Vascular Disorders of the Gastrointestinal Tract
Kisha A. Mitchell
A. Brian West
Introduction
The gastrointestinal (GI) tract performs energy-demanding functions of digestion and absorption of nutrients while maintaining a critical barrier between the internal milieu and the external (luminal) environment. It also contributes to the activities of the immune system. These functions depend on a reliable blood supply. It is not surprising, therefore, that vascular disorders have important clinical manifestations. Vascular disorders usually manifest clinically as bleeding or ischemia, both of which occur in acute and chronic forms. In this chapter, vascular disorders are discussed in the context of these two types of clinical presentation, although there is considerable overlap between them. Vascular tumors (e.g., angioma, angiosarcoma, glomus tumor, Kaposi sarcoma) are discussed in Chapter 30.
Anatomy of the GI Vascular System
The proximal esophagus derives its blood supply from the superior and inferior thyroid arteries, the middle esophagus from the bronchial and right intercostal arteries, and the distal esophagus from the left gastric, left inferior phrenic, and splenic arteries. The middle and lower esophagus also receives arterial blood directly from the aorta. Venous drainage is likewise segmental. Blood from the proximal esophagus drains into the superior vena cava, that from the middle esophagus into the azygos vein, and that from the distal esophagus through the left and short gastric veins into the portal vein. Anastomoses among the vessels that supply each of these regions result in complex arterial and venous networks. For instance, the venous network in the mucosa and submucosa of the middle and distal esophagus is the source of esophageal varices in patients with portal hypertension.
The stomach, proximal duodenum, liver, and pancreas are supplied by branches of the celiac artery. The distal duodenum is supplied by a branch of the superior mesenteric artery. As in the esophagus, there are numerous anastomoses between feeding vessels that result in a complex vascular network. The presence of extensive esophageal and gastric vascular anastomoses helps explain why ischemia and infarction occur only rarely in these organs. The gastric and duodenal veins drain into the portal system.
The remainder of the small intestine is supplied by the jejunal and ileal branches of the superior mesenteric artery, which also supplies the cecum and ascending and proximal transverse colon through its ileocolic, middle colic, and right colic branches. The inferior mesenteric artery supplies the distal transverse colon to the proximal rectum, whereas the distal rectum is supplied by the internal iliac and pudendal arteries. The arterial supply of the small and large intestines consists of numerous vascular arcades (vasa recta) with abundant anastomoses that result in a rich collateral circulation that helps protect the bowel against damage from pathologic processes in individual blood vessels. Detailed anatomic studies and isolated reports have suggested areas of the large bowel that are predisposed to vascular insult. The most commonly characterized of these are the splenic flexure and rectosigmoid regions. Reports suggest that the ileocecal region is also predisposed to ischemic injury, but to a lesser extent.1–3 Venous drainage of the colon and rectum follows the arterial supply. The vascular collaterals in the rectum provide another location in which varices may develop in patients with portal hypertension.
The anus is supplied by the superior, middle, and inferior rectal arteries. Branches of these arteries have multiple small arteriovenous anastomoses with submucosal venous plexuses. Interestingly, the blood flow to the anal canal exceeds its metabolic requirements. Venous drainage of the anus is mainly to the superior rectal vein.
Blood flow to the GI tract is normally under tight physiologic regulation. For example, the blood supply increases by as much as 100% immediately after ingestion of a meal. On the other hand, in states of shock, when it is vital to maintain blood flow to critical organs such as the central nervous system, splanchnic blood flow may be drastically reduced, sometimes beyond the critical level needed to maintain viability of the intestinal mucosa.
Upper GI Bleeding
General Comments
Bleeding from the upper GI tract is a frequent indication for endoscopy and is common worldwide. In the United States, acute GI bleeding accounts for 250,000 to 300,000 hospitalizations per year.4 As many as 14% of patients admitted on an emergency basis and as many as 28% of those who are hospitalized die of their acute GI bleeding.5,6 Upper GI tract bleeding, defined as bleeding proximal to the ligament of Treitz, is four times more common than lower GI tract bleeding.7 Upper GI bleeds occur most commonly in elderly patients, although in conditions such as hereditary hemorrhagic telangiectasia (HHT), onset of disease may occur as early as the third decade of life.8 Risk factors for upper GI tract bleeding include older patient age, alcohol use, and concomitant use of drugs such as nonsteroidal antiinflammatory drugs (NSAIDs) and anticoagulants.9
Patients often are seen with direct evidence of a GI bleed, such as hematemesis, melena, heme-positive stools, and, less commonly, hematochezia. They may also be seen with signs and symptoms of iron deficiency anemia, such as syncope, chest pain, dizziness, and shortness of breath. In severe, acute, life-threatening hemorrhage, the clinical presentation is usually a result of shock or cardiovascular collapse.
Esophagus
The most important causes of esophageal bleeding are varices that develop as a result of portal hypertension, Mallory-Weiss lacerations, or esophagitis (Table 10.1).
Table 10.1
Pathologic Features of Entities That Cause Esophageal Bleeding
| Entity | Key Clinical Features | Key Pathologic Features | Treatment |
| Gastroesophageal reflux disease | Most common symptoms are heartburn, regurgitation, and dysphagia More prevalent after age 40 yr |
Typically distal 7 cm of esophagus Background esophagitis: squamous hyperplasia, intraepithelial neutrophils, and increased eosinophils and lymphocytes |
Medical options: PPI and H2 antagonists Surgical options: fundoplication and gastropexy |
| HSV infection | Main symptom: odynophagia Common in immunocompromised patients, particularly organ and bone marrow transplant recipients Occurs in immunocompetent patients |
Vesicles early feature; coalesce to form ulcer Ground-glass nuclear inclusions in squamous cells at edge of shallow, punched-out ulcers Immunostain when in doubt |
Antivirals (e.g., acyclovir) In immunocompetent patients may resolve spontaneously |
| Candida infection | Main symptom: substernal dysphagia More common in immunocompromised patients Often coexists with other etiologies |
Patchy white mucosal plaques Neutrophils, yeast and pseudohyphae in superficial squamous epithelium Usually with desquamation: often detached fragments in biopsies Fungal stains (PAS, GMS) often helpful |
Antifungals (e.g., fluconazole, itraconazole) |
| CMV infection | Main symptom: odynophagia Common in immunocompromised patients In HIV-infected patients, more common than HSV |
Ulcers deeper, more linear than in HSV Single large nuclear and multiple amphophilic cytoplasmic inclusions in capillary endothelial or stromal cells in granulation tissue at base Immunostaining may be necessary because inclusions can be rare |
Antivirals (e.g., ganciclovir, foscarnet) |
| NSAIDs | Concomitant illnesses necessitating NSAID (e.g., chronic back pain, arthritis) Simultaneous alcohol use common |
Shallow, large ulcers with broad base, usually in middle esophagus Usually devoid of background esophagitis |
Discontinue medication Supportive therapy |
| Pill/Medication induced | Sudden onset of symptoms Pills swallowed without fluids Can be caused by many medications (e.g., tetracycline, potassium chloride, biphosphonate, quinidine) |
Nonspecific superficial desquamation of squamous epithelium, spongiosis, necrotic keratinocytes Exudate seen commonly with quinidine-induced ulcers |
Educate patient to take pills with fluids Discontinue medication or start liquid therapy if necessary Supportive therapy |
| Radiation | Usually history of head and neck, mediastinal, pulmonary, or esophageal malignancy | Fibrinoid necrosis and dilatation of blood vessels, vascular intimal proliferation, stromal fibrosis, stellate fibroblasts ± cytologic atypia and increased apoptosis | Supportive therapy Surgery if perforation or fistula develops |
| Foreign body injury | Most common in children; also seen in elderly, psychiatric patients, inmates Sudden onset of symptoms; may lead to esophagovascular fistula |
Nonspecific necrosis, inflammation, and ulceration; may progress to perforation | Endoscopic or surgical removal Resection or repair of perforation or fistula |
| Chemical injury | Similar to foreign body injury Alkaline ingestions damage esophagus more commonly |
Necrosis more common | Supportive therapy May require resection |
| Varices* | Occur in portal hypertension, cirrhotic and noncirrhotic Schistosomiasis and viral hepatitis are common causes worldwide Typically asymptomatic until rupture; rebleeding common |
Most common in distal 3-4 cm of esophagus Dilated tortuous veins of lamina propria and submucosa bulging into lumen, fresh hemorrhage and adjacent thrombosis Erosions and ulcers uncommon Thrombosis, ulceration, necrosis, and inflammation more likely after therapy |
Sclerosing and banding varices Portosystemic shunting Medical management of portal hypertension |
| Mallory-Weiss tears* | Strong association with chronic alcoholism, ASA use common Typical history (retching or forceful vomiting) in only 30% Related to upward diaphragmatic movement |
Minority involve esophagus; majority on lesser curve of proximal stomach, may be at esophagogastric junction Nonspecific mucosal breach with acute hemorrhage, seldom into submucosa Usually no acute inflammatory response |
Usually self-limited If therapy is required: balloon tamponade, embolization, medical management Surgery rare |
* Most common causes.
ASA, Acetylsalicylic acid; CMV, cytomegalovirus; GMS, Grocott-Gomori methenamine–silver nitrate; H2, histamine 2; HIV, human immunodeficiency virus; HSV, herpes simplex virus; NSAIDs, nonsteroidal anti-inflammatory drugs; PAS, periodic acid–Schiff; PPI, proton pump inhibitor.
Esophageal Varices
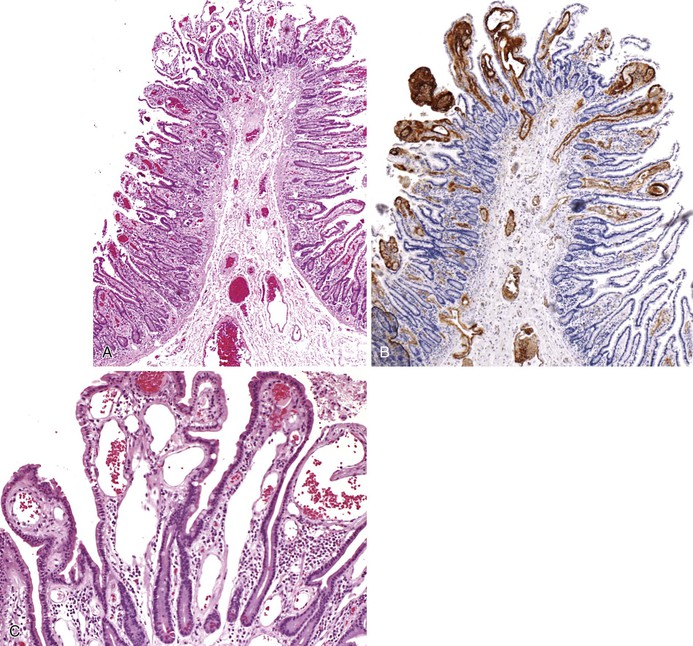
Esophageal varices are dilated, tortuous veins within the lamina propria and submucosa that bulge into the esophageal lumen because of portal hypertension and portosystemic shunting. Esophageal varices may develop in any condition that leads to portal hypertension, but they are most often associated with alcoholic cirrhosis. The demographic features of patients with esophageal varices are similar to those of patients with cirrhosis and portal hypertension (see Chapter 50). Worldwide, hepatic schistosomiasis is a common cause of esophageal varices. Varices are typically asymptomatic until they rupture into the esophageal lumen, resulting in hematemesis or melena.
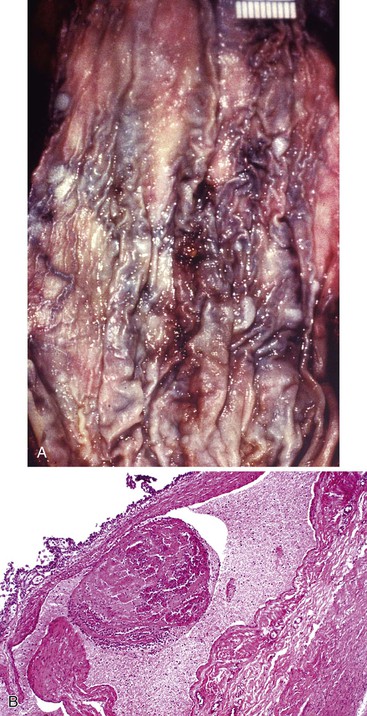
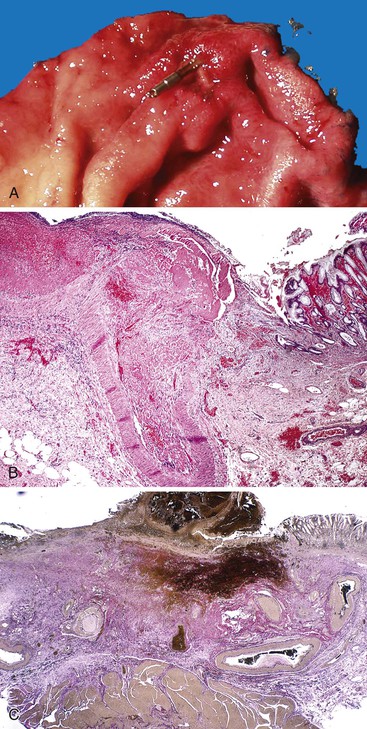
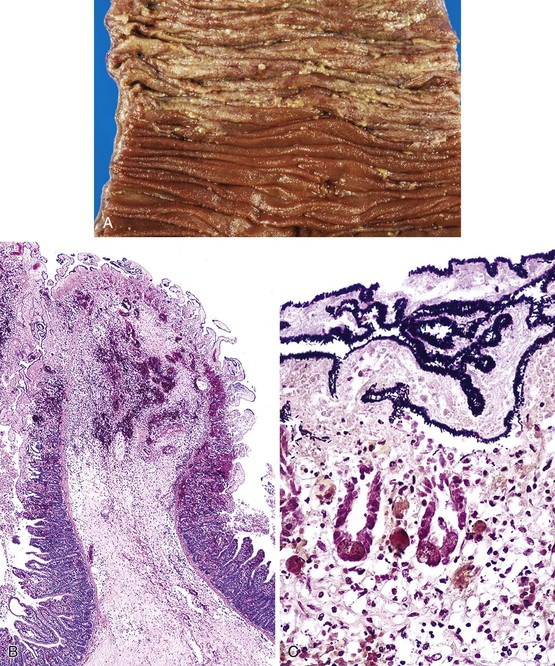
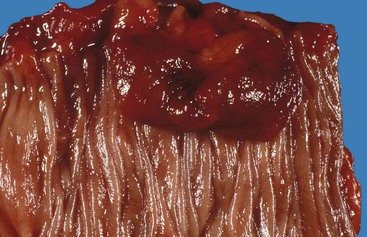
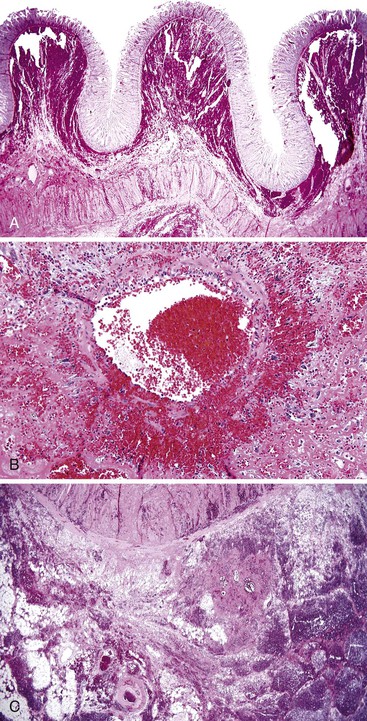
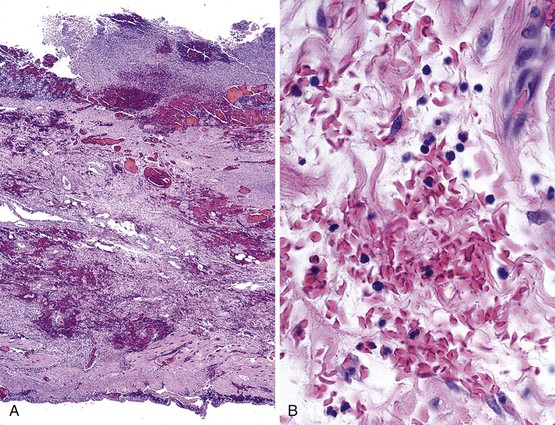
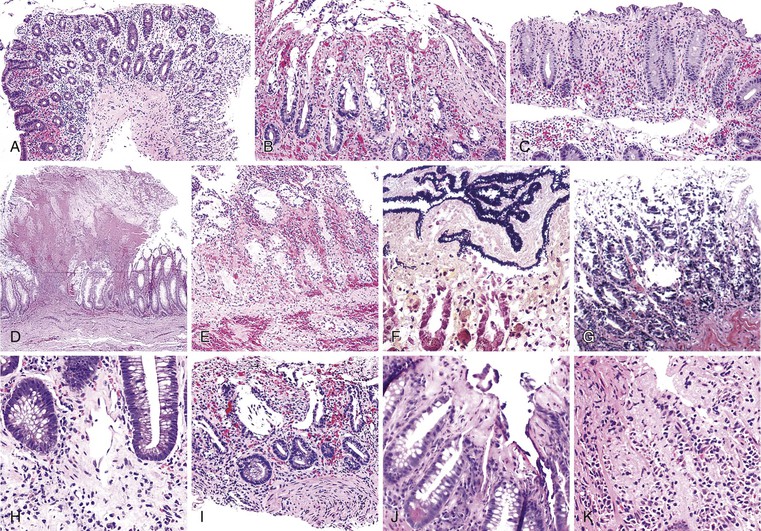
Bleeding varices are most common in the caudal portion of the esophagus; however, a discrete bleeding site is usually difficult to identify pathologically. Varices tend to collapse at the time of autopsy and therefore are best diagnosed endoscopically. In autopsy specimens, eversion and formalin fixation of the esophagus are techniques that can be used to help demonstrate varices. Grossly dilated, tortuous veins may be seen in the distal 3 to 4 cm of the esophagus when they bulge into the lumen (Fig. 10.1, A). Thrombosis may be observed in the dilated veins.
Histologically, dilated submucosal veins are characteristic, and these are usually associated with fresh hemorrhage or organizing thrombi (see Fig. 10.1, B). Surface ulcers or erosions are uncommon. In specimens examined after treatment with sclerotherapy or endoscopic ligation, findings may include thrombosis, ulceration, necrosis, inflammation, and fibrosis.10,11
Although variceal bleeding is the most important cause of hematemesis in patients with portal hypertension, other causes of upper GI bleeding are implicated in approximately 50% of patients who are seen with hematemesis (Table 10.2; see also Table 10.1).
