Chapter 51D Vascular Diseases of the Nervous System
Stroke in Children
Epidemiology
Full-Term and Near-Term Neonates
Neonates appear to be at higher risk for stroke than older children. Asymptomatic subdural hemorrhage affects almost half of term neonates and can occur in infants delivered by both vaginal and cesarean delivery (Rooks et al., 2008). Symptomatic intracranial hemorrhage affects 1 in 100 full-term neonates (Gradnitzer et al., 2002). Most estimates place the rate of arterial ischemic stroke around 1 in 4000 neonates (Lynch and Nelson, 2001), but Gunther and colleagues (2000) found a much lower rate of 1.35 per 100,000 full-term births. Laugesaar et al. (2007) looked at all prospectively and retrospectively diagnosed perinatal stroke in Estonia and found a rate of 1 in 1587 live births. DeVeber and colleagues (2001) found a rate of 0.67 cases of cerebral venous thrombosis (CVT) per 100,000 children per year, with neonates making up 43% of cases; rates were not described in relation to the number of term births.
Chapter 80 discusses cerebral vascular injury in the premature neonate.
The General Population of Children
Estimates of the incidence of all pediatric stroke in the United States and France have ranged from 2.6 to 13 cases per 100,000 children per year (Giroud et al., 1995), with some variation among studies on the inclusion of neonates, traumatic strokes, and meningitis, and whether to use 16 or 18 as the age cutoff for pediatric stroke. Disagreement exists about whether hemorrhagic or ischemic strokes predominate. Estimates of the rate of hemorrhagic stroke ranged between 1.5 and 5 per 100,000 children per year (Giroud et al., 1995), and estimates of the rates of ischemic stroke ranged between 1.2 and 8 per 100,000 children per year (Agrawal et al., 2009; deVeber, 2002; Giroud et al., 1995). A Saudi Arabian study estimated the hospital frequency rate of all pediatric strokes as 27.1 per 100,000 pediatric admissions (Salih et al., 2006a).
High-Risk Subgroups
Vascular malformations may present with intracranial hemorrhage. At the Hospital for Sick Children in Toronto, 80% of children diagnosed with arteriovenous malformations (AVMs) presented with spontaneous intracranial hemorrhage (Humphreys et al., 1996), but it is not clear what percentage of children with AVMs never have hemorrhage or other neurological signs, since neuroimaging is generally prompted by neurological symptoms. Cavernous malformations and aneurysms may be genetic and can present in childhood. Risk varies depending on the mutation involved (Kato et al., 2001; Labauge et al., 2000, 2001). Intracranial hemorrhage may lead to vasospasm and resultant ischemic stroke, but this is less common in children than adults (Menkes et al., 2000).
Children with bleeding disorders are at high risk for intracerebral hemorrhage. Estimates of the rate of intracranial hemorrhage in children with hemophilia are up to 10% (Revel-Vilk et al., 2004); approximately 3% of neonates with hemophilia experience intracranial hemorrhage (Tarantino et al., 2007). Some 4% of patients with hemophilia have intracranial bleeds, and 48% of these bleeds are intracerebral (Klinge et al., 1999).
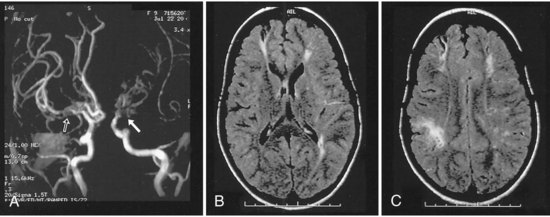
Children with sickle cell anemia are at risk for ischemic stroke because sickling red blood cells may lead to thrombosis or endothelial injury and in some patients are associated with moyamoya syndrome (Pegelow, 2001; Pegelow et al., 2002) (Fig. 51D.1). The rate of stroke in children with sickle cell anemia has dropped since the institution of transfusion therapy (see Therapies, later). In California, the incidence of first stroke in children with sickle cell anemia dropped from 0.88 per 100 person-years to 0.17 per 100 person-years (Fullerton et al., 2004); however, 17% have clinically silent infarcts (Pegelow, 2001). Kwiatkowski et al. (2009) found that 28% of asymptomatic young children (<6 years old) with sickle cell disease had silent infarcts on magnetic resonance imaging (MRI). Children with sickle cell anemia may also develop aneurysms and resultant intracranial hemorrhage, but this is more common in adults with sickle cell anemia (Pegelow, 2001).
Children treated with mechanical circulatory support are at increased risk for both intracranial hemorrhage and embolic ischemic stroke. The rates of infarction after extracorporeal membrane oxygenation (ECMO) vary dramatically among series, ranging from 0% to 26%; one study examined the brains of 44 patients who died while on ECMO and found evidence of focal ischemic infarct in 50% and intracranial hemorrhage in 52%. A recent large study of ECMO survivors found cerebral infarction in 7% and intracranial hemorrhage in 7% (Barrett et al., 2009). Stroke occurred in 7 of 17 treated children (41%) at one center that started to use the Berlin Heart EXCOR ventricular assist device (Rockett et al., 2008).
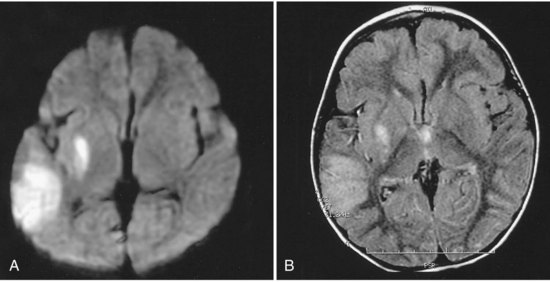
Children with complex congenital heart disease are at risk for cardioembolic stroke, thrombotic stroke, watershed infarcts from drops in perfusion pressure, and cerebral venous thrombosis (CVT) (Fig. 51D.2). The rates of stroke in children with complex congenital heart disease also vary among series, with some of the variation due to the severity of the malformation, the number of corrective surgeries required, anesthetic techniques during surgery, patient selection, and length of follow-up. A large Canadian study looked at 5526 children who underwent cardiac surgery and found a stroke rate of 5 per 1000 children (Domi et al., 2008). Mayer and associates (2002) found evidence of cerebrovascular complications in 5 of 77 (6.5%) pediatric patients with heart transplants and an average of 59.2 months of follow-up; 25 of the 77 patients (32.5%) died. The greatest risk for children with congenital heart disease occurs at the time of surgery or cardiac catheterization.
