Chapter 51C Vascular Diseases of the Nervous System
Intracranial Aneurysms and Subarachnoid Hemorrhage
An intracranial aneurysm is defined as an abnormally circumscribed dilatation of an artery which grows and ruptures over time. The term aneurysm originally comes from the Greek aneurysma—ana- meaning “across” and eurys meaning “broad.” Subarachnoid hemorrhage (SAH), mostly from rupturing of intracranial aneurysms, accounts for 5% to 10% of all stroke cases and is related to greater fatality than the other forms of strokes. Case fatality of aneurysmal SAH ranges between about 30% and 70%, with an average of approximately 50%. In more than half of SAH survivors, the level of disability is major; 60% of those patients never achieve the quality of life they enjoyed before the aneurysm ruptured (Kelly et al., 2001; Ropper and Zervas, 1984). In the United States, the estimated lifetime cost (including hospitalization, treatment, morbidity, and mortality) for patients hospitalized with unruptured intracranial aneurysms is $522 million, and $1.75 billion for patients with aneurysmal SAH (Wiebers et al., 1992). Owing to recent advancements in minimally invasive neuroimaging technology, such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), intracranial aneurysms are more frequently detected prior to rupture. Considering that intracranial aneurysm is a fairly common disease and the relatively young, productive age at which a disabling or fatal aneurysmal SAH could occur, in-depth understanding of intracranial aneurysms and their complications is very important for a modern comprehensive management of aneurysm patients.
Epidemiology
The true incidence of intracranial aneurysms remains unknown, but the reported incidence varies from as low as 0.2% to 10% of the general population, depending on whether the source of the data is an imaging study or an autopsy study. Some autopsy studies estimate that approximately 5% of the population may harbor undiscovered intracranial aneurysms (Jakubowski and Kendall, 1978; Juvela et al., 1993; Wiebers et al., 1992). Fortunately, most intracranial aneurysms do not rupture during the lifetime of a patient. Clinically, the frequency of ruptured aneurysms has been estimated at a median value of 11 per 100,000 people per year. In 1981, the Cooperative Study estimated the incidence of aneurysmal SAH in the United States at 26,000 cases per year (Sahs et al., 1981).
The prevalence of aneurysmal multiplicity ranges from 10% to 30% in large clinical series (Inagawa, 1990; Juvela, 2000; Qureshi et al., 1998). Multiple aneurysms can be mirror aneurysms or located asymmetrically on different locations in the circle of Willis. There is a strong female predominance in aneurysm multiplicity. Some studies have shown that multiple aneurysms are associated with vasculopathies such as fibromuscular dysplasia.
There is a rising occurrence of intracranial aneurysms associated with increasing age and female gender (Nakagawa and Hashi, 1994). In the United States, the peak age for aneurysm rupture is between 50 and 60 years of age (Sahs et al., 1981). Overall, the female-to-male ratio is approximately 2 : 1 in adults. Pediatric aneurysms are rare and account for about 1% to 3% of aneurysmal SAH (Wojtacha et al., 2001). In contrast to the definitive female predominance in adults, female-to-male ratio is reversed in the pediatric population. Large and giant intracranial aneurysms are more commonly reported in pediatric aneurysm series, particularly in infants (Meyer et al., 1989). These differences in clinical manifestations strongly suggest that the underlying etiology of intracranial aneurysm formation is different between pediatric and adult populations.
Pathophysiology
Saccular Aneurysm
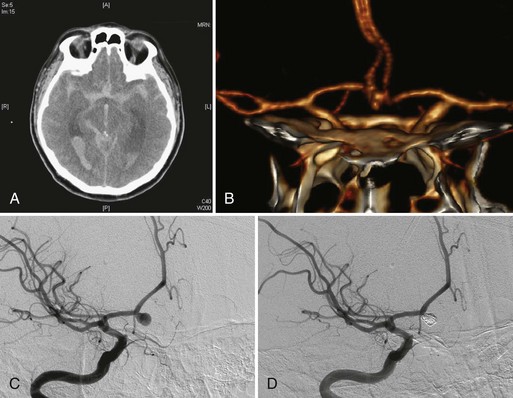
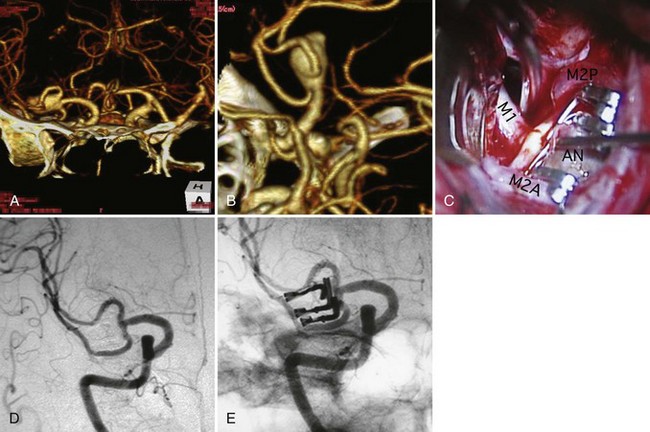
Intracranial aneurysms are classified according to their morphology, size, pathogenesis, and anatomical location (Box 51C.1).The most common intracranial aneurysm morphology is the saccular aneurysm, which is a rounded berry-like outpouching arising from first- and second-order branches in the circle of Willis (Fig. 51C.1). Saccular aneurysms account for approximately 90% of intracranial aneurysms and are responsible for most of the morbidity and mortality from SAH (Yong-Zhong and van Alphen, 1990).
Intracranial arteries are structurally unique in that they lack external elastic lamina. The disappearance of the external elastic lamina occurs in the horizontal segment of the cavernous portion of internal carotid arteries (Masuoka et al., 2010). Significant reduction or disappearance of elastic fibers in the tunica media and external elastic lamina also occurs in the vertebral arteries as they enter the skull (Wilkinson, 1972). Thus, the internal elastic lamina provides a very important structural support for the cerebral vasculature. Alterations in the internal elastic lamina or tunica media of intracranial arteries structurally weaken the arterial walls and make them less resistant to constant hemodynamic forces such as dynamic pressure, blood pressure, and shear stress. Although the etiology of intracranial saccular aneurysm formation involves multiple factors, the most significant pathogenetic factor is considered to be the degeneration of tunica media and internal elastic lamina at the branching sites of intracranial arteries in regions of chronic hemodynamic stress. In the pediatric population, the abnormalities of tunica media and internal elastic lamina may be congenital rather than the degenerative acquired changes in adults.
