Chapter 51E Vascular Diseases of the Nervous System
Spinal Cord Vascular Disease
Vascular Anatomy of the Spinal Cord
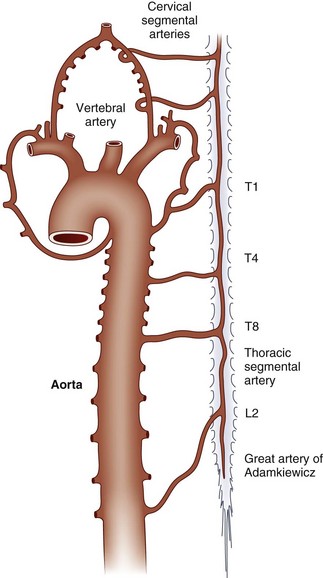
The embryonic arterial supply to the spinal cord derives from intradural vessels that enter at each spinal level and divide to follow the dorsal and ventral roots. The ventral radicular branches join along the midline to form the anterior spinal artery. Irregular anastomoses among the dorsal roots, as they enter the cord on each side, form paired posterior spinal arteries. The anterior and posterior spinal arteries constitute longitudinal arterial plexuses. Circumflex vessels (arteria vasocorona) connect the anterior and posterior arterial systems in a pial plexus around the lateral margins of the cord (Fig. 51E.1).
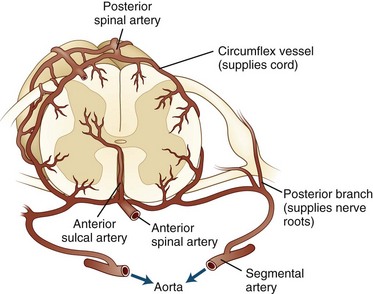
Fig. 51E.1 Arterial supply to the spinal cord and nerve roots at the level of a medullary radicular artery.
(Adapted from Henson, R.A., Parsons, M., 1967. Ischaemic lesions of the spinal cord: an illustrated review. Q J Med 36, 205-222.)
During development, a few radicular arteries become dominant and provide most of the flow to the spinal cord through the anterior spinal artery. These are variably known as medullary or radiculomedullary vessels. Unfortunately, the terms radicular, medullary, and radiculomedullary are often used interchangeably in the literature. Five to eight of these vessels typically persist in adults. The largest and most frequently identified of the anterior vessels is the arteria radicularis magna or great artery of Adamkiewicz, which courses along one of the lower thoracic or upper lumbar anterior roots to join the anterior spinal artery. It provides a major portion of the blood flow to the lower thoracic cord and the lumbar enlargement. The sacral cord, conus medullaris, and cauda equina are supplied by small lower segmental radicular arteries. In general, a larger number of smaller vessels serve the posterior spinal artery. The cervical and upper thoracic spinal cord is richly vascularized by a plexus arising from branches of the ascending cervical and vertebral arteries (Fig. 51E.2).
The main blood supply to spinal gray matter, as well as to anterior and lateral funiculi, is derived from anterior sulcal arteries. These arise from the anterior spinal artery in the midline and course into the ventral median fissure. Each anterior sulcal artery distributes blood to only the left or right half of the spinal cord. The greatest distance between sulcal arteries is in the thoracic segments; the vascularity is proportional to the numbers of neurons located throughout the cord at that level. The dorsal columns and extreme dorsal horns (approximately one-third of the cord cross-section) are supplied by penetrating branches from the posterior spinal arteries. The superficial white matter also receives blood flow via the circumflex anastomotic vessels. This arrangement leads to a clinically relevant border zone between the territories of the sulcal and superficial arterial distributions. As a result, many spinal cord infarctions do not follow the conventional boundaries between anterior and posterior arterial distributions (Ishizawa et al., 2005).
Spinal Cord Ischemia
Paraplegia complicating aortic surgery was recognized as early as 1825, though it was not attributed to spinal cord ischemia until the 1880s. By the early 20th century, cardiac embolism, atheromatous disease, and decompression sickness were also described as causes of paraplegia attributable to spinal cord ischemia. The actual prevalence of spinal cord infarction is unknown but is generally cited as representing 1% to 2% of all central neurovascular events and 5% to 8% of all acute myelopathies. The clinical presentation of spinal cord syndromes is presented in more detail in Chapter 24.