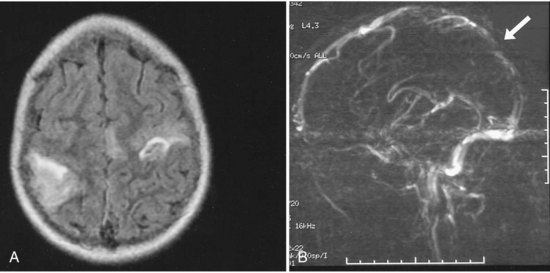
Children with cancer are at risk for both intracranial hemorrhage and ischemic infarction. Intracranial hemorrhage occurs secondary to thrombocytopenia from bone marrow suppression or bleeding within the tumors. Children with cancer may develop ischemic infarction or CVT due to leukostasis in the setting of leukemia; complications of chemotherapy such as l-asparaginase, which cause decreases in antithrombin, fibrinogen, and plasminogen; fungal or bacterial meningitis leading to arteritis; vasculopathy secondary to radiation; or complications of intracranial surgery (Fig. 51D.3). Bowers and colleagues (2002) observed 807 pediatric brain tumor patients for a total of 3224 non-perioperative years and found that 1.6% had a non-perioperative stroke. Children receiving mantle radiation for Hodgkin lymphoma may develop strokes years after treatment, possibly due to radiation-induced vascular changes (Bowers et al., 2005). The Children’s Oncology Group reviewed the literature and found that children surviving central nervous system tumors, Hodgkin lymphoma, or acute lymphoblastic leukemia treated with radiation to the head and/or neck were at increased risk of stroke from childhood into adulthood (Morris et al., 2009).
Presentations
Seizures are the most common manifestation of intraventricular hemorrhages (IVH), arterial ischemic strokes, and CVT in term neonates. Seizures are a presenting sign for 65% of term neonates with IVH, at least 80% of term neonates with arterial ischemic stroke (Volpe, 2001), and over 50% of neonates with CVT (Fitzgerald et al., 2006). Other presenting signs include apnea, irritability, jitteriness, lethargy, and bulging fontanel. The immature central nervous system may not demonstrate focal signs, and hemiparesis may not be apparent until a child is older than 6 months of age.
Children older than 6 months of age may present with seizures or focal signs similar to those seen in adult stroke, with hemiparesis, ataxia, or aphasia. Children younger than 1 year of age are more likely to present with seizures and altered mental status, whereas children older than 1 year of age are more likely to present with focal motor signs (Zimmer et al., 2007). Although severe headaches such as those in intracranial hemorrhages often prompt parents to seek medical attention immediately, most children arrive at the emergency room more than 6 hours after the event (Gabis et al., 2002). Parents may not detect focal motor weakness in a young child who has not yet started to walk or aphasia in a young child who is just starting to speak. Parents may interpret the sudden onset of focal neurological signs as behavioral rather than neurological. It can be easier to detect focal neurological signs and symptoms in older children, but these are also often missed in the first minutes to hours, possibly because many parents and children do not realize children can have strokes. Some children may have no symptoms or only gradual onset of developmental delay. While silent strokes are recognizable in the sickle cell population, and several studies have screened for them, the rates of silent infarction in other cerebrovascular disorders are unclear because radiological investigations are lacking in the absence of clinical manifestations.
Etiology
Cardiac
Complex congenital heart disease may lead to thrombosis and ischemic stroke through several mechanisms. Abnormal cardiac anatomy or associated cardiac arrhythmias lead to abnormal flow and may predispose to the formation of intracardiac thrombi. Septal defects may lead to right-to-left shunts that allow venous thrombi to cross to the arterial side and cause cerebral infarction. Surgery and cardiac catheterization can disrupt the endothelium and lead to thrombosis. Cardiac surgery itself may lead to a temporary prothrombotic state (Petaja et al., 1996). An abnormal heart valve can serve as a nidus for bacterial or fungal vegetations that may cause cardioembolic stroke. Chronic hypoxemia in severe cases of congenital heart disease may lead to polycythemia, and the increased blood viscosity may promote thrombosis. Patent foramen ovale is associated with arterial infarction in both children and adults because of presumed right-to-left shunting.
Hematological
Any hematological disorder that disrupts coagulation can place a child at risk for hemorrhagic stroke. Newborns have lower levels of coagulation factors and have a drop in the vitamin K–dependent factors in the first days of life. Bleeding due to vitamin K deficiency was more common before intramuscular or oral administration to neonates became widespread; Cornelissen and colleagues (1996) found that administering vitamin K lowered the incidence of vitamin K deficiency bleeding from 7 to 1.1 per 100,000 births per year. However, late-onset vitamin K deficiency bleeding can occur in children with undiagnosed cholestatic jaundice who received oral vitamin K and are exclusively breastfed, because they cannot absorb the vitamin, and breast milk is low in vitamin K (Ijland et al., 2008). Accidental ingestion or overdose of warfarin has the same effect as a vitamin K deficiency and may occur when a young child finds an older family member’s medications. The most common congenital coagulation factor deficiencies are deficiencies of coagulation factor VIII (hemophilia A), coagulation factor IX (hemophilia B), and von Willebrand factor. Intracranial hemorrhage is the most common cause of death from bleeding in patients with hemophilia.
A deficiency or imbalance of factors involved in regulating coagulation may place the child at risk for thrombosis. Pediatric ischemic stroke has been associated with deficiencies of protein C, protein S, and antithrombin; activated protein C resistance due to the factor V Leiden mutation; the prothrombin gene 20210A mutation; the methylene tetrahydrofolate reductase (MTHFR) gene variants; the ATG haplotype of the protein Z gene (Nowak-Gottl et al., 2009); elevated lipoprotein (a); elevated antiphospholipid antibodies (Kenet et al., 2010) and lupus anticoagulant; with elevated factor VIII levels; and with low plasminogen or high fibrinogen. The importance of the plasminogen activator inhibitor promoter polymorphism (PAI-1) in childhood stroke is controversial. Temporary hematological abnormalities and resultant thrombosis may result from intercurrent illness; for example, idiopathic nephrotic syndrome is associated with decreased antithrombin levels and CVT and/or arterial thromboembolic events (Fluss et al., 2006).
Several authors have noted the presence of multiple prothrombotic abnormalities in some children with ischemic stroke (Kenet et al., 2010). The combination of MTHFR and endothelial nitric oxide synthase gene variants may raise stroke risk more than MTHFR alone (Djordjevic et al., 2009). Children with congenital heart disease or leukemia may be at higher risk for developing thrombotic complications during hospitalization if they also have a prothrombotic abnormality.
Trauma
Trauma can injure vessels directly, leading to hemorrhage from torn vessels and thrombosis in damaged intima. Subdural hemorrhages, subarachnoid hemorrhages, and ischemic infarctions occur in head injury. Ascertaining the cause of the trauma is important; 95% of serious intracranial injuries in children younger than 1 year of age are due to abuse, and 5% of children returned to abusive parents without any intervention are killed (Johnson, 2000). Most subdural hemorrhages in infants are due to abuse (Matschke et al., 2009). In older children and adults, low admission Glasgow Coma Scale score, low systolic blood pressure, brain herniation and requirement for decompressive craniotomy are all predictors of posttraumatic cerebral infarction (Tian, 2008). Bony abnormalities of the vertebrae or abnormalities of vessel walls due to collagen-vascular disease or metabolic disease may predispose to cervicocephalic arterial dissection after mild trauma. Arterial dissection may be idiopathic in otherwise apparently normal children (Rafay et al., 2006). A prothrombotic state associated with trauma can promote thrombosis and worsen outcome; trauma patients who go into disseminated intravascular coagulation have worse outcomes than those who do not. Trauma is the most common precipitant of hemorrhages in children with hemophilia.
