Epithelial Neoplasms of the Stomach
Gregory Y. Lauwers
Introduction
Gastric cancer is the second most common type of cancer worldwide. Approximately 800,000 new cases and 650,000 deaths are reported per year, despite a steadily declining incidence during the past 50 years.1 There is wide variation in incidence on different continents, with the highest rates in Asia, central Europe, and South America.2 In the United States, gastric cancer is the seventh most frequent cause of cancer-related death. The National Cancer Institute estimates that almost 22,000 new cases of gastric cancer will be diagnosed in 2013 and that approximately 11,000 deaths will be attributed to this disease.2,3
The topographic distribution of gastric cancer has also changed in recent years. Since the early 1980s, the incidence of proximal gastric tumors has been on the rise. Carcinomas of the gastric cardia now represent approximately 30% of all gastric cancers.4,5 Changes in clinical practice have also led to diagnosis of a higher percentage of early-stage cancers. The widespread increased use of upper gastrointestinal (GI) endoscopy has led to more frequent detection of superficial cancers. Early gastric cancer (EGC) now represents almost 20% of all newly diagnosed cancers in the United States, and as many as 50% in Japan.6–9 This trend has had a dramatic impact on the mortality rate, to the point that gastric cancer is now considered potentially curable if it is detected at an early stage.
Pathogenesis
The pathogenesis of sporadic gastric carcinoma is a multifactorial process in which both environmental and host-related factors play a major role.10 The carcinogenic process involves a progression from chronic gastritis to atrophy with hypochlorhydria or achlorhydria, intestinal metaplasia, dysplasia, and, ultimately, adenocarcinoma.10,11 Intestinal metaplasia and, subsequently, dysplasia and early adenocarcinoma develop initially in the neck region of the antral or fundic glands of the stomach, supporting the hypothesis that precursor cells are located in this region.12
The well-known geographic variations in incidence data, obtained from studies of changes in dietary and sanitary conditions among immigrants, have underscored the role of environmental influences in the development of the intestinal type of gastric carcinoma, which is the most common type.13–15 Furthermore, the worldwide decrease in the incidence of intestinal-type gastric cancer has paralleled the decline in Helicobacter pylori infection, which confirms this bacterium as a major environmental cause of this type of cancer. Long-standing H. pylori infection induces chronic gastritis that gradually results in atrophy and intestinal metaplasia.16,17 There is a fourfold to ninefold increased risk of gastric lesions among patients with H. pylori infection, particularly if infection began in early childhood.18–20 Chronic acid suppression also increases the risk for development of atrophy in patients with H. pylori gastritis.21
Certain aspects of H. pylori virulence have been associated with risk of gastric cancer. For instance, strains that are positive for cytotoxin-associated gene A (CagA)produce higher levels of interleukin 8, elicit more intense inflammation, and are associated with an increased risk of gastric carcinoma.22
However, gastric cancer does not develop in most individuals who are infected by H. pylori, and, conversely, as many as 20% of patients in whom gastric cancer develops are H. pylori seronegative. Therefore, other environmental and host factors are presumed to be important in the pathogenesis of this disease.23,24 Diets that are rich in salt (e.g., dried and salted fish and meats, soy sauce, smoked fish, pickled foods) and contain low levels of micronutrients, vitamins, and antioxidants14 favor intraluminal formation of genotoxic agents, such as specific N-nitroso compounds (formed by nitrosation of ingested nitrates) and have been associated with the development of gastric cancer.25–27 In contrast, diets rich in fresh vegetables, citrus fruits, and ascorbic acid are inversely associated with risk of gastric cancer.27 Bile reflux has been associated with the development of adenocarcinoma in surgical stumps.28 With regard to host factors, polymorphisms of the interleukin 1 gene have been associated with an increased risk of gastric cancer in H. pylori–infected individuals. Furthermore, the presence of a proinflammatory interleukin 1 genotype, which plays a role in hypochlorhydria and atrophy, is clearly associated with an increased risk of the intestinal type, but not the diffuse type, of gastric cancer.29
In contrast to the intestinal type of gastric cancer, the diffuse type is more common in younger individuals and is observed with equal incidence in both high- and low-risk geographic regions. Its development is more regulated by genetic factors than is intestinal-type gastric cancer.14,30 The importance of genetic factors is also underscored by the existence of familial clustering31 and by the increased incidence of atrophic gastritis in relatives of patients with gastric cancer (see Genetic Predisposition and Hereditary Tumor Syndromes).
Histologic Precursors of Gastric Cancer
In most instances, the development of gastric adenocarcinoma represents the culmination of an inflammation–metaplasia–dysplasia–carcinoma sequence, known as the Correa cascade of multistep gastric carcinogenesis.32 Mucosal atrophy and intestinal metaplasia confer a high risk for the development of gastric cancer; however, gastric epithelial dysplasia (or adenoma, if it is a polypoid lesion) represents a direct neoplastic precursor lesion.32,33 Almost all gastric epithelial dysplasias (or adenomas) have an “intestinal” phenotype (type I), resembling colonic adenomas. Another, less common, histologic variant is hyperplastic (type II) dysplasia.34 Finally, the exceedingly rare tubule neck (or globoid) dysplasia is believed to be a precursor of diffuse-type gastric carcinoma.35
Anatomic Distribution: Cardia Cancer
There has been an important epidemiologic shift in the location and frequency of gastric cancer during the past few decades, exemplified by an increase in the incidence of adenocarcinoma of the cardia region. The cause of the apparent shift in the anatomic location of gastric cancer is controversial. Because there is a lack of widespread consensus regarding the anatomic definition of the gastric cardia, it is unclear whether previously diagnosed cases of gastric cardia cancer in fact represent distal esophageal cancer involving the gastric cardia. The International Gastric Cancer Association has endorsed a classification system of gastric tumors in which type I tumors are defined as those that arise in the distal esophagus, type II as tumors in the gastric cardia, and type III as tumors in gastric mucosa distal to the cardia.36 However, this classification system does not address the criteria to define tumors from each of these anatomic areas.
The increased incidence of carcinomas of the gastric cardia observed during the past several decades has shown a geographic restriction: Studies from Scandinavia and Japan have failed to report a similar trend.4,37,38 Some investigators have suggested that the widespread use of endoscopy and improvements in diagnostic methods are responsible for these apparent changes in the distribution of gastric cancer.39
There are well-known differences between cancers of the cardia and those in the distal stomach. Patients with cardia cancer show a higher male-to-female ratio, and whites are affected more frequently than African Americans.40 There are also similarities between cardia and esophageal adenocarcinomas,41–43 such as similar risk factors, age and distribution, and morphologic phenotypes.43,44 However, obesity, high body mass index, smoking, and alcohol intake are not universally accepted as risk factors for cardia cancer, as they are for esophageal cancer.38,44–48 Similarly, the association of cardia cancer with Barrett’s esophagus and gastroesophageal reflux disease is a subject of debate.44
In some studies, cardia cancer has been significantly associated with older patient age, H. pylori infection, and intestinal metaplasia elsewhere in the stomach.49–52 In other studies, cardia cancer has been associated with reflux disease. Some researchers suggest that both etiologies play a role in the pathogenesis of cardia cancers.53 Although intestinal metaplasia has been demonstrated in adjacent mucosa in as many as 70% of carcinomas of the cardia, the actual risk of malignant transformation in patients with intestinal metaplasia has not been determined in prospective studies.54,55 Some studies have shown that progression of intestinal metaplasia to dysplasia is slower, and less frequent, than in Barrett’s esophagus.48,51 Currently, the finding of intestinal metaplasia in the gastric cardia is not an absolute indication for periodic endoscopic surveillance.