Presentation and Initial Course
Weakness (100%), sensory loss (89%), back pain at onset (82%), and urinary complaints requiring catheterization (75%) were the most common symptoms of cord ischemia at the time of presentation in a prospectively collected series (Masson et al., 2004). In retrospective series, the same major symptoms are observed, though with minor differences in observed frequency (Kumral et al., 2010; Nedeltchev et al., 2004; Novy et al., 2006). The most common location to be affected is the mid- to low thoracic spine. Lower cervical lesions are less common, and upper thoracic spinal infarcts are rare. Quadriparesis is present in only 20% to 25% of all cases of spinal infarction.
Pain and sensory changes occur first in most cases, followed by weakness within minutes or hours. Over 80% of the back pain with spinal infarction follows a radicular pattern (Novy et al., 2006), but in cases of acute aortic disease, pain may have a more visceral character. Maximum motor disability is observed within 12 hours of onset in a majority of patients, with a trend toward longer intervals when dysfunction was less severe. Urinary retention is typical in the acute phase, but involuntary voiding or defecation may be associated with the onset of the ischemic insult.
Investigations
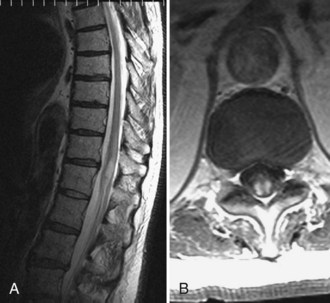
Magnetic resonance imaging (MRI) is the diagnostic procedure of choice for detecting spinal cord ischemia, although the results can be normal in up to one-third of patients (Novy et al., 2006). In the acute phase of spinal cord infarction, high diffusion-weighted imaging signal is noted and matches with low apparent diffusion coefficient signal (Thurnher and Bammer, 2006). Pencil-shaped hyperintense signal on T2-weighted images initially appear within 2 hours to several days, and may be accompanied by gadolinium enhancement (Weidauer et al., 2002). Abnormal signal and contrast enhancement may demonstrate a double-dot (“owl’s eyes”) pattern in the region of the anterior horns, an H-shaped pattern involving the central gray matter, or a more diffuse pattern involving both gray and white matter (Fig. 51E.3). The diffuse pattern may be difficult to distinguish from venous infarction. When cord infarction results from compromise of a segmental artery, branches supplying the ipsilateral half of the vertebral body may also be affected. Vertebral body infarct is best detected on sagittal T2-weighted images, usually appearing as a triangular area of increased signal near the end-plate and/or deep medullary portion of the vertebral body.
Other laboratory and radiographic studies are not diagnostic in noncompressive spinal cord ischemia. Myelography is usually normal. Cerebrospinal fluid (CSF) protein was elevated in 44% of one series, but pleocytosis was not observed (Novy et al., 2006).
The exact cause of spinal ischemia often remains occult and could not be discerned in up to half of prospective and retrospective series (Masson et al., 2004; Novy et al., 2006).
Course
The course of spinal ischemic syndromes is variable. Transient ischemic attacks have been reported to precede up to 10% of cord infarcts (Kumral et al., 2010; Novy et al., 2006). Pain is often persistent and is a major contributor to long-term disability after spinal cord infarction. A slowly progressive myelopathy attributed to chronic constriction of radiculomedullary arteries in the neck has been suggested but not established.
Prognosis
Rates of recovery vary widely among case series, which were collected by different methods. In a prospectively collected series, approximately half of patients had a favorable outcome, defined as the ability to walk with one assistive device (or none) and no need for urinary catheterization at the time of hospital discharge (Masson et al., 2004). Recent retrospective series also suggest that ambulation can be restored in about 50% of patients (Kumral et al., 2010; Novy et al., 2006). Likelihood of recovery is higher when the deficits are less severe at presentation. Over a mean follow-up period of 4 years, more than 90% of patients with the mildest severity of deficit during the acute phase were able to walk independently or with assistive devices; in contrast, nearly one-third of more severely affected patients required a wheelchair (Nedeltchev et al., 2004). Poor outcome is predicted when proprioceptive loss, gait impairment, or urinary dysfunction were present at the time of presentation. Chronic pain can be a disabling consequence of cord ischemia, but it tends to occur only in individuals with spinothalamic sensory impairment early in the course. The duration of motor dysfunction is also useful in determining prognosis. Unless significant motor recovery occurs in the first 24 hours, the likelihood of major improvement is low.