Infection
The consequences of bacterial meningitis are disseminated intravascular coagulopathy and vascular inflammation, and subsequent arterial or venous thrombosis and infarction. Group B streptococcal meningitis is an important cause of stroke in neonates and transmitted vertically from the mother or horizontally by nursery staff. During the first 2 months of life, infants are susceptible to bacteria found in maternal flora or in the local environment, including group B Streptococcus, gram-negative enteric bacilli, and Listeria monocytogenes. After 2 months of age, Streptococcus pneumoniae and Neisseria meningitides are the most common causes of bacterial meningitis. The institution of Haemophilus influenzae type b vaccination at 2 months of age has led to a dramatic drop in H. influenzae-b meningitis. In immunosuppressed patients such as those with cancer or acquired immune deficiency syndrome (AIDS), Aspergillus species may lead to vasculitis and infarction. Patients with AIDS may also develop arteriopathy of medium and small vessels or aneurysms, and although the presumed cause for most cases is direct or secondary infection, the exact pathophysiology is not always clear. Antiretroviral therapy for AIDS may itself cause vascular injury (Mondal et al., 2004). Tuberculosis leads to meningitis in 1% to 2% of cases, which may cause vasculitis and infarction (Starke, 1999). Several cases of stroke occurred in children with neurobrucellosis (Salih et al., 2006c). Lyme disease is a rare cause of infectious vasculitis and aneurysm formation. Varicella-zoster virus (VZV) may cause vasculitis by direct infection of the arterial wall or by a postinfectious inflammatory reaction that manifests weeks to months after the primary infection. In some cases, arteriopathy is associated with recent upper respiratory infection (Amlie-Lefond et al., 2009a).
Vascular Malformations/Vasculopathy/Migraine
As discussed previously, vascular malformations may present with intracerebral hemorrhage, and resulting vasospasm may lead to ischemic infarction. Arterial abnormalities such as large-artery stenosis are common in otherwise healthy children with arterial ischemic stroke (Ganesan et al., 2003). At least 10% of hemorrhagic strokes and most subarachnoid hemorrhages in children are caused by ruptured aneurysms (Jordan et al., 2009).
Primary angiitis of the central nervous system may occur in children and can be fatal if not treated with aggressive immune suppression. There are more frequently reported cases of less virulent vasculopathies in children, often occurring after varicella infection, which respond to aspirin alone and do not require immune suppression (Chabrier et al., 1998; Lanthier et al., 2001). Stroke can result from vasculopathy outside the brain. Childhood stroke can be a first manifestation of Takayasu arteritis, an inflammatory large-vessel vasculitis that affects the aorta and its branches (Brunner, 2008). A growing body of literature exists examining the role of inflammatory factors in stroke in children and adults.
Metabolic
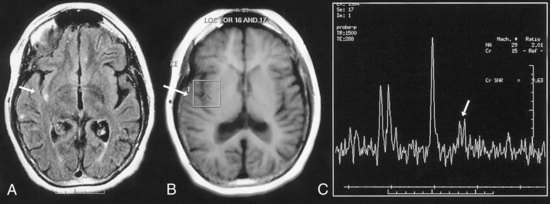
The mitochondrial diseases may lead to metabolic strokes, particularly during times of metabolic stress. MRI can demonstrate infarction in non-vascular territories (Fig. 51D.4). However, in rare cases, mitochondrial mutations have been associated with moyamoya vasculopathy (Longo et al., 2008; Papavasiliou et al., 2007).
Other metabolic diseases lead to cerebral infarction by contributing to thrombosis from arterial damage. Homocystinuria may lead to infarction, presumably through elevated homocysteine levels and subsequent vascular injury. The C677T MTHFR gene variant may be associated with childhood stroke, but it is not clear how large a role it plays; at least 10% of healthy children are homozygous for the MTHFR C677T (Gunther et al., 2000; Koch et al., 1999; Nowak-Gottl et al., 1999). Studies vary on the degree of associated risk, and carriers do not always have elevated homocysteine levels at the time of infarction. Fabry disease is an X-linked lysosomal storage disease that causes a deficiency of α-galactosidase and resultant accumulation of glycolipids in the endothelial wall. Male patients experience paresthesias of the hands and feet and cardiac abnormalities that can begin in childhood; hypohidrosis and renal dysfunction tend to occur later in the course of the disease (Ries et al., 2005). Both male and female heterozygotes are susceptible to cerebral thrombosis, possibly because of an increase in vasoreactivity in damaged vessels or endothelial and leukocyte activation. Males may be more severely affected but rarely show cerebrovascular involvement before age 23 (Schiffmann, 2001). α1-Antitrypsin deficiency may lead to decreased structural integrity of the arterial wall by disrupting the balance of activity between proteases and antiproteases. α1-Antitrypsin deficiency has been associated with aneurysms and with vascular changes consistent with fibromuscular dysplasia. The hyperlipidemias can cause atherosclerotic vascular changes in children similar to those in older adults.
Gender
Both arterial ischemic stroke and sinovenous thrombosis are more common in boys (Golomb et al., 2009). Higher testosterone levels have been associated with increased risk of stroke in boys (Normann et al., 2009). Gender may influence not just risk of stroke but recovery from stroke. A study of mortality from childhood stroke in England and Wales from 1921 to 2000 found that boys had a higher mortality rate (Mallick et al., 2009).
Differential Diagnosis
Children with stroke often present with seizures, and in the first few hours after a seizure, before cranial imaging, it can be difficult to determine whether a new hemiparesis is due to a temporary postictal Todd paresis or to infarction. Todd paresis usually does not last more than 24 hours, though in rare cases it may persist several days. Migraine may lead to infarction and permanent motor impairment, but hemiplegic migraine may lead to temporary motor impairment. A strong family history of hemiplegic migraine or documentation of a missense mutation of the CACNA1A calcium channel gene on chromosome 19p13 may help differentiation (Terwindt et al., 2002), but variation exists in mutations among families (see Chapter 69). Alternating hemiplegia of childhood is a progressive neurodegenerative condition. The origin is generally unclear, although cerebrovascular factors and mitochondrial disease may be contributory. Neuroimaging studies usually do not demonstrate pathology. Edema, bleeding, or shifting of brain tumor may cause sudden onset of neurological signs. Encephalitis or meningoencephalitis may lead to sudden onset of focal neurological symptoms. Several metabolic diseases including glutaric aciduria and carbohydrate-deficient glycoprotein syndrome (see Chapter 62) can present with stroke-like episodes, and serum and urine testing together with MRI help make the diagnosis. Acute disseminated encephalomyelitis (ADEM), multiple sclerosis, vasculitis, and the vasculitic form of Hashimoto encephalopathy (Nolte et al., 2000) are all causes of sudden onset of focal or multifocal neurological symptoms, and all may cause multiple T2 bright lesions on MRI.