Early Gastric Cancer
Clinical and Pathologic Features
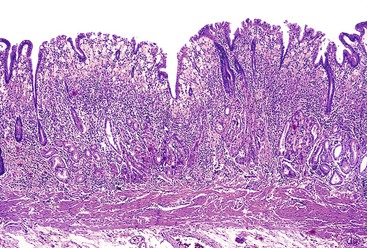
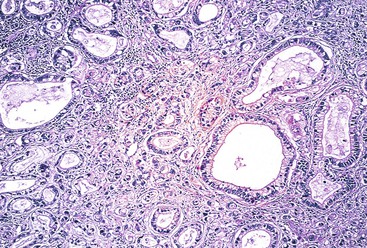
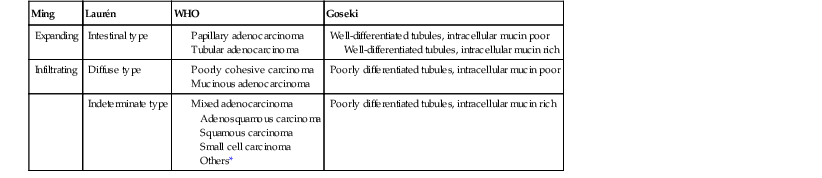
Invasive adenocarcinomas confined to the mucosa or submucosa, regardless of whether lymph node metastasis is present, are defined as EGC5 (Fig. 25.1). EGC represents an early stage in development, before invasion of the muscularis.56 Because of an increased number of upper endoscopies being performed worldwide, detection rates for this lesion are on the rise. In Western series, EGC represents 15% to 21% of all newly diagnosed gastric cancers, whereas in Japan it accounts for more than 50% of cases.6–9 A higher prevalence of gastric cancer, more liberal use of upper endoscopy and chromoendoscopy, and differences in diagnostic criteria help explain the differences between Western and Japanese studies.
Similar to dysplasia, most EGCs are diagnosed in men older than 50 years of age; this is a younger age than for advanced adenocarcinoma, reflecting the amount of time required for progression from early to advanced disease.40,57 Most patients are asymptomatic, but some complain of symptoms that mimic peptic ulcer disease.9,58 Epigastric pain and dyspepsia are the most frequently reported symptoms. They usually occur only within the last few months before diagnosis.59 Most EGCs are small, between 2 and 5 cm, and they are typically localized on the lesser curvature around the angularis region.5,60 In 3% to 13% of patients, multiple primary sites are present, and this has been shown to be associated with a worse prognosis.6,61
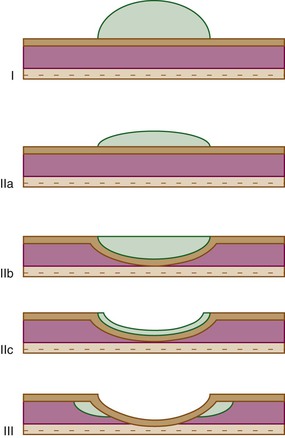
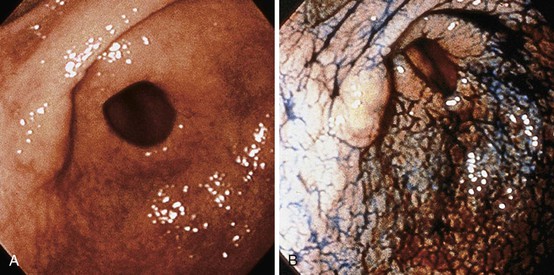
EGCs are divided into three types based on their endoscopic appearance (Fig. 25.2): protruding (type I), superficial (type II), and excavating (type III).62 Type II is further subdivided into IIa (elevated; Fig. 25.3), IIb (flat), and IIc (depressed). Superficial EGCs (type II) account for the highest proportion of cases (80%), with type IIc being most common.63 Type IIb accounts for 58% of tumors that are smaller than 5 mm.64 The endoscopic appearance of EGCs has been shown to be a good indicator of the rate of lymph node metastasis, with the lowest rates reported in type I or IIa EGCs.59
Type IIa, which is defined as a lesion that is twice as thick as normal mucosa, and type IIc, which mimics benign ulcers, are difficult to detect endoscopically, and multiple biopsies are often required for diagnosis. Subtle diagnostic signs include ease of bleeding and an irregular interface with the surrounding mucosa.65,66
Microscopic variants of EGCs have been reported. Minute EGCs measure less than 5 mm in diameter, and although most are limited to the mucosa, submucosal extension is detected in as many as 15% of cases.67,68 Superficial spreading EGCs are characterized by the presence of large, serpiginous ulcerations with neoplastic cells that spread laterally over a large area of mucosa.5
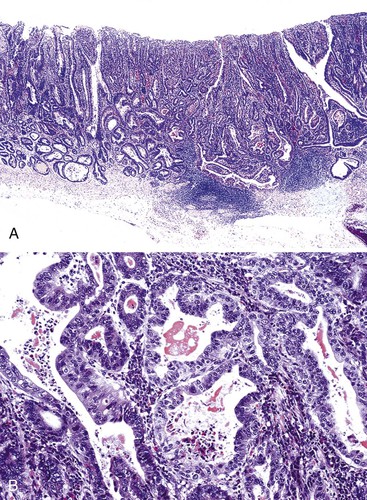
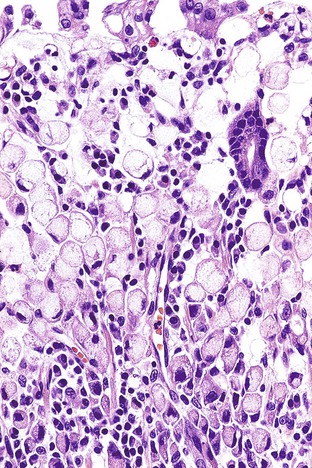
The majority of EGCs are well differentiated. Tubular and papillary variants represent 52% and 37% of cases, respectively, and may be difficult to differentiate from dysplasia because of the lack of obvious tissue invasion. Signet ring cell carcinoma (Fig. 25.4) and poorly differentiated carcinoma represent 26% and 14% of cases, respectively, and are usually depressed or ulcerated (types IIc and III).5,6,69 Diffuse-type EGCs tend to show greater depths of invasion.59
Natural History and Treatment
In a series of patients with EGC followed conservatively without surgery, 63% of EGCs progressed to advanced carcinoma during a 6- to 88-month period.70 With resection, the prognosis of EGC is excellent, with 5-year survival rates greater than 90% reported in most series.6,9,10,58,71 Size of the tumor and depth of invasion are the two major prognostic indicators. Larger tumors have a greater risk of submucosal infiltration.7,72,73 However, the risk of invasion should not be overlooked even in very small tumors. In one series, 15.5% of tumors that measured 3 to 5 mm in diameter showed invasion into the submucosa.68 Lymph node metastases have been reported in 0% to 7% of intramucosal EGCs and are associated with a 5-year survival rate of almost 100%.6,72,73 The rate of lymph node metastases for EGCs that extend into the submucosa varies between 8% and 25%, and the 5-year survival rate for these tumors is 80% to 90%.6,73
