke has been shown to increase with the use of an “acute stroke” window and level settings (see Fig. 1-15). A very narrow window width of 8 Hounsfield units (HU) and a level of 32 HU (compared with 80 and 20 HU, respectively) may increase the sensitivity of CT to approximately 70% without a loss of specificity. CT is currently used to screen patients who may be considered for treatment with intravenous recombinant tissue plasminogen activator (rt-PA) within 3 hours of onset based on guidelines from the National Institute of Neurologic Disorders and Stroke (NINDS) rt-PA trial. Beyond 3 hours, the risk of intracranial hemorrhage due to intravenous thrombolysis was shown to outweigh potential benefits. An association between larger stroke volumes (greater than one third of the middle cerebral artery territory) and reperfusion hemorrhage was initially reported. This criterion for the use of 100 mL estimated infarct volume has been commonly applied in stroke trials. The Alberta Stroke Program early CT score (ASPECTS), a 10-point topographic scoring system, was later developed to try to more easily quantify initial stroke volumes. This score has been shown to correlate with the initial National Institutes of Health stroke score (NIHSS).
Magnetic Resonance: Diffusion-weighted Imaging
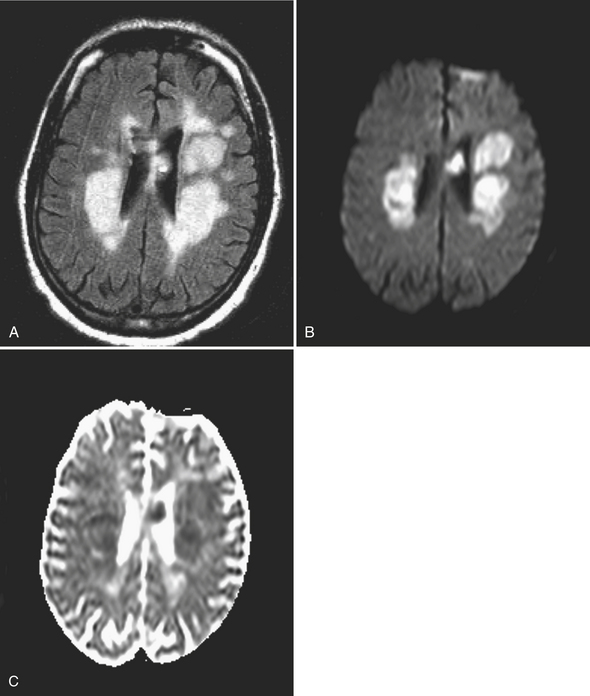
Acute ischemic infarcts may appear as hyperintense regions on DWI (see Fig. 1-15), and this can occur as quickly as 30 minutes after onset. Up to 100% sensitivity has been demonstrated in clinical studies. However, in routine practice, small lesions in the brainstem may not be perceived initially, only to be detected on a follow-up exam prompted by persistent symptoms. It is also possible that a region of ischemia (prior to completed infarction) may go undetected on an initial imaging study, resulting in a false negative result. False positives on DWI can be due to processes that mimic stroke and also cause diffusion restriction, such as certain neoplasms, multifocal metastatic disease, and abscesses. Presence or absence of associated findings on conventional MR sequences—such as loss of gray–white matter differentiation on T1WI, and hyperintense edema on FLAIR and T2WI—may help with diagnosis, although these signs may be inconspicuous for 6 to 12 hours after stroke onset. Blooming on gradient-echo sequences due to intravascular thrombus and loss of expected vascular flow voids are other useful clues.
Lacunar infarcts are generally less than 1 cm in diameter and presumed to be due to occlusion of small perforating branches due to embolic, atheromatous, or thrombotic lesions. They occur most commonly in the basal ganglia, internal and external capsules, immediate periventricular white matter (corona radiata), and, less frequently, the centrum semiovale. Occlusion of basilar artery perforators will result in lacunes in the brainstem. Diffusion imaging offers the ability to identify very small, acute infarcts even in the background of chronic white matter disease and remote lacunes (Fig. 1-16). While MR is still considered a relatively expensive technique, it has the potential to reduce the number of unnecessary hospital admissions for recurrent small vessel infarcts in many patients. It may also help to select the most appropriate pathway for patients with central embolic sources of infarcts based on detection of infarcts in different vascular territories.
Magnetic Resonance Angiography
Noninvasive imaging of the vessels of the head and neck with MR angiography (MRA) based upon time-of-flight or phase-contrast MRA techniques can be used to locate stenoses and occlusions in the extracranial and intracranial arterial systems (see Fig. 1-15). Gadolinium-enhanced MRA has become the standard of care at some institutions; this requires consideration of renal function. Complete brain MR and head and neck MRA examinations can be acquired in less than 30 minutes and have become the routine standard of care, often performed immediately or soon after completion of CT. It must be stressed that patient safety is a primary concern and therefore careful attention to screening for potential contraindications prior to MR scanning is a requisite at all times.
Computed Tomography: Perfusion Imaging
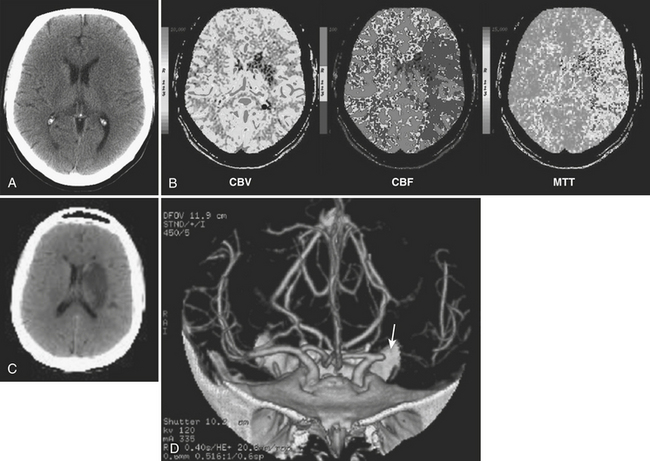
CTP applied in the setting of acute stroke has been validated with clinical outcomes and follow-up imaging and also by comparison with diffusion-weighted imaging. Wintermark and colleagues have proposed that regions with CBV less than 2.5 mL/100 g be considered the “core infarct” and regions with CBF reduction of more than 34% be defined as the penumbra. These values are based on correspondence with initial DWI abnormality and final infarct size. Schaefer and colleagues have proposed other absolute values and the use of normalized CBV and CBF ratios to help distinguish ischemic tissue likely to infarct from that likely to survive following intra-arterial recanalization therapy. Perfusion maps can be visually compared to determine if a penumbra is present but may underestimate or overestimate the extent of tissue at risk (Fig. 1-17). Software packages are available that can automatically segment the processed perfusion maps into core infarct, penumbra, and normal regions based on thresholding techniques.
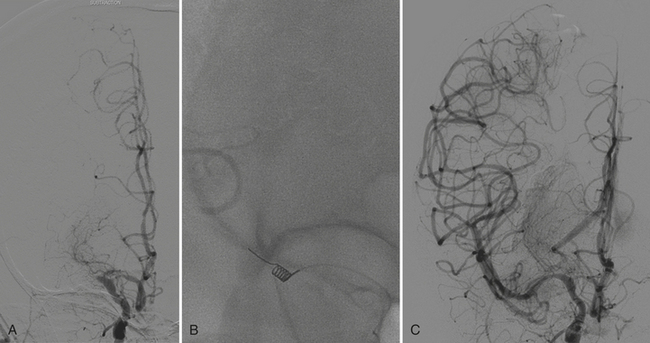
The ongoing MR RESCUE (MR and recanalization of stroke clots using embolectomy) trial is employing a similar, automated analysis of PWI data to determine eligibility for inclusion. Patients presenting with intracranial arterial occlusions within 8 hours of symptom onset and having a suitable penumbra are randomized to embolectomy with the Merci Retriever device (Fig. 1-18).
Hemorrhagic Transformation
Hemorrhagic transformation of an ischemic infarct, thought to result from reperfusion injury, can be a dreaded complication of therapy or may happen spontaneously within hours or after a period of several weeks (Fig. 1-19). If a large hematoma is present at the time of initial imaging, it may not be possible to distinguish a primary parenchymal hemorrhage from hemorrhagic transformation. The blood products generally cause substantial artifacts on DWI. However, if the hemorrhage is confined within a larger zone of restricted diffusion, the etiology may be clear. Petechial hemorrhage occurs very commonly within an ischemic infarct, is best detected by gradient-echo imaging, and does not usually lead to increased morbidity.
Cortical Laminar Necrosis
A pattern of gyriform T1 hyperintensity developing a week or two after an ischemic infarct may be attributed to cortical laminar necrosis (Fig. 1-20). It seems that gray matter is more vulnerable to ischemic necrosis than white matter (especially the third layer of the six cortical layers), and, although the signal changes may lead one to diagnose hemorrhage or calcification, in one histologic study neither was detected. The exact cause of the T1 shortening is uncertain, but it may be due to high concentrations of proteins and macromolecules.
Cerebral Venous Infarction and Sinus Thrombosis
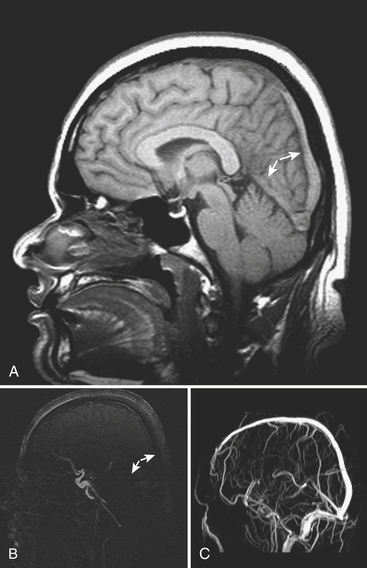
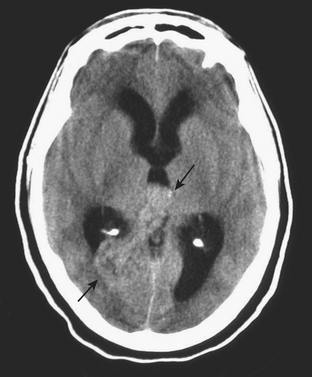
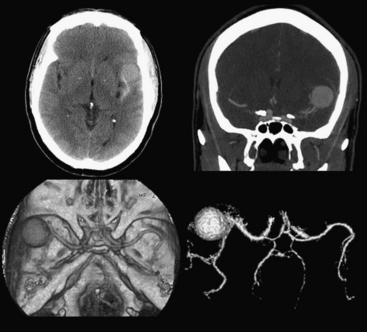
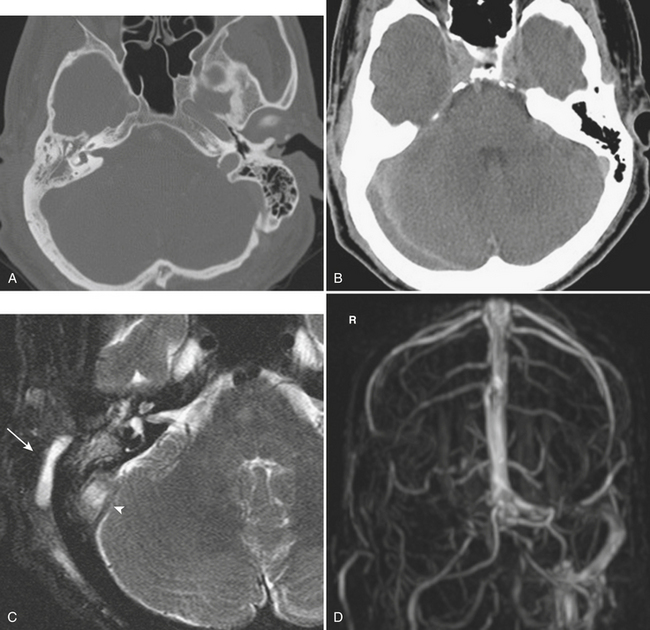
An uncommon (annual incidence estimates of less than 1 case per 100,000 population) but important cause of hemorrhage is cerebral venous thrombosis (Fig. 1-21). This may affect cortical veins and other portions of the superficial and deep venous drainage systems. Hypercoagulable states due to pregnancy and the postpartum period, or from oral contraceptive use, dehydration, regional infections, and trauma, are relatively common causes. Common presenting symptoms include headache, seizure, and focal neurologic deficits. Fluctuating symptoms and intracranial hypertension are common as well. Bilateral parenchymal hemorrhages or infarcts that do not obey usual arterial territorial borders can be clues to diagnosis. A working knowledge of the normal anatomy of the major venous structures and the common variants is necessary to avoid diagnostic pitfalls, especially false positives. On unenhanced CT, normal venous structures may appear denser than usual due to dehydration or elevated hematocrit, whereas thrombosis should appear hyperdense relative to arteries. A filling defect or occlusion may be detected on contrast-enhanced CT. CT venography can be performed with thin-section, volumetric technique allowing for creation of 2D and 3D reconstructions. Normal and thrombosed venous sinuses take on many different appearances on MR depending on scan parameters, flow velocity, and turbulence. Unexpected hyperintensity, loss of usual flow voids, and blooming on gradient-echo sequence are clues that flow-sensitive MR venography (MRV) should be performed. Time-of-flight MRV may be less sensitive than phase contrast technique due to shine-through of methemoglobin in a thrombosed sinus simulating flow in the vessel. Contrast-enhanced MRV may be more sensitive, although enhancement of chronic thrombus can be misleading. Associated findings of edema, hemorrhage, or ischemic infarct may help toward arriving at the correct diagnosis, but brain swelling without signal changes has been reported in up to approximately 40% of patients. Cavernous sinus thrombosis is discussed in relation to complex sinus and orbit infections in the section on head and neck imaging. Prompt diagnosis of cerebral thrombosis is critical, as many of the parenchymal changes may be reversible. Systemic anticoagulation and local catheter-based thrombolytic, mechanical, or rheolytic clot dissolution are treatment considerations. Intracranial hypertension and collateral formation leading to dural arteriovenous malformations are possible long-term sequelae.