Causes of Spinal Cord Ischemia
In recent series, the cause of spinal cord infarction could not be identified in up to 74% of cases. Among cases with identifiable causes, mechanical triggering movements were most common (Kumral et al., 2010; Novy et al., 2006). A high frequency of concomitant spinal column disease and the pattern of clinical findings suggest compression of radicular arteries in cases with mechanical trigger events. Most infarcts associated with spinal disease (e.g., chronic radicular pain, compression fractures) follow anterior or posterior spinal artery patterns and occur at the level of the mechanical stresses in the spine (Novy et al., 2006). Other typical causes of spinal ischemia are summarized in (Box 51E.1).
In contrast, mechanical triggering events are not associated with central or transverse infarct patterns. Systemic arterial disease was noted more frequently in patients with transverse patterns (Novy et al., 2006). Aortic pathologies with regional hemodynamic compromise accounted for 30% to 40% of cord infarcts in older case series. Complications of aortic surgery represented the largest proportion of those cases. Prolonged clamping of the aorta above the renal arteries (e.g., for more than 20-30 minutes) or operative ligation of lower thoracic intercostal vessels places the cord at risk for ischemia and infarction. Open thoraco-abdominal aortic aneurysm repairs are associated with a 5% to 20% risk of significant neurological deficits. Endovascular techniques appear to be safer, but they do not completely eliminate risks for spinal cord ischemia. Intraoperative interventions like distal aortic perfusion and CSF drainage may also contribute to lower complication rates.
Thromboembolism causes both acute and stepwise spinal cord dysfunction. Emboli arising from the mitral valve in rheumatic heart disease and from acute bacterial endocarditis may cause acute paraplegia. Similarly, thromboembolism from an atrial myxoma may cause multiple spinal cord infarcts. Myelopathy associated with decompression sickness (also known as Caisson disease [see Chapter 51A]) results from circulating nitrogen bubbles that block small spinal arteries. Spinal cord ischemia also may complicate therapeutic renal or bronchial artery embolizations.
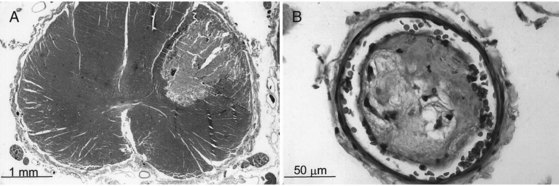
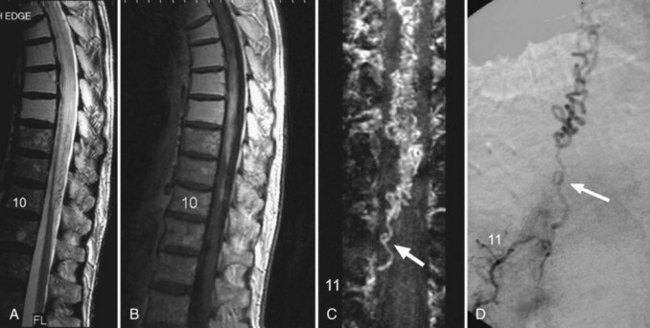
Fibrocartilaginous emboli from ruptured intervertebral disks are the cause of an ischemic syndrome unique to the spinal cord. Fragments of connective tissue material from the damaged disk are traumatically forced into bone marrow sinusoids by local fracture. The increased tissue pressure at the site of injury allows retrograde entry of emboli into the spinal vertebral plexus as well as into arterial channels, leading to cord infarction (Fig. 51E.4). Approximately half of these events are purely arterial; the rest have mixed arterial and venous involvement. The anterior portion of the cervical cord is affected in up to 70% of such cases. Women are affected twice as often as men.