Table 10.2
Pathologic Features of Entities That Cause Gastric or Duodenal Bleeding
| Entity | Key Clinical Features | Key Pathologic Features | Treatment |
| Helicobacter pylori–induced peptic ulcer disease | Most common cause Decreasing prevalence rates |
Gastritis more common in antrum Typically superficially oriented lymphoplasmacytic gastritis with neutrophil infiltrate and spiral forms of H. pylori May have reactive lymphoid follicles, intestinal metaplasia, mucosal atrophy Ulcers may be located in duodenum |
H. pylori eradication therapy Antisecretory therapy |
| Chemical-induced ulcers | NSAID use is a clear predisposing factor to gastric bleeding | Erosions or shallow ulcers More common in fundus and body Usually without background gastritis |
Discontinue medication or significantly reduce dose |
| Mucin loss with nuclear enlargement and hyperchromasia in adjacent epithelium | Supportive therapy | ||
| Stress related | Patients with multiple comorbidities; risk factors include prior PUD, CNS trauma or surgery, sepsis, multiple trauma, liver and kidney failure, and organ transplantation In hospital patients, duodenal ulcers more later in course16a Curling ulcer: following burns Cushing ulcer: following neurologic injury or surgery |
Erosions or shallow ulcers Usually without background gastritis More common in fundus and body, may also be seen in duodenum Abrupt erosions or ulcers with interstitial hemorrhage, reactive epithelial atypia Adjacent mucosa with acute hemorrhagic gastritis, diffuse mucosal hyperemia, edema |
Supportive therapy; may include blood transfusion. Prophylactic therapy to prevent additional ulcers (includes PPI, antacids, H2 antagonists, sucralfate, prostaglandin analogs) |
| Dieulafoy lesion | Most common in middle-aged and elderly men | Most common in proximal stomach on lesser curve Similar lesions reported in distal esophagus, small and large bowel Small mucosal defect with abnormally large, thick-walled artery in base Adjacent mucosa usually lacks inflammation |
Therapeutic endoscopy (e.g., clipping, electrocoagulation, injection sclerotherapy, banding) Surgical ligation or resection |
| Cameron ulcers | Associated with hiatal hernias | Linear ulcers in hiatal hernias Histologic features of a benign ulcer |
Therapeutic endoscopy to control bleeding Surgical management of hernia. |
| Varices* | Most commonly in continuity with esophageal varix with similar morphology | See Table 10.1 | |
| Portal hypertensive gastropathy and gastric vascular ectasia | See Table 10.3 | See Table 10.3 | |
| Hereditary hemorrhagic telangiectasia (HHT) | Autosomal dominant disease with variable penetrance and expression Telangiectases on skin and mucous membranes Frequently have history of GI bleeding 20% have no family history of symptoms |
More common in stomach but also seen in esophagus and duodenum Lesions include telangiectasias, arteriovenous malformations, aneurysms, venous varicosities, and arteriovenous fistulas Classically clusters or tufts of dilated, tortuous arteriolar-venular connections without intervening capillaries |
Medical therapy includes danazol, estrogen, aminocaproic acid Therapeutic endoscopy (coagulation, cautery or laser) Surgical resection |
| Hemodialysis-associated telangiectasia | Long-term hemodialysis | Morphologically similar to HHT, may appear reddish and fern-like grossly | Therapeutic endoscopy (thermal coagulation) |
* Most common cause.
CNS, Central nervous system; H2, histamine 2; NSAID, nonsteroidal antiinflammatory drug; PPI, proton pump inhibitor; PUD, peptic ulcer disease.
Esophageal varices often rebleed, and the risk of dying within 6 weeks of an initial bleed is 20% to 30% in patients with cirrhosis-related portal hypertension.12 Variceal bleeding may precipitate hepatic encephalopathy in cirrhotic patients. Treatment modalities include sclerosis and banding of individual varices, portosystemic shunting, and pharmacologic management of portal hypertension.
Mallory-Weiss Lacerations
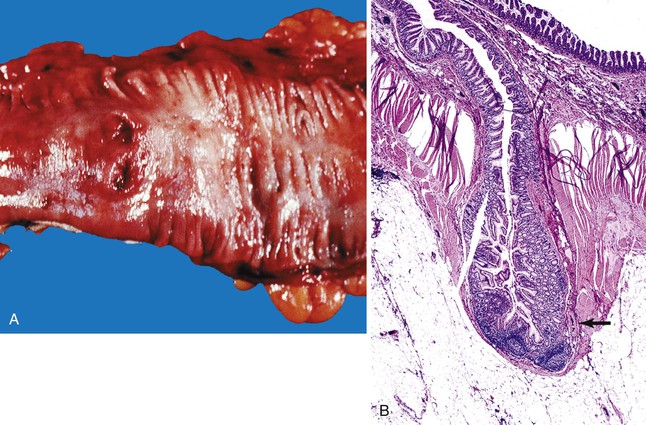
Mallory-Weiss lacerations are longitudinal tears of the mucosa located in the region of the gastroesophageal junction. These lesions may bleed profusely.13 A clinical history of retching or forceful vomiting is identified in approximately 30% of patients. Mallory-Weiss lacerations develop mainly in patients with chronic alcoholism and typically occur in the third to fifth decades of life. Concomitant aspirin use has been reported in approximately 30% of patients. Other risk factors include upward diaphragmatic movement related to coughing, heavy lifting, pregnancy, and abdominal trauma.14 Mallory-Weiss laceration is an uncommon complication of upper GI endoscopy, in association with retching and hiatal hernia. In addition, some patients are seen with melena or iron deficiency anemia.
The pathogenesis of Mallory-Weiss lacerations is related to upward movement of the diaphragm and an increase in intraabdominal pressure, which leads to protrusion of the proximal stomach into the thoracic cavity. This is often preceded by nausea, which causes distention of the stomach and reflux of gastric contents into the esophagus. When protrusion of the distended stomach is forceful, longitudinal lacerations may develop. Lacerations are more likely in patients with a hiatus hernia.
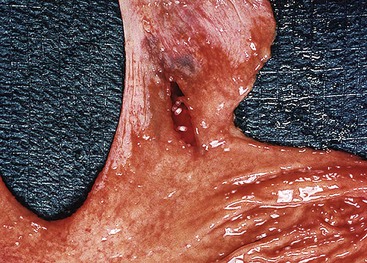
Most Mallory-Weiss lacerations are located on the gastric side of the gastroesophageal junction. Only a minority involve the tubular esophagus.13 They occur most commonly on the lesser curve of the proximal stomach (Fig. 10.2) but may also occur at the gastroesophageal junction. Histologically, Mallory-Weiss lacerations are characterized by a longitudinal breach of the mucosa that extends into the submucosa, but generally not into the muscularis propria, usually accompanied by hemorrhage, either with or without an acute inflammatory response. Healing Mallory-Weiss lacerations show granulation tissue, fibrosis, epithelial regeneration, and other features of healing ulcers.
Mallory-Weiss lacerations are usually treated supportively. In occasional patients with persistent bleeding, pharmacologic management, balloon tamponade, embolization, and, rarely, surgery may be required to establish hemostasis. Boerhaave syndrome (acute rupture of the esophagus) is a catastrophic event that is sometimes considered to be a complication of Mallory-Weiss lacerations. However, patients with Boerhaave syndrome are not typically seen with severe acute GI bleeding.15
Esophagitis and Esophageal Ulcers
Esophagitis and esophageal ulcers are most commonly caused by GERD, infection (e.g., herpesvirus, Candida, cytomegalovirus), drugs, chemical or physical injury from ingestion of toxic substances, or foreign objects. Bleeding from esophagitis is usually occult, and patients commonly are seen with anemia. Bleeding from esophageal ulcers varies in severity depending on the cause, but it is most often acute. Signs and symptoms include hematemesis, epigastric pain, and odynophagia. Most patients with acute bleeding are seen in the emergency department,16 and many of them have significant comorbid conditions.
Overall, GERD-associated ulcers are most common, and they are seen in all age groups, although they are more prevalent after the age of 40 years. Ulcers caused by infections are more likely to occur in immunocompromised patients. However, herpes simplex virus esophagitis has been documented in immunocompetent patients as well. Other causes of esophageal ulcers include Crohn’s disease and use of NSAIDs. Concomitant alcohol use has been reported in a subset of patients with NSAID-induced esophageal ulcers, suggesting that it may have a synergistic effect. Ulcers related to medication injury are common in the elderly, whereas those caused by foreign objects are more common in children. Reflux is less likely to be associated with bleeding than use of NSAIDs, which cause injury by direct mucosal contact.17 Radiation-induced injury is mediated by vascular compromise. Injury from chemical agents, such as lye, is caused by their corrosive effect on the mucosa.
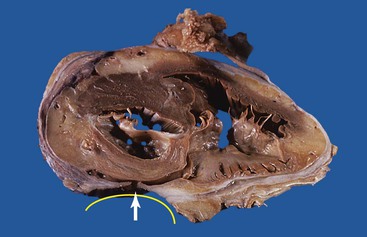
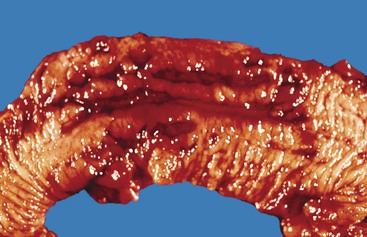
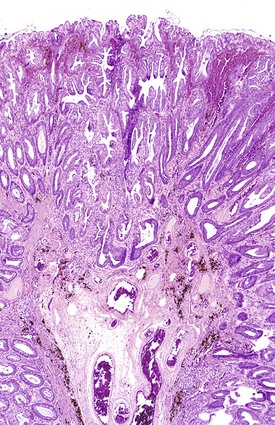
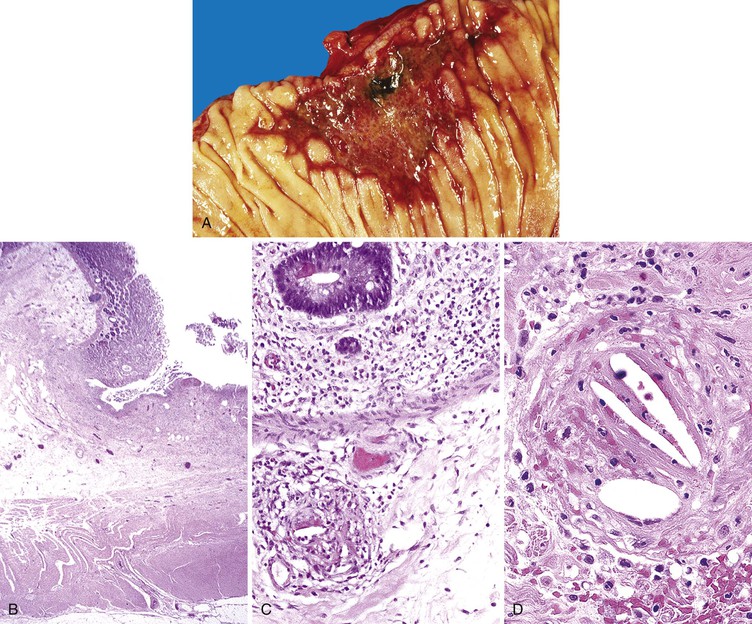
Ulcers resulting from GERD occur mainly in the distal third of the esophagus. Adjacent mucosal changes typical of reflux, such as squamous hyperplasia with prominent fibrovascular papillae and intraepithelial inflammation (eosinophils, neutrophils, and lymphocytes), are usually present. Herpesvirus inclusions are identified most readily in squamous cells at the edges of herpetic ulcers (Fig. 10.3). In contrast, cytomegalovirus inclusions are most often found in endothelial cells or stromal cells at the ulcer base. Candida should be sought wherever neutrophils accumulate in the surface epithelium, usually in association with superficial desquamation. Morphologic features of radiation injury include dilated and thickened capillaries with deposition of hyaline, prominent endothelial cells, and reactive stroma with atypical stellate fibroblasts. NSAID-induced ulcers are typically large, shallow lesions with a broad base and are most common in the middle esophagus.17 Pill esophagitis shows superficial epithelial sloughing, keratinocyte necrosis, spongiosis, and intraepithelial eosinophils. Ulcerating malignant neoplasms may also be a source of bleeding, but this is an unusual presentation of esophageal cancer.18 However, the most common indication for endoscopy in patients with a secondary malignant neoplasm of the esophagus is GI bleeding.19 Bleeding from NSAID-induced ulcers is usually active and infrequently complicated by strictures. Rarely, esophageal or gastric ulcers perforate or penetrate into contiguous vascular organs, such as the heart and aorta, leading to massive bleeding20 (Fig. 10.4). Ulcers resulting from foreign bodies are particularly prone to this complication.18,19,21,22
Esophageal Diverticula
Esophageal diverticula are rare and usually asymptomatic. However, patients with diverticula may occasionally present with upper GI tract bleeding. Bleeding may occur in association with an ulcer, entrapped pills, or use of anticoagulants.23
Stomach
Aside from bleeding resulting from varices, which commonly involves both the esophagus and the proximal stomach, gastric bleeding is most often caused by benign ulcers and erosive gastritis (see Table 10.2).
Gastric Varices
Gastric varices have clinical and pathologic features similar to those of esophageal varices. They most commonly occur on the lesser curve of the proximal stomach, in continuity with esophageal varices.24 Reports vary as to whether gastric varices, compared with esophageal varicies, are less likely24 or equally likely25 to bleed. Bleeding from gastric varices, when it does occur, is usually severe.24
Gastritis and Gastric Ulcers
Clinical Features and Etiology
An estimated 350,000 cases of acute gastric ulcers are diagnosed annually. There are many causes of acute gastric (and duodenal) ulceration. Overall, gastric ulcers are more common in men than in women, and they are seen most often in the middle-aged and elderly population.
Peptic ulcers, both acute and chronic (including those located in the duodenum), account for 20% to 50% of cases of upper GI bleeding.26 Risk factors for peptic ulcer disease include Helicobacter pylori infection, NSAIDs, and alcohol.