Fusiform Aneurysm
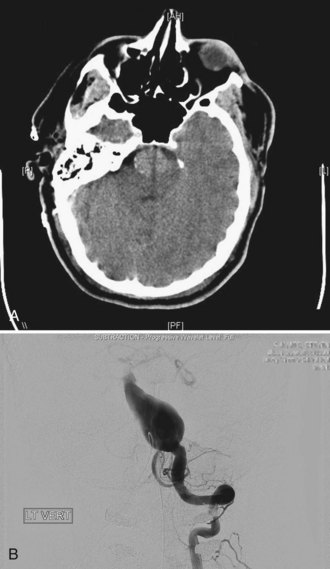
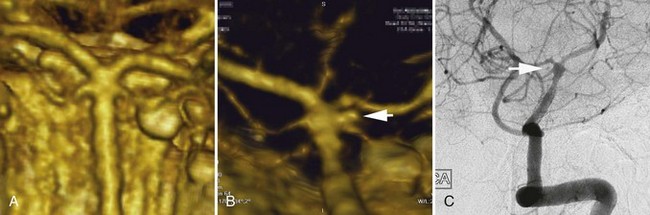
Two other morphological types of intracranial aneurysms are fusiform and dissecting aneurysms (see Box 51C.1), although the definitions of both aneurysm types may overlap. A fusiform aneurysm is defined as a circumferential dilatation in a segment of an intracranial artery. Unlike a saccular aneurysm that has a single orifice (neck) through which blood flow goes in and exits the aneurysm cavity, a fusiform aneurysm does not have a defined orifice (neck). The inflow and outflow of fusiform aneurysms are longitudinally separate, which often makes surgical or interventional treatment of these aneurysms challenging. Fusiform aneurysms are generally associated with atherosclerosis, which causes extensive damage to the media of an artery and results in arterial stretching to all sides or elongation. Although infrequent, other diseases are known to cause vessel damage resulting in fusiform aneurysms. If an arterial fusiform dilatation is accompanied by a marked elongation, it is call a dolichoectasia (dolichos, long; ectasia, distended). Dolichoectatic fusiform aneurysms are often seen in the posterior circulation and can reach several centimeters in diameter (Fig. 51C.2). Patients with dolichoectasia aneurysms characteristically present with mass effect on cranial nerves or brainstem compression, but they are not commonly associated with subarachnoid hemorrhage (Lou and Caplan, 2010).
Dissecting Aneurysm
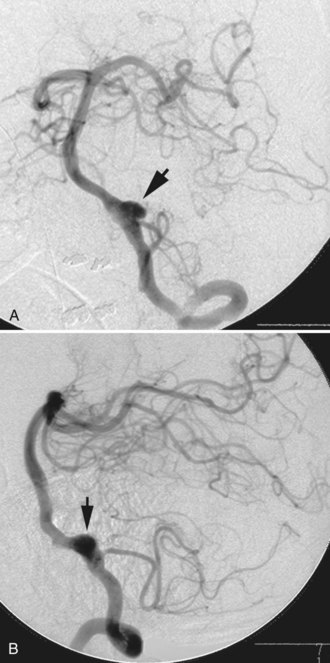
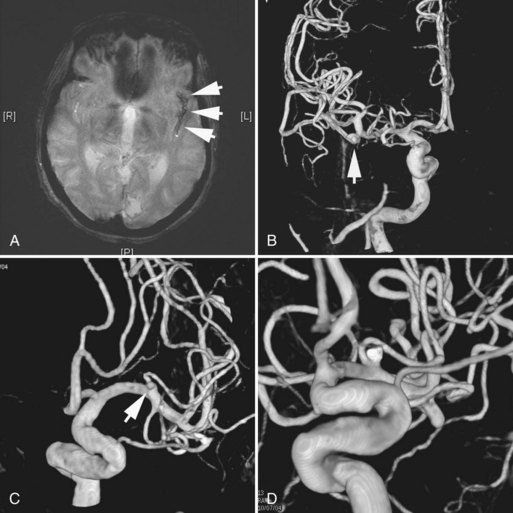
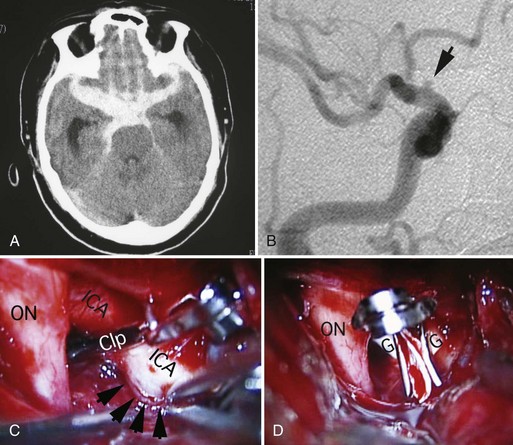
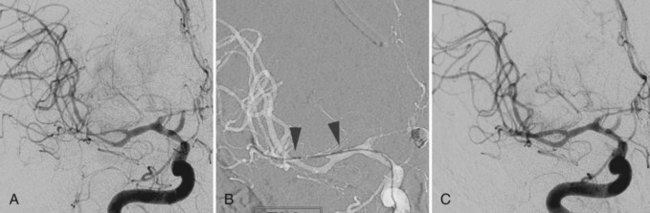
A dissecting aneurysm is formed as a result of splitting or dissection of an arterial wall by blood flow entering through a tear. This may cause a ballooning out on one side of the artery, with arterial narrowing (a classic radiographic appearance of pearl-and-string sign), an outpouching mimicking a saccular aneurysm (pseudoaneurysm or false saccular aneurysm), or a fusiform segmental dilatation. It could obstruct blood flow through the dissecting segment of an artery. Consequently, the angiographic and physical appearance of an intracranial dissecting aneurysm or arterial dissection varies widely depending on the arterial layer in which the dissection occurs and the extent of the dissection (Fig. 51C.3). Intracranial dissecting aneurysms often present with hemorrhage. In patients with severe SAH but no clear angiographic evidence of the source of the hemorrhage, very careful review of the angiograms should be conducted to search for a small dissecting aneurysm (Figs. 51C.4 and 51C.5).
Intracranial dissections can occur spontaneously, but they are commonly associated with trauma or an underlying vasculopathy such as fibromuscular dysplasia. Although controversial, it is believed that trivial trauma such as head turning, chiropractic manipulation, sneezing, or any sports activity may cause intracranial dissection or resultant dissecting aneurysm, with or without underlying vasculopathies (Smith et al., 2003). Unlike saccular aneurysms in the adult population, there is a male predominance in intracranial dissecting aneurysm (Yamaura et al., 1990).
Associated Conditions
Various hereditary and acquired risk factors are known to be associated with the formation of intracranial aneurysms. Some of the conditions listed in Box 51C.2 are controversial and still being studied. Nevertheless, any congenital or acquired condition that produces vascular wall weakening and/or increased hemodynamic stress could result in the formation of intracranial aneurysms. Approximately 5% of intracranial aneurysms are associated with heritable connective tissue disorders, the most important being Ehlers-Danlos syndrome vascular type (type IV), neurofibromatosis type 1, and autosomal dominant polycystic kidney disease (Schievink, 1997). The association of intracranial aneurysms and autosomal dominant polycystic kidney disease has been well studied. The prevalence of intracranial aneurysms in patients with polycystic kidney disease is approximately 8%, which is higher than in the normal population (Rinkel et al., 1998). Positive family history of aneurysm in a patient with polycystic kidney disease increases the risk (Pirson et al., 2002). The vascular type of Ehlers-Danlos syndrome, formally called type IV, has a defect of type-III collagen synthesis, which is a major component of distensible tissues such as vessels. A higher incidence of intracranial aneurysms, particularly in the cavernous segment of internal carotid artery, has been reported. The indication of arterial or venous puncture for catheter angiography in patients with Ehlers-Danlos syndrome should be carefully discussed because of the vascular fragility.