Evaluation
History and Physical
Physical examination should include examination of the face for signs of dysmorphic features suggestive of a genetic syndrome. Always assess head circumference. Early stroke may lead to macrocephaly due to hydrocephalus or to microcephaly due to tissue loss and poor brain growth from infarction. Examination of the skin should document signs of bruising or petechiae, livedo reticularis suggestive of Sneddon syndrome, systemic lupus erythematosus or other autoimmune disorders, café-au-lait spots suggestive of neurofibromatosis type 1, hypopigmented macules suggestive of tuberous sclerosis complex, excess skin laxity suggestive of a collagen disorder such as Ehlers-Danlos syndrome, cyanosis suggestive of heart failure and anoxia, and pallor suggestive of anemia. The head and neck should be auscultated for vascular bruits and the heart for murmurs and arrhythmias; peripheral pulses should be compared, and the abdomen should be auscultated for renal bruits suggestive of a systemic vascular disorder. Neurological examination should look for signs of focal abnormality, with the caveat that signs may not localize well in the very young child or may be difficult to assess in the frightened or uncooperative child (Table 51D.1).
| Finding | Possible Significance/Suggestive of |
|---|---|
| HEAD CIRCUMFERENCE | |
| Macrocephaly | Hydrocephalus caused by IVH, SDH, SAH, or vascular malformation |
| Microcephaly | Failure of brain growth resulting from stroke or genetic disorder |
| EYES | |
| External/Iris | |
| Epicanthal folds, Brushfield spots | Down syndrome |
| Horner syndrome | Carotid dissection (postganglionic)/vertebral dissection (central) |
| Pulsating exophthalmos | Carotid-cavernous fistula |
| Lens subluxation | Marfan syndrome, homocystinuria |
| Angioid streaks | Pseudoxanthoma elasticum |
| Xanthelasma on lids, corneal arcus | Hyperlipidemia |
| Corneal opacity | Fabry disease |
| Retina | |
| Papilledema | Increased ICP resulting from hydrocephalus, vascular malformation, or acute brain edema |
| Hemorrhages | Trauma (consider child abuse), bleeding diathesis, ruptured aneurysm, collagen disease, emboli |
| Vasculopathy | Systemic vasculitis |
| Angioid streaks | Pseudoxanthoma elasticum, Paget disease, sickle cell anemia |
| Angioma | Familial cavernous angiomatosis, von Hippel-Lindau disease |
| SKIN | |
| Bruising | Bleeding diathesis (consider child abuse) |
| Petechiae | Platelet count low or dysfunction; DIC |
| Purpura | Henoch-Schönlein purpura |
| Pallor | Anemia |
| Erythema | Polycythemia |
| Cyanosis | Complex congenital heart disease, other cause of hypoxia |
| Skin necrosis | Meningococcemia |
| Café-au-lait spots | Neurofibromatosis type 1 |
| Hypopigmented macules, shagreen patches, facial angiofibromas | Tuberous sclerosis complex |
| Yellow papules | Pseudoxanthoma elasticum |
| Premature aging | Progeria |
| Malar rash | Systemic lupus erythematosus |
| Skin laxity | Ehlers-Danlos syndrome type IV |
| Telangiectasias | Osler-Weber-Rendu disease (hereditary hemorrhagic telangiectasia) |
| Oral/genital ulcers | Behçet disease |
| Angiokeratomas | Fabry disease |
| Lentigines in non-sun-exposed areas | Predisposition to dissection or to atrial myxoma, which may lead to cardioembolic stroke |
| Discoloration of fingers with cold: white, blue, then red | Raynaud phenomenon as sign of collagen disease or systemic lupus erythematosus |
| Subcutaneous nodules on elbows, forehead, tendons | Rheumatic fever, systemic lupus erythematosus, rheumatoid arthritis |
| Erythematous macules that evolve to clear, fluid-filled vesicles | Acute varicella (chicken pox); shingles if in a dermatomal distribution |
| Multiple round, white, puckered scars | Past varicella infection |
| Needle tracks | IV drug addiction with risk for endocarditis and HIV |
| MOUTH | |
| High, arched palate | Marfan syndrome |
| Petechial hemorrhages | Infective endocarditis |
| HEART AND PERIPHERAL PULSES | |
| Murmur | Complex congenital heart disease, valve abnormality |
| Decreased pulses | Takayasu arteritis |
| Increased pulses | Hypertension |
| ABDOMEN | |
| Hepatomegaly | Infection, cancer, liver failure |
| Bruit | Renal artery stenosis |
| BACK | |
| Scoliosis, vertebral anomalies | Possible increased risk of dissection |
| HANDS | |
| Long, tapering fingers | Marfan syndrome |
| Other anomalies of bones of hands | May be seen in association with congenital heart disease |
| Clubbing of fingers | Congenital heart disease |
| JOINTS | |
| Painful, restricted movement | Arthritis due to autoimmune disease, past bleeds (hemophilia) |
| Warm | Autoimmune disease, infection |
| OVERALL SIZE | |
| Tall | Marfan syndrome, homocystinuria |
| Short | Progeria, mitochondrial disease, dwarfism (risk of vertebral anomalies) |
DIC, Disseminated intravascular coagulation; HIV, human immunodeficiency virus; ICP, increased intracranial pressure; IV, intravenous; IVH, intraventricular hemorrhage; SAH, subarachnoid hemorrhage; SDH, subdural hematoma.
Physical findings taken from Hurst, J.W., 1993. Cardiovascular Diagnosis: The Initial Examination. Mosby-Year Book, St. Louis. Information on lentigines from Neau, J.P., Rosolacci, T., Pin, J.C., et al., 1993. Cerebral infarction, cardiac myxoma and lentiginosis. Rev Neurol (Paris) 149, 289-291; and Schievink, W.I., Michels, V.V., Mokri, B., et al., 1995. Brief report: a familial syndrome of arterial dissections with lentiginosis. N Engl J Med. 332, 576-579.
Imaging Studies
Ultrasound (see Chapter 33B) is useful in assessing IVH or periventricular leukomalacia in the premature infant or carotid flow in any child, but its sensitivity for detecting arterial stroke in the neonate is probably below 50% (Golomb et al., 2001). Power Doppler ultrasound may be useful in detecting CVT in the neonate. Transcranial Doppler ultrasound is used to screen children with sickle cell anemia, and higher blood flow velocities are associated with higher stroke risk. Studies have suggested that this screening should begin in infancy (Telfer et al., 2007).
Cranial computed tomography (CT) (see Chapter 33A) is better at assessing hemorrhage in the older child and ischemic stroke in the neonate and older child, but it may not detect arterial stroke until 24 hours or more after the event. CT angiography and venography (see Chapter 33B) can image the cerebral vasculature.
MRI is the imaging tool of choice for most types of childhood stroke, and diffusion-weighted imaging (DWI) can detect brain ischemia within hours (see Chapter 33A). DWI stays bright for approximately 2 weeks in the older child or adult but may “normalize” (pseudonormalization) within days in the neonate (Mader et al., 2002). Stroke imaging at any age should include DWI, T2, and fluid-attenuated inversion recovery (FLAIR) to detect areas of early infarction. Magnetic resonance angiography (MRA) may be helpful in diagnosing vasculitis and dissection involving larger vessels.
Magnetic resonance spectroscopy (MRS) and single-photon emission CT (SPECT) demonstrate changes in regional blood flow and may be helpful in assessing patients with moyamoya disease and other sources of vasculopathy. MRS provides the earliest detection of ischemic lesions. Conventional angiography can clarify the structure of vascular malformations and is the most accurate method for detecting vasculitis and dissection, but angiography may not always be possible in the acutely ill child. Use all forms of neuroimaging in combination with other laboratory and physical findings, because no one technique has perfect sensitivity or specificity for detecting vasculopathy. Continuous arterial spin labeling MRI has been used in research settings to study regional cerebral blood flow in persons with sickle cell anemia (Van den Tweel et al., 2009). Other newer forms of imaging such as functional MRI and diffusion-weighted tensor imaging are used in research settings and may provide insights into stroke evolution and recovery but are not widely available.