Treatment
The medical management of spinal cord ischemia focuses on supportive measures and reducing risk for recurrence. Recurrence risk is managed with maintenance of adequate blood pressure, early bed rest, and reversal of proximate causes such as hypovolemia or arrhythmias. Acute thrombolysis has not been systematically studied, but treatment with intravenous recombinant tissue plasminogen activator at usual stroke doses may be beneficial in some patients (Restrepo and Guttin, 2006). The low incidence of spinal cord infarction and the variability of its natural course make systematic treatment trials of thrombolysis and antithrombotic therapies unlikely. Over the longer term, care is directed toward minimizing the complications of autonomic dysfunction and immobility. Physical and occupational therapy are useful in promoting functional recovery.
Spinal Vascular Malformations
Spinal vascular malformations consist of normal-sized to enlarged arteries and enlarged tortuous veins, without an intervening capillary network. A commonly accepted classification system (Anson and Spetzler, 1993) categorizes spinal vascular malformations into four types:
Type I: Dural arteriovenous fistula (AVF), subtypes IA (single feeding artery) and IB (multiple feeding arteries).
Type II: Intramedullary glomus-type arteriovenous malformation (AVM).
Type III: Intramedullary juvenile-type AVM, which is more extensive than a glomus-type AVM, frequently having an extramedullary component and sometimes an extradural component.
Type IV: Intradural extramedullary (perimedullary) AVF; subtypes IVA, IVB, and IVC correspond to lesions with progressively increased arteriovenous shunting, manifested as increased number, size, and tortuosity of feeding arteries.
Clinical Presentation and Course
Pain may be local, radicular, diffuse, or any combination of these. Upper motor neuron weakness, lower motor neuron weakness, or both may occur. A spinal bruit is a highly specific (though uncommon) finding that is diagnostic of a spinal AVM. Vascular malformations in the skin or paraspinal muscles are sometimes noted in conjunction with spinal AVMs. In cutaneomeningospinal angiomatosis (Cobb syndrome), a cutaneous angioma appears in the dermatome corresponding to the AVM’s spinal segment. Foix-Alajouanine syndrome (see Causes of Spinal Cord Ischemia earlier in this chapter) has been associated with end-stage dural AVF with thrombosis and venous infarction.
Vascular malformations (AVMs and dural AVFs) may cause increased local venous pressure, decreased perfusion pressure, decreased tissue perfusion, and finally tissue ischemia. This explains the coexistence of deficits in more than a single arterial territory and the symptomatic improvement that results from ligation of feeding vessels. The sometimes confusing and widely varied presentation of spinal vascular malformations results in a large differential diagnosis that includes neoplasms, herniated discs, multiple sclerosis, intracranial SAH, subacute combined degeneration, meningovascular syphilis, and transverse myelitis (see Chapter 24).
Investigations
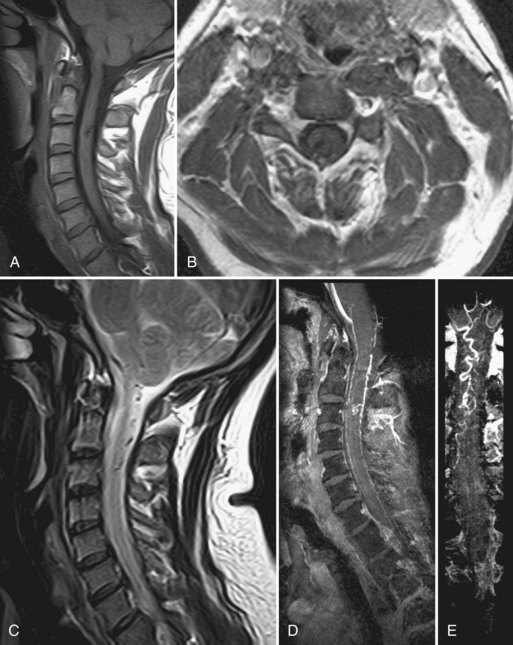
MRI can discriminate extramedullary from intramedullary lesions, document thrombosis of the malformation following ligation or embolization of the feeding vessels, and demonstrate changes in the spinal cord (edema, hemorrhage) distinct from, yet due to, the vascular malformation. Routine MRI is also sensitive in detecting intramedullary AVMs (types II and III vascular malformations). The findings include intramedullary low signal with surrounding normal cord tissue, focal cord enlargement at the location of the nidus, and serpentine signal voids within the subarachnoid space in the region of the nidus (Fig. 51E.5). MRA augments the MR study by confirming the location of the nidus and allowing better visualization of the AVM drainage to the coronal venous plexus on the surface of the spinal cord.