Endoscopic mucosal resection has become the treatment of choice for EGC. It usually is performed in association with endoscopic ultrasonography for staging. The primary criteria for identifying EGCs most amenable to endoscopic mucosal resection are (1) elevated lesion less than 2 cm in diameter, (2) depressed lesion less than 1 cm in diameter and without ulceration, and (3) absence of lymph node metastasis.74–76 Whether eradication of H. pylori improves prognosis is unclear. In a study of 132 patients with EGC who underwent endoscopic mucosal resection, no new cases of gastric cancer were observed after H. pylori eradication. In contrast, new early-stage intestinal-type gastric cancer developed in 13.5% of untreated patients.77
Advanced Gastric Carcinoma
Advanced adenocarcinoma is defined as a tumor that invades the gastric wall beyond the submucosa. Most patients are men (male-to-female ratio of 2 : 1) in their fifth to seventh decades of life. Clinically, symptoms include epigastric pain, dyspepsia, anemia, and weight loss. Hematemesis and symptoms of gastric outlet obstruction are not uncommon.5 Some patients, particularly younger ones, have intraabdominal dissemination at presentation. Metastatic ovarian lesions (Krukenberg tumors) composed of diffuse-type cancer cells may develop in female patients.5 Sixty-five percent of patients with gastric cancer in the United States are diagnosed at an advanced stage (beyond stage Ib).3
In North America, most gastric adenocarcinomas occur in the antrum or in the antropyloric region, and preferentially on the lesser curvature.3,78 Approximately half of all gastric adenocarcinomas measure between 2 and 6 cm, and 30% measure 6 to 10 cm in greatest dimension. Only 15% of gastric carcinomas are larger than 10 cm at the time of diagnosis.5 Multiple adenocarcinomas are detected in 5% of patients.61,79
Pathologic Features
Gross Features
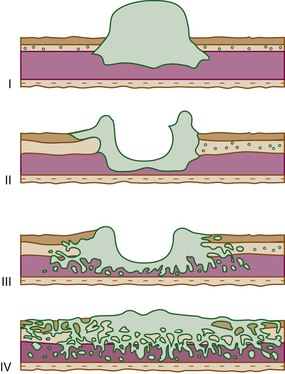
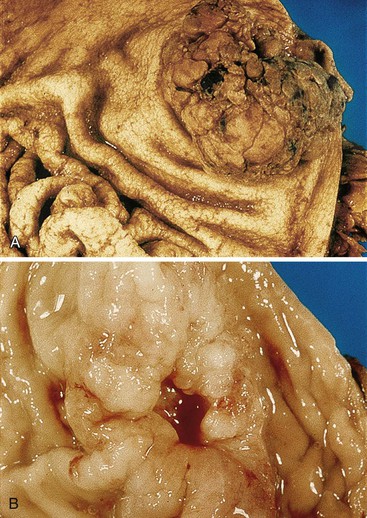
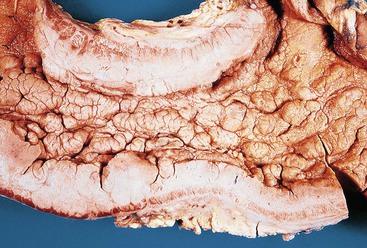
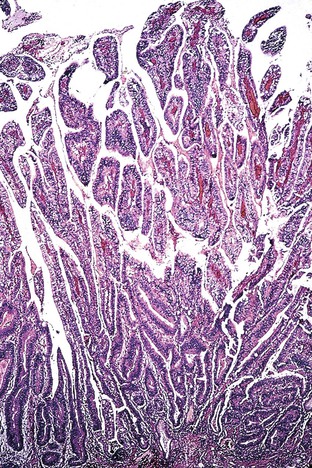
Advanced gastric carcinomas may display several different gross appearances, referred to as exophytic, ulcerated, infiltrative, and combined. The Borrmann classification remains the most widely used system. It divides gastric carcinomas into four distinct types80 (Fig. 25.5): polypoid carcinoma (type I), fungating carcinoma (type II), ulcerating carcinoma (type III), and diffusely infiltrating carcinoma (type IV) (Fig. 25.6). The latter is also referred to as linitis plastica when it involves the majority of the stomach (Fig. 25.7). Type II represents 36% of all gastric carcinomas and is frequently detected in the antrum on the lesser curvature. Types I and III each represent 25% of all advanced gastric carcinomas, and they are more common in the corpus, usually on the greater curvature.
Microscopic Features
Gastric adenocarcinomas are characterized by marked heterogeneity at both the cytologic and the architectural level, and they frequently show overlap among the four different gross patterns. Cytologically, a combination of gastric foveolar, intestinal, and endocrine cell types usually constitutes at least a portion of all tumors.81,82 Ciliated tumor cells may also be observed.83 Mucin histochemical and immunohistochemical stains (MUC1, MUC2, MUC5AC, MUC6, and CD10) may be useful in highlighting the different cellular components.81,82,84–86 In fact, on the basis of mucin immunohistochemistry, a new phenotypic classification of gastric cancer has been proposed that separates them into four phenotypes: G (gastric; MUC5AC+ and/or MUC6+; MUC2− and CD10−), I (intestinal; MUC2+ and/or CD10+; MUC5AC− and MUC6−), GI (gastric and intestinal); and N (null). Type I is more common in differentiated gastric cancers than in undifferentiated ones.87 For each histologic subtype, a shift from the gastric to the intestinal phenotype is commonly observed with tumor progression.88
Several classification systems of gastric adenocarcinoma have been proposed, most based primarily on the microscopic appearance of the tumor (Table 25.1). The three-tiered Laurén classification system is important in helping to understand the role of environmental factors and epidemiologic trends and is the system most often used by pathologists.89 This classification scheme recognizes intestinal, diffuse, and indeterminate or unclassified types. Their relative frequencies are 50% to 67%, 29% to 35%, and 3% to 21%, respectively.90
Table 25.1
Gastric Adenocarcinoma Classification Systems
| Ming | Laurén | WHO | Goseki |
| Expanding | Intestinal type |
Well-differentiated tubules, intracellular mucin rich
* Rare morphologic variants are classified under this heading.
WHO, World Health Organization.
The World Health Organization (WHO) recognizes four other major types of gastric adenocarcinoma (adenosquamous, squamous, small cell carcinoma, and other rare morphologic variants) in addition to those included in the Laurén classification. Several rare variants are also included in the WHO classification1,86 (see Table 25.1).