Coagulation Workup
The basic evaluation for prothrombotic disorders may include prothrombin time (PT) with international normalized ratio (INR) and activated partial thromboplastin time (APTT) and a complete blood cell count, including platelets, protein C, protein S and antithrombin levels, activated protein C resistance, plasminogen, fibrinogen, homocysteine, antiphospholipid antibody screen, lipoprotein (a), and a lipid panel. Testing for protein Z may become part of the evaluation in the future if other studies confirm its role in childhood stroke. Genetic testing may include screening for the factor V Leiden mutation, the prothrombin 20210A gene, and the MTHFR gene variants. Some mutations are very common, and the ratio of symptomatic to nonsymptomatic carriers may be very high in some populations; more than 40% of healthy children in a European study were either homozygous or heterozygous for the C677T MTHFR variant (Koch et al., 1999). The frequency of different prothrombotic risk factors varies among populations (Salih et al., 2006b). Unfortunately, identifying asymptomatic carriers of prothrombotic genes might adversely affect later health, life, and disability insurance status (Golomb et al., 2005).
Other Studies
Flexion and extension radiographs of the cervical spine may identify bony abnormalities predisposing to dissection. Electroencephalogram (EEG) may help localize the lesion in children who present with seizures and is helpful for evaluating cerebral function in the unresponsive and possibly locked-in patient with a brainstem stroke (see Chapter 5). Visual evoked potentials and brainstem auditory evoked potentials may be particularly helpful in evaluating the very young or somnolent patient (see Chapter 32A).
Serum and plasma studies may help identify the cause of the stroke. Thrombocytopenia, deficiencies of factors I, VII, VIII, IX, and XIII, and deficiency of von Willebrand factor are all risk factors for intracranial hemorrhage, whereas polycythemia, thrombocythemia, and anemia are all risk factors for ischemic stroke. Abnormalities in platelet count and fibrinogen may be markers for disseminated intravascular coagulation, which may lead to thrombosis and ischemic stroke. Elevated serum lactate is a marker for mitochondrial disease, but not all patients with mitochondrial disease have elevated serum lactate; genetic tests may be required to confirm the diagnosis. Muscle biopsy may help confirm mitochondrial disease (see Chapter 63). Serum studies for plasma α-galactosidase will identify Fabry disease. Serum ammonia, amino acids, and organic acids may help identify metabolic disease such as hyperhomocysteinemia and mitochondrial diseases that can lead to stroke (Menkes, 2000). Skin biopsy is often necessary to confirm the diagnosis of collagen vascular disease (Kato et al., 2001; Roach and Riela, 1995).
Treatment
The Acute Period and Initiating Chronic Therapy
Children with intracranial hemorrhage or hemorrhagic stroke require close observation in the first hours. Children with hemophilia need immediate factor replacement and may require blood transfusion. Children with large hematomas with significant mass effect may need surgery (Johnson, 2000). Any child presenting with unexplained or poorly explained intracranial hemorrhage should also be evaluated for other sites of injury and possible child abuse.
Between 2000 and 2003, fewer than 2% of children with ischemic stroke received thrombolytic therapy (Janjua et al., 2007). Some of these children were not treated in accordance with guidelines used for adults (Amlie-Lefond, 2009b; Roach et al., 2008). Few children present within the originally mandated 3 hours of infarct onset (Kwiatkowski et al., 1999). The benefit of thrombolytic therapy in children is not clear. No evidence-based guidelines are currently available on using intravenous or intraarterial thrombolytics in children. It may not be helpful in neonates, who have lower levels of plasminogen than older children (Andrew et al., 2000).
No clear guidelines are available on the use of heparin in pediatric arterial stroke and CVT patients either. Pilot studies and case series have described good results with heparin and low-molecular-weight heparin (deVeber et al., 1998; Massicotte et al., 1996). Large-scale studies with catheter-related noncranial thrombosis have shown good results (Andrew et al., 2000). Moharir et al. (2009) showed that neonates and children with sinovenous thrombosis who are not anticoagulated are at increased risk for thrombus propagation, which can be asymptomatic and lead to poor outcome. Other low-molecular-weight heparinoids such as danaparoid may be alternatives for patients with heparin-induced thrombocytopenia (Neuhaus et al., 2000; Ranze et al., 1999; Risch et al., 2006).
In sickle cell anemia, exchange transfusion is the usual treatment for acute stroke (Menkes et al., 2000). The Stroke Prevention in Sickle Cell Trial (STOP) demonstrated that in children with vasculopathy shown by transcranial Doppler ultrasound screening, regular transfusions to keep the sickle hemoglobin concentration at less than 30% reduced the risk of recurrent stroke by 90% (Adams, 2000). However, chronic transfusions carry the risk of infection and cause increases in serum ferritin (Files et al., 2002). Some children require treatment with deferoxamine chelation to prevent iron overload (Wayne et al., 2000). Stopping exchange transfusion therapy leads to increased vascular disease and stroke risk (Adams and Brambilla, 2005).
Attempts at treating and preventing metabolic stroke and cerebrovascular disease have been made by addressing the pathological defects in metabolic pathways, using coenzyme Q10 and other antioxidant vitamins (see Chapter 66). l-Arginine improves endothelial dysfunction in children with MELAS (mitochondrial encephalopathy, lactic acidosis, and strokes) and may aid in treating the stroke-like episodes (Koga et al., 2006). Folate, vitamin B6, and vitamin B12 all play a role in homocysteine metabolism, which is impaired in homocystinuria and in carriers of the MTHFR C677T gene variant. Several different enzyme abnormalities may lead to homocystinuria, and the enzyme affected determines whether supplementation with folate, vitamin B6, or vitamin B12 is helpful (Menkes, 2000). Dietary supplementation with folate lowers serum homocysteine levels in adults with the MTHFR C677T gene variant (Thuillier et al., 1998) and may be helpful in children with it. Enzyme replacement with α-galactosidase decreases the symptoms of Fabry disease and normalizes cerebrovascular reactivity but does not remove the risk of recurrent stroke (Schiffmann et al., 2006).
Patients with protein C deficiency have been treated with protein C concentrate (Ettingshausen et al., 1999). Good results are reported when using antithrombin concentrate to treat consumptive coagulopathy and antithrombin deficiency at the time of acute stroke, but it does not appear to prevent thrombosis in leukemia patients treated with l-asparaginase (Hongo et al., 2002).
