Chapter 51B Vascular Diseases of the Nervous System
Intracerebral Hemorrhage
Intracerebral hemorrhage (ICH) accounts for approximately 10% of strokes. Its clinical importance derives from its high frequency and 30-day mortality, which is close to 50%. There has been a general decline since the 1980s in the incidence of stroke, including ICH, as a result of improved detection and treatment of hypertension. However, ICH continues to be a major public health problem, especially in populations at high risk such as young and middle-aged blacks and Hispanics, in whom ICH occurs more frequently than in whites, and the medically indigent who lack hypertension treatment. A growing body of evidence suggests that genetic factors such as possessing the E2 and E4 alleles of the apolipoprotein E gene play an important role in the occurrence of certain forms of ICH such as lobar hemorrhages (O’Donnell et al., 2000). Novel potential genetic factors predisposing to ICH continue to be added by experimental and clinical studies (Gould et al., 2006). Finally, the management of ICH is controversial as the assessment of various interventions awaits the completion of prospective clinical trials.
Mechanisms of Intracerebral Hemorrhage
Hypertension
In one study of 188 patients with primary ICH (i.e., excluding patients with hemorrhage associated with ruptured arteriovenous malformations [AVMs], tumor, anticoagulant and thrombolytic therapy, and cocaine ingestion), it was determined that the cause was hypertension in 72% of patients. Further support for the importance of hypertension in the pathogenesis of ICH is the steady increase in ICH incidence with advancing age, which is also associated with an increase in the prevalence of hypertension. The role of hypertension as a cause of ICH is most relevant for the nonlobar locations, which may be due to hypertension in approximately 50% of cases (Woo et al., 2002). In both hypertensive and nonhypertensive patients, the circadian rhythm of ICH onset, with peaks at 8 am and 8 pm (Casetta et al., 2002), coincides with the physiological daily peaks of blood pressure, pointing to the importance of blood pressure rises in the pathogenesis of ICH.
The vascular lesion produced by chronic hypertension that leads to arterial rupture and ICH is probably lipohyalinosis of small intraparenchymal arteries. The role of microaneurysms of Charcot and Bouchard is uncertain, although their anatomical location at sites preferentially affected by ICH supports their causal importance. The nonhypertensive causes of ICH are listed in (Box 51B.1).
Box 51B.1 Nonhypertensive Causes of Intracerebral Hemorrhage
Vascular malformations (saccular or mycotic aneurysms, arteriovenous malformations, cavernous angiomas)
Bleeding disorders, anticoagulant and fibrinolytic treatment
Granulomatous angiitis of the central nervous system and other vasculitides, such as polyarteritis nodosa
Vascular Malformations
Because a detailed discussion of intracranial aneurysms and AVMs is provided elsewhere (see Chapter 51C), the analysis is limited here to the role of small vascular malformations in the pathogenesis of ICH. These lesions are often documented by magnetic resonance imaging (MRI), by pathological examination of specimens obtained at the time of surgical drainage of ICHs, or at autopsy. However, cerebral angiography also plays an important role in the diagnosis of these lesions. In a group of 38 young ICH patients (mean age 46 years) subjected to angiography, AVMs were documented in 23 patients and aneurysms in 9 (a total of 32 of 38, or 84%). The ICH in these 38 patients had computed tomography (CT) characteristics suggestive of an underlying structural lesion (associated subarachnoid or intraventricular bleeding, calcification, prominent vascular structures, atypical ICH location). However, in a group of 42 patients lacking these CT features, angiography still documented vascular abnormalities in 10 (AVMs in 8, aneurysm in 2) (Halpin et al., 1994).