Box 51C.2 Risk Factors and Associated Conditions for Intracranial Aneurysms
Familial intracranial aneurysms are defined as those identified in two or more first-degree relatives. Studies have shown that the risk for harboring intracranial aneurysms in such families is higher than in the general population. This is not just due to genetic factors but also could be due to common environmental exposures. The reported incidence of familial intracranial aneurysms varies but is approximately 8% in persons with two or more relatives who have had a subarachnoid hemorrhage or an aneurysm (Ronkainen et al., 1997).
The prevalence of intracranial aneurysms increases with age in both genders. This is not surprising, since chronic hemodynamic stimuli and degenerative changes in intracranial arteries play a role in aneurysm formation. Many studies have shown that current cigarette smoking is a risk factor for intracranial aneurysm formation (Inagawa, 2010), and it is suggested that cigarette smoking may increase the risk of aneurysm rupture. Cocaine use is a known risk factor for intracranial aneurysm formation. This association is likely due to the transient increase of blood flow and blood pressure. Among cocaine users, intracranial aneurysms are found in a younger population. The role of hypertension in the formation of intracranial aneurysms has been controversial in the literature. However, coarctation of the aorta, pheochromocytoma, and cocaine use have been associated with intracranial aneurysms, most likely because of the elevated blood pressures that occur in these conditions. Considering hypertension is associated with degenerative changes such as intracranial atherosclerosis, it could play a role in a subset of intracranial aneurysms.
Natural History of Intracranial Aneurysms
Surgical or endovascular treatments of unruptured incidental intracranial aneurysms remain controversial. Thorough understanding of the natural history of incidental intracranial aneurysms as well as the risks associated with their repair is mandatory for the appropriate management of aneurysm patients. The International Study of Unruptured Intracranial Aneurysms (ISUIA) group investigated the outcome of unruptured intracranial aneurysms in patients who underwent surgical treatment or no treatment (conservative observation). Two ISUIA studies, the largest series to date, were published in 1998 and in 2003, and both reported a lower incidence of aneurysm rupture and a higher incidence of procedural morbidity and mortality than most practicing clinicians expected (ISUIA Investigators, 1998, 2003). The first study published in the New England Journal of Medicine in 1998 was highly controversial for many reasons, but mainly because of its selection bias and the inclusion of many cavernous-carotid aneurysms.
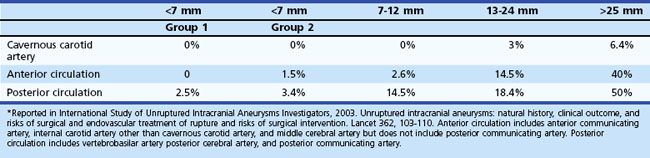
The second study published in Lancet in 2003, which was less controversial to practicing physicians, prospectively analyzed 4060 patients for treatment outcomes versus conservative observation. The untreated cohort consisted of 1692 patients with 2686 aneurysms, 1077 with no prior history of SAH (classified as group 1), and 615 with a prior history of SAH from another aneurysm (classified as group 2), with a mean follow-up period of 4.1 years. Among this cohort, 51 patients (3%) had a confirmed SAH (Table 51C.1). The study reported that larger aneurysm size was associated with higher risk of rupture in group 1 aneurysms but not in group 2, although there were only a few large aneurysms in group 2. According to their data, the rupture rate of group 1 aneurysms was extremely low (<0.1% per year). The ISUIA also found that posterior circulation aneurysms, including posterior communicating artery aneurysms, carried higher rupture rates than anterior circulation aneurysms.
It should be emphasized that the ISUIA results do not apply to all situations, not only because of their non-randomized nature and selection bias but also for other reasons. For instance, fusiform aneurysms, saccular traumatic aneurysm, and mycotic aneurysms were excluded from the ISUIA study, so the true natural history of such aneurysms remains unknown. Furthermore, saccular aneurysms with an irregular contour or an obvious bleb formation may have been included in the ISUIA very infrequently, since the enrolling physicians felt it unsafe to conservatively follow such irregular-shaped saccular aneurysms. Moreover, among small saccular aneurysms (<7 mm) that could have been included in the ISUIA study, the rupture risk of ISUIA no longer applied once unruptured aneurysms increased in size during the follow-up (Juvela et al., 2001; Miyazawa et al., 2006). The growth of an aneurysm may warrant immediate intervention regardless of its size.
The result of the second ISUIA in 2003 may lead to the conclusion that a patient with a group 1 aneurysm less than 7 mm in the largest diameter does not benefit from any treatments, including endovascular coiling. On the other hand, most ruptured aneurysms in many reports are less than 7 mm in the greatest diameter, and there have been no clear scientific explanations for this discrepancy. Also, the ISUIA results are not in direct accordance with previous natural history studies (Juvela et al., 1993; Tsutsumi et al., 2000). The cumulative risk of aneurysm rupture reported in most other clinical studies ranges between approximately 10% and 20% at 10 years, and the risk becomes higher for large (>10 mm) aneurysms.
Imaging Modalities and Diagnosis
Subarachnoid Hemorrhage
Brain computed tomography (CT) remains the gold standard to rule out a possibility of SAH (see Fig. 51C.1, A). CT scan provides other pertinent information such as the presence of early hydrocephalus. The distribution and pattern of hemorrhage on CT scan are also important in predicting the location of the rupture site. The Fisher Scale classifies the appearance of subarachnoid hemorrhage based on CT scan (Box 51C.3) and is used not just to describe the pattern of hemorrhage but also to predict the probability of vasospasm. CT scan confirms subarachnoid hemorrhage in more than 90% of patients in the acute phase. The sensitivity of CT scan in detecting SAH decreases with the time interval after the hemorrhage because of the change in density of subarachnoid blood on CT. If there is a strong clinical suspicion for aneurysm rupture and a CT scan fails to reveal SAH, lumbar puncture should be performed. The sensitivity of lumbar puncture within 12 hours of hemorrhage is high when cases of traumatic lumbar puncture are eliminated. The characteristics of cerebrospinal fluid (CSF) for patients with SAH include increased red blood cell count and xanthochromia. Approximately 2 hours after the hemorrhage, xanthochromia becomes detectable and may last as long as several weeks. In a patient where there is the clinical suspicion for SAH but negative CT and lumbar puncture, angiography including CTA should be used to search for the cause.