In cases of dural AVF (Fig. 51E.6), MR abnormalities involving the spinal cord have been observed with variable frequency: slight enlargement of the cord; cord hypointensity on T1-weighted images and hyperintensity on T2-weighted images, involving the central region of the cord and extending over several levels; scalloping of the cord contours on sagittal images; and enhancement of the cord on postcontrast T1-weighted images. Of these findings, the most consistently observed is hyperintensity within the center of the cord on T2-weighted images. In general, however, these findings are nonspecific and, like the clinical findings, can mimic those of cord neoplasm, infection, or ischemia from arterial occlusive disease. Thus, detection of blood flow–related signal abnormalities in the subarachnoid space is crucial to achieving high diagnostic accuracy for dural AVF. The detection of intradural flow voids on T2-weighted images, and the detection of intradural serpentine enhancement on T1-weighted images extending for more than three contiguous vertebral levels, are each associated with the presence of dural AVF (Saraf-Lavi et al., 2002). Contrast-enhanced three-dimensional (3D) spinal MRA provides more direct and extensive visualization of the abnormal intradural vessels (veins), and when added to standard MRI can improve detection of dural fistulas. MRA improves localization of the vertebral level of the fistula; digital subtraction angiography (DSA) studies performed after MRA required less than 50% of the fluoroscopy time and volume of iodinated contrast when the fistula level and side were identified on pre-DSA MRA (Luetmer et al., 2005).
Spinal Hemorrhage
Spinal Epidural and Subdural Hemorrhage
Both SEH and SSH are surgical emergencies. Operative treatment is directed toward relief of local pressure and repair of any underlying defect. Laminectomy with evacuation of the clot should be performed as soon as possible. The prognosis for recovery is better when the preoperative deficits are not severe; timing of surgery appears less important (Börm et al., 2004).
Anson J.A., Spetzler R.F. Spinal dural arteriovenous malformations. In: Awad I.A., Barrow D.L. Dural arteriovenous malformations. Park Ridge, Il: American Association of Neurological Surgeons; 1993:175-193.
Börm W., Mohr K., Hassepass U., et al. Spinal hematoma unrelated to previous surgery. Analysis of 15 consecutive cases treated in a single institution with a 10-year period. Spine. 2004;29:E555-E561.
Ishizawa K., Komori T., Shimada T., et al. Hemodynamic infarction of the spinal cord: involvement of the gray matter plus the border-zone between the central and peripheral arteries. Spinal Cord. 2005;43:306-310.
Kumral E., Polat F., Güllüoglu H., et al. Spinal ischaemic stroke: clinical and radiological findings and short-term outcome. Eur J Neurol. 2010. 10.1111/j.1468-1331.2010.02994 (Published Online 8 Apr 2010)
Luetmer P.H., Lane J.I., Gilbertson J.R., et al. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol. 2005;26:711-718.
Masson C., Pruvo J.P., Meder J.F., et al. Spinal cord infarction: clinical and magnetic resonance imaging findings and short-term outcome. J Neurol Neurosurg Psychiatry. 2004;75:1431-1435.
Nedeltchev K., Loher T.J., Stepper F., et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004;35:560-565.
Novy J., Carruzzo A., Maeder P., et al. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
Restrepo L., Guttin J.F. Acute spinal cord ischemia during aortography treated with intravenous thrombolytic therapy. Tex Heart Inst J. 2006;33:74-77.
Saraf-Lavi E., Bowen B.C., Quencer R.M., et al. Detection of spinal dural arteriovenous fistula with MRI and contrast-enhanced MR angiography: sensitivity, specificity, and prediction of vertebral level. AJNR Am J Neuroradiol. 2002;23:858-867.
Thurnher M.M., Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006;48(11):795-801.
Weidauer S., Nichtweiss M., Lanfermann H., et al. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology. 2002;44(10):851-857.