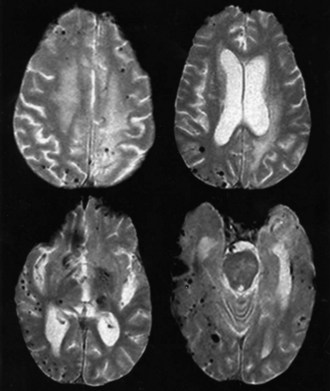
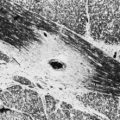
Cavernous angiomas are often recognized by MRI as a cause of ICH in the subcortical portions of the cerebral hemispheres and in the pons. This technique demonstrates a characteristic pattern on T2-weighted images, with a central nidus of irregular bright signal intensity mixed with mottled hypointensity (the “popcorn” pattern), surrounded by a peripheral hypointense ring corresponding to hemosiderin deposits (Fig. 51B.1), reflecting previous episodes of blood leakage at the edges of the malformation. These lesions are predominantly supratentorial, favoring the temporal, frontal, and parietal lobes, whereas the less frequent infratentorial locations favor the pons. They are generally single lesions, but multiplicity is not rare, especially in patients with familial cavernous angiomas. Familial clustering is common among individuals of Mexican descent, in whom cavernous angiomas are inherited in an autosomal dominant pattern linked to a mutation in chromosome 7q. Cavernous angiomas manifest with either seizures (27%-70%), ICH (10%-30%) or progressive neurological deficits (35%). ICH occurs in both the supratentorial and infratentorial varieties. A progressive course due to recurrent small hemorrhages within and around the malformation is occasionally seen in posterior fossa (especially pontine) lesions, and the deficits can evolve over protracted periods, at times suggesting a diagnosis of multiple sclerosis or a slowly growing brainstem tumor.
Intracranial Tumors
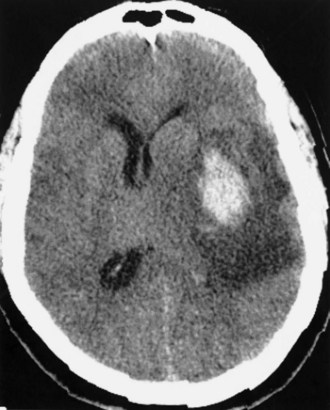
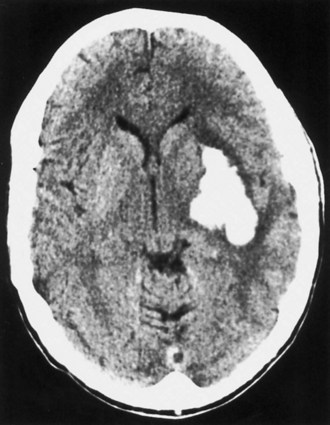
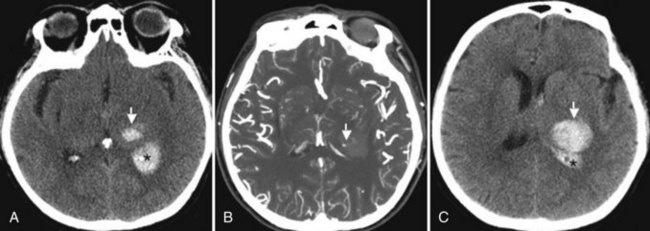
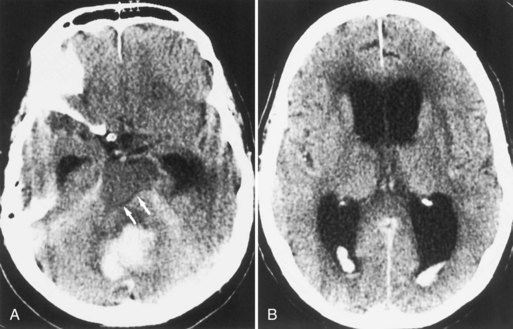
Bleeding into an underlying brain tumor is relatively rare in series of patients presenting with ICH, accounting for less than 10% of the cases. The tumor types most likely to lead to this rare complication are glioblastoma multiforme or metastases from melanoma, bronchogenic carcinoma, choriocarcinoma, or renal cell carcinoma (Fig. 51B.2). The ICHs produced in this setting may have clinical and imaging characteristics that should suggest an underlying brain tumor, including: (1) the presence of papilledema on presentation, (2) the location of ICH in sites that are rarely affected in hypertensive ICH, such as the corpus callosum, which in turn is commonly involved in malignant gliomas, (3) the presence of ICH in multiple sites simultaneously, (4) a CT scan characterized by a ring of high-density hemorrhage surrounding a low-density center in a noncontrast study, (5) enhancing nodules adjacent to the hemorrhage on contrast CT or MRI, and (6) a disproportionate amount of surrounding edema and mass effect associated with the acute hematoma (Fig. 51B.3). In these circumstances, a search for a primary or metastatic brain tumor should follow and include evaluation for systemic malignancy; if there is none, cerebral angiography and eventually craniotomy for biopsy of the wall of the hematoma cavity should be considered. Confirmation of the diagnosis of ICH secondary to malignant brain tumor carries a dismal prognosis, with a 30-day mortality rate in the 90% range.