ANEURYSMS, VASCULAR MALFORMATIONS, AND VASCULAR INJURIES
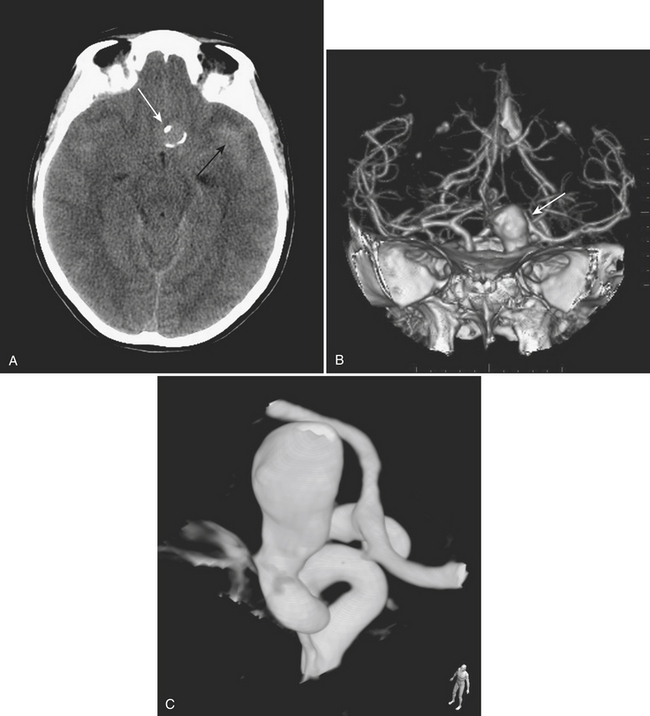
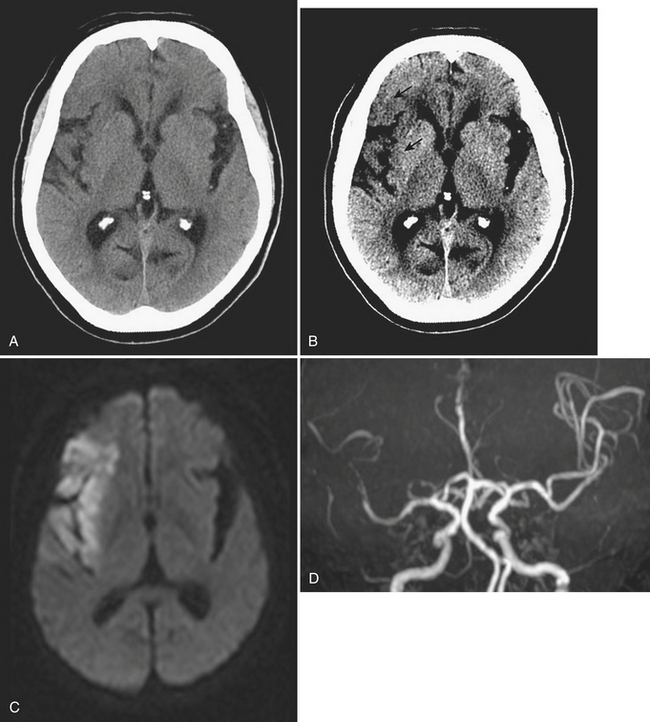
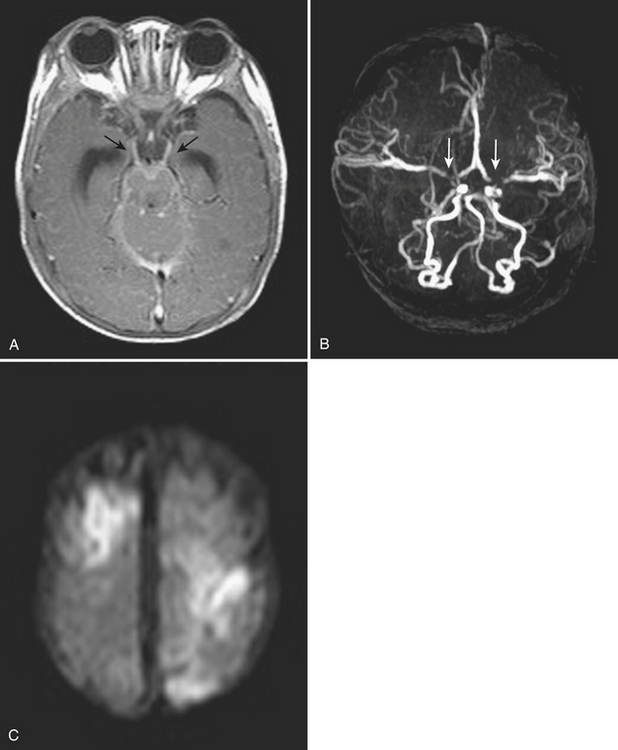
Aneurysms and cerebral vascular malformations present in various ways in the emergency setting. Subarachnoid hemorrhage resulting from a ruptured aneurysm and parenchymal hemorrhage related to an arteriovenous malformation are dramatic examples of problems that may present with headache as the chief complaint. Presenting symptoms of nausea and vomiting are common with hemorrhages arising in the posterior fossa. On routine noncontrast CT, large unruptured aneurysms may simulate other mass lesions and displace or compress adjacent structures. Arteriovenous malformations may also be conspicuous on routine CT based on abnormally enlarged feeding arteries and draining veins or internal calcifications (Fig. 1-22).
The traditional gold standard for diagnostic evaluation of vascular lesions, both spontaneous and traumatic, is digital subtraction angiography (DSA). The risk of major complication from this invasive procedure is low in experienced hands and treatment (complete or partial) with endovascular techniques is possible for many types of aneurysms and other vascular lesions. That being said, the constantly improving technology and clinical experience with CT angiography have led to a substantial decrease in the number of diagnostic angiograms (at some institutions). CTA is commonly applied in the setting of spine, facial, and skull base fractures. One study of CTA in 2004 in the setting of acute SAH reported a sensitivity of 89% and specificity of 100% for detection of aneurysms. Many centers have adopted immediate CTA in their protocol for the workup of spontaneous SAH (Fig. 1-23). If a ruptured aneurysm is detected by this method, detail may be adequate for treatment planning in certain situations. However, others have offered the opinion that the gold standard of DSA (with the recent enhancement of 3D rotational angiography) provides greater sensitivity and should not yet be replaced by CTA. This is based on recent reports of a 10% false negative rate of CTA for aneurysm detection and the belief that the greater spatial resolution of DSA is necessary for accurately determining proper triage to surgery versus endovascular coiling. Both of these reasons support the use of DSA regardless of a negative or positive result from CTA. Given its availability and lack of invasive risks, CTA will probably continue to be used as a diagnostic tool in this setting. In the setting of SAH, a negative CTA or DSA exam often requires a repeat examination, depending on the pattern of hemorrhage. DSA offers evaluation of cerebral hemodynamics, important for the diagnosis of brain and dural vascular malformations. This type of detail is not available from most current CTA techniques. On occasion, arteriovenous shunting may be inferred from the presence of dilated draining veins, or from asymmetric opacification of the cavernous sinuses, although this might just as well be due to normal physiologic variation. Evaluation of the smallest arteries is necessary for the evaluation of cerebral vasculitis, still not quite within the realm of CTA. Improvements in spatial and temporal resolution and reconstruction techniques will certainly reduce the number of false negative aneurysm hunts and increase the clinical utility of CTA for evaluation of arteriovenous malformations and vasculitis. Use of CTA in the setting of spontaneous parenchymal hemorrhage is gaining favor. One recent prospective study of CT angiography in the of acute intracerebral hemorrhage demonstrated that tiny enhancing foci within the hematoma are an independent predictor of hematoma expansion. Lack of a “spot” sign had a very high negative predictive value for worsening of hemorrhage. These findings may have important clinical value in future therapeutic trials.
CERVICOCEREBRAL ARTERIAL INJURIES
Traumatic Cervicocerebral Injuries
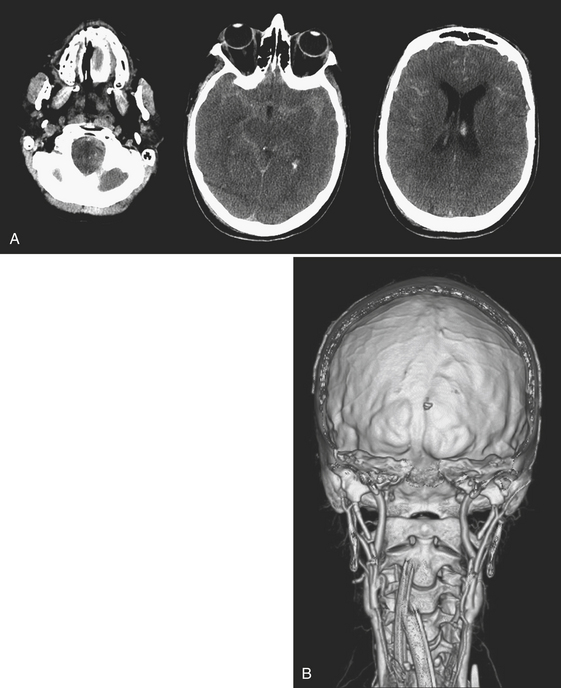
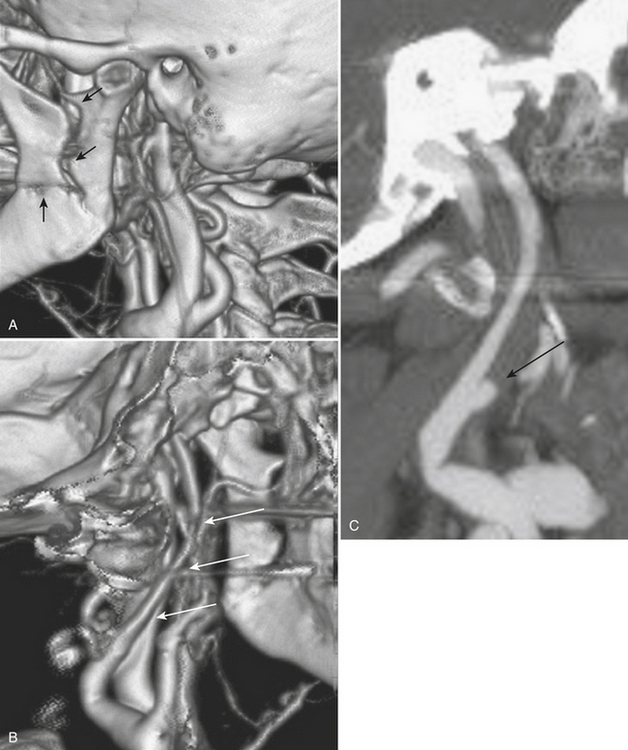
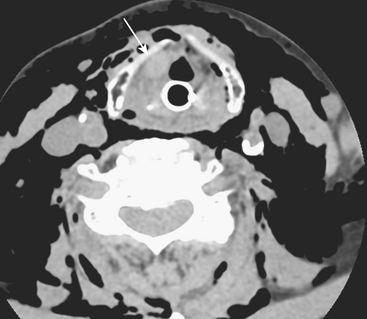
The evaluation of vascular injuries of the neck and head has undergone a dramatic transformation due to the capabilities of helical and now multidetector CT angiography. Since 2000, favorable results from the use of CTA in the setting of penetrating trauma have supported its clinical application on a routine basis. High sensitivity and specificity for the detection of vascular injuries has been reported in a number of studies. It has essentially replaced DSA, still the gold standard, as the initial screening modality of choice for penetrating neck injuries at many institutions. In many cases it has been used as a complementary technique to surgical exploration of the neck, but, more recently, a normal CTA may avert the need for exploration. CTA has been applied to the setting of blunt trauma with similar results and has resulted in a decrease in the number of DSA exams performed. Abnormalities that may be detected include stenosis, occlusion, dissection, pseudoaneurysm formation, and contrast extravasation from vessel rupture. Vascular evaluation is generally limited to the common carotid, internal carotid, and vertebral and proximal branches of the external carotid arteries. With currently available equipment, submillimeter, subsecond imaging is possible, and even minor abnormalities of the distal external carotid branches are now being diagnosed prospectively. Nondiagnostic exams may occur as a result of technical deficiencies such as extravascular contrast infiltration from intravenous catheter problems, patient motion, and so on. Using a contrast test bolus or bolus tracking techniques helps to reduce the number of poor-quality scans due to arrhythmias or compromised cardiac output. Since intravenous contrast bolus may be impeded by transient compression of the left brachiocephalic vein due to pulsations of the great vessels, a right-sided antecubital injection site is preferred. Streak artifacts from dental fillings and hardware and beam-hardening effects also take their toll on image quality. Positive findings on CTA help guide therapeutic decisions toward medical, surgical, or endovascular intervention; however, well-defined pathways do not yet exist for most vascular injuries. It is the clinical expectation that by prompt diagnosis and implementation of antithrombotic or other vascular treatments, the incidence of stroke will be reduced. As more subtle injuries will be detected with advances in CT technology, outcomes research will be necessary to help determine the most appropriate therapy. It is well known that a certain (small) percentage of patients will present with delayed formation and rupture of a post-traumatic pseudoaneurysm, yet recommendations for and timing of follow-up examination remain rather dubious (Fig. 1-24).