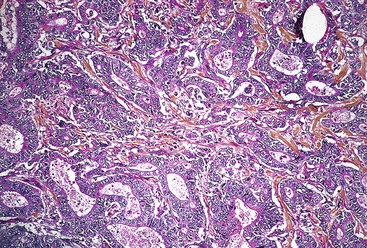
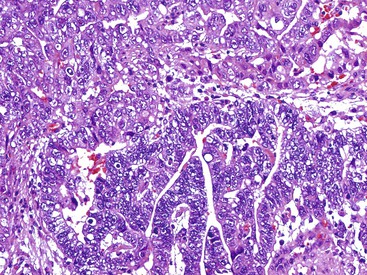
In the WHO classification, intestinal-type adenocarcinomas characteristically form glands, but with various degrees of differentiation (Fig. 25.8). They are usually diagnosed in older patients, mostly in the antrum, and are strongly linked to chronic H. pylori infection, atrophic gastritis, and intestinal metaplasia. All papillary and tubular adenocarcinomas fall into this category. These glandular carcinomas tend to form polypoid or fungating masses.86 The papillary variant is characterized by long epithelial projections scaffolded by central fibrovascular cores (Fig. 25.9). This variant accounts for 6% to 11% of all gastric carcinomas, affects older patients, occurs mainly in the proximal stomach, and is frequently associated with liver metastases.91,92 A higher rate of lymph node metastases has been reported for papillary adenocarcinoma compared with other intestinal types.93 The tubular variant is composed of distended or anastomosing, branching tubules of various sizes (Fig. 25.10). Mucin and cellular or inflammatory debris are often observed. In both the papillary and tubular variants, the cells may be columnar or cuboidal and may possess various degrees of nuclear atypia and mitoses. Combined papillotubular variants are also not uncommon.
Poorly cohesive carcinomas are composed of mostly single, or small, nests of neoplastic cells that diffusely infiltrate the gastric wall. This type is found most commonly in the gastric body and in younger patients. Although this type is also associated with H. pylori infection, the carcinogenetic sequence of the diffuse type of gastric cancer is not well characterized.94,95
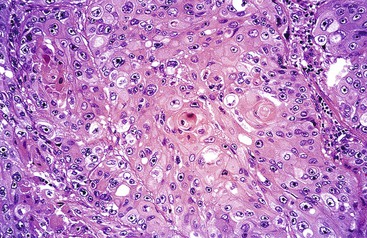
Pure signet ring cell carcinomas are included in the poorly cohesive type (Fig. 25.11). They are characterized by the presence of infiltrating single cells that contain distended cytoplasm and compressed, eccentrically displaced nuclei that form a crescent shape. Gland formation is not a normal component of this tumor; it grows in cords, tight clusters, and solid sheets.1,86 By consensus, more than 50% of the tumor should be composed of signet ring cells to warrant this designation. Other variants have also been observed, such as tumors that contain cells resembling histiocytes, deeply eosinophilic cells with neutral mucin, and anaplastic cells with little or no intracellular mucin1 (Fig. 25.12). Mitoses are typically less numerous than in the glandular type of diffuse gastric carcinoma.
Mucinous adenocarcinoma is a subtype in which pools of extracellular mucin comprise at least 50% of the tumor volume; these represent 10% of all gastric carcinomas.86 The cellular component may be formed of glands or of irregular clusters of cells that float freely in the extracellular mucin.1
Undifferentiated carcinomas lack cytologic and architectural differentiation and may resemble lymphomas, squamous cell carcinomas, or sarcomas.96 They fall into the indeterminate category of the Laurén classification. Immunohistochemical analysis (positive cytokeratin immunolabeling) is often necessary to confirm their epithelial phenotype.
From a practical standpoint, some authors recommend another three-tiered classification system based on the resemblance of the tumor to either normal gastric or metaplastic intestinal epithelium.1 In this grading system, well-differentiated adenocarcinomas are composed of well-formed glands or papillae, usually lined by mature absorptive or goblet cells. Moderately differentiated adenocarcinomas are characterized by the presence of irregularly branching glands or complex incomplete papillae. Poorly differentiated adenocarcinomas have poorly formed glands or single cells.
Other classification systems have been proposed, some of which have attempted to correlate certain pathologic features of the tumor with its prognosis. For example, Ming proposed a two-tiered classification based on the pattern of growth and degree of invasiveness of the carcinoma.97 In this system, the expanding type represents 67% of gastric cancers and reveals tumor growth by expansion of cohesive tumor masses with a well-defined tumor–stroma interface (Fig. 25.13, A). The infiltrative type of gastric carcinoma is characterized by infiltrative single cells that grow independently or are aggregated in small nests (see Fig. 25.13, B). Expanding adenocarcinomas are characteristically well-differentiated “intestinal” tumors. These correspond to the intestinal type of tumors in the Laurén classification and have a better prognosis than infiltrative carcinomas, which correspond to Laurén diffuse-type adenocarcinomas.5
Finally, Goseki and colleagues proposed a four-tiered classification based on the degree of tubular differentiation and the amount of mucin production.98 Several retrospective studies report that this system provides more accurate prognoses for advanced gastric adenocarcinomas when used with the tumor-node-metastasis (TNM) system.98,99
Morphologic Subtypes of Gastric Adenocarcinoma
Uncommon histologic variants represent approximately 5% of all gastric cancers.
Gastric Carcinoma with Lymphoid Stroma
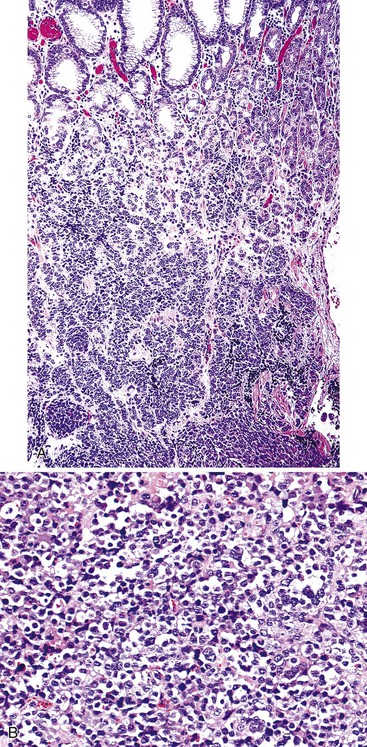
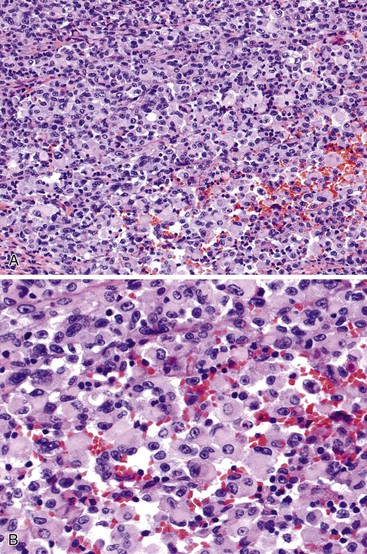
The morphologic subtype defined as gastric carcinoma with lymphoid stroma (GCLS), also known as medullary carcinoma or lymphoepithelioma-like carcinoma, is characterized by the presence of prominent lymphoid infiltration of the stroma. More than 80% of GCLS tumors are associated with Epstein-Barr virus (EBV) infection.100 When EBV-infected gastric cancers of usual histology are excluded, GCLS represents approximately 8% of all gastric carcinomas.101,102 GCLS affects men more frequently than women, particularly in the United States; Hispanics are also preferentially affected.103,104 These tumors are more common in the proximal stomach and in the remnant stomach in patients who have had a subtotal gastrectomy.105,106
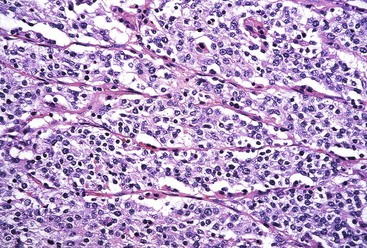
GCLS usually shows a pushing tumor border and is typically composed of irregular sheets, or syncytia, of small, polygon-shaped cells embedded within a prominent lymphocytic infiltrate, with occasional lymphoid follicles107 (Fig. 25.14). Rarely, giant cells may be observed.101 CD8+ T lymphocytes are the predominant type of inflammatory cell, although B lymphocytes and plasma cells are usually present as well. Intranuclear expression of EBV-encoded nonpolyadenylated RNA-1 can be demonstrated by in situ hybridization.