Additional Issues in Chronic Therapy
Patients with genetic chronic thrombotic abnormalities such as protein C or S deficiency, or with antiphospholipid antibody syndrome, may require long-term care with low-molecular-weight heparin or warfarin (Andrew et al., 2000). For many patients, however, the best long-term therapy is not clear. Low-molecular-weight heparin may be used for 3 to 6 months after cervicocephalic arterial dissection or CVT (Andrew and deVeber, 1999), with an Insuflon patch to make administration tolerable. Warfarin or aspirin are more often used for long-term therapy (Andrew et al., 2000). Families of children on warfarin need to be counseled about regulating vitamin K levels in the diet, bony changes, possible teratogenicity, and the risks of under- or overtreatment. Families of children taking aspirin are generally concerned about Reye syndrome, but there are no reports in children taking aspirin for stroke prophylaxis (Roach, 2000). Strater and colleagues (2001) did a prospective follow-up study of 135 children with first onset of ischemic stroke. The children received prophylactic treatment with either low-dose low-molecular-weight heparin or aspirin and were followed for a median of 36 months. Low-dose low-molecular-weight heparin was not superior to aspirin in preventing stroke recurrence. For children with sickle cell anemia, chronic treatment with hydroxyurea can increase levels of fetal hemoglobin and decrease complications of sickle cell anemia, but careful monitoring is required for cytotoxicity and genotoxicity (Khayat et al., 2006). Bone marrow transplantation with matched donors may be an option for some children (Vermylen, 2003). Surgery or interventional procedures play a role in treating some types of cerebrovascular disease. Surgical procedures such as encephalodural synangiosis or arterial bypass may be used to treat children with moyamoya disease. Other surgical procedures such as clipping, coiling, radioablation, or removal may be useful in the treatment of aneurysms or malformations.
Socioeconomic status and social stressors may play a role in family compliance with therapy and patient outcome. In a Texas study of children with sickle cell anemia, families with private insurance were more likely to be compliant with Doppler ultrasound screenings than families on Medicaid (Raphael et al., 2008).
Other Issues
The risks that accompany pregnancy in adolescence are greater for young women with a history of hemorrhagic or ischemic stroke (see Chapter 81).
Oral contraceptives carry a very small risk for the general population but may carry more risk for women with prothrombotic disorders (Glueck et al., 2001). Oral contraceptive use raises the risk of both arterial thromboembolism and CVT. These issues require explicit discussion with patients and their families, preferably before adolescence, as some oral contraceptives are used to treat acne or menstrual disorders in young adolescent women.
Summary
Children with stroke are at risk for future cognitive impairment, motor impairment, and epilepsy. In full-term neonates, it is difficult to predict outcome based on initial cranial imaging, and it is unclear whether the side on which the infarcts occur affects language development. Evidence of degeneration of corticospinal tracts and resulting asymmetry in the midbrain on MRI performed during the second 6 months of life does predict hemiplegia. About half of children with neonatal arterial ischemic stroke have feeding problems; when severe, feeding dysfunction can lead to aspiration and failure to thrive (Barkat-Masih et al., 2009). Children who present with signs of perinatal stroke after the perinatal period appear to have worse motor outcomes than children with perinatal stroke who present in the neonatal period (Lee et al., 2005). For these children, young age at presentation, fever at presentation, and right middle cerebral artery infarction have been associated with poor outcome (Cnossen et al., 2010). Bilateral hemispheric lesions and low muscle tone of affected limbs at presentation have also been associated with poor outcome (Kim et al., 2009). Studies in both infants and older children show that larger and multiple infarcts are more likely to cause cognitive and motor impairment as well as epilepsy. Overall, the intelligence of children with stroke falls within the normal range, but some require extra help in school.
The prognosis for children with progressive cerebrovascular diseases is particularly problematic. Those children with severe forms of sickle cell or moyamoya disease who are not treated or who do not respond to therapy may develop significant cognitive and motor disabilities and epilepsy (Manceau et al., 1997; Miller et al., 2000; Ohene-Frempong et al., 1998). More than 20% of children who survive childhood arterial ischemic stroke may have recurrent transitory ischemic attacks or stroke (Ganesan et al., 2002). Prothrombotic disorders and abnormalities on vascular imaging raise the risk of recurrence (Strater et al., 2002).
Adams R.J. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol. 2000;15:344-349.
Adams R.J., Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769-2778.
Agrawal N., Johnston S.C., Wu Y.W., et al. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40(11):3415-3421.
Amlie-Lefond C., Bernard T.J., Sebire G., et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119(10):1417-1423.
Amlie-Lefond C., deVeber G., Chan A.K., et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8(6):530-536.
Andrew M., deVeber G. Pediatric Thromboembolism and Stroke Protocols. Hamilton, Ontario, Canada: B. C. Decker; 1999.
Andrew M., Monagle P., Brooker L. Thromboembolic Complications During Infancy and Childhood. London: B.C. Decker; 2000.
Barkat-Masih M., Saha C., Hamby D.K., et al. Feeding problems in children with neonatal arterial ischemic stroke. J Child Neurol. 2009. Published on-line Oct. 12
Barrett C.S., Bratton S.L., Salvin J.W., et al. Thiagarajan RR. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10(4):445-451.
Bowers D.C., McNeil D.E., Liu Y., et al. Stroke as a late treatment effect of Hodgkin’s disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6508-6515.
Bowers D.C., Mulne A.F., Reisch J.S., et al. Nonperioperative strokes in children with central nervous system tumors. Cancer. 2002;94:1094-1101.
Brunner J., Armstrong D., Feldman B.M., et al. Childhood stroke as the presentation of Takayasu’s arteritis: diagnostic delay can cause catastrophic complications. J Rheumatol. 2008;35(6):1228-1230.
Chabrier S., Rodesch G., Lasjaunias P., et al. Transient cerebral arteriopathy: a disorder recognized by serial angiograms in children with stroke. J Child Neurol. 1998;13:27-32.
Cnossen M.H., Aarsen F.K., Akker S., et al. Paediatric arterial ischaemic stroke: functional outcome and risk factors. Dev Med Child Neurol. 2010;52(4):394-399.
Cornelissen E.A., Hirasing R.A., Monnens L.A. [Prevalence of hemorrhages due to vitamin K deficiency in The Netherlands, 1992-1994]. Ned Tijdschr Geneeskd. 1996;140:935-937.
deVeber G. Stroke and the child’s brain: an overview of epidemiology, syndromes and risk factors. Curr Opin Neurol. 2002;15:133-138.
deVeber G., Andrew M., Adams C., et al. Cerebral sinovenous thrombosis in children. New Engl J Med. 2001;345:417-423.
deVeber G., Chan A., Monagle P., et al. Anticoagulation therapy in pediatric patients with sinovenous thrombosis: a cohort study. Arch Neurol. 1998;55:1533-1537.
Djordjevic V., Stankovic M., Brankovic-Sreckovic V., et al. Genetic risk factors for arterial ischemic stroke in children: a possible MTHFR and eNOS gene-gene interplay? Child Neurol. 2009;24(7):823-827.
Domi T., Edgell D.S., McCrindle B.W., et al. Frequency, predictors, and neurologic outcomes of vaso-occlusive strokes associated with cardiac surgery in children. Pediatrics. 2008;122(6):1292-1298.
Ettingshausen C.E., Veldmann A., Beeg T., et al. Replacement therapy with protein C concentrate in infants and adolescents with meningococcal sepsis and purpura fulminans. Semin Thromb Hemost. 1999;25:537-541.
Files B., Brambilla D., Kutlar A., et al. Longitudinal changes in ferritin during chronic transfusion: a report from the Stroke Prevention Trial in Sickle Cell Anemia (STOP). J Pediatr Hematol Oncol. 2002;24:284-290.