Bleeding Disorders, Anticoagulants, and Fibrinolytic Treatment
Treatment with oral anticoagulants increases the risk of ICH by 8- to 11-fold compared with individuals with otherwise similar risk factors for ICH who are not receiving anticoagulants. Anticoagulant-related cases account for 9% to 11% of ICH. Potential risk factors for intracranial bleeding in patients receiving anticoagulants include advanced age, hypertension, preceding cerebral infarction, head trauma, and excessive prolongation of the International Normalized Ratio (INR). The last factor plays a major role in the pathogenesis of ICH in patients receiving oral anticoagulants. In the secondary stroke prevention trial, SPIRIT (Stroke Prevention in Reversible Ischemia Trial), 651 patients assigned to warfarin treatment were maintained at an INR of 3.0 to 4.5, resulting in 24 instances of ICH (14 fatal), in comparison with 3 ICHs (1 fatal) in the group of 665 patients treated daily with 30 mg of aspirin (The Stroke Prevention in Reversible Ischemia Trial Study Group, 1997). These data further support the recommendation that oral anticoagulation in patients with cerebrovascular disease should aim at an INR of 2 to 3 to reduce the frequency of this complication. The presence of severe leukoaraiosis on CT is an additional factor that independently increases the risk of ICH in patients on oral anticoagulants (Smith et al., 2002).
These hemorrhages have certain distinctive clinical characteristics: they tend to present with a slowly progressive course, at times over periods as long as 48 to 72 hours, in contrast with the usually more rapidly evolving presentation of hypertensive ICH; hematomas in patients receiving anticoagulants reach volumes that are, on average, larger than those occurring in hypertensive ICH, in turn resulting in the higher mortality rate of approximately 65%; signs of systemic bleeding rarely accompany ICH in this setting. Anticoagulant-related ICH may represent bleeding from vessels different from those involved in ICH of hypertensive origin (Hart et al., 1995). Certain angiopathies with bleeding potential, such as cerebral amyloid angiopathy (CAA), may play a causal role in the ICHs that occur in patients treated with anticoagulants (Rosand et al., 2000).