Stress ulcers are acute gastric ulcers that develop in patients suffering from shock, sepsis, or trauma.27 Stress ulcers have numerous synonyms, such as acute hemorrhagic gastritis. Curling ulcers develop after severe burns. Cushing ulcers develop after severe head trauma. Steroid ulcers develop because of steroid use. Risk factors for stress ulcers include respiratory failure, coagulopathy, recent major surgery, major trauma, severe burns, hepatic or renal disease, sepsis, and hypotension.28
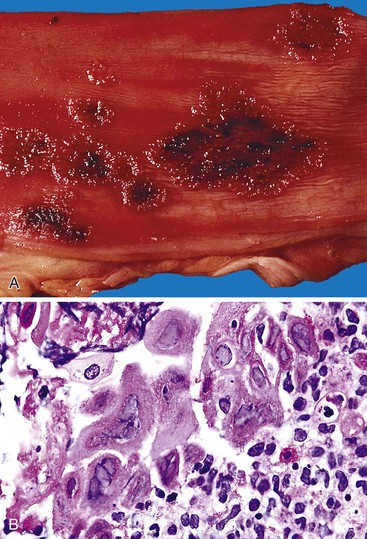
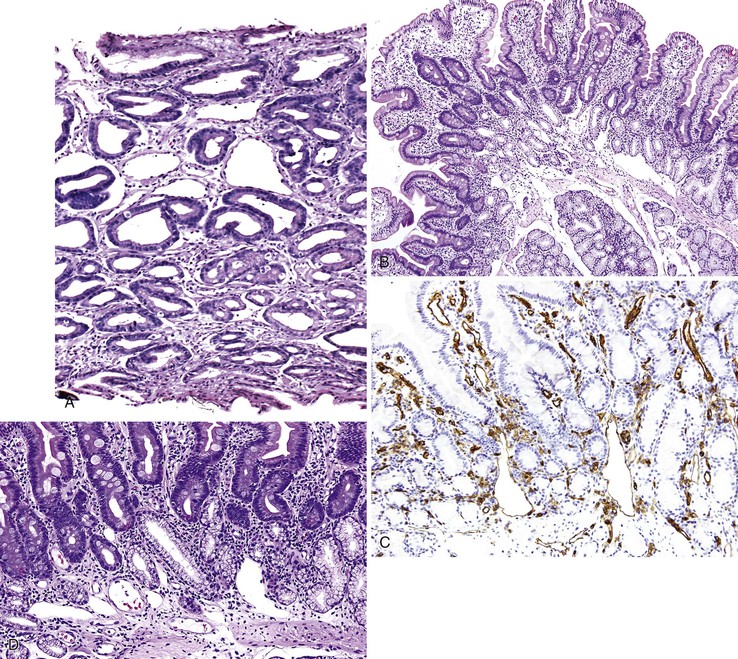
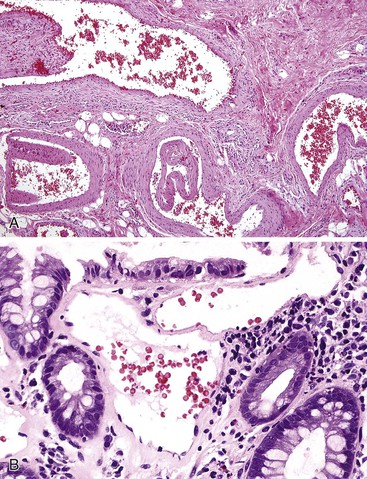
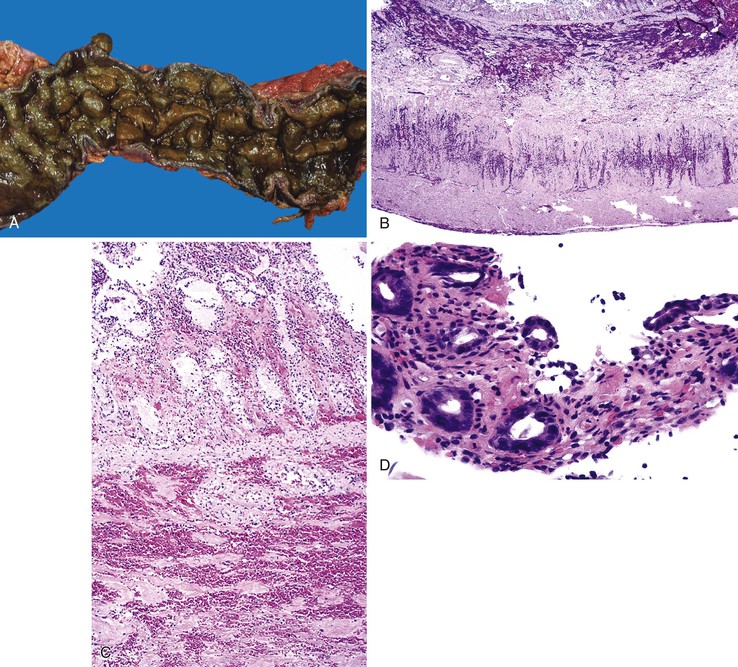
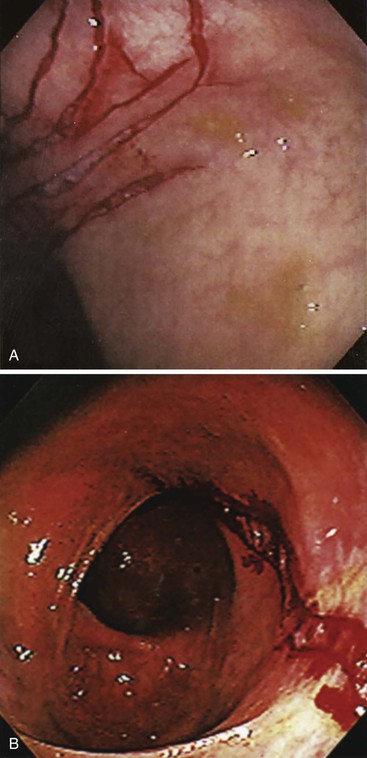
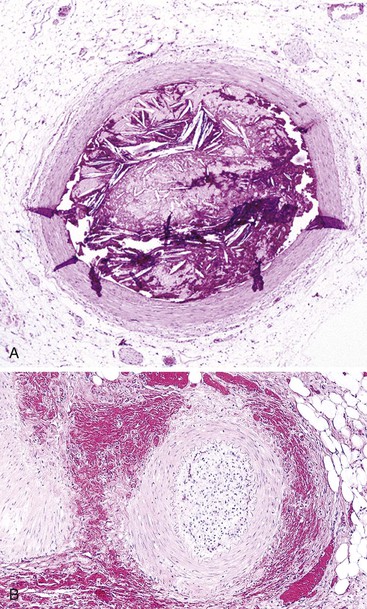
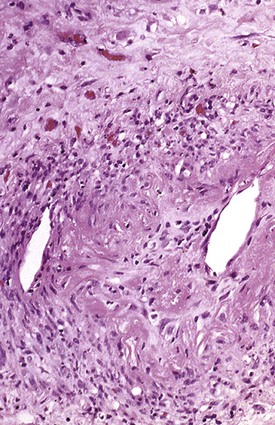
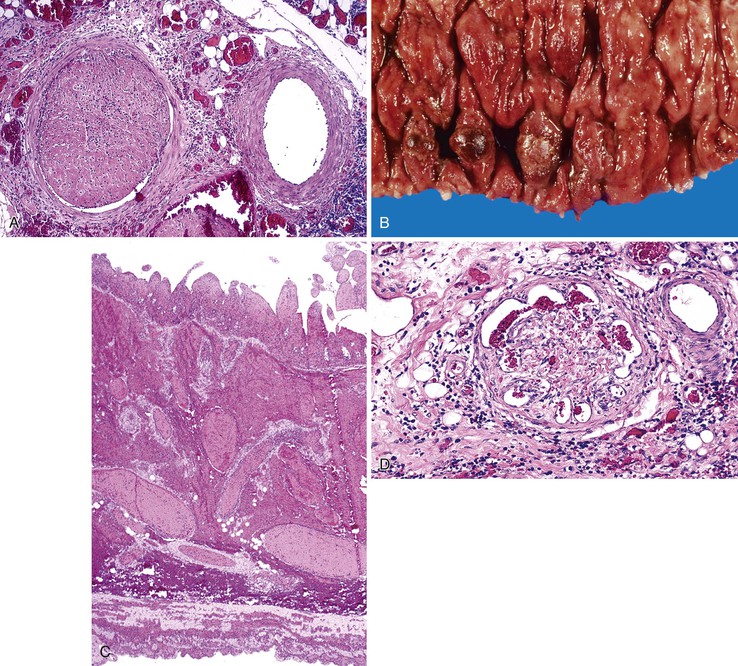
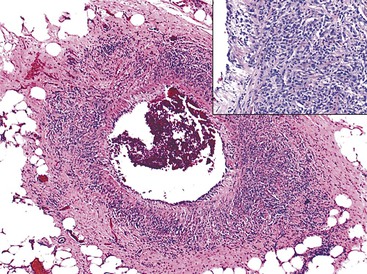
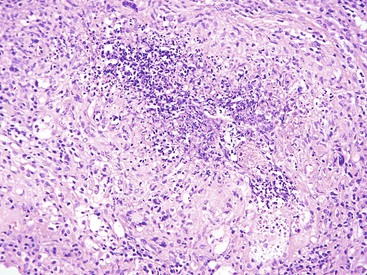
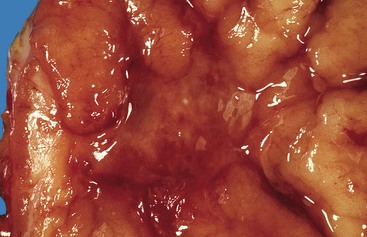
Bleeding from acute gastritis may occur in long-distance runners and is of uncertain pathogenesis.29,30 Cocaine can cause gastric erosions and bleeding.31 Bleeding may also occur when an ulcer erodes into an underlying blood vessel. Dieulafoy lesions (also called caliber-persistent artery) are bleeding ulcers that occur most commonly in the stomach but can also occur in the small and large intestines and, rarely, in the esophagus.32 Dieulafoy lesions are characterized by the presence of a single, unusually large-diameter mural arteriole that penetrates the submucosa (Fig. 10.5, A). These large vessels erode through the mucosa and cause massive bleeding (see Fig. 10.5, B and C). Cameron ulcers are linear ulcers that form in a sliding hiatal hernia; they also can cause gastric bleeding.
Mass lesions, located either in the mucosa or deeper within the gastric wall, may erode the overlying mucosa and cause chronic low-grade bleeding (Fig. 10.6). Fundic gland polyps, gastric adenomas, ectopic pancreas, malignant tumors (e.g., primary gastric adenocarcinomas, lymphomas, endocrine tumors), GI stromal tumors, and metastatic tumors (e.g., melanomas, breast carcinomas) can all cause upper GI bleeding. Gastric bleeding may also be iatrogenic in origin, such as after surgery for obesity.33
Pathologic Features
Gastric ulcers consist of full-thickness loss of the gastric mucosa. Gastric erosions, in contrast, are characterized by partial loss of mucosa, with preservation of the muscularis mucosae. On gross examination, erosions appear as small, focal, erythematous areas of mucosa. Gastric ulcers are sharply demarcated, usually circular depressions in the luminal surface. Acute ulcers may have an erythematous base covered with clotted blood. Chronic ulcers have a distinctive gross appearance resulting from scarring of the underlying tissue, which results in the formation of radiating cicatricial folds in the mucosa surrounding the ulcer. Histologically, erosions show loss of superficial mucosa, scant fibrinopurulent exudate, and reactive changes in the surrounding epithelium (loss of mucin, nuclear hyperchromasia, and increased mitotic activity). Acute ulcers show necrosis, granulation tissue, and hemorrhage. Chronic ulcers have a granulation tissue base with underlying fibrosis that often disrupts the muscularis propria.
Portal Hypertensive Gastropathy
Portal hypertensive gastropathy occurs in as much as 90% of patients with cirrhosis-related portal hypertension. It is characterized endoscopically by the presence of a mosaic mucosal pattern, with or without petechial hemorrhages, usually of the proximal stomach. Portal hypertensive gastropathy may also occur in patients without cirrhosis. It is usually detected in patients with portal hypertension who are being evaluated for variceal bleeding. Portal hypertensive duodenopathy has been described in 51% of portal hypertensive patients and is an uncommon cause of occult GI bleeding. Endoscopic features include erythema, erosions, ulcers, telangiectases, and duodenal varices.34
Altered gastric blood flow has been implicated in the pathogenesis of portal hypertensive gastropathy. Other postulated mechanisms include nitric oxide production, tumor necrosis factor-α synthesis, and sensitivity to prostaglandin inhibition.35
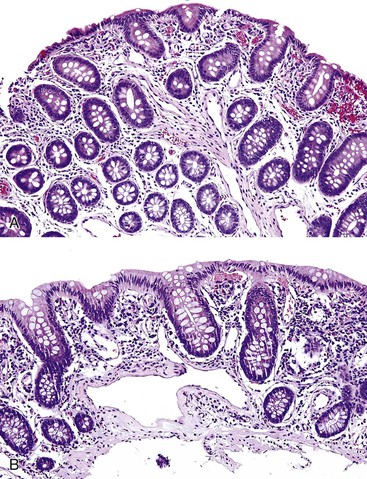
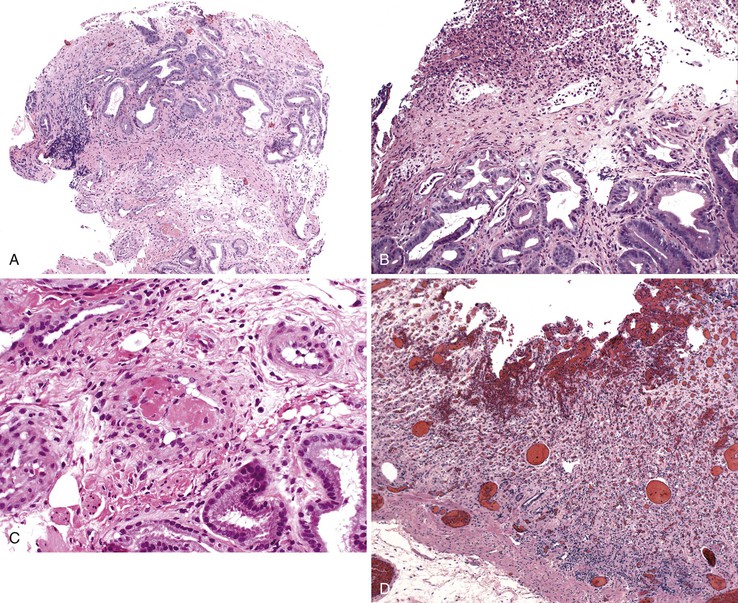
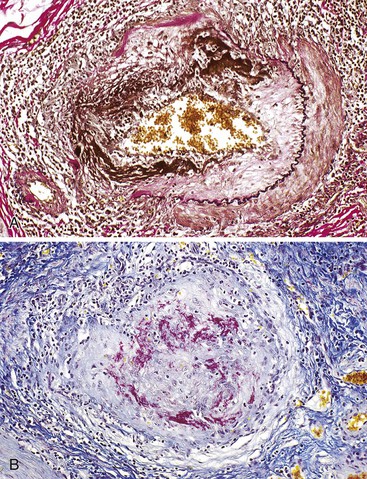
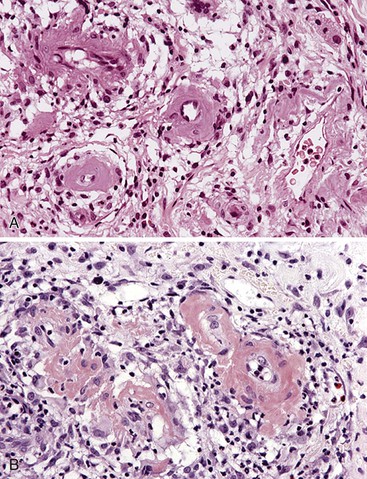
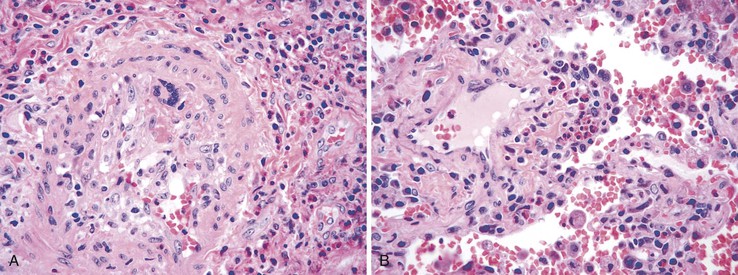
Portal hypertensive gastropathy occurs most commonly in the fundus and body of the stomach. Histologic features include the presence of numerous ectatic capillaries and venules in otherwise normal mucosa and submucosa. Fibrin thrombi, inflammation, mucosal fibrosis, and fibrohyalinosis are not histologic components of portal hypertensive gastropathy35–38 (Fig. 10.7, A). There is no association with atrophic gastritis, CREST syndrome (calcinosis, Raynaud phenomenon, esophageal involvement, sclerodactyly, and telangiectases), or bone marrow transplantation (Table 10.3). Portal hypertensive duodenopathy usually occurs in association with gastropathy; it is characterized by capillary congestion, capillary angiogenesis, and edema in the duodenal mucosa (see Fig. 10.7, B through D) but only minimal inflammation.34
Table 10.3
Comparative Features of Portal Hypertensive Gastropathy and Gastric Antral Vascular Ectasia
| Features | Portal Hypertensive Gastropathy | Gastric Antral Vascular Ectasia |
| Clinical | ||
| Age | Adults and children | Middle aged to elderly |
| Gender | Male predominance | Female : male = 4 : 1 |
| Cirrhosis | ~95% | ~40% |
| Portal hypertension | 100% | ~40% |
| Iron deficiency anemia | Uncommon | Typical |
| Atrophic gastritis | Not associated | Associated |
| CREST syndrome | Not associated | Associated |
| Bone marrow transplantation | Not associated | Associated |
| Endoscopic | ||
| Location | Proximal stomach | Antrum |
| Red spots | Present | Present |
| Mosaic pattern | Present | Absent |
| Watermelon pattern | Absent | Often present |
| Pathologic | ||
| Background mucosa* | Oxyntic | Antral |
| Ectatic mucosal vessels | Present | Present |
| Fibrin thrombi* | Absent | Present |
| Spindle cell proliferation* | Absent | Present |
| Fibrohyalinosis* | Absent | Present |
| Treatment | ||
| Endoscopic ablation | Not required | Effective |
| Control of portal hypertension | Effective | May also be necessary |
* Most important distinguishing features.
CREST, Calcinosis, Raynaud phenomenon, esophageal involvement, sclerodactylyl, and telangiectases.
Bleeding from portal hypertensive gastropathy is usually chronic and often controllable by medical or surgical management of the underlying cause of the patient’s portal hypertension.
Gastric Antral Vascular Ectasia
Gastric antral vascular ectasia (GAVE) affects mainly women of middle age, frequently causes iron deficiency anemia, and is associated with portal hypertension in about 30% to 40% of cases. It is sometimes associated with achlorhydria, atrophic gastritis, and the CREST syndrome. Autoimmune disorders are present in approximately 62% of patients with GAVE.35 At endoscopy, GAVE is characterized by a linear pattern of mucosal hyperemia on the antral rugae that appears similar to the stripes of a watermelon; hence, the synonym, watermelon stomach.39 This endoscopic appearance tends to be more diffuse in cases of cirrhosis but usually involves only the gastric antrum. The pathogenesis of GAVE is believed to be altered gastric motility.
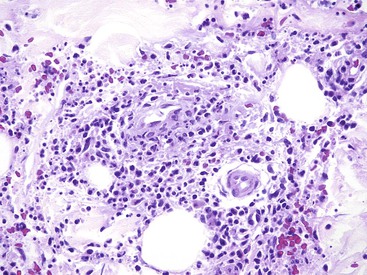
Histologically, GAVE is characterized by the presence of dilated capillaries in the mucosa and submucosa, often containing microthrombi. Fibromuscular hyperplasia of the intervening lamina propria and focal hyalinosis are characteristic features, along with edema, congestion, and reactive changes of the foveolar epithelium37,38,40 (Fig. 10.8). Inflammation is typically minimal. Surface erosion is uncommon.
In mucosal biopsies, both portal hypertensive gastropathy and GAVE should be distinguished histologically from chronic radiation gastritis, which also shows ectatic mucosal capillaries. In contrast, radiation injury is characterized by prominent endothelial cells and hyaline thickening of dilated capillaries and small blood vessels, sometimes associated with thrombosis (Fig. 10.9). Features that differentiate portal hypertensive gastropathy and GAVE are summarized in Table 10.3.
Endoscopic mucosal coagulation is the treatment of choice for most patients with GAVE. Antrectomy is reserved for patients with uncontrollable bleeding.
Hereditary Hemorrhagic Telangiectasia (Rendu-Osler-Weber Disease)
HHT, also referred to as Rendu-Osler-Weber disease, is an autosomal dominant condition in which telangiectases involve the mucous membranes of the oral cavity, but also any portion of the GI tract, where they may bleed.41 The disorder affects both sexes equally and occurs in all races. The typical clinical triad consists of telangiectasia and recurrent epistaxis in a patient with a family history of the disorder. The clinical features are variable. About 20% of patients do not reveal a family history of either telangiectasia or recurrent bleeding.8 Most patients express at least two of the following four manifestations: epistaxis, telangiectasia, visceral lesions (including GI telangiectasia), and a positive family history.42 At least two gene loci (HHT1 and HHT2) have been implicated in HHT. GI bleeding is more likely in patients with HHT1 mutations.41 Upper GI bleeding is common and can be difficult to distinguish from epistaxis; the two are the most common manifestations of HHT. GI bleeding is often difficult to control. Blood transfusion requirements in excess of 100 units have been reported.