Box 51C.3 Computed Tomography Scan Classification of Subarachnoid Hemorrhage (Fisher Scale)
Group 2: Diffuse deposition or thin layer of blood, with all vertical layers of blood (interhemispheric fissure, insular cistern, ambient cistern) <1 mm thick
Group 3: Localized clots or vertical layers of blood 1 mm or greater in thickness
Group 4: Diffuse or no subarachnoid hemorrhage but with intraparenchymal or intraventricular clots
Multimodal magnetic resonance imaging (MRI), including fluid-attenuated inversion recovery (FLAIR) and gradient echo (GRE) sequences, is becoming an important diagnostic tool to detect acute and chronic SAH. Recent data suggest that the GRE sequence demonstrates chronic blood, which is not apparent on CT (Kidwell et al., 2004). Although GRE overestimates the true volume of the hemorrhage because of the blooming artifact, it is very useful in detecting subacute or chronic SAH (Fig. 51C.6).
Aneurysm and Other Sources of Hemorrhage
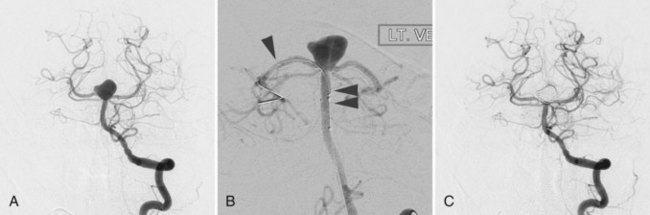
Current imaging modalities used to reveal and characterize the size, location, and morphology of intracranial aneurysms include MRA, CTA, and conventional catheter angiography. For the purpose of screening or as an initial method for evaluating unruptured intracranial aneurysms, less invasive imaging modalities, either MRA or CTA, are preferred. Noncontrast enhanced time-of-flight MRA is the least invasive modality to visualize intracranial vasculatures and is the best tool for screening, but it has lower sensitivity in detecting aneurysms with slow blood flow. The use of 3 tesla (3 T) MRA with contrast administration provides very clear visualization of the intracranial vasculature. Longer acquisition times and higher susceptibility to patient motion than CTA make it unsuitable as a first-line imaging modality in acute SAH. Technically, adequate four-vessel conventional catheter angiography (both two-dimensional [2D] and rotational three-dimensional [3D] angiography) is an important modality in the assessment of SAH, although there are some publications that reported success with CTA as the only diagnostic modality. However, in a case of clear SAH with negative CTA, four-vessel conventional catheter angiography with 3D reconstruction has to be considered. If four-vessel angiography and/or CTA does not reveal any source of the hemorrhage, six-vessel angiography (including external carotid injections) should be performed to rule out rare causes of hemorrhage such as a dural fistula or venous pathology. Use of 3D rotating catheter angiography is also essential to delineate the clear relationship of a saccular aneurysm to its parent and daughter arteries (Fig. 51C.7). Catheter angiography with carotid compression (Matas test and Alcock test) is used to visualize cross-flow via the anterior and posterior communicating arteries and is required if a parent artery occlusion must be considered as a treatment option.
In a patient with multiple aneurysms, identifying the rupture site requires thorough evaluation of all available information, including the distribution of SAH, clinical signs, and angiographic findings (Nehls et al., 1985). CT findings such as a focal parenchymal or cisternal hematoma adjacent to an aneurysm strongly suggest the rupture site. A larger and irregularly shaped aneurysm is more likely to be the rupture site. Focal spasm is also a reliable sign but relatively uncommon.
In approximately 10% to 15% of patients with SAH, no aneurysm or other source of hemorrhage can be detected. If the amount of SAH is small and its distribution is perimesencephalic, the diagnosis of benign perimesencephalic SAH may be established, and the prognosis is often good. It is believed that benign perimesencephalic SAH results from spontaneous rupture of perimesencephalic veins. In a patient with an extensive SAH but negative angiography, repeating the imaging studies, including catheter angiography or CTA, is mandatory. The repeat study should be performed 1 to 2 weeks after the initial study, paying particular attention to identifying small dissections. CTA with intravenous administration of contrast is more sensitive in identifying very slow-flowing lesions such as pseudoaneurysms from a small dissection (see Fig. 51C.5).
Symptoms
The majority of intracranial aneurysms remain asymptomatic until the time of rupture. The most common presentation of an intracranial aneurysm is SAH. Aneurysmal SAH is characterized by the sudden onset of severe headache of atypical quality, which patients often describe as “the worst headache of my life.” Other common symptoms associated with SAH include alteration in consciousness, nausea and vomiting, meningeal irritation, blurred vision, diplopia, photophobia, focal neurological deficits, and seizures. In patients with significant SAH in the posterior fossa, respiratory dysfunction and cardiovascular instability occur. Autonomic disturbances and hormonal dysfunction are not common symptoms, depending on the distribution of SAH. Thus the rupture of aneurysm and resultant SAH could elicit a variety of clinical symptoms. Depending on the clinical symptoms at the time of admission, the severity of SAH can be measured by using grading methods. The most widely used grading methods are summarized in Table 51C.2. These methods are known to correlate with clinical outcomes.
Table 51C.2 Most Commonly Used Clinical Grading Scales for Subarachnoid Hemorrhage
| HUNT AND HESS SCALE | ||
Despite the characteristic symptoms such as the sudden onset of headache, SAH patients with very mild or common symptoms are frequently misdiagnosed. A recent study showed that 1 in 20 SAH patients were misdiagnosed during emergency department visits (Vermeulen and Schull, 2007). By carefully interviewing patients with SAH, a history of an intense sentinel headache may be obtained in 30% to 60% (Okawara, 1973; Waga et al., 1975). Most sentinel headaches are attributed to warning leaks, partial ruptures, hemorrhage into the wall of an aneurysm, and/or expansion and stretching of an aneurysm wall. A retrospective clinical study found that more than 90% of patients initially presenting as grade 1 or 2 SAH achieved good or excellent outcome, whereas only 53% of patients with initial misdiagnosis could achieve the same clinical outcome (Mayer et al., 1996). Deterioration before correct diagnosis was the major reason for a poor or worse final outcome. Employing a full diagnostic workup to rule out an intracranial aneurysm among patients complaining of headache would be expensive and invasive. Nevertheless, it has to be emphasized that such patients with sentinel headaches or very mild SAH provide the best and possibly only opportunity to treat their aneurysms with good or excellent outcomes. When a patient complains of any unusual intense headache, a sentinel headache or very mild SAH has to be included in the differential diagnosis.
It is known that intracranial aneurysms, particularly internal carotid artery–posterior communicating artery aneurysms, present with a third cranial nerve palsy. Usually, unilateral ptosis, outward eye deviation, and a dilated pupil are the classic presentation of aneurysm-induced third nerve palsy. The nerve fibers controlling the pupils are located very superficially, so the ipsilateral pupil is often affected by the mass effect from an aneurysm or blood leakage from the aneurysm. Some textbooks suggest that pupil-sparing third nerve palsies are less likely to be associated with an intracranial aneurysm and that they often resolve uneventfully within a few weeks. However, a small series of 26 patients with third cranial nerve palsies due to intracranial aneurysms had one pupil-sparing patient whose deficit was caused by an aneurysm (Fujiwara et al., 1989). Thus, pupil-sparing third cranial nerve palsy does not exclude the possibility of an intracranial aneurysm. The same series also showed that retrobulbar pain was frequently observed in the patients with third nerve palsy due to unruptured intracranial aneurysms.