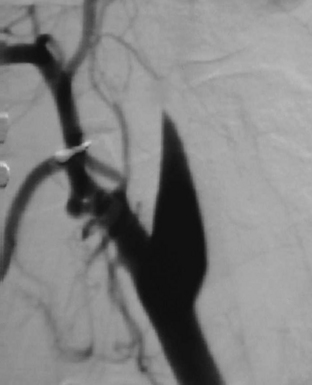
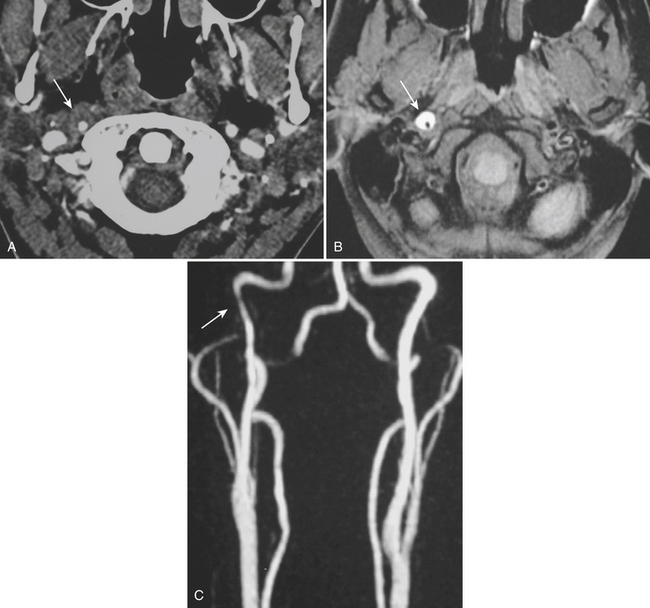
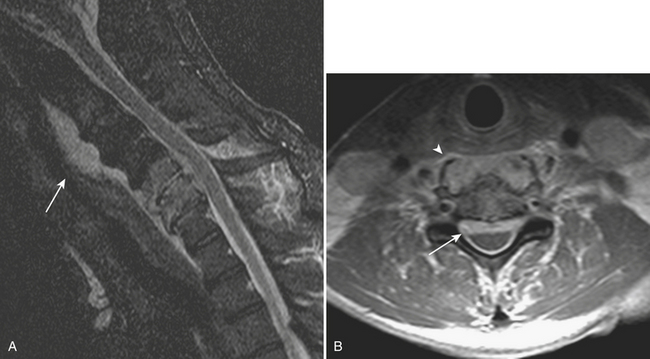
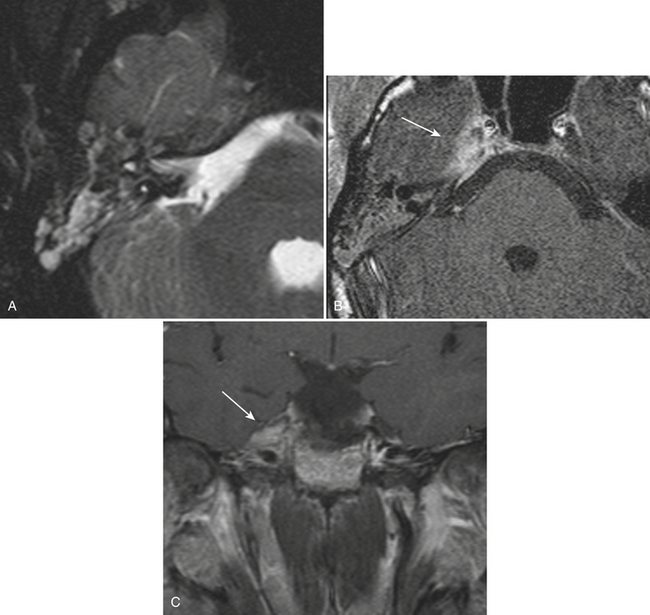
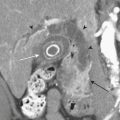
Whether performed for screening or based on symptoms, the workup and treatment of dissection continue to evolve. As with other vascular disorders, catheter angiography was once the only method available and is still used for confirmation if other studies are equivocal. The most common finding is a smoothly or mildly irregular tapered mid-cervical narrowing. Dissections that result in occlusion may show a “rat tail” or “flame-shaped” lumen; this may help distinguish other causes such as thromboembolism or atherosclerotic disease from dissection (Fig. 1-25). Saccular or fusiform aneurysmal dilatation (pseudoaneurysm) may also be identified. Presence of an intimal flap or a false or double lumen is unusual in the cervicocerebral vessels. In the internal carotid, the dissection is typically found a few centimeters beyond the carotid bifurcation or a few centimeters below the skull base. The most common sites of vertebral artery dissection are at the entry into the C6 foramen transversarium and at the C1-C2 level.
The combination of MR and MRA is considered by many to be the preferred technique. MRA should be able to demonstrate the morphologic features of the vessel in a fashion similar to DSA. In addition, MR images may demonstrate an eccentrically located narrowed lumen, a crescentic or circumferential intramural hematoma, and an increase in the external artery diameter. Fat-suppressed T1-weighted images are recommended to improve sensitivity for detection of the intramural hematoma (Fig. 1-26). However, in the acute setting, the clot should not be expected to appear hyperintense, as it may take a few days for conversion to methemoglobin to take place. The reported sensitivity and specificity for detection of carotid dissection are very high, approximately 95% and 99%, respectively. Sensitivity is lower for vertebral dissection (approximately 60%) due to the smaller size of the native vessel and relatively high incidence of hypoplasia. One must remember that lack of flow-related signal due to slow flow in a vessel may simulate occlusion. These noninvasive techniques are very useful for follow-up of dissection due to the lack of ionizing radiation or need for contrast injection.
OTHER NONTRAUMATIC INTRACRANIAL EMERGENCIES
Hydrocephalus
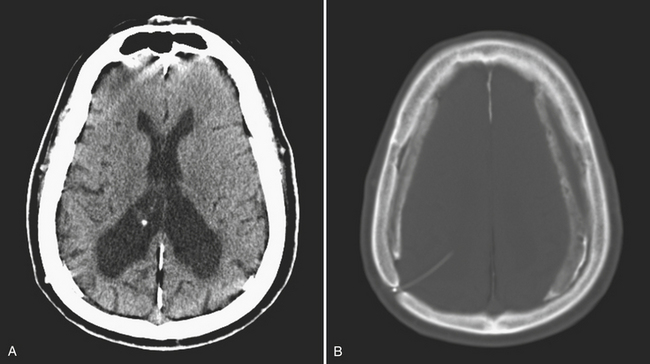
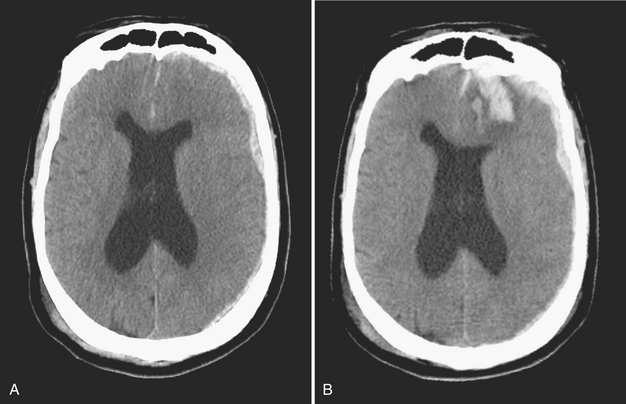
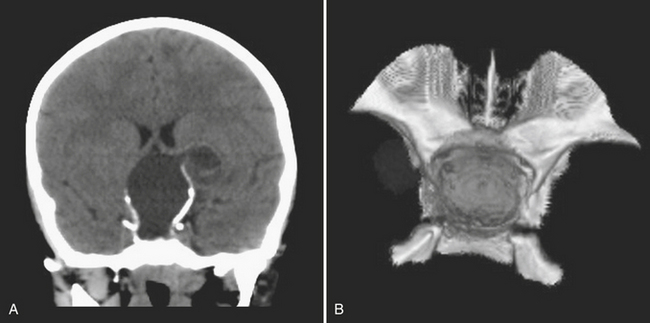
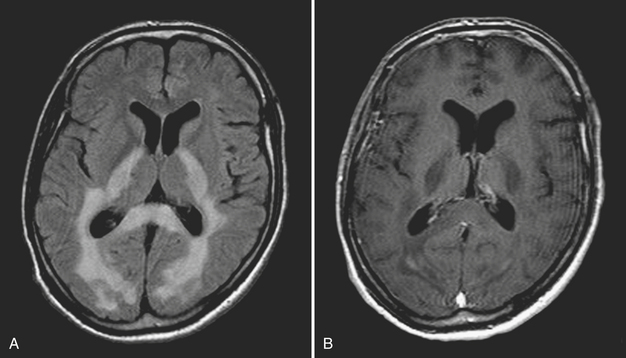
Disturbance of the usual pattern of CSF flow or production/absorption may result in dilatation of the ventricular system. Hydrocephalus may occur acutely or may be of chronic duration, and the distinction between the two forms may not be entirely clear based on imaging alone. Hypodensities/T2 hyperintensities in the periventricular white matter of the frontal and periatrial regions may be a sign of transependymal flow of CSF and may be seen with acute hydrocephalus. Chronically compensated hydrocephalus is less likely to demonstrate this finding. Terminology can be confusing: obstruction at the level of foramen of Monro, cerebral aqueduct of Sylvius, or foramina of Magendie and Luschka is considered noncommunicating hydrocephalus, whereas obstruction at the level of the arachnoid granulations is considered communicating hydrocephalus. One should always remember the value of prior exams, since long-standing, compensated hydrocephalus is generally not a cause for alarm even if encountered in the emergency setting. Ventricular obstruction may develop as a result of SAH, IVH, intraventricular mass, aqueductal stenosis, and any lesion that may cause extrinsic mass effect. Analysis should include evaluation of basal cisterns and search for possible complications that may result from herniation. Ideally, treatment of hydrocephalus will prevent such complications. Recurrence of symptoms due to ventricular shunt malfunction is a common problem that may result from catheter/tubing obstruction (intrinsic or in the peritoneal cavity), disconnection, or migration (Fig. 1-27).
Infections
Meningitis
Suspected meningitis is a very common cause for imaging, not necessarily for diagnosis, but as a precaution prior to performance of a lumbar puncture. In adults with suspected meningitis, clinical features can be used to identify those who are unlikely to have abnormal findings on CT of the head. However, many practitioners still rely on CT prior to lumbar puncture in order to exclude unsuspected mass effect and lesions that might result in rapid increases in intracranial pressure; this is especially true for patients who are immunocompromised or older than 60 years. The majority of exams are normal, but detection of findings such as brain edema possibly leading to herniation, hydrocephalus, or other complication will alter management. On FLAIR images, sulci may appear hyperintense due to proteinaceous exudates; however, SAH or high concentration of inspired oxygen may also cause the same appearance. In the vast majority of cases, meningitis is aseptic (generally of viral origin, commonly enteroviruses) and is self-limited. Bacterial meningitis is more likely to result in severe disease, and in some cases the source of meningitis may evident on imaging, such as a sinus or ear infection. On intravenous contrast-enhanced CT and MR exams, diffuse enhancement within the sulci (leptomeningeal) may be detected. Imaging is also indicated for those patients who do not respond to antibiotic treatment in hope of detecting a drainable source such as subdural empyema or parenchymal abscess. Gadolinium-enhanced MR is generally preferred to CT due to its relatively higher soft tissue contrast. Neonatal meningitis due to Citrobacter species is another indication for imaging since brain abscess develops in 80% to 90% of cases. Meningoencephalitis (encephalomeningitis) is the term applied to brain parenchymal infection in association with meningitis. Other complications of meningitis include infarcts, venous thrombosis, subdural empyema or hygroma, and obstructive or communicating hydrocephalus (Fig. 1-28). Subdural empyema and epidural abscess are illustrated in the section on head and neck imaging.