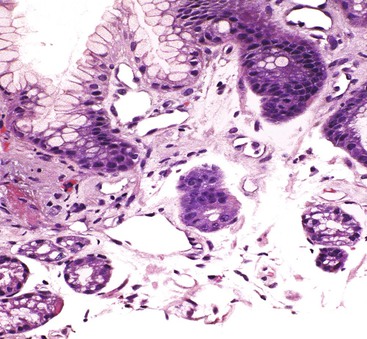
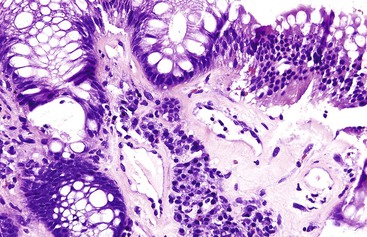
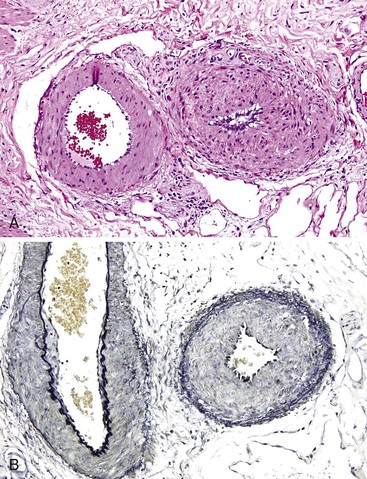
GI tract lesions include telangiectases, arteriovenous malformations (AVMs), and angiodysplasias. Telangiectases, which are the most common lesions, appear grossly as bright red spots on the mucosa. These lesions are composed of a tuft of dilated venules and arterioles that communicate directly with one another, thereby bypassing capillaries (Fig. 10.10). The precise vascular architecture is often difficult to appreciate in routine histologic sections, but evaluation of serial sections may help.43
Telangiectasia may also occur in patients with scleroderma, as part of the CREST syndrome. The morphology of telangiectases associated with scleroderma is similar to that in HHT, and distinction therefore requires clinical and serologic correlation (rheumatoid factor, antinuclear antibody, SCL-70 antibody, and anticentromeric protein antibody). Telangiectases may also develop in patients with chronic renal failure after hemodialysis.44
Treatment options for GI hemorrhage associated with HHT include drug therapy with estrogen, danazol, or aminocaproic acid; endoscopic coagulation, cautery, or laser therapy; and surgical resection.
Lower GI Bleeding
Lower GI tract bleeding refers to any type of bleeding from a source located distal to the ligament of Treitz.45 Hemodynamically significant bleeding from the lower GI tract is most commonly caused by colonic diverticula and angiodysplasia (angiectasia). Other causes are listed in Table 10.4. Colorectal causes are more prevalent than small intestinal causes. Chronic low-grade bleeding is often not visible to the patient and may be detected only by fecal occult blood testing.
Table 10.4
Pathologic Features of Entities That Cause Lower Gastrointestinal Bleeding
| Entity | Key Clinical Features | Key Pathologic Features |
| Diverticular disease* | Most frequent in elderly Accounts for 30-40% of cases of significant lower GI hemorrhage |
Most common in sigmoid colon Thickened muscularis propria, exaggerated mucosal folds Pseudodiverticula extend through muscularis at points of entry of vasa recta Inflammation of diverticula may lead to vascular erosion and bleeding |
| Angiodysplasia* | Common in elderly patients Bleeding chronic and recurrent Common cause of bleeding in renal failure |
Usually in right colon Dilated, tortuous, thin-walled submucosal veins, capillaries, and arterioles with arteriovenous anastomoses Most commonly submucosal, may involve mucosa |
| Arteriovenous malformation | Developmental defects but may present at any age | Most common in sigmoid colon and rectum Arterialized veins with thick muscular walls are associated with a knot of tortuous, dilated veins and arteries |
| Dieulafoy lesion | See Table 10.2 | See Table 10.2 |
| Varices (rectal, stomal) | Associated with portal hypertension and mesenteric and splenic vein obstruction48a | Occur at portosystemic anastomoses in rectum and at enterocolic-cutaneous stomas Histologic features similar to upper GI varices |
| Portal hypertensive colopathy | Seen in portal hypertension Strong association with cirrhosis but no correlation with degree |
Dilated tortuous mucosal and submucosal capillaries correspond to red spots seen at endoscopy |
| Hereditary hemorrhagic telangiectasia | See Table 10.2 | See Table 10.2 |
| Hemodialysis-associated telangiectasia | See Table 10.2 | See Table 10.2 |
| Hemorrhoids | Common in adults, increase in prevalence after third decade | Dilated, submucosal and mucosal blood vessels, usually with thrombosis and hemorrhage |
| Infections | See Table 10.5 | See Table 10.5 |
| Mucosal prolapse | History of prior lower GI tract trauma or surgery Diverticular disease |
Reactive, hyperplastic mucosa with muscularization of lamina propria and inflammation |
| Meckel diverticulum | GI bleed most common presentation in children | True diverticulum on antimesenteric border of ileum Heterotopic oxyntic mucosa with peptic ulcer in 65% |
| Stercoral ulcers | Acute GI bleed in elderly patients Typically a history of constipation Common in renal patients treated for hyperkalemia |
Sharply demarcated ulcers in rectosigmoid, formed as pressure sores caused by hard feces High risk of perforation |
| Ulcerative colitis | Intermittent rectal bleeding, bloody diarrhea, and abdominal pain | Typically continuous colonic involvement proceeding proximally from rectum with mucosal architectural distortion, a lymphoplasmacytic infiltrate, basal plasmacytosis, cryptitis, crypt abscesses, and ulceration |
| Crohn’s disease | More variable symptoms than ulcerative colitis, including abdominal pain, fatigue, weight loss, and fever | Typically patchy transmural involvement of small and/or large bowel by deep ulcers and lymphoplasmacytic inflammation with reactive lymphoid follicles, variable degrees of architectural distortion, pyloric gland metaplasia, cryptitis and crypt abscesses, with or without granulomas |
| Ischemic enterocolitis | See Table 10.9 | See Table 10.9 |
| Radiation enterocolitis | History of prostate cancer common in men, cervical cancer in women Rare cause of lower GI bleeding |
Dilated, tortuous mucosal capillaries with prominent endothelial cells in mucosa Hyalinized vessels with prominent endothelial cells, stellate fibroblasts, and fibrosis in submucosa |
| Collagenous colitis | Watery diarrhea Female predominance: 4 : 1 Rare cause of lower GI bleeding |
Marked subepithelial collagenous deposition and submucosal fibrosis, increased lamina propria lymphoplasmacytic inflammation and intraepithelial lymphocytes; subepithelial collagen may mimic hyalinosis of ischemic colitis |
| Diversion colitis | History of previous bowel resection with fecal stream diversion May cause bloody rectal discharge |
Friable mucosa with normal architecture, mucin depletion, a lymphoplasmacytic infiltrate most dense in the upper mucosa, rare cryptitis, Paneth cell metaplasia, and prominent reactive lymphoid follicles |
| Diaphragm disease | History of use of NSAIDs | Transverse stenosing membrane composed of submucosa lined on both sides by mucosa, with central aperture |
| Ehlers-Danlos syndrome, vascular type | Acute hemorrhage and bowel perforation in young patients due to deficiency of collagen type 3 | Most commonly affects the sigmoid colon Thinned muscularis propria and diminished submucosa Proliferating fibroblasts surrounding fat Frayed, degenerated vascular and stromal collagen |
* Most common causes.
NSAIDs, Nonsteroidal antiinflammatory drugs.
Lower GI bleeding occurs most commonly in the seventh decade of life. Although it is less common than upper GI bleeding, patients with lower GI bleeding are more likely to require surgery. The most common clinical presentation of patients with lower GI bleeding is passage of bright red blood per rectum (hematochezia). Similar to upper GI bleeding, lower GI bleeding may result in melena, anemia, or hemodynamic instability. Investigative modalities useful for identification of the source of lower GI bleeding include colonoscopy, angiography, nuclear scan, barium enema, and push enteroscopy.46
Diverticulosis
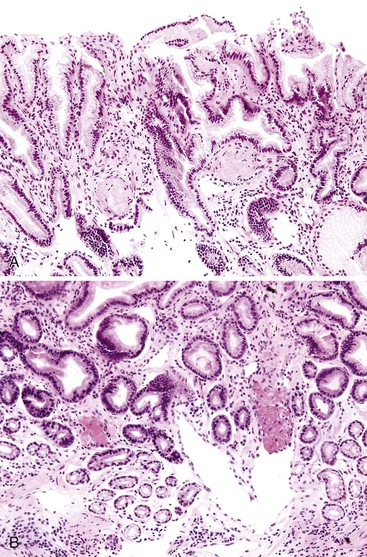
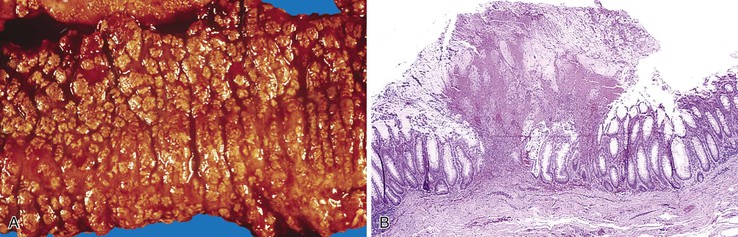
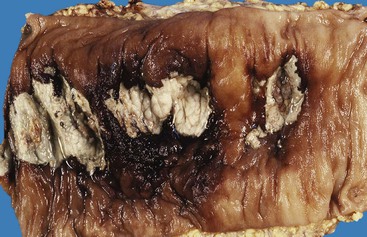
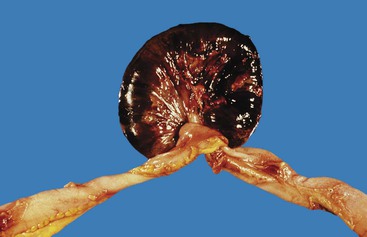
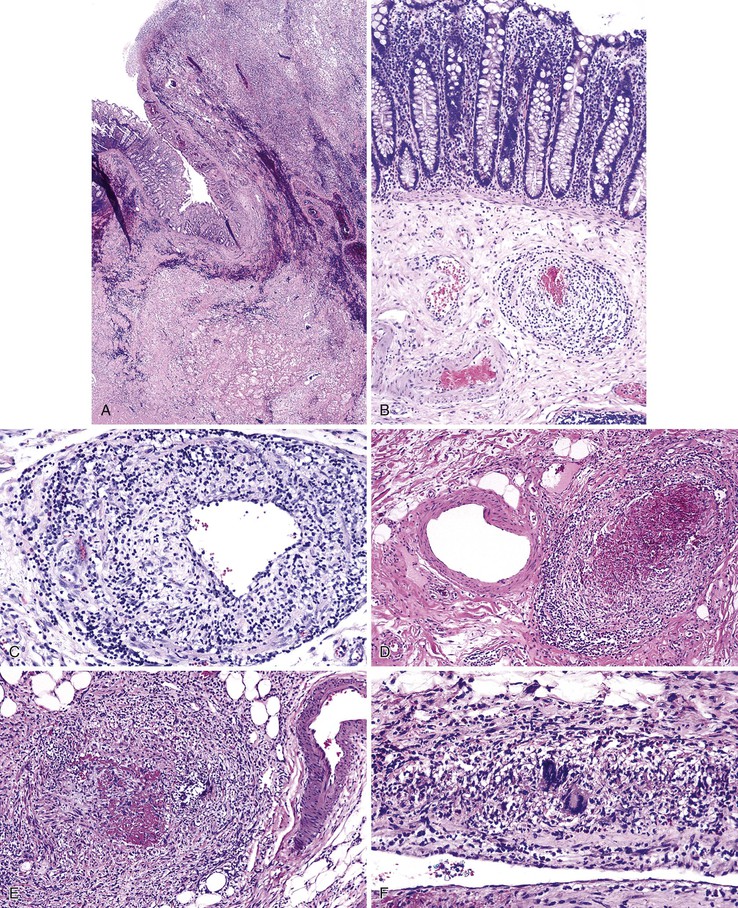
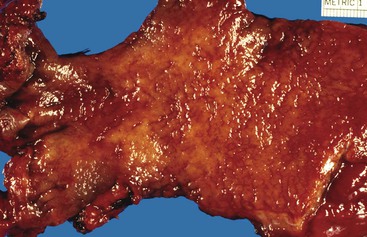
Diverticulosis accounts for 30% to 40% of cases of significant lower GI hemorrhage.47 Left-sided diverticula are acquired outpouchings of the colonic mucosa that protrude through the muscularis propria at the points of entry of the vasa recta (Fig. 10.11). They are usually situated in intimate contact with penetrating blood vessels, where inflammation and ulceration of diverticula can lead to erosion of the vessel wall and bleeding. Diverticular bleeding may be slow and chronic if venous in origin or acute and severe if an artery is involved. Right-sided diverticula are not usually associated with penetrating vessels. GI bleeding in Meckel diverticula is usually from foci of gastric heterotopia that cause peptic ulceration of the adjacent mucosa (Fig. 10.12).
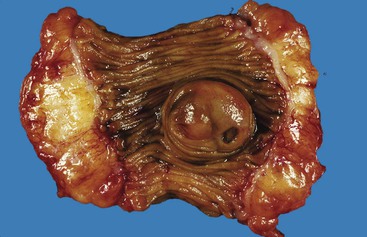
Most episodes of bleeding caused by colonic diverticula resolve spontaneously. However, as much as one third of patients require transfusion or therapeutic intervention. Treatment options include contact coagulation, epinephrine injection, hemoclip application, fibrin sealant, and surgical resection.
Angiodysplasia
Angiodysplasia (angiectasia) is the second most common cause of lower GI bleeding in elderly patients.48 Angiodysplasia is characterized by the presence of a cluster of abnormally dilated blood vessels in the submucosa and mucosa of the lower GI tract. Bleeding from angiodysplasia is usually chronic and recurrent, but massive hemorrhage occurs in approximately 10% to 15% of affected patients.
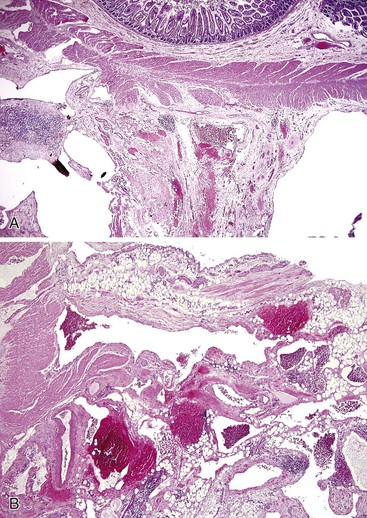
Angiodysplasias are acquired lesions associated with aging. They are usually located in the right colon but may occur anywhere in the large or small intestine. The incidence of angiodysplasia increases with age. This increase is thought to be related to changes in the composition and structure of the extracellular matrix of the bowel wall. Although usually readily seen by colonoscopy and angiography, angiodysplasia is often extremely difficult to detect on gross examination of a resection specimen without the use of specific injection techniques.49 By performing vascular injection studies of resected colons, Boley and Brandt determined that angiodysplasia develops as a result of intermittent partial obstruction of small veins that drain the colonic mucosa and submucosa as they course through the muscularis propria. They postulated that obstruction was caused by muscle contraction and increased tension within the bowel wall, which is highest in the region of colon with the greatest diameter (i.e., the cecum).48 With time, obstruction of the penetrating veins in the muscularis propria leads to dilation and tortuosity of the submucosal veins and, consequently, the venules and capillaries that drain them. Dilation of capillaries ultimately leads to loss of capillary sphincter function, which creates arteriovenous fistulas and secondary effects on the structure of the feeding arteries.
Angiodysplasias are often difficult to diagnose in pathologic specimens. In resection specimens examined in the fresh state, one may see only a small focus of enhanced vascular markings and erythema, and even these subtle signs may be absent. In specimens examined after formalin fixation, the lesions are usually not visible on the mucosal surface. Few laboratories are equipped to perform injection studies, which require processing of fresh specimens. With fixed resection specimens, slicing the bowel wall with a sharp blade at the site of suspected mucosal abnormalities helps reveal the lesion. If a vascular lesion is detected, histologic examination usually reveals a discrete cluster of dilated, tortuous veins and venules within the submucosa (Fig. 10.13, A), some associated with dilated capillaries in the overlying mucosa as well (see Fig. 10.13, B).
Diagnosis of angiodysplasia in biopsy specimens is also problematic. The main histologic component of angiodysplasia is usually situated in the submucosa, which may not be sampled in superficial endoscopic biopsies. When capillary dilation involves the mucosa, biopsy specimens may show only one or two ectatic capillaries, and these may collapse when the specimen is immersed in formalin. In more advanced lesions with more extensive mucosal involvement, clusters of dilated capillaries may distort the architecture of the mucosa, displacing glands and separating the crypts from each other.
In most instances, bleeding can be controlled adequately by therapeutic endoscopy. Angiographic techniques not only enable precise localization of angiodysplasia but allow for treatment by superselective embolization. Surgical resection is reserved for patients with uncontrolled bleeding.
Arteriovenous Malformation
In contrast to angiodysplasia, which is an acquired lesion that develops mainly in elderly patients, arteriovenous malformation (AVM) develops during embryologic or fetal life and is typically present at birth. AVM also differs from angiodysplasia in that it results in an abnormal direct communication between arteries and veins. Because of this communication, arterial blood flows directly into the venous system at higher-than-normal pressure, bypassing the (high-resistance) capillary bed. AVMs can manifest clinically with bleeding at any age.
In the GI tract, AVMs occur most often in the sigmoid colon and rectum and are usually located external to the muscularis propria—that is, in the subserosa. The malformation consists of a tangled mass of tortuous, variably dilated arteries, veins, and vessels with intermediate characteristics. Veins in AVMs undergo “arterialization” in response to exposure to elevated (arterial) blood pressure. They develop a thick, muscular wall as a result of myointimal hyperplasia (Fig. 10.14). It has been proposed that idiopathic myointimal hyperplasia of the mesenteric veins (discussed later) is an example of venous arterialization as a complication of AVM. Histologically, AVMs are characterized by the presence of complex clusters of tortuous, dilated vascular channels and vessels that appear intermediate in structure between arteries and veins. However, the vascular architecture is more easily deciphered by angiographic studies.
Most AVMs of the GI tract are treated angiographically by superselective embolization. Large lesions may require surgical resection.
Hemorrhoids
Hemorrhoids are very common. Clinically, most patients are seen in middle age with anal pain or bleeding.50–52 Hemorrhoids arise from the anal cushions, which are normal anatomic structures of the anorectal canal. The anal cushions are composed of tufts of anastomosing arterioles and venules in the submucosa; they are embedded within compact, dense, submucosal collagenous and elastic fibrous stroma and covered by anorectal mucosa. Hemorrhoids develop as a result of degenerative changes that occur in the supporting stroma or as a result of locally increased intravascular pressure. The blood vessels become engorged and the cushions prolapse, strangulate, thrombose, ulcerate, and bleed. Histologic sections of hemorrhoids show tufts of engorged, dilated veins and arteries with thrombi and hemorrhage, in a dense stroma, covered by anal or rectal mucosa, which often is ulcerated. Treatment options for hemorrhoids include dietary modification, sclerotherapy, photocoagulation, diathermy, banding, laser ablation, cryotherapy, and surgical hemorrhoidectomy.