Fitzgerald K.C., Williams L.S., Garg B.P., et al. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006;63:405-409.
Fluss J., Geary D., deVeber G. Cerebral sinovenous thrombosis and idiopathic nephrotic syndrome in childhood: report of four new cases and review of the literature. Eur J Pediatr. 2006;165:709-716.
Fullerton H.J., Adams R.J., Zhao S., et al. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004;104:336-339.
Gabis L.V., Yangala R., Lenn N.J. Time lag to diagnosis of stroke in children. Pediatrics. 2002;110:924-928.
Ganesan V., Chong W.K., Cox T.C., et al. Posterior circulation stroke in childhood: risk factors and recurrence. Neurology. 2002;59:1552-1556.
Ganesan V., Prengler M., McShane M.A., et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167-173.
Giroud M., Lemesle M., Gouyon J.B., et al. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343-1348.
Glueck C.J., Fontaine R.N., Wang P. Interaction of heritable and estrogen-induced thrombophilia: possible etiologies for ischemic optic neuropathy and ischemic stroke. Thromb Haemost. 2001;85:256-259.
Golomb M.R., Dick P.T., MacGregor D.L., et al. Cranial ultrasound has a low sensitivity for detecting acute infarct in term neonates (abstract). Ann Neurol. 2001;50:S120.
Golomb M.R., Fullerton H.J., Nowak-Gottl U., et al. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40(1):52-57.
Golomb M.R., Garg B.P., Walsh L.E., et al. Perinatal stroke in baby, prothrombotic gene in mom: does this affect maternal health insurance? Neurology. 2005;65:13-16.
Gradnitzer E., Urlesberger B., Maurer U., et al. [Cerebral hemorrhage in term newborn infants—an analysis of 10 years (1989-1999)]. Wien Med Wochenschr. 2002;152:9-13.
Gunther G., Junker R., Strater R., et al. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors, [erratum appears in Stroke 2001 32, 279]. Stroke. 2000;31:2437-2441.
Hongo T., Okada S., Ohzeki Z., et al. Low plasma levels of hemostatic proteins during the induction phase in children with acute lymphoblastic leukemia: a retrospective study by the JACLS. Japan Association of Childhood Leukemia Study. Pediatr Int. 2002;44:293-299.
Humphreys R.P., Hoffman H.J., Drake J.M., et al. Choices in the 1990s for the management of pediatric cerebral arteriovenous malformations. Pediatr Neurosurg. 1996;25:277-285.
Ijland M.M., Pereira R.R., Cornelissen E.A. Incidence of late vitamin K deficiency bleeding in newborns in the Netherlands in 2005: evaluation of the current guideline. Eur J Pediatr. 2008;167(2):165-169.
Janjua N., Nasar A., Lynch J.K., et al. Thrombolysis for ischemic stroke in children: data from the nationwide inpatient sample. Stroke. 2007;38(6):1850-1854.
Johnson C.F. Abuse and neglect of children, Nelson WE Textbook of Pediatrics. In, Behrman R.E., Kliegman R.M., Jenson H. Abuse and neglect of children. New York: WB Saunders Company. 2000:2414.
Jordan L.C., Johnston S.C., Wu Y.W., et al. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke. 2009;40(2):400-405.
Kato T., Hattori H., Yorifugi T., et al. Intracranial aneurysms in Ehlers-Danlos syndrome type IV in early childhood. Pediatr Neurol. 2001;25:336-339.
Kenet G., Lutkhoff L.K., Albisetti M., et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121(16):1838-1847.
Khayat A.S., Antunes L.M., Guimaraes A.C., et al. Cytotoxic and genotoxic monitoring of sickle cell anaemia patients treated with hydroxyurea. Clin Exp Med. 2006;6:33-37.
Kim C.T., Han J., Kim H. Pediatric stroke recovery: a descriptive analysis. Arch Phys Med Rehabil. 2009;90(4):657-662.
Klinge J., Auberger K., Auerswald G., et al. Prevalence and outcome of intracranial haemorrhage in haemophiliacs—a survey of the paediatric group of the German Society of Thrombosis and Haemostasis (GTH). Eur J Pediatr. 1999;158:S162-S165.
Koch H.G., Nabel P., Junker R., et al. The 677T genotype of the common MTHFR thermolabile variant and fasting homocysteine in childhood venous thrombosis. Eur J Pediatr. 1999;158:S113-S116.
Koga Y., Akita Y., Junko N., et al. Endothelial dysfunction in MELAS improved by l-arginine supplementation. Neurology. 2006;66:1766-1769.
Kwiatkowski J.L., Zimmerman R.A., Pollock A.N., et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146(3):300-305.
Kwiatkowski T.G., Libman R.B., Frankel M., et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340:1781-1787.
Labauge P., Brunereau L., Laberge S., et al. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology. 2001;57:1825-1828.
Labauge P., Brunereau L., Levy C., et al. The natural history of familial cerebral cavernomas: a retrospective MRI study of 40 patients. Neuroradiology. 2000;42:327-332.
Lanthier S., Lortie A., Michaud J., et al. Isolated angiitis of the CNS in children. Neurology. 2001;56:837-842.
Laugesaar R., Kolk A., Tomberg T., et al. Acutely and retrospectively diagnosed perinatal stroke: a population-based study. Stroke. 2007;38(8):2234-2240.
Lee J., Croen L.A., Lindan C., et al. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58:303-308.
Longo N., Schrijver I., Vogel H., et al. Progressive cerebral vascular degeneration with mitochondrial encephalopathy. Am J Med Genet A. 2008;146(3):361-367.
Lynch J.K., Nelson K.B. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13:499-505.
Mader I., Schoning M., Klose U., et al. Neonatal cerebral infarction diagnosed by diffusion-weighted MRI: pseudonormalization occurs early. Stroke. 2002;33:1142-1145.
Mallick A.A., Ganesan V., O’Callaghan F.J. Mortality from childhood stroke in England and Wales, 1921-2000. Arch Dis Child. 2009;95(1):12-19.
Manceau E., Giroud M., Dumas R. Moyamoya disease in children. A review of the clinical and radiological features and current treatment. Childs Nerv Syst. 1997;13:595-600.
Massicotte P., Adams M., Marzinotto V., et al. Low-molecular-weight heparin in pediatric patients with thrombotic disease: a dose finding study. J Pediatr. 1996;128:313-318.
Matschke J., Voss J., Obi N., et al. Nonaccidental head injury is the most common cause of subdural bleeding in infants <1 year of age. Pediatrics. 2009;124(6):1587-1594.
Mayer T.O., Biller J., O’Donnell J., et al. Contrasting the neurologic complications of cardiac transplantation in adults and children. J Child Neurol. 2002;17:195-199.
Menkes J. Metabolic diseases of the nervous system. In: Menkes J.H., Sarnat H.B. Child Neurology. Sixth ed. Philadelphia: Lippincott Williams & Wilkins; 2000:33-169. Ch 1
Menkes J., Fink B., Hurvitz C.C.H., et al. Neurologic manifestations of systemic disease. In: Menkes J.H., Sarnat H.B. Child Neurology. Sixth ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1093-1154. Ch 15
Miller S.T., Sleeper L.A., Pegelow C.H., et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342:83-89.