Many other infectious agents may cause meningitis, including Lyme disease (Borrelia) and, especially in the immunocompromised setting, HIV, toxoplasmosis, cryptococcosis, tuberculosis, syphilis, cytomegalovirus, and a variety of fungi (see Fig. 1-12).
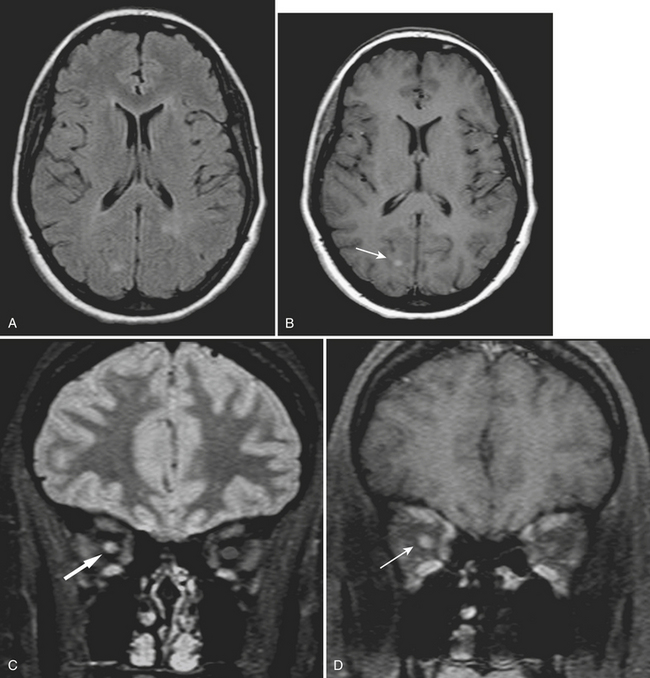
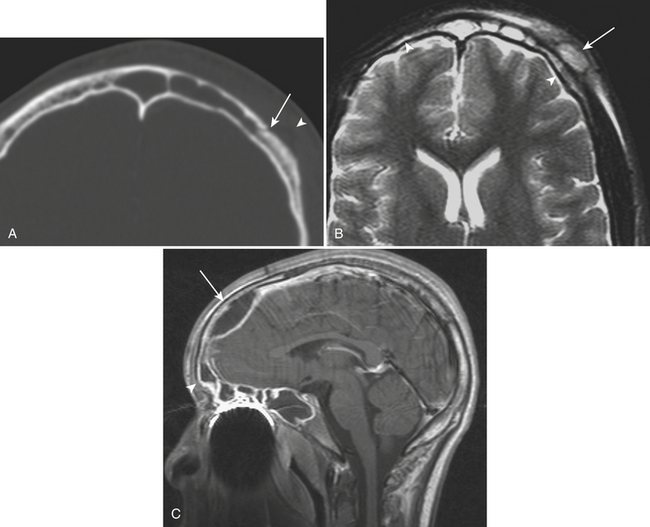
Diffuse thickened enhancement of the dura is a sign of pachymeningitis. This can be due to carcinomatous, granulomatous, and noninfectious causes including idiopathic intracranial hypotension (Fig. 1-29). Tuberculosis has a predilection for causing basal meningitis with resultant stroke due to involvement of arteries at the base of the brain.
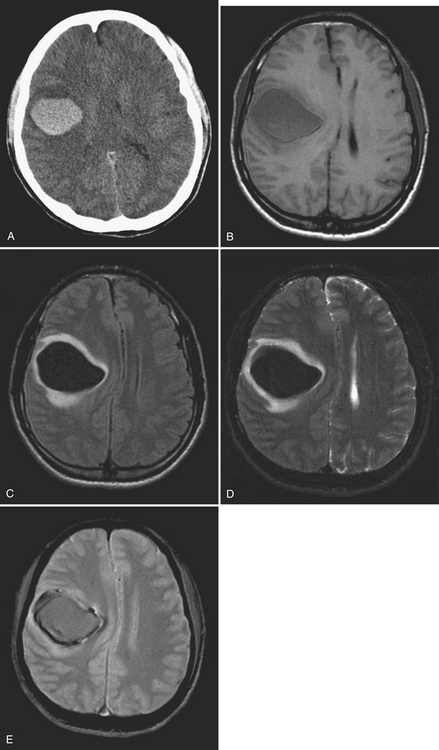
Brain Parenchymal Infection
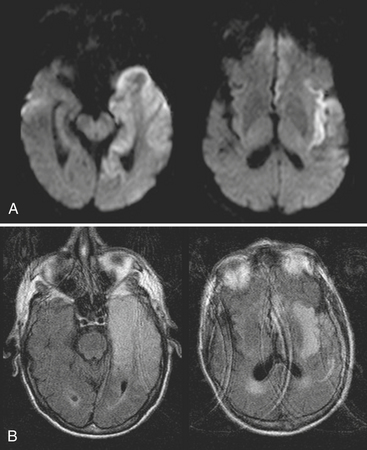
The term encephalitis refers to inflammation of the brain and is usually applied in the setting of viral infection. Cerebritis is often used interchangeably; however, it should be reserved for the cerebrum with cerebellitis added when necessary. Usually the findings are nonspecific, with simultaneous involvement of various structures. A certain pattern may suggest a particular organism, such as the classic findings of T2 hyperintensity, restricted diffusion, and minimal enhancement within the temporal lobe(s) and limbic system due to herpes simplex type 1 infection (Fig. 1-30). Nonspecific patchy T2 hyperintensities, variable diffusion, and enhancement characteristics may lead to a differential diagnosis including ischemia, infiltrating neoplasm, status epilepticus, and toxic/metabolic causes. Rather than identify the exact agent, imaging findings may be able to suggest infection and exclude other possibilities.
Abscess
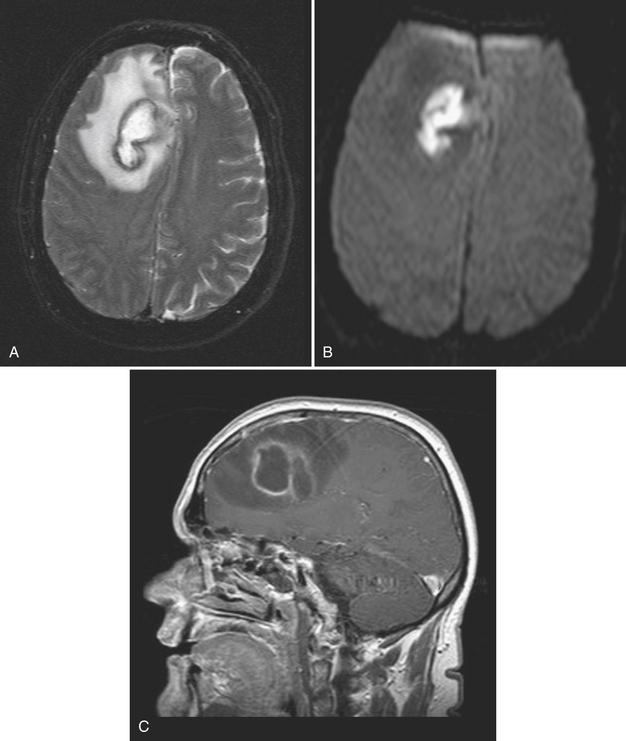
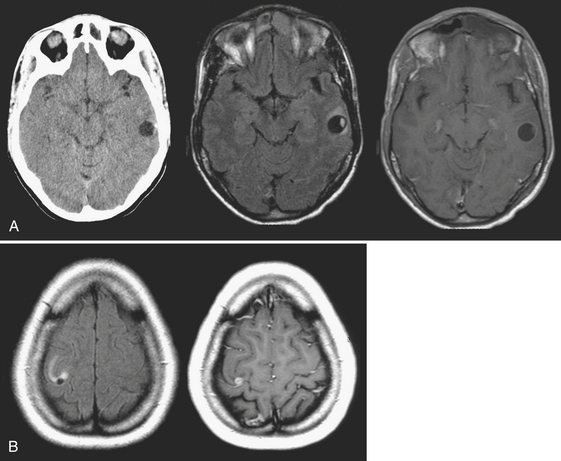
Abscess is a focal parenchymal infection due to bacteria, fungi, or parasites. The imaging characteristics reflect the phase of the infection as it evolves from early cerebritis to late cerebritis, then to early and finally late encapsulated stages. If diagnosed early, it often appears as a low-density (on CT), T2 hyperintense region with faint enhancement and a mild amount of surrounding edema. Once in the capsule stage, a typical fluid collection with a thin rim of enhancement and larger amount of edema becomes evident (Fig. 1-31). Over time (with treatment), the lesion may decrease in size and develop a thicker rim of enhancement and surrounding edema will wane. The rim is typically T2 hypointense and thinner along the deep margin. This may predispose cerebral lesions to rupture into the ventricular system, leading to ventriculitis and a dramatic clinical decline. A bacterial abscess typically shows diffusion-weighted hyperintensity; this may help to distinguish a bacterial abscess from a necrotic tumor. Lesions in the differential diagnosis other than neoplasm might include demyelination, subacute infarct, and subacute hematoma. In the setting of immune suppression, fungal abscess should be considered. In the setting of AIDS, the differential for single or multiple ring-enhancing lesions includes toxoplasmosis versus lymphoma (Fig. 1-32). Also prevalent in the AIDS population is M. tuberculosis and Cryptococcus, which can present as either meningitis or focal parenchymal lesions. To further complicate matters, another classic presentation of cryptococcosis is that of nonenhancing, gelatinous pseudocysts that distend the perivascular spaces.
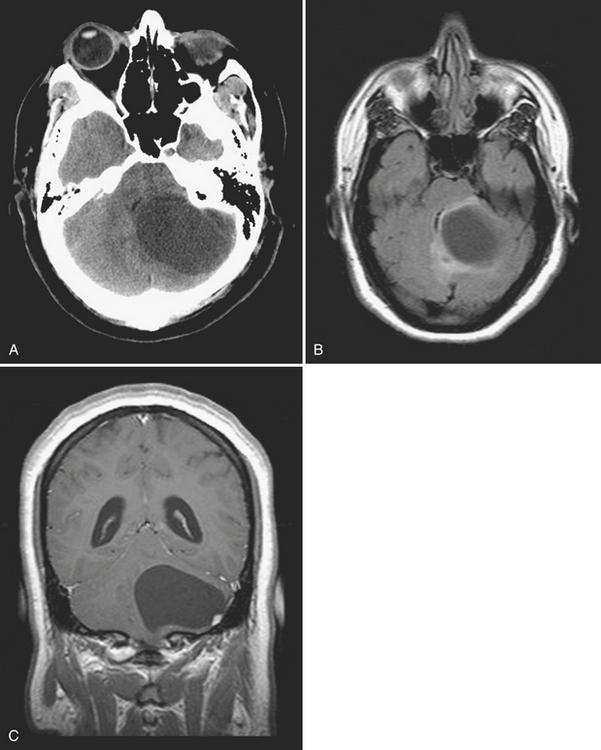
Single or multiple abscesses may develop from septic emboli due to endocarditis. Initially, these appear similar to other cardioembolic infarcts but in time develop relatively more surrounding edema and enhancement due to the inflammatory response (Fig. 1-33). If cardiac valve replacement is considered, screening for mycotic aneurysms may be requested prior to surgery.
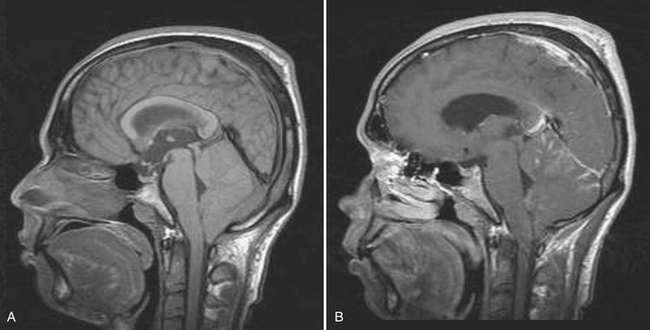
Neurocysticercosis is the intracranial infection by the pork tapeworm, Taenia solium, which is endemic in many parts of the world. It is the leading cause of seizures worldwide and has a unique life cycle and imaging After ingestion of contaminated food or water, larvae migrate from the gastrointestinal tract to the brain and skeletal muscle. Once intracranial, the larvae develop into cysticerci, and these cysts may be located in the subarachnoid spaces, brain parenchyma, or ventricular system. The cysts then progress through four stages with distinct imaging features based on the lesion and the host response. In the vesicular stage, the lesions appear as thin-walled, fluid-filled cysts, possibly with mild rim enhancement, but without surrounding edema. Lesions are generally small (less than 1 cm), and often an eccentric scolex of a few millimeters can be detected. In the colloidal vesicular stage, in which the larva starts degenerating, a thicker rim of enhancement and surrounding edema develops. It is in this stage that the appearance is similar to any other brain abscess. Next is the granular nodular stage in which the cyst involutes, the wall thickens, the lesion begins to calcify, and edema decreases. In the final nodular calcified stage, only a small calcification persists and edema resolves completely. Since lesions may progress at different rates, it is not uncommon to find more than one type of lesion. Identification of cysts in different compartments, presence of a scolex, variable amounts of edema, and small calcifications in the same patient offer great sensitivity and specificity. It is not often that the can show such confidence in diagnosis, and therefore neurocysticercosis deserves special recognition (Fig. 1-34). One might not expect this to be common in the United States; however, due to travel and immigration, it presents fairly commonly in patients presenting to our ED with new or recurrent seizures.