Whether EBV plays a direct role in carcinogenesis or is simply a secondary infection is a subject of debate.107 However, infection occurs early in the carcinogenetic sequence, because EBV can also be found in surrounding noninvasive (dysplastic) epithelium.108 The frequent loss of chromosomes 4p, 11p, and 18q seems to indicate a pathogenetic pathway different from that of most other usual types of gastric carcinoma.102 EBV-positive GCLS tumors have been shown to posses a CpG island methylator phenotype, with frequent aberrant methylation of multiple genes.109 The prognosis of GCLS is considered better than that of ordinary adenocarcinomas, with survival rates of approximately 77% after 5 years, although this figure is somewhat controversial.5,105,110
AFP Producing Carcinomas
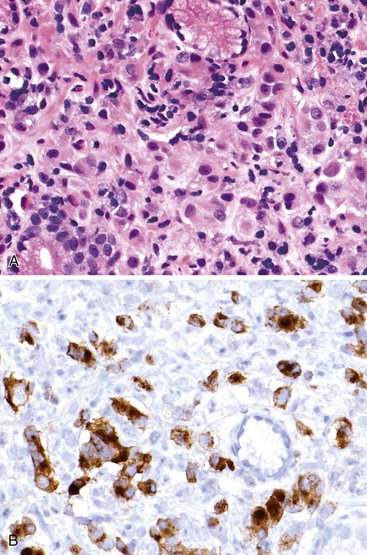
The reported incidence of hepatoid and α-fetoprotein (AFP)-producing carcinomas ranges from 1.3% to 15% of all gastric cancers.111 The recognition of two histologic types of AFP-producing tumors helps explain the wide variation in incidence previously reported. Hepatoid adenocarcinomas are composed of large, polygonal-shaped cells with prominent eosinophilic cytoplasm—features that resemble hepatocellular carcinoma.112,113 Hepatoid areas are frequently interspersed with areas of more typical adenocarcinoma. Bile and periodic acid–Schiff (PAS)-positive, diastase-resistant intracytoplasmic eosinophilic globules can be observed. The diagnosis is usually straightforward in terms of the primary tumor, but it can be more challenging when one is evaluating liver metastases. In such cases, negativity for Hep-Par 1 and positivity for cytokeratin 19 (CK19) and CK20 are considered helpful in excluding a primary hepatocellular carcinoma.114
The second type of AFP-producing gastric cancer consists of well-differentiated papillary or tubular adenocarcinomas with clear cytoplasm115 (Fig. 25.15). A combination of these two types may be seen in some cases. Immunohistochemical and in situ hybridization studies have documented albumin, AFP, α1-antichymotrypsin, and bile production within tumor cells.86
A high level of AFP can also be detected in the serum of affected patients. This subtype of gastric carcinoma is particularly aggressive, showing a 5-year survival rate of only 12%.86 Whether these tumors are CDX2 positive is currently unknown.
Adenosquamous and Squamous Cell Carcinoma
Adenosquamous carcinoma, which accounts for 0.5% of all gastric cancers, is defined as a tumor in which the neoplastic squamous component comprises at least 25% of the tumor volume.8,116 These tumors are usually deeply penetrating and associated with lymphovascular invasion, and they carry a relatively poor prognosis.117 However, a few cases limited to only the mucosa and submucosa, as well as some exhibiting a positive response to aggressive chemotherapy, have been reported.69,118
Pure squamous cell carcinomas represent from 0.04% to 0.09% of all gastric carcinomas and affect men four times more often than women.86,119,120 The degree of differentiation varies, from moderately differentiated with keratin pearl formation to poorly differentiated (Fig. 25.16). The pathogenesis of this tumor is unknown. The squamous component may arise from squamous metaplasia of adenocarcinoma cells, from a focus of heterotopic squamous epithelium, or from multipotential stem cells that show bidirectional differentiation.69,119 A case of adenosquamous carcinoma with EBV infection has been reported.121 For tumors of the cardia region, caudal extension of a primary esophageal squamous cell carcinoma should be excluded. Gastric squamous cell carcinomas are often diagnosed at a late stage, and their prognosis is generally poor despite response to chemotherapy.69,117,119,122
Choriocarcinoma
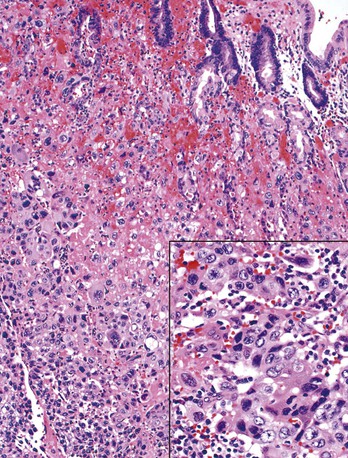
Pure gastric choriocarcinomas are rare. Most cases demonstrate a combination of syncytiotrophoblast and cytotrophoblast elements within an otherwise variably differentiated adenocarcinoma123 (Fig. 25.17). Yolk sac and hepatoid carcinoma components may be seen as well.124 However, two cases of pure gastric yolk sac tumor have been reported.125 Gastric choriocarcinomas are usually exophytic and characterized by prominent necrosis and hemorrhage at both the macroscopic and the microscopic level.126,127 Human chorionic gonadotropin can be detected by immunohistochemistry, and serum levels of this hormone can also be used as a marker of prognosis.86 Secondary endocrine effects of β-human chorionic gonadotropin secretion have been reported.128 The commonly accepted pathogenetic explanation is that these tumors represent choriocarcinomatous differentiation or transformation of a typical adenocarcinoma.129 Hematogenous and lymphatic dissemination with metastases is common; affected patients have a poor prognosis.