Treatment of Incidental Intracranial Aneurysms
Whether asymptomatic unruptured intracranial aneurysms found incidentally should be treated or managed conservatively has been debated for decades (ISUIA Investigators, 1998, 2003; Juvela et al., 1993; Murayama et al., 1999; Tsutsumi et al., 2000). Nevertheless, the prophylactic treatment of intracranial aneurysms has been a regular practice for many years, despite insufficient clinical data. After the ISUIA study, more small incidental intracranial aneurysms were conservatively followed than before. The ISUIA study suggested that the risk of aneurysm rupture increased as a function of the diameter of the aneurysm. As described in Natural History of Intracranial Aneurysms, risks and benefits of conservative management and aneurysm treatment must be carefully evaluated and discussed with the patient. The risks should not be limited to the annual or lifetime rupture rates from large clinical studies; other conditions such as a known vascular fragility, family history, cigarette smoking, and other comorbidities have to be considered as well. Cigarette cessation and adequate maintenance of normal blood pressure are the first steps in managing incidental intracranial aneurysms, regardless of the overall treatment plan.
The goal in treating patients with incidental intracranial aneurysms is to isolate the aneurysm from the circulation. Two present therapeutic alternatives to achieve this goal include direct surgical clipping and endovascular aneurysm occlusion using various embolic materials (Guglielmi et al., 1991; Johnston et al., 2000). Both approaches have their place in the treatment of unruptured intracranial aneurysms. Depending on the patient’s condition, aneurysm configuration, aneurysm size, and the vascular anatomy in the circle of Willis and proximal cervical vessels, the most suitable treatment modality should be selected for each patient.
Open Surgical Treatment
Direct surgical clipping has a longer history than endovascular coiling and more robust clinical data. A variety of surgical approaches have been established, and each approach is tailored to the specific anatomy and location of the intracranial aneurysm. Advances in the development of microsurgical techniques and extensive research on microsurgical anatomy allows safe surgical clipping procedures for true incidental aneurysms (Rice et al., 1990; Sano, 2010). A surgical clip is usually placed across the aneurysm neck, with preservation of the parent artery. Multiple surgical clips may be placed on an aneurysm that has branches so that the parent artery and its branches remain patent (Fig. 51C.8). Regardless of the aneurysm geometry, surgical clipping has proven to be highly effective, with reported rates of complete aneurysm occlusion of approximately 90% to 95%. When a clip cannot be safely applied at the neck of an aneurysm, alternative modalities such as wrapping or trapping may be performed (Fig. 51C.9). In principle, conventional surgery, particularly neck clipping, is definitive and highly effective in obliterating intracranial aneurysms. However, it may carry a significant risk of procedural morbidity and mortality. Many past reports, including the ISUIA study, document such risk (ISUIA Investigators, 1998, 2003). The surgical treatment of intracranial aneurysms of the posterior circulation, such as the upper basilar region, are associated with higher risks. Other known risk factors for surgery are advanced patient age, large or giant aneurysms, a past history of ischemic cerebrovascular disease, and symptomatic aneurysms other than rupture.
Endovascular Treatment
Endovascular treatment of intracranial aneurysms has evolved rapidly since the introduction of Guglielmi detachable coils (Guglielmi et al., 1991). In the endovascular procedure, a microcatheter is placed in the dome of an aneurysm, and it is tightly packed with a variety of coils to induce thrombosis (see Fig. 51C.1, C-D). Initially, endovascular coiling was performed only for those aneurysms not amenable to surgical clipping. Now many aneurysms with a relatively small neck are coiled. The difficulties of endovascular treatment of intracranial aneurysms are less dependent on their location and more dependent on their anatomical configuration. This nature of endovascular treatment is particularly beneficial to treat aneurysms located at the upper basilar and paraclinoid regions, where the surgical exposure of aneurysm is challenging (Tateshima et al., 2000). Conversely, endovascular treatment of middle cerebral artery aneurysms raises more technical difficulty because of the high incidence of wide-necked configurations, whereas surgical treatment of middle cerebral artery aneurysms is less difficult than other sites because they are closer to the brain surface and accessible with limited brain retraction (Suzuki et al., 2009).
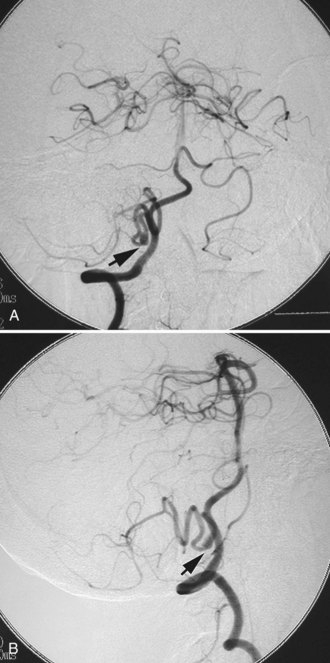
Some studies suggest that endovascular treatment of intracranial aneurysms may carry less procedural morbidity and mortality than conventional surgical treatment (ISUIA Investigators, 2003). There is, however, an intrinsic limitation in the current endovascular treatment of intracranial aneurysms, which is aneurysm recanalization. It is unlikely that a completely coiled small-necked aneurysm will recanalize. Wide-necked aneurysms which can not be completely embolized and have remaining aneurysm space in the neck or dome can have rates of aneurysm recurrence as high as 25% (Murayama et al., 2003). The invention and introduction of complex-shaped coils and intracranial stents have improved the success rate of endovascular coiling for fusiform or wide-necked aneurysms, as well as the long-term durability of the treatments (Mocco et al., 2009; Piotin et al., 2010) (Fig. 51C.10). A new treatment approach using the flow-diversion stent is one solution for aneurysm recurrence (Lylyk et al., 2009). Nevertheless, the issue of aneurysm recanalization remains, and a treating physician needs to take this fact into account when the treatment is for relatively young aneurysm patients. Endovascular treatment is more suitable for older patients because age was not a predictive factor for higher procedure morbidity and mortality rates (ISUIA Investigators, 2003).
Management of Subarachnoid Hemorrhage
As soon as a diagnosis of SAH is made, it is recommended that the patient be referred to a high-volume center where multidisciplinary care can be provided. The first important step in the management of SAH patients is to assess important variables such as neurological condition and cause of the hemorrhage. Depending on the patient’s neurological condition, the severity of SAH is measured using widely accepted grading systems (see Table 51C.5). A poor neurological condition may be due to direct damage to the brain tissue caused by the SAH, acute hydrocephalus secondary to intraventricular blood, and/or thick blood in the posterior fossa. Placement of a ventricular drainage catheter should be considered to treat hydrocephalus. Intraparenchymal hematoma may accompany SAH, which can also be the cause of poor neurological condition.