Tumors
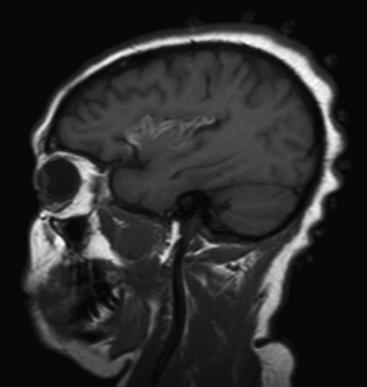
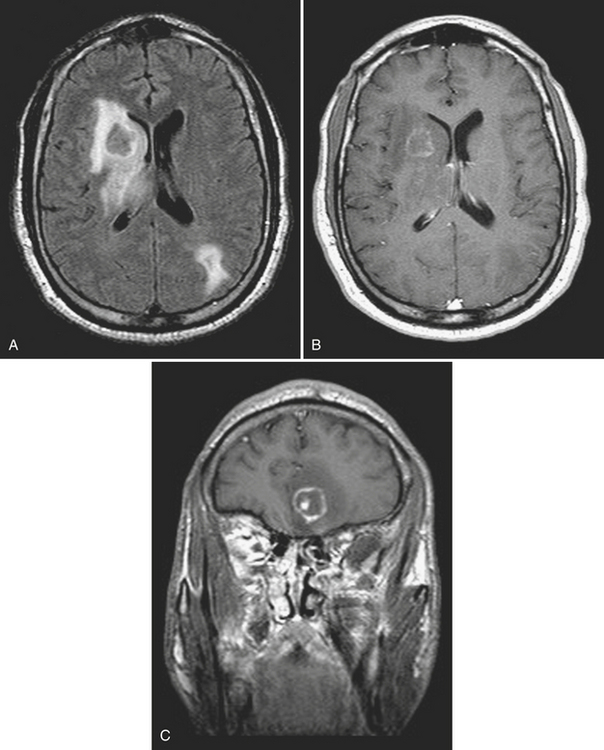
Metastases account for approximately half of all intracranial neoplasms in adults. They may present as multiple small enhancing lesions at the gray–white junction or as a single large lesion with extensive surrounding edema. Lesions are usually found in the cerebral hemispheres, and may be solid, cystic, calcified, or hemorrhagic, and approximately 50% will present as a solitary lesion. The most common primary tumors are lung, breast, and melanoma. Calcification may imply a tumor of mucinous origin (gastrointestinal tract), and hemorrhage may indicate hypervascular primaries such as choriocarcinoma or renal or thyroid primaries in addition to the other most common culprits. The World Health Organization classification of primary central nervous system neoplasms is generally based on either cell type of origin or other location (e.g., sellar). Knowledge of this classification system is important for accurate communication among clinicians from different subspecialties, especially pathology, neuro-oncology, and neurosurgery. In addition, the radiologist should be familiar with the typical imaging characteristics, locations, and demographics of the common lesions in each category. As in any field, expertise requires effort and experience. Although it may be very satisfying to correctly identify the tumor histology (or at least narrow the differential diagnosis to a select few), the major goal in the emergency setting is to recognize subtle lesions, bring attention to those that may soon result in significant complications, and provide reassurance when intervention is not indicated. Perhaps the first step in this process should be to try to determine whether a mass is intra-axial or extra-axial. While not always clear, this distinction helps to guide the differential diagnosis toward the proper category. Location of the lesion, supratentorial versus infratentorial; relation to the skull base and cisterns (anterior or middle fossa, sellar/parasellar, pineal region, cerebellopontine angle cistern, intraventricular, etc.); and multiplicity are also important details. Demographics and clinical presentation may be equally important. One must also be aware that the differential for many primary neoplasms includes metastasis, as well as infection, infarct, demyelination, inflammatory conditions, and congenital/developmental anomalies. Although space limitations preclude even the most basic description of each of the intracranial tumors, a few examples are presented to illustrate common imaging features to be recognized during workup of patients in the emergency setting (Figs. 1-35 to 1-39).
Disorders of White Matter
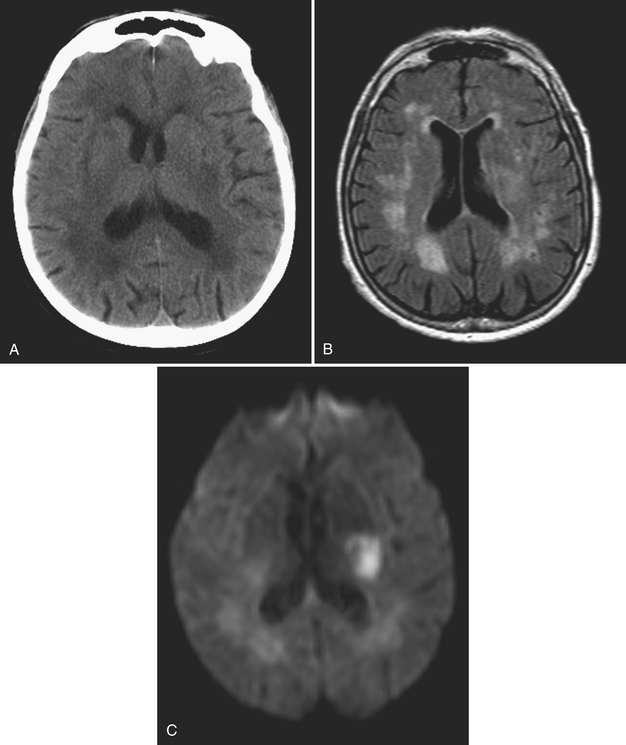
Multiple Sclerosis
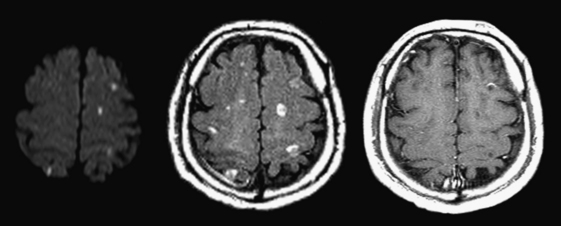
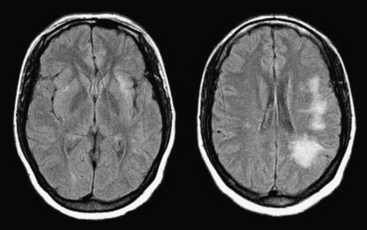
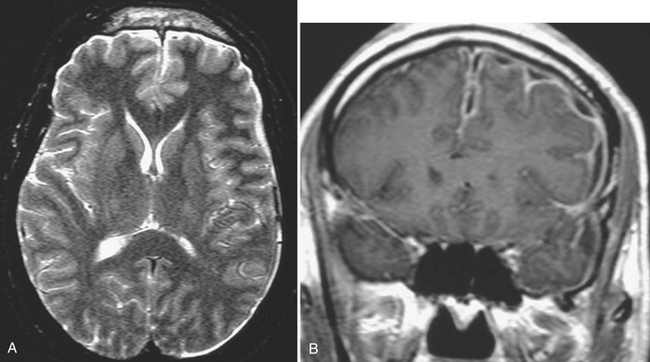
Patients presenting with a clinically isolated syndrome (first episode of neurologic dysfunction) often undergo imaging in the emergency setting mainly to exclude infarction or other process. White matter lesions detected by CT or MR may raise the suspicion of a demyelinating disorder. The diagnosis of multiple sclerosis (MS) can be complex, requiring dissemination of lesions in time and space and exclusion of other causes such as Lyme disease, vasculitis, neurosarcoidosis, lupus, and others. The Revisions to the McDonald Diagnostic Criteria for MS imaging are a standardized set of diagnostic guidelines that are applied clinically and to clinical trials; these are based on number, location, and enhancement of lesions. Classically, hypodense/T2 hyperintense lesions are located in a periventricular distribution, in the corpus callosum (callosal-septal interface), and also the brainstem, cerebellum, and spinal cord. Transient disruption of the blood-brain barrier will lead to solid or rim enhancement of active plaques (Fig. 1-40). Active demyelination may appear hyperintense on DWI. Magnetization transfer and MR spectroscopy techniques may demonstrate abnormalities even in normal-appearing white matter. Genetic and environmental factors and female to male ratios of almost 2 to 1 (adults) and greater than 5 to 1 (children) have been found. As the name implies, the unusual tumefactive variety simulates a neoplasm, often leading to biopsy. Another autoimmune-mediated disorder that is generally monophasic is acute disseminated encephalomyelitis (ADEM) and may be indistinguishable from MS on initial imaging (Fig. 1-41). Another presumably autoimmune disorder that mimics MS and ADEM is Susac syndrome. This rare syndrome has a clinical triad of encephalopathy, branch retinal artery occlusions, and hearing loss.
Progressive Multifocal Leukoencephalopathy
In the immunocompromised host (especially due to AIDS), nonenhancing T2 hyperintensities (often confluent) in the parietal and occipital lobes should raise suspicion of progressive multifocal leukoencephalopathy (Fig. 1-42). This demyelinating process due to infection with the JC virus was originally thought to affect only white matter, but cases with both gray and white matter lesions and variable enhancement have been reported. The progressive nature of the disorder is also less certain due to current, highly active, antiretroviral regimens.
Posterior Reversible Encephalopathy Syndrome
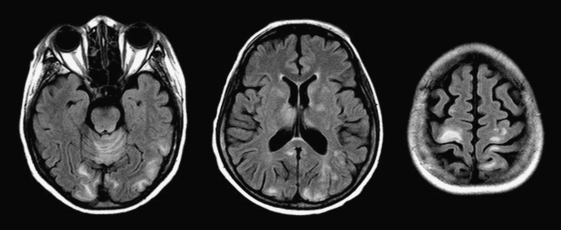
Posterior reversible encephalopathy (and seizure) syndrome (PRES) has a number of common causes such as (pre)eclampsia, hypertensive crisis, lupus and other causes of nephropathy, and drug toxicity (including immunosuppressants and erythropoietin). In the workup of new seizures, the classic features of T2 hyperintensity in a rather symmetric distribution within the occipital, parietal, and posterior frontal lobes should bring this diagnosis to mind (Fig. 1-43). Although white matter is primarily affected, gray matter structures in the basal ganglia, brainstem, and cerebellum may also be involved. Diffusion imaging is most helpful to differentiate this process from acute infarction. Small amounts of hemorrhage (and infarcts) may occur within the background of vasogenic edema resulting from abnormal vascular autoregulation. Differentiation from acute or subacute stroke is important in directing the search toward an offending agent rather than initiating the stroke clinical pathway.
Toxic Encephalopathy
Whether due to accidental or intentional exposure/ingestion, a variety of well-known toxins may cause encephalopathy. Symmetric bilateral hypodensities/T2 in the globus pallidus are the hallmark of hypoxic damage due to carbon monoxide poisoning; hemispheric white matter may also be involved. Methanol toxicity typically affects the putamen and can be hemorrhagic. In addition to cerebellar degeneration, chronic alcoholism may lead to the development of Wernicke encephalopathy. It can also be the result of thiamine deficiency and a variety of other, less common, systemic conditions, presenting with lesions of the hypothalamus, mammillary bodies, dorsal medial thalamus, and periaqueductal gray matter. Ischemic and hemorrhagic complications related to illicit drug use have already been discussed. Toxic encephalopathy may also result from drug abuse. The pattern of white matter abnormality that results from inhalation of heroin vapor (known as “chasing the dragon”) can be quite dramatic (Fig. 1-44). Encephalopathy may also be iatrogenic and related to chemotherapy (e.g., methotrexate) or radiation therapy, and generally affets the periventricular white matter in a diffuse fashion.