Diaphragm Disease
Diaphragm disease is a rare cause of GI bleeding linked to chronic use of NSAIDs. This entity was first described in the small intestine in 1988.53,54 Similar abnormalities have been described in the large intestine as well.55,56 GI bleeding associated with diaphragm disease is usually occult. Obstructive symptoms tend to dominate the clinical picture. The pathogenesis of this disorder is unclear. Some suggest that inhibition of the cyclooxygenase pathway leads to erosions in the GI tract, modulation of nuclear factor-κB and peroxisome proliferator–activated receptor-γ, and increased intestinal permeability, which lead to mucosal injury. Alternatively, NSAID-associated circumferential ulceration, followed by the development of submucosal fibrosis and mucosal regeneration, may result in diaphragm formation.
Diaphragms may be single or multiple. They are composed exclusively of mucosa and submucosa, without involvement of the muscularis propria or adventitia. Histologically, the diaphragm represents a disc of submucosa lined by mucosa, with a central opening. Typically, the submucosa is fibrotic. The connective tissue fibers are usually oriented perpendicular to the lumen of the bowel and are often associated with haphazardly arranged nerves, blood vessels, and smooth muscle fibers54 (see Chapter 16 for photograph).
Ehlers-Danlos Syndrome, Vascular Type (Type IV)
Ehlers-Danlos syndrome (EDS) is a heterogeneous group of inherited disorders characterized by defective collagen and connective tissue synthesis. Of these, EDS type IV (vascular type) causes GI tract pathology, leading to significant complications in as much as 25% of patients by the age of 20 years and as much as 80% by age 40 years.57 Patients usually succumb to complications of this disease by the fourth or fifth decade of life. The diagnosis is established by the presence of two or more of the following features: distinctive facial features (slender face, prominent facial bones, bulging eyes with telangiectasia, and thin, pinched nose), translucent skin, excessive bleeding or hematomas, and blood vessel or visceral organ rupture. In most cases, this disorder is inherited in an autosomal dominant manner, although many cases are sporadic. EDS type IV is caused by mutations in the COL3A1 gene, which result in defective synthesis of type III procollagen and diminished extracellular type III collagen. The result is weakening of the supporting connective tissue stroma, including the submucosa and blood vessels.
The sigmoid colon is most commonly affected, but the small bowel, stomach, and even the esophagus may also be involved in rare cases.58 EDS type IV causes thinning of the muscularis propria and submucosa of the GI tract. Complications include diverticula, perforation, and hemorrhage. Microscopically, vascular and stromal collagen is often frayed and fragmented. The submucosal blood vessels may be thin, thick, or nodular in appearance. The outer layers of the blood vessel walls may appear discontinuous. The muscularis propria often shows secondary ischemic changes. Inflammation is uncommon. Diminished expression of type III collagen (vascular and extravascular) can be demonstrated by immunohistochemical staining.
As many as 8% of patients die of bowel rupture and sepsis.57 Bleeding may occur as a result of rupture of blood vessels in the bowel wall. Treatment is by surgical resection of the affected segment. As much as one third of patients have recurrent perforations.
Miscellaneous Entities
Chronic radiation injury in the GI tract is characterized by the presence of dilated, tortuous capillaries with hyaline thickening and prominent capillary endothelial cell nuclei (Fig. 10.15). Dilated capillaries located near the mucosal surface may rupture and cause hemorrhage. Histologically, this condition should be distinguished from portal hypertensive colopathy in patients with portal hypertension. In that condition, the dilated capillaries typically lack the hyalinization and prominent endothelial cell nuclei typical of radiation injury (Fig. 10.16). In resection specimens with chronic radiation injury, sclerosis of submucosal and extramural arteries is characteristic and is associated with fibrosis and stenosis, the presence of atypical stellate fibroblasts, and secondary chronic ischemic changes.
Many types of inflammatory conditions can cause lower GI bleeding. Microbial agents that can cause GI bleeding include cytomegalovirus (Fig. 10.17), which predominantly affects immunocompromised patients; Salmonella; Shigella; enterohemorrhagic Escherichia coli (Fig. 10.18); Clostridium difficile (Fig. 10.19); Clostridium perfringens (Fig. 10.20); and Klebsiella oxytoca59 (Table 10.5). Certain GI parasites can also cause bleeding, such as Entamoeba histolytica, Strongyloides, and hookworms. Active ulcerative colitis (Fig. 10.21) or Crohn’s disease (Fig. 10.22), mucosal ulceration or injury caused by mucosal prolapse, use of NSAIDs, and endoscopic procedures may lead to bleeding as well. In some patients with collagenous colitis, the thickened subepithelial collagen layer may render the colon susceptible to tearing of the mucosa during colonoscopy60 (Fig. 10.23). Stercoral ulcers in the rectum of elderly patients are a common cause of lower GI bleeding (Fig. 10.24).
Table 10.5
Infections That May Result in Lower Gastrointestinal Bleeding
| Agent | Clinical Presentation | Pathologic Findings |
Normal mucosal architecture with superficial edema
Epithelium with mucin depletion and reactive changes
Lymphoplasmacytic infiltrate in lamina propria with variable neutrophil content and focal cryptitis
Focal erosions or shallow ulcers
Shigella: mainly affects distal colon
Salmonella: May mimic ulcerative colitis
Ulcerated malignant neoplasms, both primary and metastatic, cause bleeding that is usually chronic and often clinically silent. Primary lymphomas, endocrine tumors, and adenocarcinomas are the major culprits in the small bowel, whereas adenocarcinomas predominate in the large intestine (Fig. 10.25). Other less common causes include GI stromal tumors, Kaposi sarcomas, and malignant melanomas. Benign polypoid lesions, such as those of the solitary rectal ulcer (mucosal prolapse) syndrome (Fig. 10.26), can also ulcerate and bleed, particularly those with a long stalk that may undergo torsion61 (e.g., polypoid prolapsing mucosal folds of diverticular disease; Fig. 10.27).
Upper GI Ischemia
Ischemic injury to the GI tract is common, particularly in elderly persons. As the percentage of the population older than 50 years increases, the incidence of GI ischemia is expected to rise. There are three general clinicopathologic patterns of ischemic injury: transient, acute, and chronic. In transient injury, the damage is usually confined to the mucosa and is reversible. An example of transient ischemia is that caused by hypotension and hypoperfusion. Acute ischemia is often fulminant, and transmural necrosis may develop. Common causes of acute fulminant ischemic injury include sudden thromboembolic occlusion of the mesenteric arteries. Chronic (recurrent) ischemia typically results in mural fibrosis and strictures. Ischemic injury is uncommon in the esophagus and stomach; it is far more common in the small and large intestines.
Esophagus
Esophageal ischemia is uncommon because of the rich blood supply provided by multiple anastomoses between esophageal blood vessels. However, esophageal ischemia may occur in patients with severe atherosclerosis or vasculitis (e.g., Wegener granulomatosis, Behçet disease) and, rarely, as a complication of trauma or surgical intervention. Acute necrotizing esophagitis has been attributed to ischemia,62 but its cause is unknown. In fact, recovery rates are higher than one might expect if the disorder were caused by ischemia.63
Stomach
As in the esophagus, the presence of multiple anastomoses between blood vessels provides a rich vascular supply to the stomach, and for that reason, ischemia is uncommon. When ischemia does occur in the stomach, there are often multiple contributing factors. Causes of gastric ischemia are listed in Box 10.1.
In gastric volvulus, constriction of the blood vessels leads to venous obstruction. This causes congestion, arterial compromise, and ischemic necrosis. Atherosclerosis may result in gastric necrosis if it is severe and involves multiple vessels simultaneously. Atherosclerotic debris in the aorta may dislodge spontaneously or as a result of instrumentation and propagate into the distal submucosal or mucosal blood vessels as atheroemboli, leading to local ischemic mucosal necrosis and surface erosions.64 Mild erosive gastritis may develop as a result of transient hypoperfusion. Chronic vascular insufficiency may lead to gastroparesis that is typically reversible if vascular perfusion is reestablished.65 Iatrogenic causes of gastric ischemia are rare. However, ischemic complications of bariatric surgery are not uncommon.66
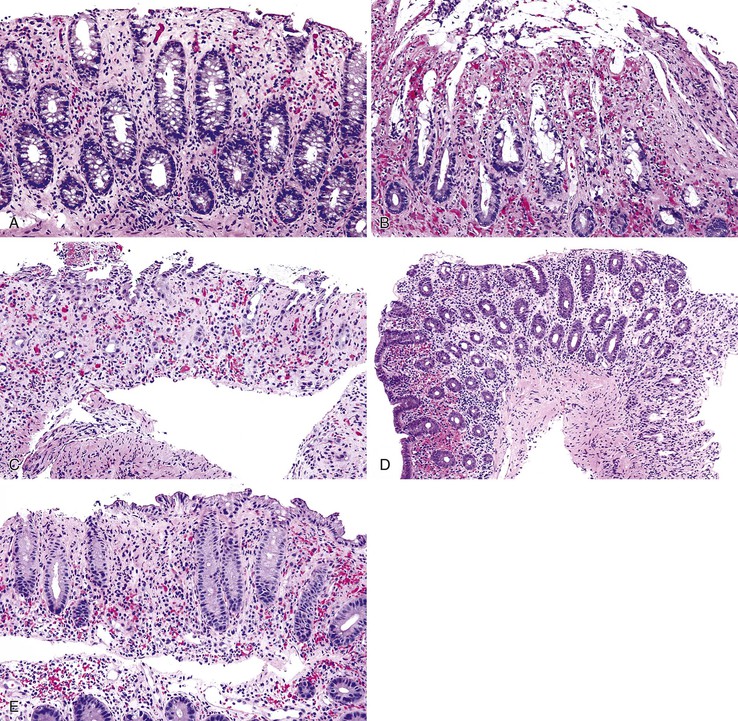
The gross appearance of ischemic gastritis depends on the severity, extent, and duration of ischemia. Early lesions reveal only acute erosions and superficial ulcers. In patients with gastric volvulus, the entire stomach may be necrotic and show marked, diffuse congestion and hemorrhage. Histologically, early ischemic changes of the gastric mucosa include capillary dilation, edema, congestion, and superficial necrosis (Fig. 10.28, A and B). With further progression, coagulative necrosis of the mucosal surface occurs. The adjacent uninvolved epithelium exhibits reactive changes such as mucin depletion, reactive nuclear hyperchromasia and enlargement, and increased mitoses. Full-thickness mucosal necrosis may follow (see Fig. 10.28, C and D), and the ulcer may penetrate deeper into the gastric wall.
The gastric mucosa has considerable reparative ability. Ischemic lesions that extend no deeper than the mucosa often heal completely, provided that perfusion is reestablished. Deep lesions may heal, but they usually resolve with fibrous scarring. Chronic ischemia, however, may lead to gastroparesis and mural fibrosis.
Lower GI Ischemia
Arterial Insufficiency
Arterial insufficiency of the large and small bowel is the most common cause of intestinal ischemia (Table 10.6). Patients with acute mesenteric ischemia commonly present with abdominal pain and hematochezia. Paradoxically, elderly patients (who are the most prone to ischemia from arterial insufficiency) often experience little or no pain until the disease is far advanced, and sometimes this leads to disastrous consequences. Patients with chronic mesenteric ischemia, who constitute fewer than 5% of all those with intestinal ischemia, often suffer from postprandial abdominal pain. This pain usually begins about 30 minutes after a meal, peaks during the following hour, and then typically resolves within 3 hours. Arterial insufficiency may be classified as nonocclusive or occlusive. See Box 10.2 for a list of nonocclusive and occlusive causes of ischemia.
Table 10.6
Frequency of Different Causes of Acute Mesenteric Ischemia
| Cause | Frequency (%) |
| Mesenteric artery embolism | 50 |
| Nonocclusive mesenteric ischemia | 25 |
| Mesenteric artery thrombosis | 10 |
| Mesenteric venous thrombosis | 10 |
| Focal segmental ischemia | 5 |
From Brandt LJ. Intestinal ischemia. In: Sleisenger MH, Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. Philadelphia: Saunders; 2006:2563-2885.
Nonocclusive Arterial Insufficiency
Nonocclusive or central arterial insufficiency is characterized by failure to deliver sufficient oxygen to the tissues, as a result of inadequate blood flow, in the absence of arterial obstruction. Poor oxygenation of the blood may be an important contributing factor. Common causes of nonocclusive arterial insufficiency are hypotension, shock of any cause (e.g., cardiogenic, hemorrhagic, septic, traumatic), dehydration, and cardiac dysrhythmias. Vasoconstrictor drugs such as digitalis, vasopressin, and propranolol can also cause arterial insufficiency. In elderly patients, atherosclerotic disease often causes nonocclusive stenosis of the major arteries, which increases their susceptibility to ischemic injury if there is a reduction of arterial pressure (Fig. 10.29).
In nonocclusive ischemia, a critical feature that determines outcome is the duration of arterial insufficiency. The intestinal tract is remarkably tolerant of hypoxia. This property allows shunting of the splanchnic circulation to the central nervous system in situations of severe shock. Among the layers of the intestinal wall, the mucosa is most susceptible to hypoxia, but it also has the greatest regenerative capacity. Therefore, complete recovery from severe mucosal ischemic damage is possible.
Occlusive Arterial Insufficiency
Occlusive or peripheral arterial insufficiency is characterized by the presence of an obstruction to arterial blood flow; the cause may be intraluminal, intramural, or extramural. Thrombi and emboli are the most common causes of intraluminal occlusion67 (Fig. 10.30). Radiologically placed coils, beads, and gels are potential iatrogenic causes. Intramural causes include atherosclerosis, dissecting aneurysms, radiation injury (Fig. 10.31), amyloidosis, and diabetes. Mesenteric vascular involvement in polyarteritis nodosa (Fig. 10.32), Wegener granulomatosis, Behçet disease, rheumatoid arthritis, systemic lupus erythematosus (SLE), scleroderma, syphilis, and other arteritides are considered intramural causes of ischemia as well. Certain drugs and toxins cause arterial occlusion by inducing vasospasm, including potassium salts, cocaine, and some forms of snake and scorpion venom. Cocaine, in addition to causing vasospasm, may cause fragmentation of the internal elastic lamina and “subelastic” edema, and may induce thrombosis and platelet aggregation68,69 (Fig. 10.33). Extramural causes include compression by tumors or adhesion bands, volvulus (Fig. 10.34), torsion, and intussusception.
In occlusive disorders, some important considerations that determine outcome are the size of the obstructed vessel and its degree of collateral circulation. Atheroembolic occlusion of terminal arterioles in the intestinal submucosa often leads to focal mucosal ulceration.70,71 Because the extramural vessels have a richer collateral supply, occlusion or compromise of several of these vessels is usually necessary to induce ischemic damage.
Pathologic Features of Arterial Insufficiency
The pathologic features of occlusive and nonocclusive ischemic enteritis or colitis are similar, except that in occlusive disease ischemia is strictly segmental in distribution and uniform within the affected region, whereas in nonocclusive cases it is patchy, variable in severity, and often widespread. Segmental ischemic changes may occur anywhere in the large and small bowel but most commonly involve the colon in the vicinity of the splenic flexure. Histologic evaluation of ischemic injury should include examination of mesenteric and serosal vessels whenever possible.
In early acute ischemia, when examined by endoscopy or in resection specimens, the mucosa is typically friable, swollen (edematous), and erythematous and may bleed quite easily on palpation. Histologic features of early-stage lesions include surface epithelial degeneration, necrosis and sloughing, loss of epithelium in the superficial portions of the glands (“withering” crypt), dilation and congestion of mucosal capillaries, lamina propria hemorrhage, and hyalinization of the lamina propria caused by leakage of plasma proteins from injured capillaries, which may contain thrombi. Residual viable epithelial cells often show reactive changes with mucin loss, hyperchromatic nuclei, and increased mitoses (Fig. 10.35). Initially, acute inflammation is minimal. In the small intestine, ischemic changes develop initially at the tips of the intestinal villi. With ongoing ischemia, mucosal necrosis extends through the entire length of the villi and involves the bases of the crypts. Empty spaces within the mucosa, bounded by viable basement membrane material, may represent the only remnants of the intestinal crypts (“crypt ghosts”). The submucosa is usually edematous, with engorged veins, with or without hemorrhage, but the muscularis propria may be viable unless the ischemic insult is near-complete or complete and relatively acute in its onset. With time, tissue damage and subsequent reperfusion lead to neutrophil infiltration in the mucosa and submucosa, ulceration, and necroinflammatory membrane debris. Transmural necrosis (infarct) and perforation occur in patients with sudden complete occlusion of arteries if blood flow to the organ is not restored. At this stage, the bowel wall appears thickened and edematous. As the muscularis propria undergoes necrosis, the bowel wall becomes thin, friable, and easily disrupted. On occasion, superimposed bacterial infection (e.g., clostridia) may lead to the formation of gas bubbles within the bowel wall, termed intestinal pneumatosis.
In contrast, chronic ischemia, which is typically low grade and recurrent, is characterized by fibrosis. The fibrosis is usually circumferential and involves all layers of the bowel wall, including the mucosa. At this stage, hemosiderin deposits may be present, indicating previous episodes of hemorrhage. Strictures may cause intestinal obstruction. Linear ulcers are commonly associated with strictures.
Transient ischemia occurs mostly as a result of nonocclusive insults. The initial histologic changes are similar to those of early acute occlusive ischemia and are typically confined to the mucosa. On restoration of arterial function, mucosal regeneration is typical. Of all the intestinal epithelial cells, those in the proliferative zones of the crypts are the most resistant to ischemic injury. If these cells survive an ischemic episode, on restoration of the blood supply they act as a reservoir for epithelial regeneration and contribute to restitution of mucosal morphology, which may be complete. Severe transient ischemia can cause deeper mural injury, perhaps also affecting the submucosa and muscularis propria. This usually results in the development of fibrosis and strictures similar to those seen in patients with chronic ischemia.