Carcinosarcoma
Gastric carcinosarcomas are rare tumors composed of varying amounts of adenocarcinomatous and sarcomatous elements. Sarcomatous elements may consist of uncommitted cells or may demonstrate light microscopic or immunohistochemical features of chondrosarcoma, osteosarcoma, rhabdomyosarcoma, or leiomyosarcoma differentiation.60,130–132 Tumors with adenosquamous and neuroendocrine components have also been reported.133–135 Most gastric carcinosarcomas are large, polypoid tumors and are associated with a poor outcome.136 A single case of gastric adenosarcoma composed of benign tubular and cystic glands embedded in an otherwise typical leiomyosarcoma stroma has been reported.137
Small Cell Carcinoma
Approximately 100 cases of small cell carcinoma (oat cell carcinoma or neuroendocrine carcinoma) have been reported in the stomach.138 Because these tumors are frequently diagnosed at an advanced stage, the prognosis is quite poor, with most patients dying within 1 year after diagnosis.139,140 However, long-term survival has been observed with aggressive adjuvant therapy.141 The morphology of these tumors is reminiscent of their pulmonary counterpart, showing a sheetlike infiltrative growth pattern, frequent rosette-like arrangements and basal palisading of nuclei, molding of nuclei, and lack of nucleoli (Fig. 25.18). Immunohistochemically, these tumors are usually negative for carcinoembryonic antigen (CEA)142 but are often positive for neuron-specific enolase (NSE), synaptophysin, and chromogranin A. Electron microscopy can demonstrate the characteristic neurosecretory granules in these tumors.96,143
Parietal Cell Carcinoma and Oncocytic Carcinoma
Rare examples of parietal cell carcinoma have been reported. These tumors are composed of solid sheets of polygonal cells with abundant, finely granular, eosinophilic cytoplasm that stains with phosphotungstic acid–hematoxylin (PTAH). Immunohistochemically, the tumor cells are positive for parietal cell–specific antibodies to H+,K+-adenosine triphosphatase and human milk fat globule 2. Ultrastructural evaluation reveals abundant mitochondria and intracellular canaliculi.144–148 It has been suggested that parietal cell carcinomas have a better prognosis than usual gastric adenocarcinomas.144 Ten cases of oncocytic adenocarcinoma, which is morphologically similar to parietal cell carcinoma but negative for anti–parietal cell antibodies, have also been reported.149
Paneth Cell Carcinomas
Very few cases of gastric mucoepidermoid or Paneth cell carcinoma have been reported. Mucoepidermoid carcinomas show a mixture of mucus-producing and squamous epithelium.150,151 One case was shown to arise from submucosal ectopic glands.151 The prognosis is reportedly poor. Paneth cell carcinomas are characterized by tumors with a predominance of cells with Paneth cell differentiation, characteristically showing eosinophilic cytoplasmic granules that are positive for lysozyme by immunohistochemistry.146,147
Malignant Rhabdoid Tumor
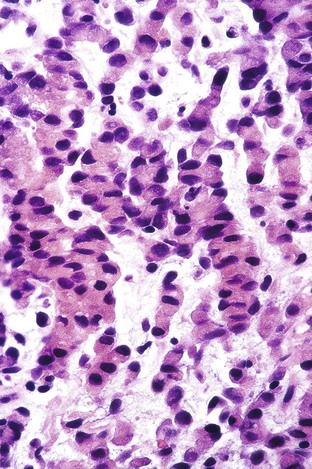
Gastric malignant rhabdoid tumors represent approximately 0.1% to 0.2% of all gastric carcinomas. They characteristically show areas of strong immunoreactivity for vimentin.152,153 Histologically, the poorly cohesive tumor cells are round to polygonal in shape, with eosinophilic or clear cytoplasm and large nuclei with prominent nucleoli (Fig. 25.19). These rhabdoid-appearing cells can also show cytokeratin, epithelial membrane antigen, and focal NSE positivity, but they are negative for CEA.153,154 The prognosis of these tumors is dismal.
Metastatic Carcinomas
Metastases to the stomach are uncommon. They are reported in fewer than 5.4% of individuals in autopsy series of patients with cancer.155,156 They may be completely asymptomatic,157 or they may manifest clinically as a large bleeding ulcer mimicking a primary carcinoma (39% of cases) or as a submucosal tumor (51% of cases).156 Between 65% and 80% of gastric metastases diagnosed endoscopically are solitary lesions. In some studies, there is a predilection for involvement of the middle and proximal stomach.155,156 Malignant melanoma and lung and breast carcinomas are the most commonly reported primaries.156,158,159 However, primary tumors originating from the kidney, pancreas, esophagus, skin, testis, cervix, and colon have been reported as well.155,156 In 50% of cases, concomitant metastases are observed in other organs.160
In patients with metastasis, infiltration of the deep layers of the gastric wall, combined with a reactive hyperplastic appearance of the overlying mucosa, may mimic benign hypertrophic gastritis. Metastatic lobular breast carcinoma deserves special attention because its typical single-file growth pattern can resemble diffuse-type carcinoma, signet ring cell carcinoma, or linitis plastica. In this circumstance (Fig. 25.20), an immunohistochemical profile consisting of gross cystic disease fluid protein 15 (GCDFP-15), estrogen and progesterone receptors, and CK7 (all positive in breast carcinoma) and CK20 (negative in breast carcinoma) have proved useful in establishing a correct diagnosis.161–163 More recently, negativity for CDX2, MUC2, MUC5AC, MUC6, and DAS1 (negative in breast cancer and variably positive in gastric cancer) has been useful in diagnosing metastatic breast carcinoma.161,164,165
Gastric Carcinoma in Special Clinical Circumstances
Gastric Stump Carcinoma
Gastric surgery is associated with an increased risk for the development of gastric cancer, usually after a 15- to 25-year period.166,167 Male patients who have undergone a Billroth II subtotal gastrectomy have a 3.3-fold higher incidence than the general population. Most carcinomas are diagnosed in the distal residual stomach and preferentially involve the gastric stoma.166,168 However, dysplasia may be observed elsewhere in the residual pouch in as many as 33% of affected patients.168 Although stump carcinomas are similar to usual gastric adenocarcinomas with regard to morphology, they probably develop through a different carcinogenetic process. Animal models suggest that enterogastric reflux of bile and pancreatic secretions may play an important role in the pathogenesis of these tumors.169,170 Histologic lesions of the remnant stomach that predate the development of cancer include intestinal metaplasia, atrophy, foveolar hyperplasia, cystic dilation of the glands, and dysplasia.171,172 However, the extent of intestinal metaplasia and the incidence of H. pylori infection in uninvolved mucosa are significantly less than in usual gastric adenocarcinomas.168EBV infection, in association with marked chronic inflammation, has been observed in gastric remnant adenocarcinomas.173 The pattern of lymph node metastases differs from that of primary gastric cancer, showing an increased risk of hematogenous and liver metastases,174 perhaps because of changes in lymphatic or vascular flow resulting from the patient’s original Billroth surgery.
Gastric Carcinoma in Young Patients
Between 2% and 10% of all gastric carcinomas are diagnosed in patients younger than 40 years of age.175,176 The presenting symptoms are usually similar to those in older patients. An equal sex distribution, or even a female predominance, has been reported in young patients with gastric carcinoma.153,175 Most cases are of the diffuse type and are not associated with gastric atrophy and intestinal metaplasia. However, H. pylori infection (particularly with CagA-positive strains) is thought to be a risk factor.176,177 Approximately 10% to 25% of young patients with gastric cancer have a positive family history, suggesting that genetic factors are of etiologic importance. A different genomic profile has been demonstrated in younger versus older patients with gastric cancer. In the former group, alterations of chromosomal regions 11q23.3 and 19p13.3 were common, which likely reflects different pathogenetic mechanisms than those in older patients.178
Genetic Predisposition and Hereditary Tumor Syndromes
Only a minority of gastric cancers (1% to 3%) are attributable to high-penetrance familial syndromes.179 However, a number of families with hereditary diffuse gastric cancer carry unknown genetic mutations.