HEAD AND NECK TRAUMA
Skull Fractures
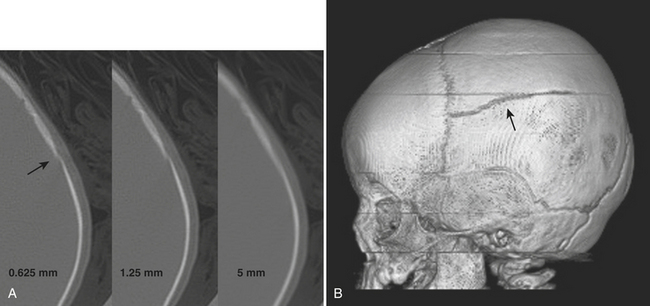
The workup of head trauma used to include skull radiographs, but those days are gone. The use of CT has become the standard of care for rapid assessment of the skull and intracranial contents. In the early days of CT, it was estimated that more than half of skull fractures were not detected, since they were oriented almost parallel to the imaging plane and therefore volume averaged with adjacent normal bone. With recent decreases in section thickness and multidetector-row technique, this limitation has effectively been overcome (Fig. 1-45). Careful review of millimeter or submillimeter thickness sections and 3D volume-rendered images and familiarity with normal sutural anatomy and variants may be necessary to accurately detect fractures. The radiologic evaluation should start with the scalp, as soft tissue swelling and scalp hematoma are very helpful indicators of possible underlying fracture. Skull fractures may be linear or comminuted and either type may be depressed. Diastasis is the term applied to separation of linear fracture fragments or sutures. Diastatic fractures occur more commonly in children. If the arachnoid membrane gets trapped within a diastatic fracture, a “growing fracture” (leptomeningeal cyst) may develop over time. Bullet wounds may leave behind a wake of comminuted bone and metallic fragments and burst fractures at the point of exit. Identification of a fracture should lead to the dedicated search for other important findings, such as intracranial hemorrhage. Fractures through the skull base may lead to other complications such as vascular or cranial nerve injuries or CSF leak. Intracranial air should prompt the search for fracture through an aerated paranasal sinus or pneumatized portion of the temporal bone, as antibiotic therapy may be indicated for prophylaxis against meningitis. One should not be alarmed by all bubbles of air, since reflux into the cavernous and other dural sinuses often occurs following insertion of intravenous catheters and injections of saline, medications, or contrast material with lax technique. The presence of fluid within the sphenoid sinuses, middle ear cavities, or mastoid air cells should also prompt a search for fractures through these regions. Temporal bone fractures may result in damage to the facial nerve, ossicles, and otic capsule. Screening for potential vascular injury in the setting of fracture of the central skull base may be accomplished with CTA. This was discussed in the section on cerebrovascular emergencies. (see also Fig. 1-54).
Orbital Blow-out Fractures
Isolated trauma to the orbit, a common cause of ED visits, plays a role in many cases of head trauma. Nontraumatic orbital emergencies are discussed later in this chapter. Fractures and injuries to the orbital soft tissues come in many different varieties. A blow to the eye causes an increase in intraorbital pressure, which is transmitted to the thin walls of the orbit. Blow-out fractures may involve the orbital floor, the medial wall, or both; the orbital rim should not be involved (Fig. 1-46). Even though the medial wall (lamina papyracea) is thinner, the network of ethmoid sinus septations acts as a support, and therefore isolated orbital floor fractures are more common. In up to about half of floor fractures, medial wall fractures will also occur. Orbital emphysema and herniation of orbital contents are possible associated findings. Coronal images are very useful to assess the degree of displacement of fracture fragments. Sagittal reformatted images help to define the anteroposterior extent of orbital floor fractures. Fragment size and displacement help to determine conservative versus operative management. Numbness of the upper cheek due to injury to the infraorbital nerve and diplopia may be presenting symptoms. Persistent diplopia and enophthalmos are possible long-term complications. Fractures that affect the orbital apex may be surgical emergencies. A bone fragment or hematoma can compress the optic nerve leading to acute decrease in visual acuity. An afferent papillary defect should also prompt careful review of this region with bone and soft tissue algorithms and display settings. While extraocular muscle entrapment is a clinical diagnosis, certain imaging features may offer guidance to the surgeon. If the inferior rectus muscle is displaced and does not retain its normal flattened shape on coronal images, then the fascial sling of the globe may be disrupted. Herniation of the muscle into the maxillary sinus can occur in the absence of a visible defect in the floor, implying that a “trap-door” fragment has sprung back into place, and prompt repair will clearly be necessary. Hematomas within the orbital soft tissues may occur in association with fractures, including retrobulbar and subperiosteal locations.
Zygomaticomaxillary Complex Fractures
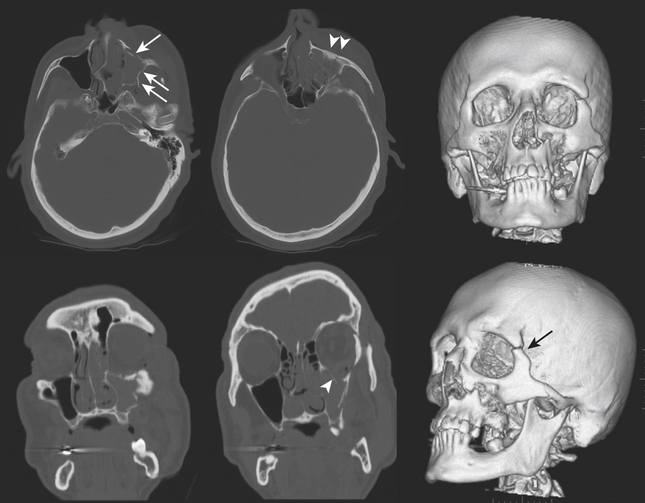
The more common injury to the zygoma involves the three major attachments (zygomaticomaxillary, zygomaticofrontal, and zygomaticosphenoid) and therefore has acquired the term tripod fracture. The preferred term may be zygomaticomaxillary complex (ZMC) fracture as it is more aptly considered a quadripod fracture due to involvement of the posteriorly located zygomaticotemporal attachment. Specific components include the infraorbital rim, orbital floor, and lateral wall, as well as the attachments to the skull base (sphenoid and temporal bones). Impaction and rotation of a large fracture fragment will result in significant deformity of the cheek and orbit (Fig. 1-47). Fractures of the pterygoid plates or greater wing of the sphenoid may sometimes be seen with severe ZMC fractures.
Le Fort Fractures
The Le Fort classification of midface fractures applies to separations of the maxilla from the skull base. The three-tier classification was developed by Le Fort in 1901 based on cadaveric experiments. These fractures rarely occur in their pure forms and while originally described as being bilateral, common usage allows for unilateral and combined bilateral types. The Le Fort I type is a fracture involving the maxillary antra, crossing midline above the hard palate. If unilateral, a sagittally oriented fracture of the palate will be present. Fractures of the pterygoid plates may occur, although some consider this a requirement by definition. With this injury, the hard palate will be allowed to separate from the rest of the face, and this will often be diagnosed by physical exam. In Le Fort II type, fractures involve both maxillary antra and inferior orbital rims crossing at the nasion (either at the nasofrontal junction or frontal process of the maxilla). The maxilla will be separated from the rest of the face. In Le Fort III type, also termed craniofacial dissociation, the inferior orbital rims are intact but the lateral orbital walls and zygomatic arches are fractured. This allows separation of the face from the rest of the skull. Variations and combinations of Le Fort and ZMC fractures are common (Fig. 1-48).
Smash Fractures
The term facial smash is a general term applied to severely comminuted fractures of the facial bones that generally occur in association with fractures of the calvarium. Intracranial hemorrhage and traumatic brain injury occur commonly with these types of fractures. The frontal type commonly involves the anterior and posterior walls of the frontal sinus (Fig. 1-49). The naso-orbitoethmoid complex can be considered in this category. Central skull base types include fractures of the sphenoid bone. Vascular injuries related to skull base fractures are discussed in the sections on temporal bone fractures and cerebrovascular emergencies.
Mandibular Trauma
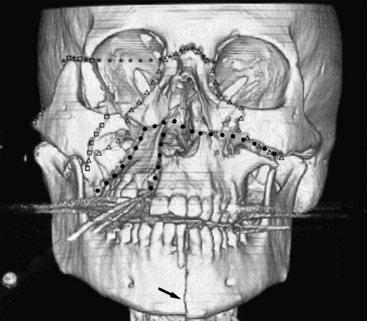
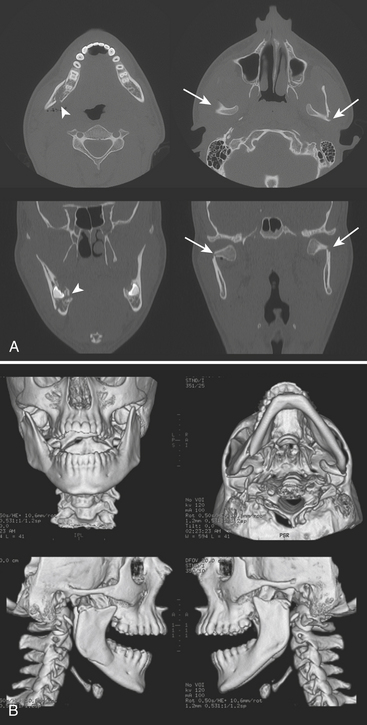
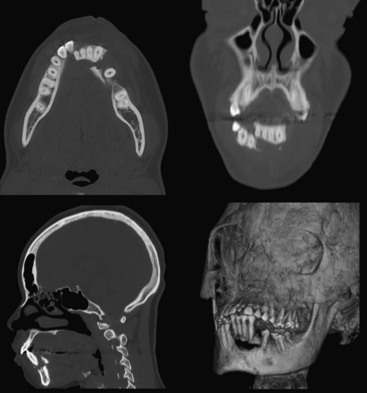
Airway compromise may result from bilateral parasymphyseal fractures since the symphysis becomes a free fragment, allowing the tongue to obstruct the oral cavity. Fractures across the mandibular canal may cause injury to the inferior alveolar nerve (branch of mandibular division of the trigeminal nerve) and result in paresthesia of the chin. Fractures affecting the teeth are considered open and require antibiotic prophylaxis. Fractured or avulsed teeth can pose a risk for aspiration, particularly in the unresponsive patient. A dedicated search for radiopaque foreign bodies in the airway on all other imaging studies is therefore necessary. Muscular forces may act favorably or unfavorably, resulting in nondisplaced or displaced fractures, respectively. Displacement may occur in either the vertical or horizontal direction. Reduction with maxillomandibular fixation or open reduction and internal fixation may be required to restore occlusion and allow proper masticatory function, speech, and facial contour. As with other facial fractures, multiplanar reconstruction and 3D volume-rendered images are helpful in comprehending the injury, especially the orientation of fracture fragments and relationships to the coronoid processes and condyles, as well as aid in surgical planning (Figs. 1-50 and 1-51).
Temporal Bone Fractures
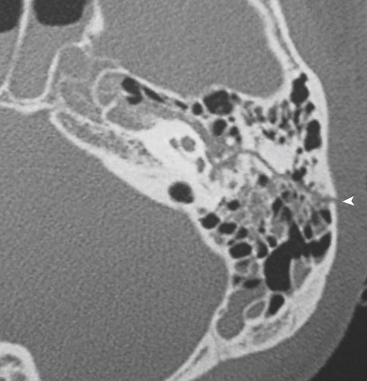
Temporal bone fracture should always be suspected when opacification of the mastoid air cells and/or middle ear compartments is present in the trauma setting. Slice thickness of 1 mm or less may be necessary to clearly identify and fully characterize the fracture. Temporal bone fracture usually occurs secondary to blunt head injury and is classically characterized as either longitudinally or transversely oriented relative to the long axis of the petrous bone. The other components of the temporal bone, namely, the mastoid, styloid, squamous, and tympanic, can be involved as well. Temporomandibular joint dislocation and styloid process fracture are discussed with mandibular fractures in the section on facial trauma. In practice, however, fractures through this region tend to be complex, and more typically than not demonstrate both longitudinal and transverse fracture characteristics (Fig. 1-52). In these cases, it is more important to characterize whether or not the otic capsule (the bony housing of the inner ear structures including cochlea and semicircular canals) and internal auditory canal are involved.