A patient with SAH needs to be under careful continuous observation (vital signs, electrocardiogram [ECG] monitoring, neurological condition, electroencephalogram [EEG] if necessary). However, bed rest and blood pressure control alone are not sufficient to decrease the risk of rebleeding; more definitive measures must be taken. The risk of rebleeding without any treatment for the ruptured aneurysm is estimated to be about 40% in the month after the initial rupture. The highest rate of rebleeding is observed within the first several hours of the initial bleed (Inagawa et al., 1987; Kassel and Torner, 1983). Only direct interventions including conventional open surgery and endovascular treatment prevent rebleeding, and a multidisciplinary approach is required to achieve this goal.
Treatment of Ruptured Aneurysms
There was debate in the past about the timing of surgical intervention. Early surgery has been shown to carry higher procedural morbidity and mortality. Nevertheless, it prevents devastating rebleeding and enables aggressive hypervolemia/hypertension/hemodilution (triple-H) therapy for cerebral vasospasm. Current evidence supports that either conventional open surgery or endovascular treatment should be performed as soon as possible after the onset of SAH unless contraindicated (Egge et al., 2002; Vinuela et al., 1997). The indication for open surgery is decided based on the grade of SAH (see Table 51C.5). Overall clinical condition of the patient, aneurysm location, aneurysm size, age of the patient, and presence of parenchymal hematoma are also determining factors. For a patient with a ruptured aneurysm in the anterior circulation and Hunt and Hess or World Federation of Neurosurgeons (WFNS) grades 1 to 3, surgical treatment is usually considered. Surgical treatment for a patient with Hunt and Hess or WFNS grades 4 and 5 is controversial owing to the overall poor outcome regardless of the surgery. Such poor-grade patients may be good candidates for intentionally delayed surgery or endovascular treatment.
After the introduction of Guglielmi detachable coils, endovascular treatment of ruptured aneurysms was performed for patients with poor-grade SAH or surgically challenging aneurysms. Unlike open surgery, the complications related to endovascular treatment for ruptured aneurysms are less dependent on the timing of intervention or aneurysm location (Vinuela et al., 1997). Endovascular treatment has gained acceptance as a safe alternative to open surgery, and it is being performed for patients with good-grade SAH or surgically treatable aneurysms.
Controversy remains about whether a ruptured aneurysm should be endovascularly coiled or surgically clipped, and the International Subarachnoid Aneurysm Trial (ISAT) was conducted to answer that question (Molyneux et al., 2002). ISAT is a multicenter prospective randomized controlled trial of endovascular coiling versus neurosurgical clipping in patients with ruptured cerebral aneurysms suitable for either therapeutic modality. A total of 9559 patients were assessed for eligibility, and 2143 (22.4%) were randomized to coiling (1073) versus clipping (1070). The study was terminated prematurely after a planned interim analysis found a 23.7% rate of modified Rankin Scale 3 to 6 (dependency or death) in the coiling cohort versus a 30.6% rate in the clipping cohort. This represents 22.6% relative risk reduction and 6.9% absolute risk reduction in the coiling cohort. Despite the slightly higher aneurysm recurrence or rebleeding risk in the coiling cohort, continued follow-up from ISAT has confirmed the superiority of coiling (Molyneux et al., 2005).
The results of ISAT do not apply to the whole SAH population and are only valid when a patient with a ruptured aneurysm qualifies for either treatment. In ISAT, a majority of patients (7416 out of 9559) were not enrolled. Among these, 2737 underwent endovascular treatment and 3615 surgical clipping. In ISAT there were more good-grade SAH patients and fewer posterior circulation aneurysms than in other large clinical series. This suggests that practitioners in the participating centers already favored the endovascular treatment for poor-grade patients and posterior circulation aneurysms. The less invasive nature of endovascular coiling may be favorable for older-age patients and those with serious comorbid medical conditions (Gonzalez et al., 2010).
There has been a debate about whether the incidence of symptomatic vasospasm differs following surgical clipping or endovascular coiling. Theoretically, surgical clipping has an advantage in this regard because the clot (a presumed cause of vasospasm) surrounding the ruptured aneurysm can be removed. Nevertheless, various clinical studies failed to show the benefit of surgical clot removal. Despite the fact that endovascular coiling leaves a subarachnoid clot in place, the incidence of symptomatic vasospasm does not differ between these two modalities (Dumont et al., 2010). It is conceivable that surgical manipulation, including the exposure of major arteries, retraction of brain, and the disruption of 3D integrity of subarachnoid space, may have an adverse effect resulting in the development of vasospasm. Based on current knowledge, it is reasonable to state that the amount of subarachnoid blood is not a major determining factor in selecting the appropriate treatment modality for patients with ruptured aneurysms.
Vasospasm and Delayed Cerebral Ischemia
Cerebral vasospasm, a delayed and sustained contraction of cerebral arteries, continues to be a leading cause of morbidity and mortality in patients with SAH after aneurysm rupture (Fig. 51C.11, A). It is not surprising that once blood leaks out of blood vessels, the blood or its breakdown products immediately irritate the adjacent vessels and induce a vasospasm. This is an important part of hemostasis and is part of the mechanisms in a human body that stop bleeding. However, what is unique about the cerebral vasospasm after SAH is the delayed occurrence after the hemorrhage and its relatively long duration. Cerebral vasospasm generally occurs in the first or second week after the hemorrhage and is therefore also called delayed cerebral ischemia. Vasospasm is initiated by the release of oxyhemoglobin, one of the blood breakdown products. However, the exact mechanism of cerebral vasospasm after SAH is not completely understood. The mechanism involves multifactorial processes and chemicals such as free radicals, lipid peroxidation, and the release of endothelin-1. Past studies on vasospasm have demonstrated prolonged smooth-muscle contraction in affected arteries. Hypertrophy, fibrosis, wall degeneration, and inflammatory changes were also observed.
Angiographic vasospasm is estimated to occur in as high as 70% of patients with aneurysmal SAH. Nevertheless, it becomes clinically symptomatic in about 30% of those patients (Romner and Reinstrup, 2001). Symptomatic vasospasm is a clinical manifestation resulting from cerebral ischemia produced by this arterial narrowing (Rusy, 1996). Patients who have only angiographic evidence of vasospasm do not have to be treated but require careful monitoring. Despite recent advancements in neuromonitoring and neuroimaging technologies, clinical observation in the neurointensive care unit plays a fundamental role in monitoring for cerebral vasospasm. New neurological signs or worsening of preexisting neurological deficits suggest symptomatic vasospasm. In a poor-grade SAH patient, subtle neurological worsening or new signs may be easily masked by preexisting deficits. In addition to clinical observation, transcranial Doppler (TCD) is frequently used as a noninvasive monitoring methodology for vasospasm. Daily TCD monitoring can provide useful information at the bedside in this setting. An elevation of blood flow velocity on TCD and/or high pulsatility index suggest cerebral vasospasm, although absolute TCD values may not always be reliable, particularly in the setting of triple-H therapy. Also, the quality of TCD monitoring is largely dependent upon the skill of the operator.