Familial Diffuse Gastric Carcinoma
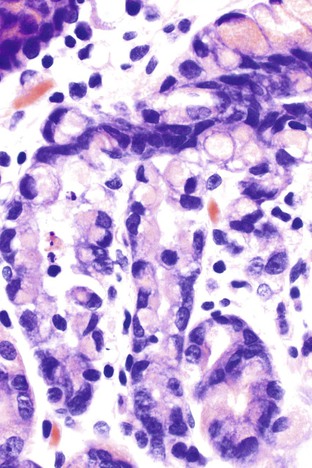
Germline mutations in the E-cadherin gene (CDH1) represent the molecular basis for a particular type of familial gastric cancer syndrome.180–183 Approximately 50 families have been reported to date.184 In addition to high risk for the development of diffuse gastric carcinoma, an increased risk of lobular breast carcinoma has been reported in affected women.183 The penetrance of the gene varies from 70% to 80%, and the average age at diagnosis of cancer is 37 years.185 The lifetime risk of gastric cancer is approximately 67% in men and 83% in women.186 Genetic counseling and testing for E-cadherin germline mutations are recommended for patients who have a positive family history, and in such cases prophylactic gastrectomy, which is associated with prolonged survival, should be strongly considered.187 In these instances, surgical specimens may detect in situ signet ring cell carcinoma, representing pagetoid spread of signet ring cells insinuated between the base of foveolar cells and the basement membrane187 (Fig. 25.21).
Hereditary Nonpolyposis Colorectal Cancer Syndrome
Gastric carcinoma, usually of the intestinal type, is frequently seen in patients with the hereditary nonpolyposis colorectal cancer syndrome, accounting for 5% to 11% of all carcinomas. The lifetime risk is 10% for patients of Western ancestry and as much as 30% for patients of Asian ancestry.188–191 A microsatellite instability (MSI) phenotype is noted in 65% of these cases.
Familial Adenomatous Polyposis Coli
Patients with familial adenomatous polyposis coli (FAP) frequently develop multiple gastric polyps, most commonly fundic gland polyps, which can undergo neoplastic changes as a result of somatic mutations of the APC gene.192 However, the development of carcinoma in these polyps is rare.193–196 Multiple adenomas, which also arise in patients with FAP at a young age, are far less common. There is a significantly higher risk of neoplastic transformation in the stomach of Asian patients with FAP compared with Western patients.197
Li-Fraumeni Syndrome
Germline mutations of the TP53 (formerly p53) gene are present in 50% to 70% of patients with Li-Fraumeni syndrome. The most common neoplasms are sarcoma, breast cancer, and brain tumors. GI tract tumors are relatively infrequent, accounting for fewer than 10% of all neoplasms. Among patients with GI tumors, however, gastric carcinomas (which may be multiple) are more frequent than colon cancer and represent more than 50% of GI cases.198,199
Peutz-Jeghers Syndrome
Characteristic hamartomatous polyps develop in patients with Peutz-Jeghers syndrome. These patients have an apparent increased risk of gastric cancer, although the exact degree of risk is a subject of debate.75,200 Mutation of the serine/threonine–protein kinase 11 (STK11) gene, located on chromosome 19p13.3, is responsible for this syndrome.201
Gastric Hyperplastic Polyposis
Gastric hyperplastic polyposis is an inherited autosomal dominant syndrome characterized by the presence of hyperplastic gastric polyposis, severe psoriasis, and a high incidence of gastric cancer of the diffuse type.202,203 Gastric hyperplastic polyposis has been associated with colorectal adenocarcinoma, possibly as a result of secondary hypergastrinemia.204
Natural History and Prognosis of Gastric Adenocarcinoma
Gastric adenocarcinomas can spread by direct extension, metastasis, or peritoneal dissemination. Well-differentiated tumors with an intestinal phenotype preferentially disseminate hematogenously and show a high rate of hepatic metastasis. Diffuse carcinomas are more likely to spread to the peritoneum (peritoneal seeding).205,206 Carcinomas that exhibit both intestinal and diffuse components possess the metastatic capabilities of each of these tumors and, as a result, have a worse prognosis.207
The anatomic stage (with special reference to extension to the serosa and lymph nodes) remains the strongest independent prognostic indicator in patients with gastric cancer (Table 25.2). For example, 5-year survival rates for carcinomas that extend into the muscularis propria are 60% to 80%, but they decrease to 50% in cases with serosal involvement.208 Thorough gross and microscopic examination and, ultimately, accurate staging are important in the pathologic examination of gastrectomy specimens. The “N” classification of gastric carcinomas was revised in the late 1990s to recommend examination of a minimum of 15 lymph nodes for appropriate pathologic staging.209–211 However, in 2007, the National Cancer Database reported that only one third of patients had appropriate staging in U.S. hospitals.3
Table 25.2
American Joint Committee on Cancer TNM Staging of Gastric Carcinomas
| PrimaryTumor (T) | Stage Grouping | ||||
| TX | Primary tumor cannot be assessed | Stage 0 | Tis | N0 | M0 |
| T0 | No evidence of primary tumor | Stage IA | T1 | N1 | M0 |
| Tis | Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria (i.e., high grade dysplasia) | Stage IB | T2 | N0 | M0 |
| T1 | Tumor invades lamina propria (T1a), muscularis mucosae (T1a), or submucosa (T1b) | T1 | N1 | M0 | |
| T2 | Tumor invades muscularis propria | Stage IIA | T3 | N0 | M0 |
| T3 | Tumor penetrates submucosal serosa without invasion of visceral peritoneum or adjacent structures*†‡ | T2 | N1 | M0 | |
| T4 | Tumor invades serosa (visceral peritoneum) (T4a) or adjacent structures (T4b)†‡ | T1 | N2 | M0 | |
| Regional Lymph Nodes (N) | Stage IIB | T4a | N0 | M0 | |
| NX | Regional lymph node(s) cannot be assessed | T3 | N1 | M0 | |
| N0 | No regional lymph node metastasis§ | T2 | N2 | M0 | |
| N1 | Metastasis in 1 to 2 regional lymph nodes | T1 | N3 | M0 | |
| N2 | Metastasis in 3 to 6 regional lymph nodes | Stage IIIA | T4a | N1 | M0 |
| N3 | Metastasis in 7 or more regional lymph nodes | T3 | N2 | M0 | |
| Distant Metastasis (M) | T2 | N3 | M0 | ||
| M0 | No distant metastasis | Stage IIIB | T4b | N0 | M0 |
| M1 | Distant metastasis | T4b | N1 | M0 | |
| T4a | N2 | M0 | |||
| T3 | N3 | M0 | |||
| Stage IIIC | T4b | N2 | M0 | ||
| T4b | N3 | M0 | |||
| T4a | N3 | M0 | |||
| Stage IV | Any T | Any N | M1 | ||
* A tumor may penetrate the muscularis propria with extension into the gastrocolic or gastrohepatic ligaments, or into the greater or lesser omentum, without perforation of the visceral peritoneum covering these structures. In this case, the tumor is classified T2. If there is perforation of the visceral peritoneum covering the gastric ligaments or the omentum, the tumor should be classified T3.
† The adjacent structures of the stomach include the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum.
‡ Intramural extension to the duodenum or esophagus is classified by the depth of the greatest invasion in any of these sites, including the stomach.
§ A designation of positive N0 (pN0) should be used if all examined lymph nodes are negative, regardless of the total number removed and examined.
TNM, Tumor-node-metastasis classification system.