Venous Insufficiency
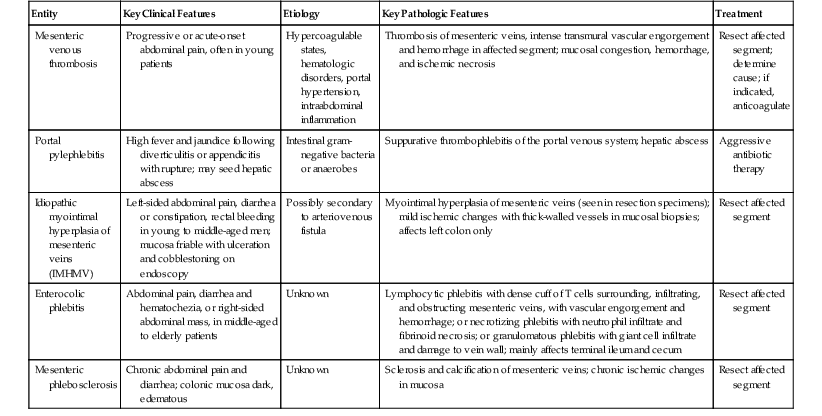
Venous insufficiency is less common than arterial insufficiency as a cause of intestinal ischemia. It accounts for approximately 5% to 15% of cases of intestinal ischemia overall72,73 (see Table 10.6). Similar to patients with acute arterial ischemia, those with venous insufficiency usually are seen with abdominal pain. However, venous insufficiency usually affects younger patients, including children and young adults, in whom a diagnosis of ischemia may not be suspected. The pathogenesis of venous insufficiency may be related to external venous compression, thrombosis (intraluminal), or pathologic intramural processes (Box 10.3). External venous compression and mesenteric venous thrombosis are the most common causes. Mural processes include several relatively uncommon but distinctive entities (e.g., idiopathic myointimal hyperplasia of mesenteric veins, enterocolic lymphocytic phlebitis) that are probably underrecognized. The histologic features and differential diagnoses of these entities are summarized in Table 10.7.
Table 10.7
Histologic Features and Differential Diagnosis of Disorders That Cause Mesenteric Venous Insufficiency (Excluding Venous Compression)
| Entity | Key Clinical Features | Etiology | Key Pathologic Features | Treatment |
| Mesenteric venous thrombosis | Progressive or acute-onset abdominal pain, often in young patients | Hypercoagulable states, hematologic disorders, portal hypertension, intraabdominal inflammation | Thrombosis of mesenteric veins, intense transmural vascular engorgement and hemorrhage in affected segment; mucosal congestion, hemorrhage, and ischemic necrosis | Resect affected segment; determine cause; if indicated, anticoagulate |
| Portal pylephlebitis | High fever and jaundice following diverticulitis or appendicitis with rupture; may seed hepatic abscess | Intestinal gram-negative bacteria or anaerobes | Suppurative thrombophlebitis of the portal venous system; hepatic abscess | Aggressive antibiotic therapy |
| Idiopathic myointimal hyperplasia of mesenteric veins (IMHMV) | Left-sided abdominal pain, diarrhea or constipation, rectal bleeding in young to middle-aged men; mucosa friable with ulceration and cobblestoning on endoscopy | Possibly secondary to arteriovenous fistula | Myointimal hyperplasia of mesenteric veins (seen in resection specimens); mild ischemic changes with thick-walled vessels in mucosal biopsies; affects left colon only | Resect affected segment |
| Enterocolic phlebitis | Abdominal pain, diarrhea and hematochezia, or right-sided abdominal mass, in middle-aged to elderly patients | Unknown | Lymphocytic phlebitis with dense cuff of T cells surrounding, infiltrating, and obstructing mesenteric veins, with vascular engorgement and hemorrhage; or necrotizing phlebitis with neutrophil infiltrate and fibrinoid necrosis; or granulomatous phlebitis with giant cell infiltrate and damage to vein wall; mainly affects terminal ileum and cecum | Resect affected segment |
| Mesenteric phlebosclerosis | Chronic abdominal pain and diarrhea; colonic mucosa dark, edematous | Unknown | Sclerosis and calcification of mesenteric veins; chronic ischemic changes in mucosa | Resect affected segment |
In patients with venous obstruction, the arterial flow to the affected segment of the bowel is usually unobstructed; hence, the tissue becomes progressively and severely engorged until either drainage is achieved through unobstructed collateral veins or the resistance of the tissue exceeds arterial pressure. At that point, arterial flow ceases, and the affected segment of bowel undergoes necrosis in a manner similar to arterial insufficiency. Therefore, the initial phases of venous insufficiency are characterized by severe vascular congestion, hemorrhage, and edema, before tissue necrosis develops.
Segmental swelling of the bowel may be quite extreme. The mucosa appears boggy and hemorrhagic, and the plicae circulares or plicae semilunares often appear markedly thickened. On histologic examination, one sees intense venous congestion of the bowel wall, dilation of engorged veins, marked tissue edema, and intramural hemorrhage. Evidence of venous thrombi, phlebitis, or other forms of venopathy should be sought.
External Mesenteric Venous Compression
The most common cause of mesenteric venous insufficiency is external mesenteric venous compression. This may be related to volvulus, torsion, adhesions, intussusception, or trauma. Because of the normal low intraluminal pressure of veins, these structures are easily occluded externally. If the occlusion is of sufficient duration, thrombosis may occur. Volvulus, torsion, and bowel entrapment may cause closed-loop obstruction, in which the lumen of the bowel is sealed off at the point of compression. As a result, luminal secretions accumulate, which facilitates bacterial proliferation, toxin production, inflammation, and necrosis (Fig. 10.36). Mesenteric venous compression by extraabdominal trauma is rare but has been attributed to a tight seat belt during prolonged plane travel74 (Fig. 10.37).
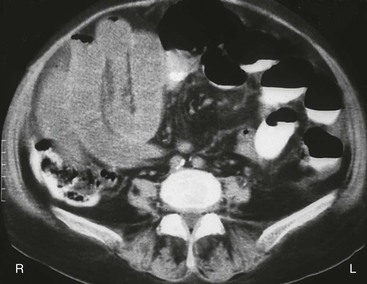
Mesenteric Venous Thrombosis
Mesenteric venous thrombosis usually manifests as progressive, severe abdominal pain of acute onset. It may occur in individuals of any age, but patients tend to be younger than those with arterial insufficiency. Until recently, mesenteric venous thrombosis was most often diagnosed at laparotomy, in resection specimens, or at autopsy. However, improved radiologic techniques, including computed tomographic angiography and magnetic resonance imaging, now enable many cases to be diagnosed radiologically. Thus, increasing numbers of patients are being treated medically with thrombolytic therapy, rather than surgery.75 Furthermore, owing to advances in understanding of hypercoagulable states and myeloproliferative disorders, the cause of mesenteric venous thrombosis is known in as many as 80% of cases.
In the absence of external venous compression, an intramural pathologic process, or a localized cause of thrombosis such as an intraabdominal abscess, acute diverticulitis, acute pancreatitis, or venous invasion by tumor (Fig. 10.38), most patients with mesenteric venous thrombosis have an underlying predisposition to thrombosis. Therefore, it is important to investigate these patients clinically for predisposing conditions that may lead to recurrence or have clinical implications for relatives who carry the predisposition to thrombosis. Hypercoagulable states include factor V Leiden mutation, the presence of antiphospholipid antibody, and deficiency of protein C or S (see Box 10.3). Myeloproliferative disorders, such as polycythemia vera, have also been found to be causative. The JAK2 V617F mutation has been detected in some patients with mesenteric venous thrombosis before the development of an overt myeloproliferative disorder.76
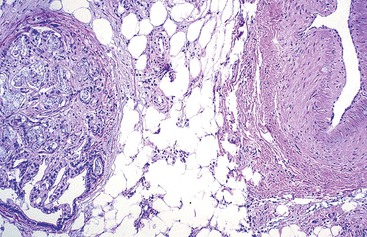
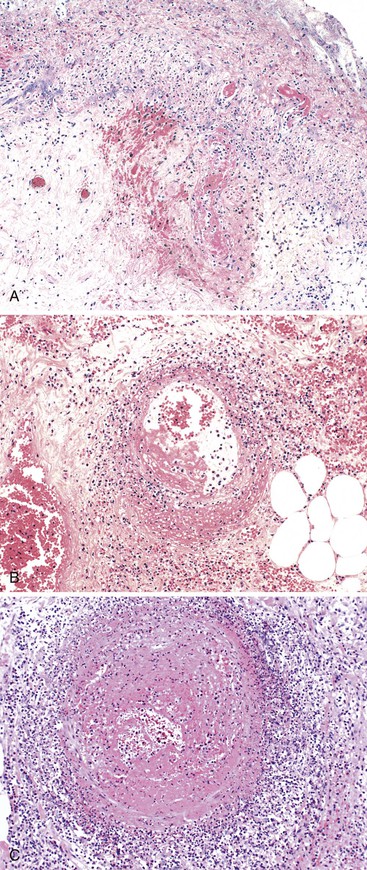
The gross and microscopic appearances of the affected segment of bowel are similar to those described in cases of mesenteric venous insufficiency. In addition, thrombi are typically found in veins located in the submucosa, adventitia, and mesentery (Fig. 10.39, A), as well as in those distant from areas of necrosis. The splenic, portal, and hepatic veins may be involved in some cases. Typically, arteries are not affected. Blood vessels located proximal to the thrombus are typically engorged and dilated. Mucosal and intramural congestion and hemorrhage, with submucosal edema and extravasation of erythrocytes, are common (see Fig. 10.39, B and C). Ischemic necrosis of the mucosa may be seen on microscopic examination. When the area of thrombosis is localized, it usually begins within large vessels and propagates proximally toward smaller ones. However, if the underlying cause of ischemia is a hypercoagulable state, thrombosis begins in the small-sized vessels and propagates centrally toward the larger ones.73 Patients may present with acute, subacute, or chronic symptoms and sometimes with Budd-Chiari syndrome. However, even in patients with an acute presentation, it is common to detect thrombi at various stages of organization (see Fig. 10.39, D).
Intramural Disorders
Portal Pylephlebitis
Portal pylephlebitis may develop as a complication of diverticulitis or perforated appendicitis. Patients typically are seen with a high fever that is sometimes accompanied by jaundice. Pylephlebitis is a form of septic (often suppurative) thrombophlebitis of the portal venous system, usually associated with gram-negative enteric aerobes and anaerobes. Some cases of pylephlebitis are believed to arise from areas of mesenteric venous thrombosis that have become secondarily infected.77 Pylephlebitis is most often diagnosed on the basis of clinical and radiologic findings but may be encountered by the pathologist on evaluation of resection specimens or at autopsy. It may also be inferred in patients with a hepatic abscess and an appropriate clinical history.
The pathognomonic histologic lesion is an acutely inflamed vein of the mesenteric/portal system, associated with bacteria or fungi and a recent or organizing thrombus. Pylephlebitis identified in a surgical resection specimen from a patient with a ruptured appendix or diverticular disease–associated abscess should alert the pathologist to the possibility of spread of infected emboli to the liver.
Pylephlebitis usually requires aggressive antimicrobial therapy. If left untreated, it may lead to the development of septic emboli. Hepatic abscesses occur in as much as 50% of cases. The mortality rate exceeds 30%.75,78 Early diagnosis and therapy improve overall outcome.
Mesenteric Phlebitis
Mesenteric phlebitis occurs in several types of systemic vasculitides that affect both arteries and veins. These disorders include Behçet disease, Buerger disease, SLE, rheumatoid arthritis, and Wegener granulomatosis. This condition is discussed more thoroughly later (see Vasculitis).
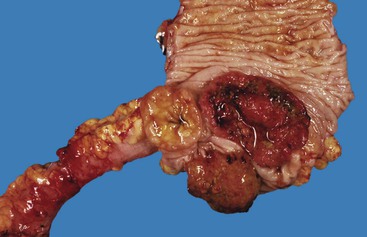
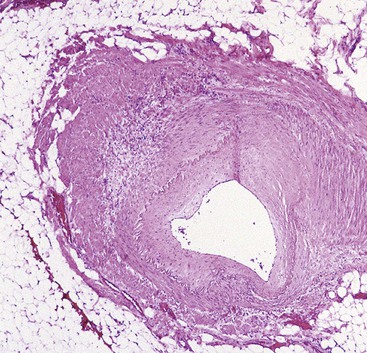
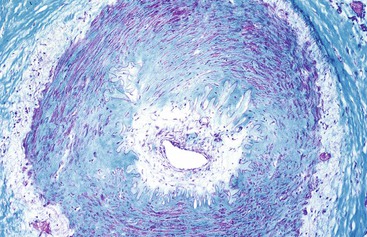
Idiopathic Myointimal Hyperplasia of Mesenteric Veins
Idiopathic myointimal hyperplasia of mesenteric veins (IMHMV) is a rare condition. It has been reported mainly in young to middle-aged men who are seen with abdominal pain, diarrhea or constipation, and rectal bleeding. The left colon is most often involved. The endoscopic findings of mucosal erythema, ulceration, friability, granularity, and cobblestoning often result in a presumptive endoscopic diagnosis of inflammatory bowel disease. However, mucosal biopsies do not show features of Crohn’s disease or ulcerative colitis but instead show mild ischemic changes. Abnormally thick-walled mucosal capillaries may sometimes be observed. A diagnosis of IMHMV may rarely be suspected on clinical grounds, but it is usually established only on evaluation of a resection specimen. The disorder is localized to the veins of discrete segments of the intestine, most commonly the sigmoid colon, and therefore is not considered a systemic form of “vasculitis.” Resection of the affected segment of bowel is curative. Disease recurrence has not been reported.
The pathognomonic histologic features of IMHMV are seen in the mural blood vessels; they consist of a concentric proliferation of smooth muscle cells in the intima and media of small to medium-sized intramural veins. This causes stenosis, and sometimes occlusion, of the veins but without an associated inflammatory infiltrate (Fig. 10.40). The arteries are normal, but the veins become enlarged and muscular so that they can easily be mistaken for arteries.79 In contrast to acute mesenteric venous ischemia, cases of IMHMV do not show mucosal congestion, vascular engorgement, or hemorrhage into the bowel wall. Rather, the mucosa shows dilated capillaries, abnormal small muscular vessels, and vessels with thickened hyaline walls. The epithelium may reveal mucin depletion and mild atrophic changes. Features typical of ischemia may also be present (i.e., loss of surface epithelium, reactive changes in residual crypt epithelium, and hyalinosis of the lamina propria). On the basis of a similarity of the pathologic changes in the mural and extramural veins to those in saphenous veins that have been used in coronary artery bypass surgery, Genta and Haggitt, in the initial description of this entity, postulated that the disorder may be a consequence of arteriovenous fistulization.80 Subsequent studies have supported this theory.81 Small, localized foci of pathologic changes similar to IMHMV have been demonstrated in intestinal specimens resected for unrelated conditions, but they contained surgical anastomoses or other forms of healed (traumatic) injury, in which arteriovenous communications are expected to occur.82 An alternative pathogenetic theory suggests that IMHMV represents the end stage of “phlebitis,” because lesions morphologically similar to IMHMV have been observed in cases of lymphocytic, granulomatous, and necrotizing phlebitis.83
Enterocolic (Lymphocytic, Granulomatous, or Necrotizing) Phlebitis
Enterocolic phlebitis is a term that was introduced to encompass a variety of related disorders termed lymphocytic, granulomatous, or necrotizing phlebitis. This name was proposed on the basis that granulomatous phlebitis and necrotizing phlebitis almost always occur in association with lymphocytic phlebitis, and not uncommonly all three forms are present together in the same pathologic specimen.83 Saraga and Bouzourenne included IMHMV within the spectrum of enterocolic phlebitis,83 but in this chapter it is considered separately, primarily because it occurs predominantly in the left colon, whereas enterocolic phlebitis occurs typically in the ileocecal region. In addition, the age and sex distributions of patients with these entities differ (see Table 10.7). Furthermore, whereas foci of IMHMV may be seen in association with enterocolic phlebitis, as well as other unrelated forms of intestinal injury, most clinically evident cases of clinical IMHMV show no evidence of enterocolic phlebitis.82
The term mesenteric inflammatory veno-occlusive disease (MIVOD) has been largely abandoned. It was originally used to describe seven cases, of which four were lymphocytic phlebitis, two necrotizing phlebitis, and one mixed lymphocytic and granulomatous phlebitis. Three of the seven cases also had foci of IMHMV.84
Similar to IMHMV, enterocolic phlebitis is a localized process rather than a systemic one, and resection of the affected segment of the intestinal tract is considered curative. Patients with lymphocytic phlebitis are typically middle-aged to elderly and are seen with abdominal pain caused by ischemia of the right colon or terminal ileum or with a right-sided abdominal mass caused by cecal edema or intussusception.83,85–87
The ischemic features of enterocolic phlebitis are typical of mesenteric venous obstruction, showing engorgement of the veins of the bowel wall, mural hemorrhage, edema, and mucosal necrosis (Fig. 10.41, A). Histologically, the lymphocytic phlebitis form of enterocolic phlebitis is characterized by a diffuse infiltrate of small lymphocytes within the walls of intramural veins, which also forms dense perivenular cuffs (see Fig. 10.41, B and C). The lymphocyte cuffs are typically circumferential. However, in tissue sections distant from areas of ischemia, a crescentic pattern of inflammation may be seen.88 The inflammatory infiltrate is composed of CD3+ CD8+ T cells, some of which have cytotoxic characteristics, admixed with smaller numbers of B cells. Some affected veins may be thrombosed (see Fig. 10.41, D).
In enterocolic phlebitis, areas of necrotizing phlebitis (in which the mural infiltrate is composed mainly of neutrophils and is associated with fibrinoid necrosis of the vessel walls) and thrombosis are usually found underlying, or adjacent to, areas of mucosal necrosis (see Fig. 10.41, E). Granulomatous phlebitis, in which the mural infiltrate contains histiocytes and giant cells associated with damage to the walls of veins (see Fig. 10.41, F), may also be seen focally.89 Enterocolic phlebitis has also been reported in association with lymphocytic enteritis and colitis.87,90 A single report of gastroduodenal lymphocytic phlebitis illustrates that this pattern of inflammatory vasculopathy may not be restricted to the distal small bowel and colon.90
Mesenteric Phlebosclerosis
Mesenteric phlebosclerosis is an entity in which calcification and sclerosis of the mesenteric veins leads to chronic progressive ischemia of the colon.91–93 Patients typically are seen with chronic abdominal pain and diarrhea that may be misdiagnosed as inflammatory bowel disease. At endoscopy, however, the colonic mucosa appears edematous and dark with prominent blue-black vessels and shallow ulcers, in contrast to the appearance of the colonic mucosa in inflammatory bowel disease.94 In resection specimens, the bowel wall is typically fibrotic, causing ischemic stenosis of the lumen. The pathognomonic histologic feature is sclerosis and calcification of the mesenteric veins, which is detectable on plain abdominal radiographs. The etiology of this disorder is unknown. The only cases reported have been from Japan. Most cases were treated by resection of the affected region of the bowel and were without recurrence or other adverse consequences.
Microvascular Insufficiency
Several disease processes may affect small blood vessels (arterioles, capillaries, and venules) in the intestinal submucosa and mucosa. Depending on the distribution and extent of microvascular injury, tissue damage may be diffuse, affecting large areas of intestinal mucosa, or discrete and localized. Clinically, tissue injury may be silent, or it may be associated with abdominal pain. Bleeding occurs commonly and may be of the chronic low-grade type, leading to anemia. If the process is widespread, there may be extensive mucosal injury and loss, resulting in the development of an acute abdomen clinically.