Although complete understanding of the mechanism of vasospasm is lacking, there has been some progress made in the treatment of patients with vasospasm. The main goal of treatment is to prevent permanent deficits from ischemia, not to treat angiographic vasospasm. Extensive clinical studies have been performed on the use of calcium channel antagonists to prevent symptomatic vasospasm by counteracting the contraction of smooth muscle cells in the affected arteries. As a result, nimodipine is approved in United States for treating vasospasm, including for prophylactic purposes. Studies have shown that nimodipine reduces morbidity from delayed ischemia due to vasospasm (Pickard et al., 1989). On the other hand, those studies also demonstrated that nimodipine did not reduce the actual frequency of vasospasm itself. Explanations could be the neuroprotective effect of nimodipine or the dilatation of smaller distal arterioles. Another calcium channel blocker, nicardipine, has been shown to reduce the frequency of vasospasm but not to affect clinical outcome.
Management of fluid and electrolyte balance is essential in preventing vasospasm and reducing its severity if it occurs. Hypomagnesemia is relatively common after SAH and known to be associated with a higher rate of cerebral vasospasm (van den Bergh and MASH Study Group, 2005). It is important to prevent hypovolemia by carefully monitoring fluid balance and central venous pressure. A past non-randomized study showed that hypervolemia reduced the frequency of delayed cerebral ischemia (Rosenwasser et al., 1983). When necessary, aggressive triple-H therapy is used to improve cerebral perfusion in those patients whose ruptured aneurysms are treated by either surgical clipping or endovascular coiling. Induced hypertension and increased cardiac output are beneficial and prevent or diminish neurological deficits resulting from symptomatic vasospasm. The optimal degree of hypervolemia to enhance cerebral perfusion is yet to be determined. It is, however, controversial whether lowering blood viscosity by hemodilution decreases the vascular resistance from distal small arteries including arterioles. Prophylactic triple-H therapy for asymptomatic vasospasm patients remains controversial. Aggressive triple-H therapy is not a risk-free treatment modality, and known complications include cardiac failure, pulmonary edema, hemorrhagic transformation of infarcted tissue, and worsening of cerebral edema (Solenski et al., 1995). Further clinical trials should be done to determine which groups of patients benefit from prophylactic triple-H therapy.
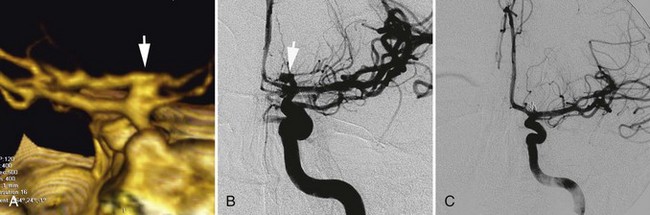
As neurointerventional technology has improved, endovascular treatment of cerebral vasospasm has become a treatment option. There are two types of endovascular treatment: angioplasty using a microballoon and superselective injection of vasodilator into the affected arteries (see Fig. 51C.11). The effect of balloon angioplasty lasts longer than vasodilator injections, and it is most useful in treating a severe spasm in relatively proximal arteries. On the other hand, superselective injection of vasodilators is most useful for distal spasm. A variety of vasodilators (e.g., papaverine, verapamil, fasudil hydrochloride) have been used (Elliott et al., 1998; Feng et al., 2002). Small clinical series clearly show that endovascular treatment of vasospasm improves the angiographic appearance of vasospastic vessels. However, no large clinical trials have demonstrated that endovascular treatment improves clinical outcome. Considering the rare but potentially very severe complications associated with endovascular treatment, it should not be a first-line treatment option.
There are some conditions that mimic symptomatic vasospasm. Hyponatremia is one of those conditions, and it is commonly seen after SAH. Hyponatremia does not just mimic the clinical symptoms of cerebral vasospasm but also is known to be associated with a higher incidence of vasospasm. The cause of hyponatremia is cerebral salt wasting in most cases. Unlike the treatment of dilutional hyponatremia, fluid restriction may harm the patient by increasing blood viscosity, aggravating ischemic symptoms from vasospasm. Thus, careful monitoring of sodium levels as well as fluid volume status is mandatory. Administration of 3% saline may be considered in hyponatremic patients with symptomatic vasospasm (Suarez et al., 1999).
Hydrocephalus Associated with Subarachnoid Hemorrhage
Unlike noncommunicating hydrocephalus in the acute phase, communicating hydrocephalus occurs in 5% to 30% of SAH patients 2 to 6 weeks after the initial hemorrhage (Germanwala et al., 2010). Location of the ruptured aneurysm, amount of subarachnoid blood, initial clinical grade of SAH, and patient age influence the development of chronic hydrocephalus. The mechanism of the development of chronic hydrocephalus is not yet understood. It has been believed that the blockage of arachnoid villi by blood breakdown products leads to insufficient absorption of CSF and results in chronic hydrocephalus. A single-center study suggested that the incidence of chronic hydrocephalus could be lowered by lamina terminalis fenestration on CSF dynamics (Tomasello et al., 1999). Thus, the etiology of chronic hydrocephalus might be multifactorial. Any disturbance of normal CSF dynamics in the ventricular system and subarachnoid space could contribute to the pathophysiology. It is not just a simple CSF absorption problem but also increased resistance in the CSF pathway due to the adhesion of arachnoid membrane secondary to SAH that could contribute to the development of chronic hydrocephalus. There was a debate about whether endovascular coiling of ruptured aneurysms might be associated with a higher incidence of chronic hydrocephalus. However, several studies suggested that the incidence of shunt-dependent hydrocephalus is not affected by the primary treatment modalities (Sethi et al., 2000).
Dumont A.S., Crowley R.W., Monteith S.J., et al. Endovascular treatment of neurosurgical clipping of ruptured intracranial aneurysms: effect on angiographic vasospasm, delayed ischemic neurological deficits, cerebral infraction, and clinical outcome. Stroke. 2010;41:2519-2524.
Egge A., Romner B., Waterloo K., Iet a.l. Results of surgery for aneurysmal subarachnoid haemorrhage in northern Norway: a retrospective study with special focus on timing of surgery in a rural area. Acta Neurol Scand. 2002;106:355-360.
Elliott J.P., Newell D.W., Lam D.J., et al. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998;88:277-284.
Feng L., Fitzsimmons B.F., Young W.L., et al. Intraarterially administrated verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol. 2002;23:1284-1290.
Fujiwara S., Fujii K., Nishio S., et al. Oculomotor nerve palsy in patients with cerebral aneurysms. Neurosurg Rev. 1989;2:123-132.
Germanwala A.V., Huang J., Tamargo R.J. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:263-270.
Gonzalez N.R., Dusick J.R., Duckwiler G., et al. Endovascular coiling of intracranial aneurysms in elderly patients: report of 205 treated aneurysms. Neurosurgery. 2010;66:714-721.
Guglielmi G., Vinuela F., Dion J., et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75:8-14.
Inagawa T. Multiple intracranial aneurysms in elderly patients. Acta Neurochir (Wien). 1990;106:119-126.
Inagawa T., Kamiya K., Ogasawara H., et al. Rebleeding of ruptured intracranial aneurysms in the acute stage. Surg Neurol. 1987;28:93-99.
Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010;73:155-164.