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Sixth Edition (2002) published by Springer Science and Business Media LLC, www.springerlink.com.
The prognosis of advanced gastric adenocarcinoma is poor, particularly in the West. The overall survival rate at 1 year after diagnosis is 63%, and the 5-year survival rate ranges from 26% to 35% after curative resection.212,213 The 10-year relative survival rate is approximately 10%. However, in Japan, the overall 5-year survival rate for patients with advanced gastric cancer who are surgically treated with an extended (D2) dissection is greater than 50%.214,215 Whether distal adenocarcinomas have a better prognosis compared with proximal carcinomas is a matter of debate.78,216,217 Saito and colleagues reported a 5-year survival rate of 61.6% for patients with carcinoma of the cardia, compared with 82.6% for those with carcinoma of the lower third of the stomach.218 In another series, however, the prognoses were equally grim, with survival of 28% and 29%, respectively.216 Aside from location, the depth of invasion is critical; for example, survival at 5 years for T1, T2, and T3 distal carcinomas is 76%, 53%, and 26%, respectively. However, lymph node status is the single best indicator of prognosis: For positive N1 (pN1) tumors, the 5-year survival rate is 40%, dropping to 31% for pN2 tumors and 11% for pN3.217,219 A survival advantage has been associated with female sex and Japanese ethnicity.78 In addition, variation in tumor location, higher frequency of early-stage carcinoma, more accurate staging, and surgical expertise, may help explain improved patient survival rates in Japanese compared with Western medical centers.3,217 After curative resection, recurrence is locoregional in 40% and systemic in 60% of cases.220 The former includes the surgical resection margins, the resection bed, and regional lymph nodes.221 The predominant sites of systemic recurrence are the liver and peritoneum.222
Complete surgical resection with adjacent lymph node dissection represents the best chance for long-term survival. Distal tumors are usually adequately treated by subtotal gastrectomy, whereas proximal tumors can be approached with either a total gastrectomy or a proximal subtotal gastrectomy. Many surgeons consider linitis plastica to be a contraindication to potentially curative resection. Another controversial area in the surgical management of gastric cancer is the optimal extent of lymph node dissection. Japanese surgeons routinely perform an extended lymphadenectomy, a practice that may account for better survival rates in Asian compared with Western series.217 D1 lymphadenectomy refers to a limited dissection of the perigastric lymph nodes, whereas a D2 lymphadenectomy involves removal of lymph nodes along the hepatic, left gastric, celiac, and splenic arteries, as well as those in the splenic hilum. A D3 dissection includes lymph nodes within the porta hepatis and periaortic regions. Despite increased morbidity and mortality, arguments in favor of an extended lymphadenectomy include the fact that a larger number of lymph nodes results in more accurate pathologic staging and that failure to remove these lymph nodes leaves tumor behind in as many as one third of patients.223,224 Operative success and perioperative mortality rates are also highly dependent on the surgeon’s experience.225
Molecular Pathology of Gastric Cancer
Gastric adenocarcinoma develops as a result of a combination of predisposing environmental conditions (H. pylori infection) and genetic and epigenetic abnormalities, changes that affect oncogenes, tumor suppressor genes, and DNA mismatch repair genes. The genetic alterations result in deregulation of cellular proliferation, adhesion, differentiation, signal transduction, telomerase activity, and DNA repair. All of these play an important role in the development and progression of gastric cancer.226–230 Although most of the genetic alterations that have been reported are observed in both intestinal and diffuse gastric cancers, it has become apparent that these two tumor types result from different genetic pathways (Table 25.3).
Table 25.3
Frequency of Genetic Alterations in Gastric Carcinomas
| Genes and Alterations | Intestinal (Well Differentiated) | Diffuse (Poorly Differentiated) |
| Telomerase activity | +++ | +++ |
| CD44 (abnormal transcript) | +++ | +++ |
| TGFA (overexpression) | ++ | ++ |
| DNA repair error | ++ | ++ |
| TP53 (p53; LOH, mutation) | ++ | ++ |
| CTNNB1 (β-catenin; mutation) | + | ++ |
| CDKNA2 (p16; reduced expression) | ++ | + |
| MET (c-Met; amplification) | + | ++ |
| VEGF (overexpression) | ++ | + |
| EGF (overexpression) | ++ | + |
| EGFR (overexpression) | ++ | + |
| APC (LOH, mutation) | ++ | + |
| DCC (LOH) | ++ | − |
| BCL2 (LOH) | ++ | − |
| FGFR2 (K-sam; amplification) | − | ++ |
| CDH1 (E-cadherin; mutation) | − | ++ |
| KRAS (K-ras; mutation) | + | + |
| Cyclin E (amplification) | + | + |
| ERBB2 (c-erbB-2; amplification) | + | − |
LOH, Loss of heterozygosity.
Tumor Suppressor Genes
Numerous tumor suppressor genes have been implicated in gastric carcinogenesis, including TP53, CDKNA2 (formerly p16), APC, and TGFB. Mutation or loss of heterozygosity of the TP53 locus has been reported in up to 40% of all gastric cancers.231 CDKNA2, an important cell cycle regulator, is also lost in many cancers, particularly those that originate from the cardia.232 Although mutations of the APC gene are present in 20% to 76% of gastric adenomas and flat dysplasia, their importance in the development of gastric cancer is less clear compared with colorectal cancer. One report demonstrated an inverse relationship between the presence of APC gene mutations and the development of adenocarcinoma.233 Mutation of the transforming growth factor-β (TGF-β) type 2 receptor, which leads to loss of growth inhibition, is also an important feature of gastric carcinogenesis.234
Oncogenes
The MET (formerly c-Met) protooncogene is frequently overexpressed in gastric cancer, and it is seen in both the diffuse and intestinal types.235,236 Alternatively, epidermal growth factor receptor genes such as ERBB2 (formerly c-erbB-2) are preferentially expressed in the intestinal type of gastric cancer. Conversely, FGFR2 (formerly K-sam, of the family of fibroblast growth factor receptors) is frequently overexpressed in diffuse-type gastric cancer but not in intestinal-type cancers.235,237 KRAS (formerly K-ras) oncogene mutations, which are common in many other types of GI malignancies, occur only very rarely in gastric cancer.
As in colorectal cancer, genetic alterations of the DNA mismatch repair pathway play a role in gastric carcinogenesis. These tumors display MSI. Wu and associates demonstrated that a subset of sporadic MSI-high gastric cancers showed a distinct clinicopathologic and genetic profile compared with MSI-low or microsatellite-stable gastric cancers.238 However, somatic mutations of DNA mismatch repair genes, such as MLH1 or MSH2, are extremely rare in sporadic gastric cancer.
In addition to the genetic alterations discussed previously, other molecules have been shown to play a role in gastric cancer. Among these, cyclooxygenase-2 is frequently upregulated in gastric adenocarcinoma, possibly induced by H. pylori infection.239 Several members of the Bcl-2 family of proteins (Bax and Bcl-2, among others) have also been implicated in gastric carcinogenesis by promoting resistance to apoptosis.95,240
Finally, with regard to the specific role of H. pylori, bacterial factors such as CagA, a pathogenicity island that includes approximately 20 genes, and the vacuolating cytotoxin (encoded by the vacA gene), play important modulating roles in the development of complications after H. pylori infection, including the development of gastric cancer.241,242