Common causes of microvascular insufficiency are listed in Table 10.8. In many cases, the pathologic features are diagnostic. Capillary lesions such as thrombi associated with disseminated intravascular coagulation or thrombotic thrombocytopenic purpura, erythrocyte blockage in sickle cell disease (Fig. 10.42), and ectatic capillaries associated with chronic irradiation injury may be detected in mucosal biopsies. In contrast, leukocytoclastic vasculitis of Henoch-Schönlein purpura, as well as angiopathies associated with diabetes mellitus and amyloidosis, may not be evident in the mucosa and are more easily diagnosed in specimens that contain submucosal tissue. Atheromicroemboli may be seen in mucosal biopsies, but they are usually more numerous, and more easily detected, in the submucosa.
Table 10.8
Microvascular Causes of Intestinal Ischemia
| Causes | Presentation | Main Vessels Affected | Pathologic Characteristics | Distribution Of Injury |
| Disseminated intravascular coagulation | Acute | Capillaries | Thrombi | Widespread |
| Thrombotic thrombocytopenic purpura | Acute | Capillaries | Platelet thrombi | Widespread |
| Sickle cell disease | Acute, recurrent | Capillaries, venules | Sickle cell blockage | Widespread |
| Henoch-Schönlein purpura | Acute | Arterioles, venules | Leukocytoclastic vasculitis, immunoglobulin A deposits | Widespread |
| Behçet disease | Acute, recurrent | Arterioles, venules | Vasculitis | Widespread |
| Diabetes mellitus | Chronic | Arterioles, capillaries | Glycosylation product deposits in walls and basement membranes | Widespread |
| Amyloidosis | Chronic | Arterioles | Amyloid deposits in walls | Widespread |
| Chronic radiation injury | Chronic | Arterioles, capillaries | Subendothelial fibrosis, gaping capillaries, hyalinized vessels | Localized |
| Yttrium microspheres from selective internal radiation therapy | Acute | Arterioles, capillaries | Glass microspheres 15-35 mm diameter eliciting fibrosis, giant cell reaction, erosion or ulcer | Localized |
| Atheromicroemboli | Acute | Arterioles | Acicular clefts in obstructed vessels | Localized |
| Cytomegalovirus infection | Acute | Capillaries | Viral inclusions in endothelial cells at ulcer base | Localized |
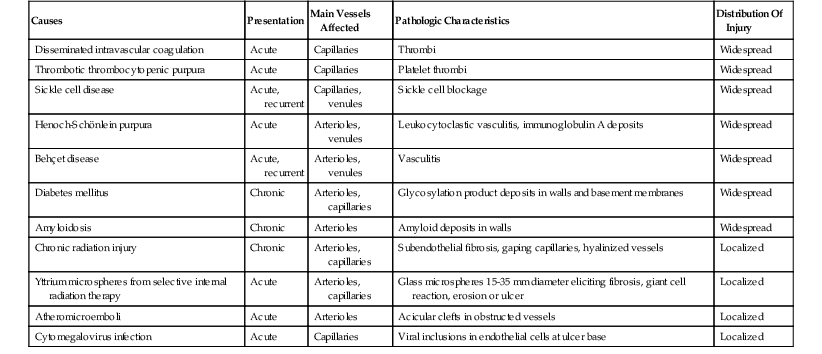
If the mucosal capillaries are obstructed, as in disseminated intravascular coagulation, thrombotic thrombocytopenic purpura, and sickle cell disease, foci of mucosal ischemia, lamina propria hemorrhage, and superficial erosions may be seen. If extensive, they may lead to the development of large areas of mucosal necrosis.
Obstruction of arterioles by atheromicroemboli may be extensive, yet without obvious signs of mucosal injury, because of the well-developed collateral circulation of the intestinal mucosa. When atheroembolic obstruction of submucosal vessels is sufficient to cause localized mucosal ischemia, punched-out areas of shallow mucosal ulceration may develop, with sharply defined edges and a flat granulation tissue base at the level of the muscularis mucosae (Fig. 10.43, A). If sufficient tissue sections of the ulcer have been obtained, atheromicroemboli can usually be recognized by the presence of sharp-edged, cigar-shaped spaces surrounded by a few occluding inflammatory cells in the obstructed lumina of small submucosal arterioles. Often, a few eosinophils may be seen surrounding the space previously occupied by the cholesterol crystals before their removal by histologic processing (see Fig. 10.43, B through D). Atheromicroembolism frequently results from instrumentation of the aorta in patients with severe atherosclerosis.70,71
Stenosis of submucosal arterioles as a result of deposition of abnormal products in the vessel walls tends to cause chronic, often progressive, ischemia. However, when this condition is exacerbated by an episode of central hypotension, at which time the systolic pressure is insufficient to irrigate the already compromised intestinal vascular bed, the clinical presentation is frequently acute. Insudation of glycosylation products in patients with diabetes mellitus, deposition of amyloid proteins in the walls of small submucosal vessels in patients with amyloidosis95 (Fig. 10.44), and progressive vascular fibrosis in patients with chronic radiation injury may all compromise the vascular supply to the mucosa in this manner.
In Behçet disease, which is a type of vasculitis that affects mainly larger blood vessels, arterioles and venules may also be involved. Because arterioles and venules are most likely to be sampled in mucosal and submucosal biopsies, a diagnosis of intestinal Behçet disease may be suggested in mucosal biopsy specimens.
Another entity that falls within the realm of microvascular insufficiency is angioedema of the intestines.96 Typically in this condition, patients are seen with one or more episodes of otherwise unexplained severe, cramping abdominal pain that may last for several days, and radiologic studies show reversible segmental edema of the small or large intestine without evidence of arterial compromise.97 Although it may be confined to the intestines initially, angioedema commonly involves other organs, including the skin of the mouth and face and the upper respiratory tract. Hereditary forms are commonly caused by mutations in the gene for C1 esterase inhibitor that result in either low levels or low activity of the protein.98 Acquired forms have several causes, notably use of angiotensin-converting enzyme (ACE) inhibitors.99 Both forms result in elevated levels of bradykinin, which increases capillary permeability with leakage of intravascular fluid into the extracellular space. The diagnosis is made on clinical and radiologic grounds correlated with quantitative and qualitative evaluation of C1 esterase inhibitor levels. Few cases have been biopsied, and, to our knowledge, the morphologic appearances of affected areas of the colonic mucosa have not been described.
Vasculitis
The classification of vasculitides (see also Chapter 6), many of which affect the GI tract, is controversial and regarded by some as having limited clinical utility.100,101 Of the several schemes in use, the Chapel Hill classification is the one used here, but with the addition of Behçet disease and Buerger disease. The classification scheme and the particular characteristics of each type of vasculitis are summarized in Table 10.9.
Table 10.9
Primary Vasculitides of the Gastrointestinal Tract
| Vessel Size | Disorder | Clinical Characteristics, Etiology, and Comments | Key Histologic Features |
| Medium | Affects celiac, mesenteric, and hepatic arteries; no glomerulitis or pulmonary capillaritis | Necrotizing arteritis | |
| Polyarteritis nodosa (PAN) | Often associated with hepatitis B | Transmural fibrinoid necrosis; early neutrophil infiltrate, later monocytes, macrophages, lymphocytes; pseudoaneurysms | |
| Kawasaki disease | Febrile disease of children <5 yr old; often involves coronaries; mucocutaneous lymph node syndrome | Less fibrinoid necrosis, more mural edema than PAN; infiltrate of monocytes, macrophages, lymphocytes | |
| Small | Affects capillaries and venules; variable involvement of arterioles; no larger vessel involvement | Necrotizing or leukocytoclastic vasculitis | |
| Wegener granulomatosis | Granulomatous inflammation of respiratory tract; necrotizing glomerulonephritis often present; 90% ANCA positive | Necrotizing vasculitis | |
| Churg-Strauss syndrome | Associated with asthma and peripheral eosinophilia; eosinophil-rich and granulomatous inflammation of respiratory tract; commonly ANCA positive | Eosinophil-rich necrotizing vasculitis | |
| Henoch-Schönlein purpura | IgA deposits in small vessels; involves skin, gut, glomeruli; arthralgias or arthritis; ANCA negative | Leukocytoclastic angiitis | |
| Cryoglobulinemic vasculitis | Cryoglobulinemia with deposits in small vessels; skin and glomeruli involved; associated with hepatitis C and hypocomplementemia; ANCA negative | Leukocytoclastic angiitis | |
| Lupus, rheumatoid, and other immune complex vasculitides | Immune complex deposits in small vessels; ANCA negative | Necrotizing vasculitis | |
| Unclassified | |||
| Behçet disease | Onset in 20s or 30s; oral and genital ulceration, ocular inflammation, arthritis | Neutrophil or lymphocyte infiltrate of small veins and arteries; ulcers, transmural inflammation, strictures; may mimic Crohn disease | |
| Buerger disease | Affects tobacco users, most male and <45 yr old; generally affects arteries to extremities; GI involvement rare; immune deposits not detected; ANCA negative | Occlusive inflammatory thrombi with neutrophils, microabscesses, giant cells in medium-sized arteries and veins; progressive organization of thrombi; vessel wall and elastic lamina remain intact | |
| Kohlmeier-Degos disease | Affects all sexes and all ages; systemic manifestations preceded by erythematosus, red or pink papules, mainly on trunk, arms, and legs | Occlusive noninflammatory arteriopathy with intimal hyperplasia, endothelial cell proliferation, and progressive sclerosis with consequent zonal tissue infarction |
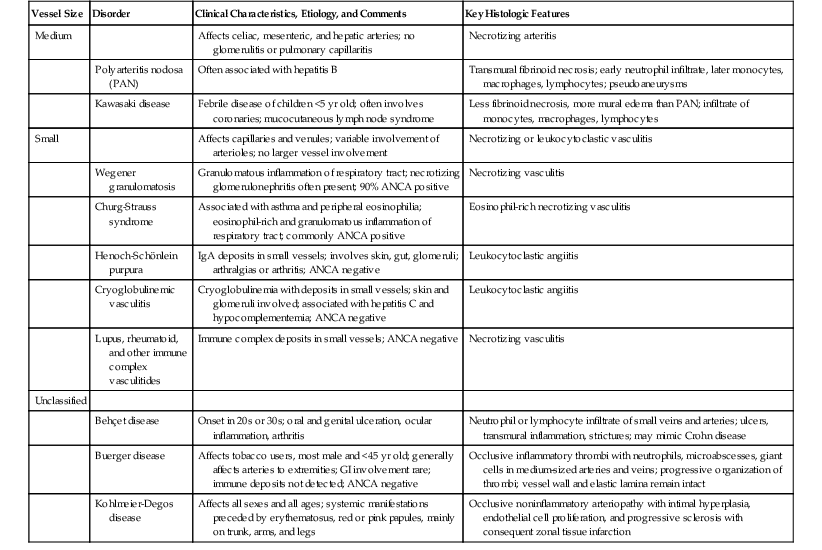
ANCA, Antineutrophil cytoplasmic antibody; IgA, immunoglobulin A.
Adapted from Jennette JC, Falk RJ. The role of pathology in the diagnosis of systemic vasculitis. Clin Exp Rheumatol. 2007;25(suppl 44):552-556.
Although many types of vasculitis affect the GI tract and liver, they are usually diagnosed on the basis of a combination of clinical features, radiologic studies, and serologic tests. Diagnostic biopsies are most commonly obtained from the skin or kidney. Small-vessel vasculitis, encountered in mucosal biopsies of the GI tract, and medium-vessel vasculitis, found in specimens resected for intestinal ischemia, should always elicit a more extensive search for possible systemic involvement. The current classifications are devoted solely to primary vasculitis; however, infection, myeloproliferative disorders, and other malignancies are among the other disease associations that should be excluded.
Medium-Vessel Disease
Medium-vessel vasculitides affect the main visceral arteries, including the celiac, mesenteric, and hepatic arteries. These disorders are considered to be within the spectrum of necrotizing vasculitis. An example of medium-vessel vasculitis is shown in Figure 10.45.
Polyarteritis Nodosa
In approximately 50% of patients with polyarteritis nodosa, the celiac and mesenteric arteries are involved, and this may result in small intestinal ischemia. The disease process less commonly affects the colon, liver, and pancreas.102 Histologically, polyarteritis nodosa is characterized by transmural fibrinoid necrosis of arteries, accompanied by a neutrophil infiltrate in the early stages of disease and monocytes, macrophages, and lymphocytes in the later stages (see Fig. 10.32). Pseudoaneurysms are common and are responsible for the characteristic nodularity of the blood vessels, which is implied by the term nodosa. Polyarteritis nodosa is commonly associated with hepatitis B infection and occasionally with hepatitis C.
Kawasaki Disease
Kawasaki disease usually occurs in children younger than 5 years of age and is characterized by the presence of mucocutaneous lymphadenopathy. In the fully developed disorder, patients have fever, skin rash on the palms and soles, cutaneous desquamation, strawberry tongue, and conjunctival congestion.103 The necrotizing arteritis associated with this condition shows a predilection for the coronary arteries, which may develop aneurysms, thrombosis, or rupture. Many patients have intestinal involvement, which manifests as abdominal pain, nausea, vomiting, diarrhea, small-bowel bleeding, and ischemic perforation.
Small-Vessel Disease
Small-vessel vasculitides are characterized by necrotizing or leukocytoclastic injury to capillaries and venules, with less involvement of arterioles and small arteries. Larger blood vessels are not affected. These disorders fall into two groups, separated by the presence or absence of immune globulin deposition and antineutrophil cytoplasmic antibodies (ANCA).
Wegener Granulomatosis
Wegener granulomatosis is a granulomatous disease of the respiratory tract that is often accompanied by nasal sinus and renal involvement. It is associated with paucity of immunoglobulin deposition. Ninety percent of patients are ANCA positive. In addition to the characteristic destructive granulomas, necrotizing small-vessel vasculitis (Fig. 10.46) is typically present. Wegener granulomatosis may affect the GI tract, particularly the mesenteric arteries, which can lead to ischemic injury to the intestines.104 Granulomatous inflammation of the stomach or ileum may cause diagnostic confusion with Crohn’s disease.105
Churg-Strauss Syndrome
Churg-Strauss syndrome is a form of allergic angiitis characterized by the presence of asthma, peripheral eosinophilia, granulomatous inflammation of the respiratory tract, and eosinophil-rich necrotizing vasculitis (Fig. 10.47) that usually responds to steroid therapy. Approximately 50% of patients experience abdominal pain and bleeding resulting from GI ischemia.106 This disorder is associated with a paucity of immunoglobulin deposition. Patients are commonly ANCA positive.
Henoch-Schönlein Purpura
In Henoch-Schönlein purpura, immunoglobulin A–predominant immune complexes are deposited within small-sized blood vessels that exhibit leukocytoclastic angiitis. The process affects small vessels of the skin, kidneys, joints, and, in 75% of cases, the GI tract. Children in the age range of 4 to 7 years are most often affected, and the condition usually develops after an upper respiratory tract infection. GI involvement results in ischemia with abdominal pain, bleeding, and intramural hemorrhage, which is the GI counterpart of the palpable purpura commonly observed in skin.107 Histologic features include hemorrhage and edema in the mucosa, submucosa, and bowel wall, accompanied by leukocytoclastic vasculitis. Serologic tests for ANCA are typically negative.
Leukocytoclastic Vasculitis
Although the term leukocytoclastic vasculitis is sometimes used to describe a specific disease entity, it simply refers to a pattern of small-vessel inflammation characterized by leukocyte fragmentation and fibrinoid necrosis of arterioles, capillaries, and venules (Fig. 10.48). This pattern of injury may be seen in a variety of vasculitides. The term is also occasionally used synonymously with hypersensitivity vasculitis, which is a disorder of adults associated with skin rash and leukocytoclastic vasculitis. GI involvement in these cases is usually mild and limited.108 Hypersensitivity vasculitis bears morphologic similarity to Henoch-Schönlein purpura; however, there is some evidence that they are, in fact, two distinct entities.109
Cryoglobulinemic Vasculitis
Cryoglobulinemic vasculitis refers to a type of small-vessel immune complex vasculitis occurs in patients with hepatitis C and cryoglobulinemia. The skin and kidneys are more commonly involved than the intestines, but intestinal ischemic injury has occasionally been reported.110
Systemic Lupus Erythematosus and Other Immune Complex Diseases
Approximately 50% of patients with SLE show GI involvement by small-vessel immune complex vasculitis. The clinical manifestations are variable. Patients may experience nausea, vomiting, abdominal pain, diarrhea, malabsorption, pancreatitis, protein-losing enteropathy, and segmental ischemic necrosis of the intestines.111 GI involvement is seen in only approximately 10% of patients with rheumatoid arthritis. Symptoms include abdominal pain, nausea, and vomiting. Ischemic injury may lead to bowel necrosis and perforation.112 Resection specimens may show small-vessel vasculitis with hemorrhage and edema in the mucosa, submucosa, and bowel wall, as well as varying degrees of tissue necrosis (Fig. 10.49).
Behçet Disease
Behçet disease, which is a vasculitis of unknown etiology, is linked to the presence of human leukocyte antigen (HLA) B51. It is most common in countries situated along the ancient Silk Road, particularly Turkey, but also occurs elsewhere, especially in Japan.113 Clinical features, which usually develop in the third or fourth decade of life, are diverse and include oral and genital ulceration, retinitis and uveitis, erythema nodosum and other skin disorders, and arthritis.
GI involvement develops in 3% to 26% of patients and is most common in the terminal ileum and right colon, where it may mimic Crohn’s disease or ulcerative colitis.114 In resection specimens, small-vessel phlebitis, either with or without arteritis, is seen in the submucosa, often with ulceration. Ulcers have been reported to be associated with lymphoid follicles and Peyer patches115 (aphthous lesions); although small, they have a tendency to penetrate deeply within the bowel wall and cause perforation. Large vessels are less commonly involved than small ones, and their involvement may lead to ischemia or infarction.. The vascular inflammation in the GI tract may be predominantly lymphocytic116 or predominantly neutrophilic,117 possibly reflecting different stages of the disease or a response to immunosuppressive therapy.
GI involvement in Behçet disease often necessitates resection of the affected segment of bowel. However, disease recurrence is common, and further intervention is required in approximately 50% of cases. The differential diagnosis of GI Behçet disease includes Crohn’s disease, ulcerative colitis, and chronic ischemic enterocolitis, but the diagnosis is usually made on the basis of both the clinical and the serologic features.