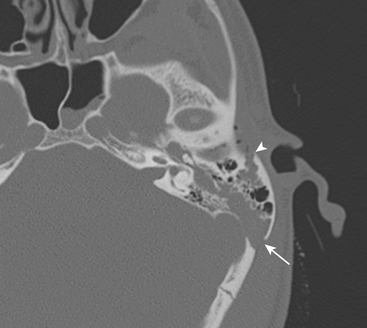
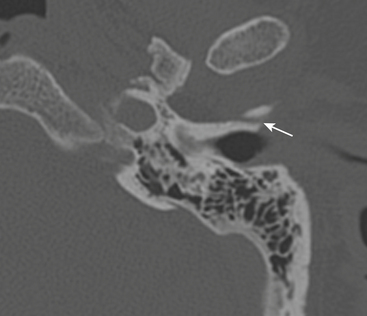
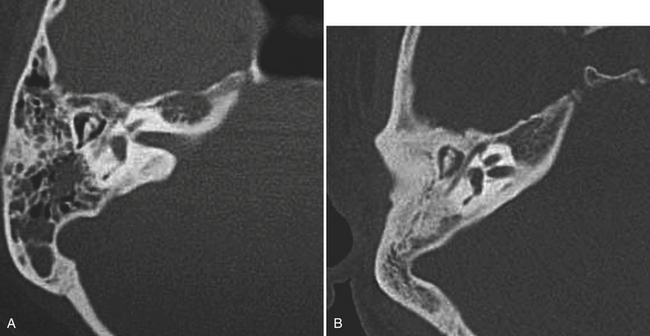
Longitudinal fractures parallel the long axis of the petrous bone and are more common than transverse temporal bone fractures (Fig. 1-53). Fracture components can secondarily involve the external auditory canal, tympanic cavity, and squamosal portions of the temporal bone. Blood and fracture fragments in the external auditory canal and tympanic cavity and ossicular disruption can account for the common clinical presentation of conductive hearing loss. Longitudinally oriented fracture tend to extend anteriorly toward the Eustachian tube and middle cranial fossa avoiding the bony labyrinth (anterior subtype). The glenoid fossa can be involved, and disruption of the middle meningeal artery can result in concomitant epidural hematoma. Less often the fracture plane may extend posteriorly behind the bony labyrinth to involve the jugular foramen and posterior fossa (posterior subtype). In these instances, careful attention should be paid to evaluate for involvement of the foramen lacerum and the sphenoid bone, which puts the traversing internal carotid artery at risk for traumatic injury. If the fracture plane is demonstrated to approximate the carotid canal, or if fractures extend into the carotid canal, CT angiography or conventional angiography can be performed to evaluate for possible internal carotid artery injury at the skull base (Fig. 1-54).
Given the complex anatomy of the temporal bone, normal sutures and channels can often be mistaken for fracture planes. Normal petro-occipital, temporo-occipital, and occipitomastoid sutures can demonstrate irregular, coarse margins, raising concern for possible fracture (Fig. 1-55). Diastasis of sutures (equivalent to a fracture) can be seen without apparent fracture. Intrinsic sutures to the temporal bone itself, including tympanosquamous, tympanomastoid, and petrotympanic sutures, also can be confused with acute fractures (Fig. 1-56). Small channels in the middle and inner ear such as the cochlear and vestibular aqueducts, singular canal, and subarcuate fossa can also convey an appearance similar to fracture planes. An understanding of normal temporal bone anatomy is essential to avoid these errors. Symmetry, lack of opacification of mastoid air cells and middle ear compartments, absence of overlying soft tissue swelling, and the clinical context can also provide reassurance that no fracture is present.
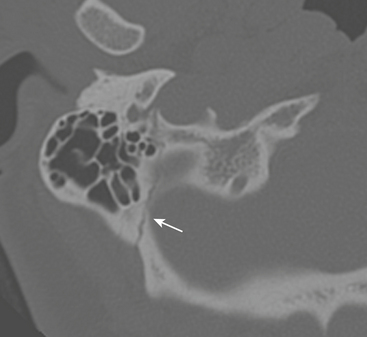
Figure 1-55 Normal occipitomastoid suture. Note jagged but well-corticated margins along suture plane.
Airway and Pharyngeal Injuries
Injuries to the airway may be accompanied by injury to the larynx and vocal cords. Presence of laryngeal hematoma and vocal cord injury should be suspected if there is asymmetry in these regions when comparing right and left (Fig. 1-57). Patients with such injury typically present with hoarseness soon after the acute event. Again, if such injury is suspected, direct visualization by endoscopy is indicated. It should be remembered that patient phonation or phase of respiration can result in apposition of the vocal cords, creating an appearance of airway obstruction. Lack of associated soft tissue swelling and clinical correlation can help in minimizing the false positive diagnosis of laryngeal injury in these cases.
INFECTIONS
Retropharyngeal Infection
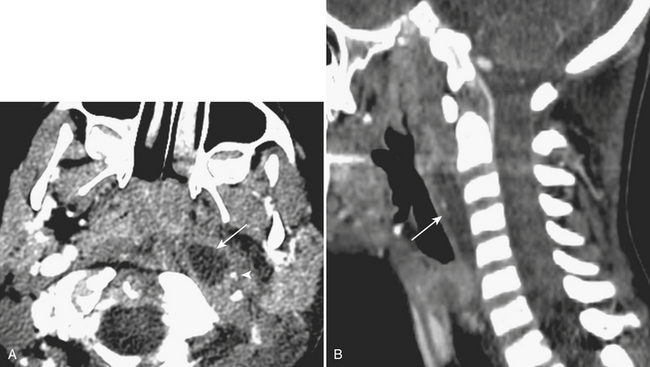
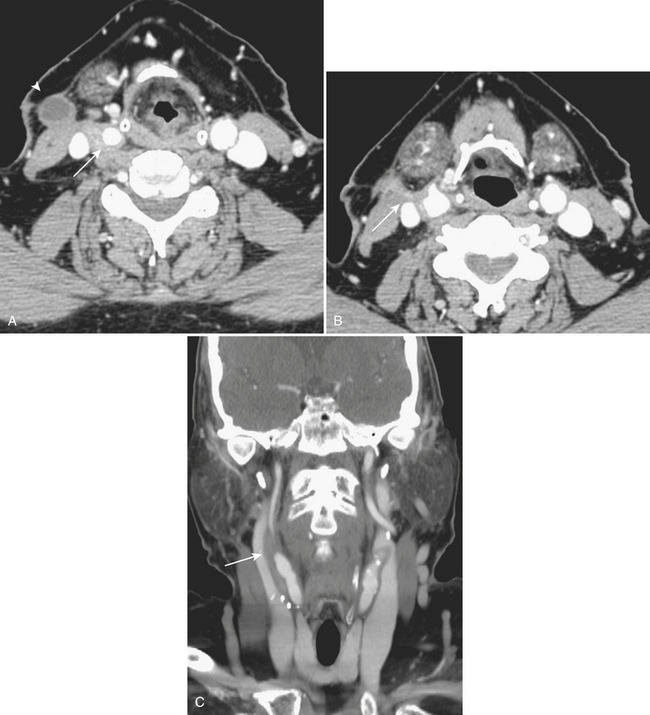
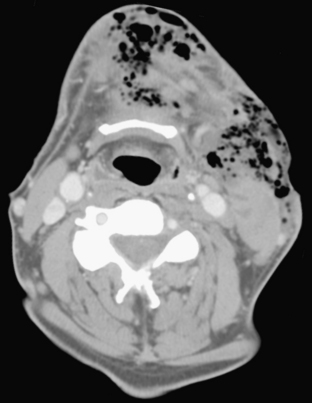
Spread of pharyngeal infection to the medial or lateral (Rouvière) retropharyngeal nodes may lead to cellulitis or development of suppurative adenitis (enlarged, rim-enhancing node). Associated edema of the retropharyngeal soft tissues is quite common, noted by expansion and diffusely increased density of the retropharyngeal fat. Extracapsular spread of nodal infection may result in a true retropharyngeal abscess typically identified as a rim-enhancing bow-tie-shaped fluid collection. Retropharyngeal space infections may quickly spread into the mediastinum (Fig. 1-58), which is a serious complication with high mortality and morbidity. Immediate surgical drainage of a retropharyngeal abscess may be indicated. Imaging plays an important role in determining the indication and access route for surgical drainage. Vascular complications of infection include spasm, arteritis and mycotic aneurysm of the internal carotid artery, and thrombophlebitis of the internal jugular vein (Lemierre syndrome). The latter condition was more common in the preantibiotic era, with rare cases still encountered in the present day. On postcontrast CT or MR imaging, a filling defect in the internal jugular vein with enhancement of the vessel wall can be demonstrated. Early diagnosis is critical, as the untreated thrombus can propagate systemically, resulting in septicemia and pulmonary emboli/infarcts.
Prevertebral Infection/Inflammation
Prevertebral collections in the neck typically occur in the setting of discitis and osteomyelitis of the cervical spine (Fig. 1-59). An associated prevertebral abscess can progress in size to potentially compromise the airway. In these cases, urgent intubation and surgical drainage may have to be considered. Retropharyngeal inflammation is often seen secondary to prevertebral space infection. Diagnosing the primary site of infection is important for appropriate patient management.
Salivary Gland Disorders
Inflammation of the submandibular or parotid glands demonstrated by glandular enlargement and surrounding fat stranding could be related to distal obstruction in Wharton’s or Stensen’s ducts, respectively, often due to obstructing calculus. Of the salivary glands, the submandibular gland is most prone to sialolithiasis, due in part to stasis from the descending then ascending course of its duct and the relatively more alkaline nature of its glandular secretions. Bacterial and viral infections can also result in salivary gland inflammation. Bacterial inflammation is most commonly seen in debilitated patients or neonates, caused by localized Staphylococcus aureus infection, and is typically unilateral. Viral inflammation occurs in children, tends to be related to systemic infection, most commonly mumps, and is usually bilateral. The inflamed gland is enlarged, hyperenhancing, with stranding of adjacent fat reflecting cellulitis, or may contain a discrete abscess (Fig. 1-60). Enlarged heterogeneous parotid glands with solid and cystic changes may reflect lymphoepithelial lesions seen in the setting of HIV disease. Similar lesions can be seen in Sjögren syndrome and other autoimmune disorders. In the chronic phase of this condition, atrophy of the parotid gland with parenchymal calcifications can be seen.
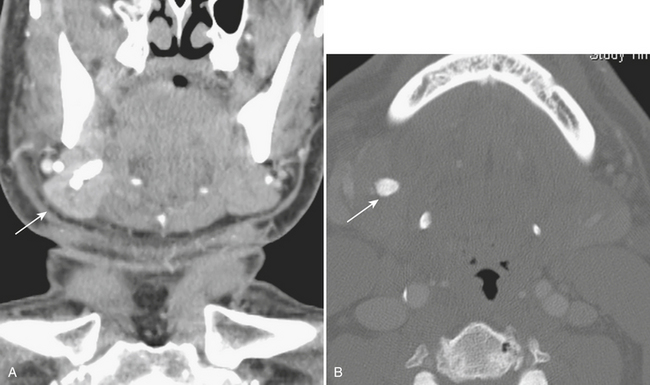
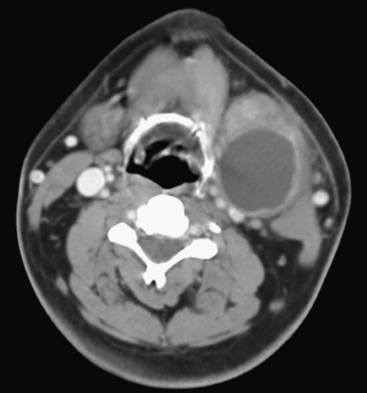
Branchial Cleft Cysts
Branchial cleft cysts are congenital epithelial-lined cystic lesions in the neck originating anywhere from the level of the mandible (first branchial cleft) to the supraclavicular region (fourth branchial cleft). These can become superinfected and present as an acutely enlarging neck mass. The second branchial cleft cyst is most common, present in the submandibular region, anteromedial to the sternocleidomastoid muscle and anterolateral to the carotid vessels (Fig. 1-61). An infected branchial cleft cyst will demonstrate peripheral rim enhancement. Necrotic infectious and metastatic lymphadenopathy may present similarly and should be considered in the differential diagnosis, particularly in adult patients.
Superficial Abscesses
Multiple bilateral superficial neck abscesses can be seen in intravenous drug abusers or “skin poppers.” Broken needles may occasionally be found. Following routine operations on the neck, fluid collections may develop in the superficial or deep soft tissues. In these cases, careful evaluation of adjacent vascular structures is warranted to assess for potential injury (pseudoaneurysm, dissection, thrombosis, or occlusion) or extension of inflammation (Fig. 1-62).
Croup
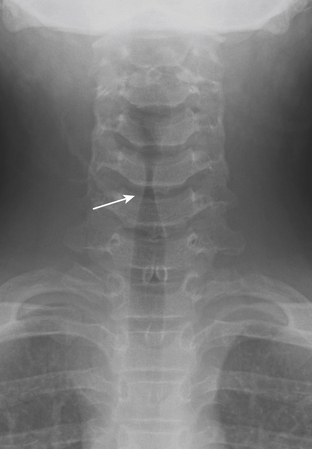
In the pediatric patient population, laryngotracheobronchitis, or “croup,” presents clinically with a characteristic cough. The condition usually occurs in children younger than 3 years of age, and is thought to be most often caused by parainfluenza virus, although other respiratory viruses and Mycoplasma have also been implicated. Croup is the most common pediatric infection causing stridor, accounting for approximately 15% of clinic and ED visits for pediatric respiratory infections. The cough is a consequence of subglottic edema and manifests classically on plain radiographs with symmetric, subglottic narrowing, the so-called “steeple” sign (Fig. 1-63). Coronal CT reformats can now replicate this appearance, although cross-sectional imaging is typically not necessary for this particular indication.