Moharir M.D., Shroff M., Stephens D., et al. Anticoagulants in pediatric cerebral sinovenous thrombosis: a safety and outcome study. Ann Neurol. 2009;67(5):590-599.
Mondal D., Pradhan L., Ali M., et al. HAART drugs induce oxidative stress in human endothelial cells and increase endothelial recruitment of mononuclear cells: exacerbation by inflammatory cytokines and amelioration by antioxidants. Cardiovasc Toxicol. 2004;4:287-302.
Morris B., Partap S., Yeom K., et al. Cerebrovascular disease in childhood cancer survivors: A Children’s Oncology Group Report. Neurology. 2009;73(22):1816-1817.
Neuhaus T.J., Goetschel P., Schmugge M., et al. Heparin-induced thrombocytopenia type II on hemodialysis: switch to danaparoid. Pediatr Nephrol. 2000;14:713-716.
Nolte K.W., Unbehaun H., Sieker T.M., et al. Hashimoto encephalopathy: A brainstem vasculitis? Neurology. 2000;54(3):769-770.
Normann S., de, Veber G., Fobker M., et al. Role of endogenous testosterone concentration in pediatric stroke. Ann Neurol. 2009;66(6):754-758.
Nowak-Gottl U., Frohlich B., Thedieck S., et al. Association of the protein Z ATG haplotype with symptomatic nonvascular stroke or thromboembolism in white children: a family-based cohort study. Blood. 2009;113(10):2336-2341.
Nowak-Gottl U., Strater R., Heincecke A., et al. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 1999;94:3678-3682.
Ohene-Frempong K., Weiner S.J., Sleeper L.A., et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288-294.
Papavasiliou A., Bazigou-Fotopoulou H., Ikeda H. Familial moyamoya disease in two European children. J Child Neurol. 2007;22(12):1371-1376.
Pegelow C.H. Stroke in children with sickle cell anaemia: aetiology and treatment. Paediatr Drugs. 2001;3:421-432.
Pegelow C.H., Macklin E.A., Moser F.G., et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014-3018.
Petaja J., Peltola K., Sairanen H., et al. Fibrinolysis, antithrombin III, and protein C in neonates during cardiac operations. J Thorac Cardiovasc Surg. 1996;112:665-671.
Rafay M.F., Armstrong D., Deveber G., et al. Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol. 2006;21:8-16.
Ranze O., Ranze P., Magnani H.N., Greinacher A. Heparin-induced thrombocytopenia in paediatric patients—a review of the literature and a new case treated with danaparoid sodium. Eur J Pediatr. 1999;158:S130-S133.
Raphael J.L., Shetty P.B., Liu H., et al. A critical assessment of transcranial Doppler screening rates in a large pediatric sickle cell center: opportunities to improve healthcare quality. Pediatr Blood Cancer. 2008;51(5):647-651.
Revel-Vilk S., Golomb M.R., Achonu C., et al. Effect of intracranial bleeds on the health and quality of life of boys with hemophilia. J Pediatr. 2004;144(4):490-495.
Ries M., Gupta S., Moore D.F., et al. Pediatric Fabry disease. Pediatrics. 2005;115:e344-355.
Risch L., Huber A.R., Schmugge M. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;118(1):123-135.
Roach E.S. Stroke in children. Curr Treat Options Neurol. 2000;2:295-304.
Roach E.S., Riela A.R. Pediatric Cerebrovascular Disorders. London: Futura Publishing; 1995.
Roach E.S., Golomb M.R., Adams R., et al. Management of stroke in infants and children. A scientific statement from a special writing group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644-2691.
Rockett S.R., Bryant J.C., Morrow W.R., et al. Preliminary single center North American experience with the Berlin Heart pediatric EXCOR device. ASAIO J. 2008;54(5):479-482.
Rooks V.J., Eaton J.P., Ruess L., et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol. 2008;29(6):1082-1089.
Salih M.A., Abdel-Gader A.G., Al-Jarallah A.A., et al. Stroke in Saudi children. Epidemiology, clinical features and risk factors. Saudi Med J. 2006;27:S12-S20.
Salih M.A., Abdel-Gader A.G., Al-Jarallah A.A., et al. Hematologic risk factors for stroke in Saudi children. Saudi Med J. 2006;27:S21-S34.
Salih M.A., Abdel-Gader A.G., Al-Jarallah A.A., et al. Infectious and inflammatory disorders of the circulatory system as risk factors for stroke in Saudi children. Saudi Med J. 2006;27:S41-S52.
Schiffmann R. Natural history of Fabry disease in males: preliminary observations. J Inherit Metab Dis. 2001;24:15-17. discussion pp. 11-12
Schiffmann R., Ries M., Timmons M., et al. Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant. 2006;21:345-354.
Starke J.R. Tuberculosis of the central nervous system in children. Semin Pediatr Neurol. 1999;6:318-331.
Strater R., Becker S., von, Eckhardstein A., et al. Prospective assessment of risk factors for recurrent stroke during childhood—a 5-year follow-up study. Lancet. 2002;360:1540-1545.
Strater R., Kurnik K., Heller C., et al. Aspirin versus low-dose low-molecular-weight heparin: antithrombotic therapy in pediatric ischemic stroke patients: a prospective follow-up study. Stroke. 2001;32:2554-2558.
Tarantino M.D., Gupta S.L., Brusky R.M. The incidence and outcome of intracranial haemorrhage in newborns with haemophilia: analysis of the Nationwide Inpatient Sample database. Haemophilia. 2007;13(4):380-382.
Telfer P., Coen P., Chakravorty S., et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica. 2007;92(7):905-912.
Terwindt G., Kors E., Haan J., et al. Mutation analysis of the CACNA1A calcium channel subunit gene in 27 patients with sporadic hemiplegic migraine. Arch Neurol. 2002;59:1016-1018.
Thuillier L., Chadefaux-Vekemans B., Bonnefont J.P., et al. Does the polymorphism 677C-T of the 5,10-methylenetetrahydrofolate reductase gene contribute to homocysteine-related vascular disease? J Inherit Metab Dis. 1998;21:812-822.
Tian H.L., Geng Z., Cui Y.H., et al. Risk factors for posttraumatic cerebral infarction in patients with moderate or severe head trauma. Neurosurg Rev. 2008;31(4):431-436. discussion 6-7
van den Tweel X.W., Nederveen A.J., Majoie C.B., et al. Cerebral blood flow measurement in children with sickle cell disease using continuous arterial spin labeling at 3.0-Tesla MRI. Stroke. 2009;40(3):795-800.
Vermylen C. Hematopoietic stem cell transplantation in sickle cell disease. Blood Rev. 2003;17:163-166.
Volpe J.J. Neurology of the Newborn. Philadelphia: W. B. Saunders; 2001.
Wayne A.S., Schoenike S.E., Pegelow C.H. Financial analysis of chronic transfusion for stroke prevention in sickle cell disease. Blood. 2000;96:2369-2372.
Zimmer J.A., Garg B.P., Williams L.S., et al. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol. 2007;37(3):171-175.