In 2009, Cheon and colleagues proposed an algorithm to distinguish Behçet disease from Crohn’s disease. The basis of the algorithm lay in categorizing the typical (not pathognomonic) Behçet ulcer as fewer than five oval, deep, discrete ileocecal ulcers.118 With this definition, patients were classified as “definite BD” if they had concurrent systemic Behçet disease, “probable intestinal BD” if they had an oral ulcer only, or “suspected BD” if they had neither systemic BD nor an oral ulcer. All other ulcers were considered atypical and nondiagnostic in the absence of systemic Behçet disease, although they were categorized as probable intestinal BD if there was a concurrent history of systemic Behçet disease. This algorithm produced greater than 95% sensitivity and greater than 80% specificity in detecting definite, probable, or suspected BD. Although the algorithm has not been validated by another series, there appears to be some value in the recognition that Behçet disease is most often associated with well-defined, discrete, oval, deep ulcers that usually number less than five.
Buerger Disease
Buerger disease, also termed thromboangiitis obliterans, is a vasculitic disorder that affects mainly men younger than 50 years of age who have a long history of cigarette smoking. The disorder results in impairment of the vascular supply to the lower extremities, which causes claudication and rubor, but occasionally the small mesenteric vessels are involved with acute inflammation and thrombosis, resulting in intestinal ischemia.119 The thrombi tend to be inflammatory, but the vessel walls usually escape the effects of severe injury. Typically, the internal elastic lamina remains intact.
Malignant Atrophic Papulosis (Kohlmeier-Degos Disease)
Malignant atrophic papulosis is a rare type of occlusive arteriopathy that occurs in patients of any age and affects both sexes. The epidemiology of this disorder is unclear. However, familial clustering has been reported, with a young male predisposition.120 Atrophic papulosis may exist as a purely cutaneous, benign form or as a systemic, “malignant” form that affects the GI tract and central nervous system. Cutaneous red or pink papules are typically found on the trunk, arms, and legs and commonly precede systemic manifestations. Patients may also be seen with abdominal pain and features of peritonitis or with manifestations of central nervous system involvement.121 Endoscopic findings include infarcts and ulcers.
Malignant atrophic papulosis may involve any region of the GI tract. However, the small bowel is most commonly involved. Lesions are characterized by progressive sclerosis of small and medium-sized arteries with zonal tissue infarction. Serosal white plaques with red borders may be seen grossly.122 Characteristic histologic features include intimal hypercellularity with endothelial cell proliferation of small and medium-sized submucosal arteries and superimposed arterial thrombosis, but typically with only minimal or no inflammation. Vascular changes are associated with progressive intestinal necrosis, which may be full-thickness. The GI manifestations may be similar to other types of vasculitis, particularly SLE, a disease of which malignant atrophic papulosis has been considered a variant.122
In patients with systemic involvement, the most common cause of death is intestinal perforation and peritonitis as a consequence of infarction.122 Enterocutaneous fistulas have also been reported. In addition to surgical management, medical therapy includes anticoagulants, antiplatelet drugs, and immunosuppressive agents.
Segmental Arterial Mediolysis
Segmental arterial mediolysis (SAM) is discussed here although it is not a true vasculitis. SAM is a rare lesion that was initially described in 1976 as segmental mediolytic arteritis and is best characterized as a noninflammatory, nonatherosclerotic arteriopathy that predominantly affects the abdominal arteries.123 In a review of 27 patients,124 Inada and colleagues supported the observations of Slavin123 of partial or total mediolysis of large muscular arteries of the abdomen, most commonly the middle colic artery. In this review, SAM occurred in patients who ranged from 44 to 88 years of age, with a male predisposition, although cases have been reported in younger patients.125 The most common presenting symptom was intraabdominal bleeding. On angiography, lesions may mimic the nodular appearance of PAN.
In addition to the middle colic artery, other involved abdominal arteries may include the gastric, gastroepiploic, inferior pancreaticoduodenal and hepatic126 arteries. Reportedly, SAM may also affect cerebral, coronary, and pulmonary arteries.127 Pathologically, these arteries undergo total or partial mediolysis, medial degeneration, and eventually aneurysm formation, hemorrhage, and rupture.
Patients require surgical intervention and may worsen with immunosuppressive therapy, rendering distinction from PAN important128 Rarely, SAM may complicate PAN.129
Localized Vasculitis of the GI Tract
In a small subset of patients, vasculitis occurs in the GI tract without systemic involvement. Reports have described patients with isolated GI tract involvement by necrotizing vasculitis,130–132 emphasizing that vasculitis is not always systemic. These patients commonly undergo surgical intervention with variable outcomes, generally more favorable with involvement of the gallbladder, pancreas, and appendix.132
Differential Diagnosis of Ischemia in Lower GI Biopsies
The features of acute ischemic colitis described in the preceding sections are summarized as follows: mucosa of normal architecture with necrosis and sloughing of the surface epithelium, loss of epithelium in the superficial aspects of the crypts (with or without ghosts of crypts), mucin depletion and reactive changes in the residual crypt epithelium with nuclear hyperchromasia and increased mitoses, paucity or complete absence of acute inflammatory cells, and the presence of hyalinosis in the lamina propria (see Fig. 10.35, A through E; Table 10.10). However, some of these histologic features are not specific for ischemic injury. The differential diagnosis of ischemia in colonic biopsies includes a number of entities with a potential for overlapping histologic findings (Fig. 10.50).
Table 10.10
Key Histologic Features of the Mucosa in Acute Ischemic Enterocolitis and its Differential Diagnosis
| Entity | Key Clinical Features | Etiology | Key Pathologic Features | Treatment |
| Acute intestinal ischemia | Acute crampy abdominal pain, followed by bloody or maroon diarrhea | Arterial (occlusive or nonocclusive), venous, or microvascular insufficiency | Loss of surface epithelium with hyalinization of lamina propria; progressive loss of gland epithelium from surface down; mucin depletion, reactive epithelial atypia in crypts, crypt cell mitoses; scant acute inflammation initially | Conservative treatment (parenteral fluids, food restriction, broad-spectrum antibiotics) unless surgical exploration is indicated (perforation or gangrene) |
| Pseudomembranous enterocolitis | Acute onset diarrhea with loose, watery stools; or fever, leukocytosis, and crampy abdominal pain | Toxigenic Clostridium difficile infection in susceptible host, 2 days to 12 wk after antibiotic use | Type I lesion: Superficial mucosal necrosis with erupting spray of fibrinopurulent exudate Type II: Desquamated epithelium in groups of crypts with plaques of inflammatory exudate that cover mucosal surface Type III: Full-thickness mucosal necrosis with hemorrhage and overlying confluent pseudomembrane |
Stop precipitating antibiotics and treat with metronidazole or vancomycin |
| Hemorrhagic (infectious) enterocolitis | Watery, nonbloody diarrhea and abdominal cramps, progressing to bloody diarrhea; nausea, vomiting, fever; can cause HUS in children or TTP in adults | Enterohemorrhagic Escherichia coli, Klebsiella oxytoca; bacterial toxins damage capillary endothelium | Lamina propria hemorrhage occurs early, followed by superficial mucosal necrosis with loss of surface epithelium and superficial gland epithelium. Neutrophils infiltrate lamina propria and glands. With resolution, lymphocytes and plasma cells infiltrate | Parenteral hydration, rest bowel, avoid antibiotic use (increases risk of HUS and TTP) |
| Enteritis necroticans | Acute abdominal pain with bloody diarrhea, usually after binge eating; also when high-protein diet is introduced after starvation | Clostridium perfringens, Clostridium septicum toxins | Usually affects small bowel; coagulative necrosis of mucosa with scant exudate, surface colonization by clostridia, submucosal neutrophil infiltrate and edema; progressing to transmural necrosis | Resection of affected segment of intestine |
| Neutropenic enterocolitis | Acute abdominal pain with bloody diarrhea in a setting of neutropenia | Clostridium septicum, Clostridium perfringens | Usually affects cecum; coagulative necrosis of mucosa with little or no exudate; paucity of neutrophils at viable edges; progresses to transmural necrosis | Resection of affected segment of intestine |
| Acute radiation injury | Crampy abdominal pain, diarrhea, and nausea during course of radiation | Therapeutic ionizing radiation | Loss of surface and gland epithelium, increased cellularity of lamina propria, lamina propria edema and hemorrhage, hyalinized vessels, dilated capillaries, atypical fibroblasts | Resolves spontaneously after course of radiation |
| Kayexalate injury | Abdominal pain and diarrhea; uremic patients particularly susceptible | Sorbitol component of the Kayexalate preparation | Rhomboid or triangular basophilic nonpolarizing crystals adherent to epithelium or in inflammatory exudates; ischemic pattern of mucosal injury; distinguish from cholestyramine by mosaic pattern in H&E stain and red (not pink) color in Ziehl-Neelsen stain | Cease treatment |
| Glutaraldehyde colitis | Severe acute crampy abdominal pain beginning 3-6 hr after colonoscopy; bloody diarrhea | Glutaraldehyde sterilant retained in endoscope channels and sprayed on mucosa during endoscopy | Identical to those of acute intestinal ischemia | Parenteral fluids if necessary; resolves spontaneously |
| Collagenous colitis | Chronic watery diarrhea, primarily in middle-aged women; may have associated cramping abdominal pain | Etiology unclear; abnormal collagen metabolism, bacteria, and medication-mediated damage have been implicated | Increased lamina propria lymphoplasmacytic inflammation and intraepithelial lymphocytes; marked subepithelial collagenous deposition that may mimic hyalinosis of ischemic colitis; superimposed acute inflammation and necroinflammatory pseudomembrane may mimic pseudomembranous colitis | Steroid therapy, cholestyramine, bismuth, and 5-aminosalicylate derivatives and immunosuppressives are therapeutic options; surgical management in selected cases (very rarely) |
| Amyloidosis | Most common with systemic amyloidosis (usually reactive or secondary AA type); diarrhea, steatorrhea, and weight loss; may have gastrointestinal bleeding or motility disorder | Abnormally pleated protein deposited in extracellular tissues | Lamina propria pink, homogenous amyloid deposition, usually with normal crypt or surface epithelium with or without coexisting capillary, venular, and arteriolar amyloid deposits | Treatment of underlying cause; supportive therapy, sometimes steroid therapy |
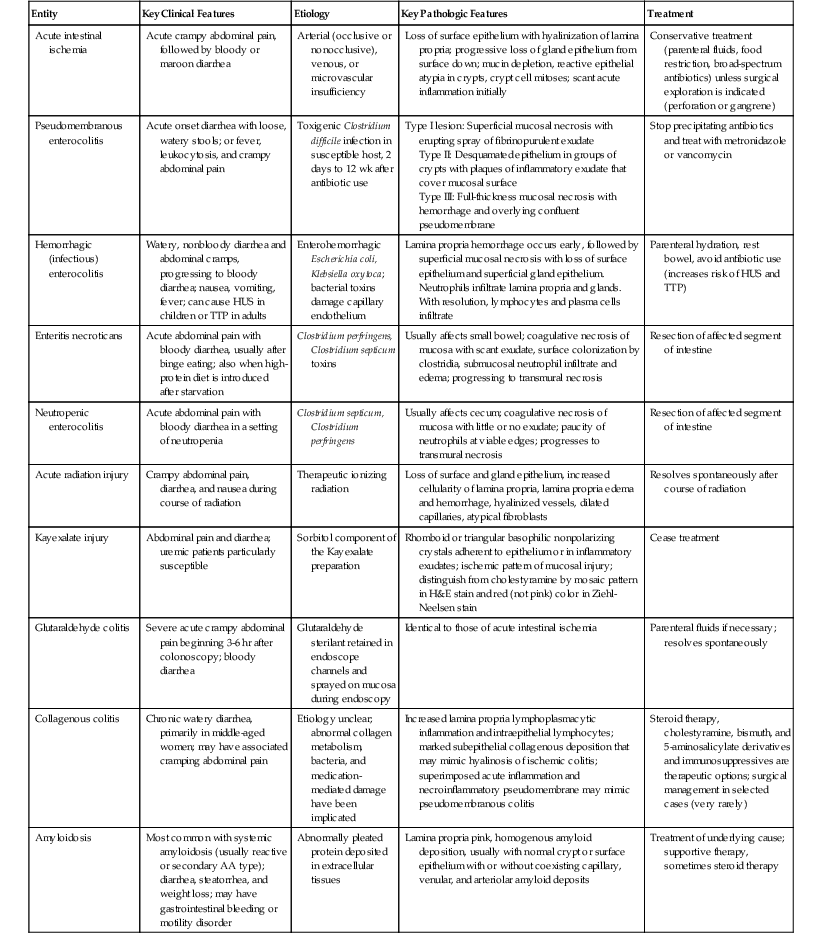
H&E, Hematoxylin and eosin stain; HUS, hemolytic-uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
Certain phases of C. difficile–induced pseudomembranous colitis can mimic ischemic colitis. Although early lesions, characterized by the presence of a volcanic (eruptive) inflammatory exudate, are distinctive, late (type II or III) lesions are characterized by mucosal necrosis and the presence of a necroinflammatory pseudomembrane that can mimic ischemic colitis. The presence of lamina propria hyalinosis, attenuated crypts with loss of superficial epithelium, and reactive epithelial changes (“microcrypts”) are features supportive of ischemia. Continuous (as opposed to patchy) involvement of mucosa in the affected region, prominent lamina propria hemorrhage, and full-thickness mucosal necrosis are also more common in ischemia compared with pseudomembranous colitis.133
Acute hemorrhagic (infectious) colitis, such as that caused by E. coli O157:H7 and certain other bacteria, including K. oxytoca,59 may mimic ischemia by showing architecturally normal mucosa, intramucosal hemorrhage, and capillary fibrin thrombi, especially in the early stages of infection before significant infiltration of neutrophils has occurred. Abundant acute inflammation and hemorrhage, with relative preservation of mucosal integrity and without withering (attenuated) crypts, are features indicative of acute hemorrhagic colitis. However, clinical, endoscopic, and bacteriologic correlation may be required to distinguish these two entities.
Enteritis necroticans is a rare type of necrotizing enterocolitis usually caused by C. perfringens. Toxins produced by the bacteria induce coagulative necrosis of the mucosa that mimics acute ischemia. However, in contrast to ischemia, enteritis necroticans, which typically affects the small bowel, usually shows coagulative necrosis with only scant or negligible neutrophilic exudate, exhibits surface colonization by clostridial organisms, and may be associated with intestinal pneumatosis. Clinically, the rapid development of toxemia and multiorgan failure helps distinguish this condition from ischemic colitis.
Neutropenic enterocolitis, an infectious disorder most often caused by C. septicum, develops in patients who are neutropenic, commonly because of chemotherapy. Toxins produced by this organism are normally degraded in the presence of neutrophils but in their absence may cause coagulative necrosis of the mucosa and submucosa, mimicking ischemia. The mucosa in patients with neutropenic enterocolitis typically reveals coagulative necrosis, marked edema, and hemorrhage in the absence of an acute inflammatory reaction. The diagnosis of neutropenic enterocolitis is often made clinically in patients who are known to be neutropenic as a result of chemotherapy, but it may be unsuspected in patients with other conditions, such as undiagnosed cyclic neutropenia.
Both acute and chronic radiation colitis may mimic ischemic colitis. The histologic and pathologic effects of radiation therapy vary with the interval between completion of radiation treatment and onset of symptoms.134 Acute radiation injury (within 2 to 3 days after radiation treatment) is characterized by surface epithelial damage, nuclear atypia with bizarre mitoses, attenuation or loss of crypt epithelium, increased apoptosis, and increased eosinophils and eosinophilic crypt abscesses. In this phase, features of ischemic colitis such as lamina propria hyalinosis, withering crypts and crypt atrophy, and superficial mucosal necrosis are not normally present. In the chronic phase of radiation injury, superimposed episodes of ischemia or the presence of mucosal or submucosal fibrosis can mimic primary acute or chronic ischemic colitis.135 However, the presence of dilated, thickened, and hyalinized small and medium-sized blood vessels, prominent reactive or bizarre-shaped endothelial cells, myofibroblasts and fibroblasts, and foamy cell plaques within arteries are distinctive features of chronic radiation injury.136
Several types of drugs, including oral contraceptives, potassium chloride, NSAIDs, pseudoephedrine, agonists of 5-hydroxytryptamine, Kayexalate (sodium polystyrene), and even glutaraldehyde, which is used as a sterilizing agent for endoscopic equipment, can produce a pattern of injury in the colon that mimics primary acute ischemic colitis.137 For instance, Kayexalate may cause an ischemic pattern of mucosal damage often associated with a mild inflammatory exudate.138 Kayexalate-induced colitis can be distinguished from ischemia by the presence of diagnostic triangular or rhomboidal, basophilic, nonpolarizing crystals adherent to the epithelium or within the inflammatory exudate. Kayexalate crystals should be distinguished from cholestyramine by the presence of a mosaic pattern on hematoxylin and eosin (H&E) stain and a red color on Ziehl-Neelsen stain. Patients with glutaraldehyde colitis often complain of severe lower abdominal pain that develops within a few hours after an endoscopic procedure. Biopsies at this stage may show mucosal necrosis, sloughing of the surface and superficial crypt epithelium, and hyalinosis of the lamina propria in the absence of a neutrophilic infiltrate—histologic features that are virtually indistinguishable from those of primary acute ischemic injury.
Other disorders that may, rarely, cause confusion with ischemia are collagenous colitis and colonic amyloidosis. In biopsy specimens from patients with collagenous colitis, detachment of the surface epithelium and the presence of a thickened and irregular subepithelial collagen band can mimic ischemia-related hyalinosis. However, in contrast to ischemia, collagenous colitis is characterized by the presence of a dense mononuclear infiltrate in the lamina propria, increased intraepithelial lymphocytes, and positive immunohistochemical staining for type III collagen. Furthermore, clinically, patients with collagenous colitis usually have nonbloody, watery diarrhea and are overwhelmingly female. A trichrome stain can often help distinguish true fibrosis in cases of collagenous colitis (positive) from hyalinosis related to ischemic colitis (negative).
Similarly, amyloid deposition in the lamina propria can mimic ischemia-related hyalinosis.139 However, in amyloidosis, the mucosa is usually otherwise within normal limits, without crypt or surface epithelial injury. On occasion, vascular involvement by amyloidosis causes superimposed ischemic colitis as a result of the underlying vascular pathologic process. A Congo red, Sirius red, or crystal violet stain can be useful in this setting to highlight the presence of amyloid.