Epiglottitis
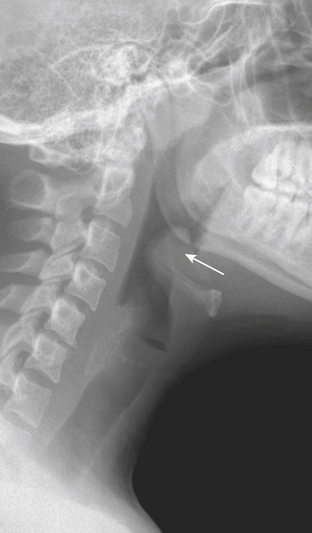
Epiglottitis is now less commonly seen in the pediatric emergency room setting, due to childhood immunization against the offending agent, H. influenzae. However, epiglottitis can occur at any age, and there has been a recent increase in incidence in the adult population, which is often underappreciated. Patients who have not been immunized can present with acute airway compromise and may require urgent intubation. On lateral radiograph, the classic “thumb” (or “thumbprint”) sign is produced by the thickened, inflamed epiglottis (Fig. 1-64). The clinical picture and radiographic imaging are usually so characteristic that cross-sectional imaging is generally unnecessary. CT imaging can provide greater diagnostic detail and can demonstrate the inflamed epiglottis along with symmetrically thickened and inflamed aryepiglottic and pharyngoepiglottic folds. However, management should be based on clinical and radiographic findings, and obtaining a CT examination might delay proper treatment.
Sinus and Orbital Infections
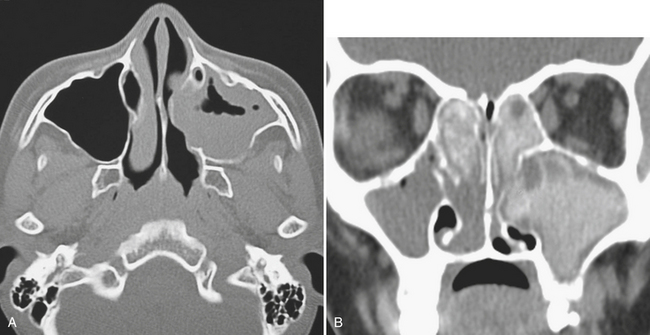
Uncomplicated acute sinusitis is typically clinically diagnosed and managed. On imaging, findings of acute sinusitis are not very specific. An air–fluid level within a sinus can suggest acute sinusitis; however, lack of this finding does not mean a patient does not have acute sinusitis. Occasionally, hyperdense opacification in the paranasal sinuses can be seen; the hyperdensity can reflect hemorrhage related to trauma, trapped proteinaceous debris, or fungal infection. Fungal infection is an important diagnostic consideration, since steroids probably should be incorporated into the treatment regimen if the cause is related to allergic disease (Fig. 1-65).
CT is particularly useful in establishing the integrity of paranasal sinus walls and can effectively demonstrate areas of bony dehiscence. Intracranial or orbital spread of infection can occur directly through areas of bony dehiscence or indirectly by perivascular extension, most commonly secondary to frontal or ethmoid sinus disease. Perivascular spread more commonly occurs in the pediatric setting. Intracranial extension is usually seen in the setting of frontal sinus infection, with involvement of the anterior cranial fossa and frontal lobes (Fig. 1-66). Imaging can demonstrate associated dural enhancement, epidural or subdural collections, and meningoencephalitis with abscess formation. There may be concomitant swelling of the soft tissues of the forehead, known as Pott’s puffy tumor (Fig.1-67). This does not imply neoplastic involvement, but rather describes osteomyelitis with extracranial soft tissue abscess formation. The presence of pachymeningeal enhancement does not necessarily imply that brain parenchyma is involved, and may only reflect dural reaction. Epidural and subdural collections can be demonstrated on either CT or MR as extra-axial fluid, possibly compressing the subjacent brain parenchyma. Subdural collections can cross sutures and generally maintain a crescentic shape, whereas epidural collections are restricted by sutures and may have a lentiform (biconvex) shape. While a reactive subdural effusion may be associated with smooth pachymeningeal enhancement, an infected subdural or epidural collection can demonstrate restricted diffusion and thickened, more irregular dural enhancement on MR. Leptomeningeal involvement is demonstrated as curvilinear enhancement extending into sulci. This may be accompanied by FLAIR hyperintensity in a corresponding distribution, which is a sensitive but nonspecific finding. Parenchymal involvement can be demonstrated by FLAIR hyperintensity and enhancement. Frank abscess formation can be demonstrated by CT or MR as a peripherally enhancing fluid collection. Restricted diffusion in the collection can support this impression.
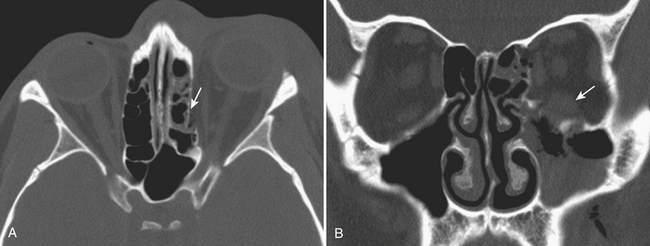
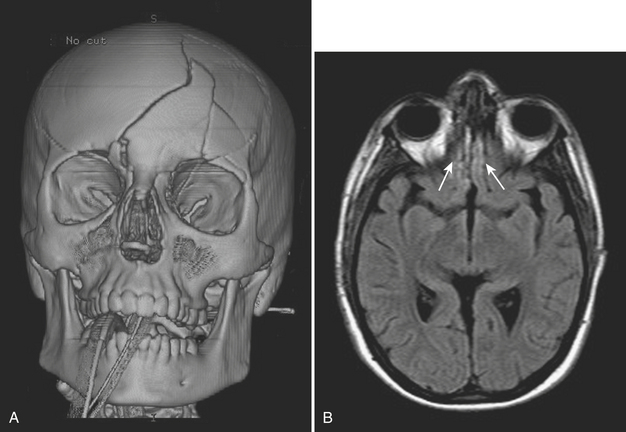
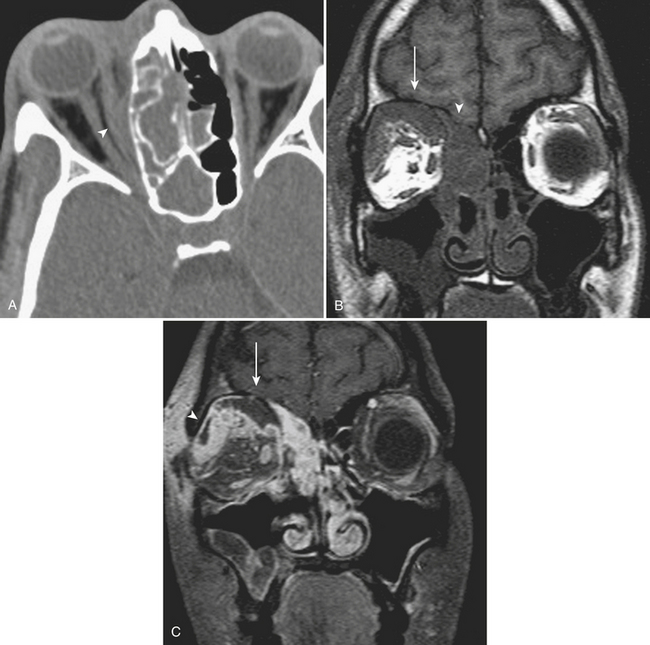
While posterior ethmoid disease can also lead to intracranial involvement, more commonly, untreated ethmoid sinusitis can extend into the medial aspect of the orbit (Fig. 1-68). Infection can spread in a subperiosteal fashion along the medial wall of the orbit, appearing as an elliptical shaped phlegmon or fluid collection. This may cause displacement of extraocular muscles. Without treatment, the phlegmon or abscess can then break through the periosteum and extend directly into the orbit and into the extraocular muscles. Depending on the size of the inflammatory process, there can be displacement of extraocular muscles and the globe, along with distortion, stretching, and compression of the optic nerve. Such findings constitute an ophthalmologic emergency, as prolonged mass effect on the optic nerve can result in permanent blindness.
Ear Infections
Much like uncomplicated paranasal sinus disease, external and middle ear infections can be diagnosed and followed clinically. Acute and chronic otomastoiditis can occasionally be picked up on imaging and should be reported since these conditions may be clinically unsuspected (Fig. 1-69). If there is clinical concern for a more complicated infectious process, CT and MR can be very useful adjuncts in diagnosis. CT imaging of the temporal bone includes the entire auditory system. At our institution it is performed with overlapping 0.625-mm-thick sections with coronal and sagittal reformats in bone and soft tissue algorithms. If there is clinical concern for intracranial involvement, contrast-enhanced CT using the same imaging parameters can provide additional diagnostic detail. MR imaging can be particularly useful in the setting of cranial nerve involvement. Our technique includes high spatial resolution T2, and pre- and postcontrast axial and coronal images from the level of the orbits to the base of the brain, in addition to routine brain imaging.
Malignant otitis externa is typically seen in diabetic and immunocompromised patients, and is a rare but serious complication of external ear infection. The inflammation can quickly progress to involve the entire ear, external auditory canal, and middle and inner ear structures. Evaluation of the stylomastoid foramen for extension of inflammation with fat stranding is important, particularly in patients with facial nerve symptoms. Associated osteomyelitis is seen as a locally aggressive destructive process of the temporal bone. Urgent treatment is indicated usually with surgical débridement, as intracranial extension of infection can develop rapidly. Cranial nerve involvement from untreated middle ear infection can occur secondarily by spread of inflammation into the cavernous sinus, typically involving cranial nerve V within Meckel’s cave and cranial nerve VI within the cavernous sinus (Gradenigo syndrome) (Fig. 1-70). Additional cranial nerve involvement can quickly ensue. Intracranial extension can result from dehiscence of the temporal bone, with direct spread into the middle cranial fossa. As with complicated paranasal sinus infection, extension into the middle cranial fossa can result in epidural abscess, subdural empyema, meningoencephalitis, or brain abscess. Transverse sinus, sigmoid sinus, and jugular vein thrombosis are other possible complications (Fig. 1-71).
Complicated Dental Disease
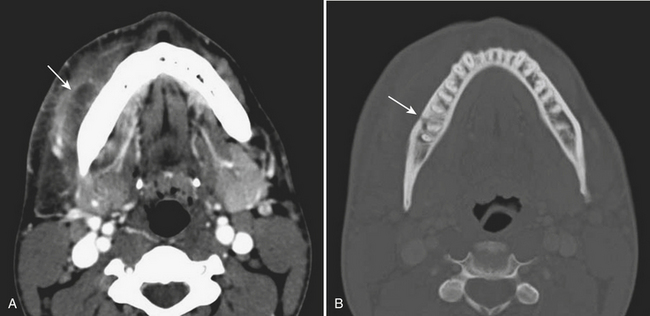
Periapical lucencies around individually infected teeth are often incidentally detected on routine CT images of the mandible and maxilla performed for other reasons. Occasionally, the infection can progress to the medullary bone, eventually breaking through the overlying buccal or lingual cortex. In these cases, this sinus tract acts as a conduit for spread of infection into the adjacent soft tissues (Fig. 1-72). With time, an abscess can develop, and the patient may present to the emergency room with pain, swelling, and elevated white blood cell count. Fluid collections in the face should therefore be carefully evaluated for a possible dental source, since the soft tissue infection will not be cured by drainage alone. Rapid progression of infection into other spaces, including the orbit and intracranial compartment, is a serious and potentially complication. Ludwig’s angina is characterized by extensive facial swelling and hardened cellulitis centered in the submandibular space, classically bilaterally, related to periodontal disease (Fig. 1-73).
Al-Nakshabandi N.A. The swirl sign. Radiology. 2001;218:433.
Filley C.M., Kleinschmidt-DeMasters B.K. Toxic leukoencephalopathy. N Engl J Med. 2001;345:425-432.
Grossman R.I., Yousem D.M. Neuroradiology: The Requisites, 2nd ed. Philadelphia: Elsevier, 2003.
Johnston S.C. Clinical Practice. Transient ischemic attack. N Engl J Med. 2002;347:1687-1692.
Osborn A.G., Blaser S., Salzman K. Diagnostic Imaging. Brain. Salt Lake City, UT: Amirsys, 2004.
van der Vorp H.B., van Gijn J. Acute ischemic stroke. N Engl J Med. 2007;357:572-579.
SUGGESTED READINGS: HEAD AND NECK
Dolan K.D., Jacoby C.G., Smoker W.R. Facial fractures. Radiographics. 1984;4:577-663.
Freeman J. Temporal bone fractures and cholesteatoma. Ann Otol Rhinol Laryngol. 1983;92:558-560.
McKennan K.X., Chole R.A. Post-traumatic cholesteatoma. Laryngoscope. 1989;99:779-782.
Peralta R., Hurford W.E. Airway trauma. Int Anesthesiol Clin. 2000;38:111-127.
Schuknecht B,Graetz K: Radiologic assessment of maxillofacial, mandibular, and skull base trauma. Eur Radiol 15:560-568. Epub 2005 Jan 21.
Shah S., Sharieff G.Q. Pediatric respiratory infections. Emerg Med Clin North Am. 2007;25:961-979.