International Study of Unruptured Intracranial Aneurysms (ISUIA) Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment of rupture and risks of surgical intervention. Lancet. 2003;362:103-110.
International Study of Unruptured Intracranial Aneurysms (ISUIA) Investigators. Unruptured intracranial aneurysms–risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
Jakubowski J., Kendall B. Coincidental aneurysms with tumors of pituitary origin. J Neurol Neurosurg Psychiatry. 1978;41:972-979.
Johnston S.C., Wilson C.B., Halbach V.V., et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol. 2000;48:11-19.
Juvela S., Porras M., Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg. 1993;79:174-182.
Juvela S., Poussa K., Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001;32:485-491.
Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000;31:392-397.
Kassell N.F., Torner J.C. Aneurysmal rebleeding: a preliminary report from the cooperative aneurysm study. Neurosurgery. 1983;13:479-481.
Kelly P.J., Stein J., Shafqat S., et al. Functional recovery after rehabilitation for cerebellar stroke. Stroke. 2001;32:530-534.
Kidwell C.S., Chalela J.A., Saver J.L., et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823-1830.
Lou M., Caplan L.R. Vertebrobasilar dilative arteriopathy (dolichoectasia). Ann N Y Acad Sci. 2010;1184:121-133.
Lylyk P., Miranda C., Ceratto R., et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632-642.
Masuoka T., Hayashi N., Hori E., et al. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol Med Chir Tokyo. 2010;50:179-182.
Mayer P.L., Awad I.A., Todor R., et al. Misdiagnosis of symptomatic cerebral aneurysms. Prevalence and correlation with outcome at four institutions. Stroke. 1996;27:1558-1563.
Meyer F., Sundt T., Fode N., et al. Cerebral aneurysms in childhood and adolescence. J Neurosurg. 1989;70:420-425.
Miyazawa N., Akiyama I., Yamagata Z. Risk factors for growth of unruptured intracranial aneurysms: follow-up study by serial 0.5-T magnetic resonance angiography. Neurosurgery. 2006;58:1047-1053.
Mocco J., Snyder K.V., Albuquerque F.C., et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2009;110:35-39.
Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Molyneux A.J., Kerr R.S., Yu L.M., et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
Murayama Y., Nien Y.L., Duckwiler G., et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003;98:959-966.
Murayama Y., Viñuela F., Duckwiler G.R., et al. Embolization of incidental aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207-214.
Nakagawa T., Hashi K. The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg. 1994;80:217-223.
Nehls D.G., Flom R.A., Carter L.P., et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg. 1985;63:342-348.
Okawara S.H. Warning signs prior to rupture of an intracranial aneurysm. J Neurosurg. 1973;38:575-580.
Pickard J.D., Murray G.D., Illingworth R., et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid hemorrhage: British Aneurysm Nimodipine Trial. BMJ. 1989;298:636-642.
Piotin M., Blanc R., Spelle L., et al. Stent-assisted coiling of intracranial aneurysms. Clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010;41:110-115.
Pirson Y., Chauveau D., Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:269-276.
Qureshi A.I., Suarez J.I., Parekh P.D., et al. Risk factor for multiple intracranial aneurysms. Neurosurgery. 1998;43:22-27.
Rice B.J., Peerless S.J., Drake C.G. Surgical treatment of unruptured aneurysms of the posterior circulation. J Neurosurg. 1990;73:165-173.
Rinkel G.J., Djibuti M., Algra A., et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251-256.
Romner B., Reinstrup P. Triple H therapy after aneurysmal subarachnoid hemorrhage: a review. Acta Neurochir Suppl. 2001;77:237-241.
Ronkainen A., Hernesniemi J., Puranen M., et al. Familial intracranial aneurysms. Lancet. 1997;349:380-384.
Ropper A.H., Zervas N.T. Outcome 1 year after SAH from cerebral aneurysm. Management morbidity, mortality, and functional status in 112 consecutive good-risk patients. J Neurosurg. 1984;60:909-915.
Rosenwasser R.H., Delgado T.E., Buchheit W.A., et al. Control of hypertension and prophylaxis against vasospasm in cases of subarachnoid hemorrhage: a preliminary report. Neurosurgery. 1983;12:658-661.
Rusy K.L. Rebleeding and vasospasm after subarachnoid hemorrhage: a critical care challenge. Crit Care Nurse. 1996;16:41-48. February
Sahs A.L., Nibbelink D.W., Torner J.C. Aneurysmal Subarachnoid Hemorrhage: Report of the Cooperative Study. Baltimore: Urban & Schwarzenberg; 1981.
Sano H. Treatment of complex intracranial aneurysms of anterior circulation using multiple clips. Acta Neurochir Suppl. 2010;107:27-31.
Schievink W.I. Genetics of intracranial aneurysms. Neurosurgery. 1997;40:651-663.
Sethi H., Moore A., Dervin J., et al. Hydrocephalus: comparison of clipping and embolization in aneurysm treatment. J Neurosurg. 2000;92:991-994.
Smith W.S., Johnston S.C., Skalabrin E.J., et al. Spinal manipulative therapy is an independent risk factor for vertebral artery dissection. Neurology. 2003;60:1424-1428.
Solenski N.J., Haley E.C.Jr., Kassell N.F. Medical complications of aneurysmal subarachnoid haemorrhage: a report of the multicenter, cooperative aneurysm study. Crit Care Med. 1995;23:1007-1017.
Suarez J.I., Qureshi A.I., Parekh P.D., et al. Administration of hypertonic (3%) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:178-184.
Suzuki S., Tateshima S., Jahan R., et al. Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery. 2009;64:876-889.
Tateshima S., Murayama Y., Gobin Y.P., et al. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurg. 2000;47:1332-1342.
Tomasello F., d’Avella D., de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage? Neurosurgery. 1999;45:827-832.
Tsutsumi K., Ueki K., Morita A., et al. Risk of rupture from incidental cerebral aneurysms. J Neurosurg. 2000;93:550-553.
van den Bergh W.M., MASH Study Group. Magnesium sulfate in aneurysmal subarachnoid hemorrhage. A randomized controlled trial. Stroke. 2005;36:1011-1015.
Vermeulen M.J., Schull M.J. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke. 2007;38:1216-1221.
Vinuela F., Duckwiler G., Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475-482.
Waga S., Otsubo K., Handa H. Warning signs in intracranial aneurysms. Surg Neurol. 1975;3:15-20.
Wiebers D.O., Torner J.C., Meissner I. Impact of unruptured intracranial aneurysms on public health in the United States. Stroke. 1992;23:1416-1419.
Wilkinson I.M.S. The vertebral artery. Extracranial and intra-cranial structure. Arch Neurol. 1972;27:392-396.
Wojtacha M., Bazowski P., Mandera M., et al. Cerebral aneurysms in childhood. Childs Nerv Syst. 2001;17:37-41.
Yamaura A., Watanabe Y., Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183-188.
Yong-Zhong G., van Alphen H.A. Pathogenesis and histopathology of saccular aneurysms: review of the literature. Neurol Res. 1990;12:249-255.