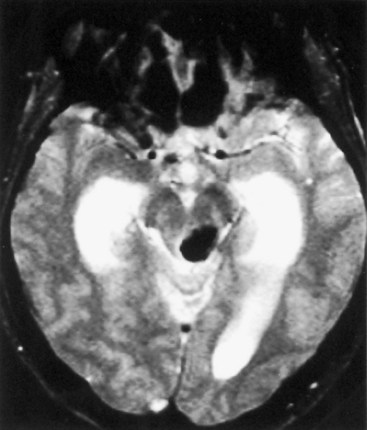
In addition to the anticoagulants, other substances with the potential for altering clot formation mechanisms are occasionally associated with ICH. These include drugs with fibrinolytic properties, such as streptokinase and tissue plasminogen activator (tPA). There is evidence to suggest that this complication of thrombolytic therapy may be favored by preexisting vasculopathies with bleeding potential such as CAA. Recombinant tPA for the treatment of acute ischemic stroke was complicated by ICH in 6.4% of cases (The National Institute of Neurological Diseases and Stroke [NINDS] rtPA Stroke Study Group, 1995), which is 10 times the rate found in the placebo group. Risk factors for ICH in this setting include a severe neurological deficit at presentation and documentation of hypodensity or mass effect on CT before treatment (The NINDS tPA Stroke Study Group, 1997). Intraarterial thrombolysis with prourokinase for middle cerebral artery occlusion leads to improved clinical outcomes but is associated with an 11% rate of early symptomatic ICH (Furlan et al., 1999). These hemorrhages occur at the site of the preceding cerebral infarct, are generally large (Fig. 51B.4), and carry a dismal prognosis (Kase et al., 2001). Hyperglycemia at pretreatment baseline has been identified as a potential risk factor for ICH in patients treated with either intraarterial prourokinase (Kase et al., 2001) or intravenous (IV) tPA (Bruno et al., 2002) for acute ischemic stroke. Another potential risk factor for ICH after intraarterial thrombolysis is the presence of incidental “microhemorrhages” (Fig. 51B.5), which can be easily detected with gradient echo (or “susceptibility-weighted”) MRI sequences (Kidwell et al., 2002). However, it is not yet clear whether these lesions are risk factors for postthrombolysis ICH; a large observational study failed to document such a relationship (Kakuda et al., 2005). Microhemorrhages are correlated with advancing age, ischemic stroke and ICH, as well as other manifestations of microangiopathy such as lacunar infarcts, leukoaraiosis, CAA, and the genetic disorder, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (Viswanathan and Chabriat, 2006). The role of microhemorrhages in causing ICH after use of anticoagulants, antiplatelet agents, and thrombolytics has not been clearly defined by prospectively collected data. As a result, no recommendations can be given at present for using or withholding these treatment options based solely on the presence of these often incidentally detected lesions (Koennecke, 2006).
Granulomatous Angiitis of the Central Nervous System and Other Vasculitides
Granulomatous angiitis of the CNS, also referred to as isolated angiitis of the CNS, is characterized by mononuclear inflammation with giant cell formation in the media and adventitia of small and medium-sized intracranial arteries and veins (see Chapter 51E). An associated element of intimal hyperplasia leads frequently to cerebral infarcts and occasionally to ICH.
Among the vasculitides, the other variety that is known to present with ICH is polyarteritis nodosa. As opposed to granulomatous angiitis of the CNS, this form of necrotizing vasculitis depicts prominent signs of systemic involvement including fever, malaise, weight loss, anemia, elevated erythrocyte sedimentation rate, and renal impairment with hypertension (see Chapter 49A).
Sympathomimetic Agents
Sympathomimetic agents can cause ICH after IV, oral, or intranasal use (see Chapter 58). The hemorrhages usually occur within minutes to a few hours after drug use, and the majority are located in the subcortical white matter of the cerebral hemispheres. In approximately half of reported cases, transient hypertension has been documented, as well as multifocal areas of spasm and dilatation (“beading”) of intracranial arteries on angiography. Although the latter is frequently referred to as a vasculitis or arteritis, histological proof is lacking, and this angiographic picture probably represents multifocal spasm secondary to the drug. The decongestant and appetite-suppressant, phenylpropanolamine, has been associated with ICH in young patients (median age in the early 30s), predominantly women (Kernan et al., 2000), usually without a history of hypertension but with acute hypertension on admission in a third of patients. Beading of intracranial arteries is frequent on angiography.
Cocaine (see Chapter 58) has become the most common sympathomimetic agent associated with ICH. Both ICH and subarachnoid hemorrhage can occur within short periods (generally minutes) of the use of both the alkaloid (free-base) form of cocaine and its precipitate form, known as crack. The ICHs favor the subcortical white matter but occasionally occur in the deep portions of the hemispheres (Fig. 51B.6). There may be multiple simultaneous ICHs, both deep and superficial, the mechanism of which remains unknown. In some instances, the origin of the ICH can be traced to a coexistent AVM or aneurysm, whereas the remainder are probably associated with either cocaine-induced vasoconstriction followed by reperfusion, heavy alcohol intake, or (rarely) a drug-induced cerebral vasculitis.
Hemorrhagic Infarction
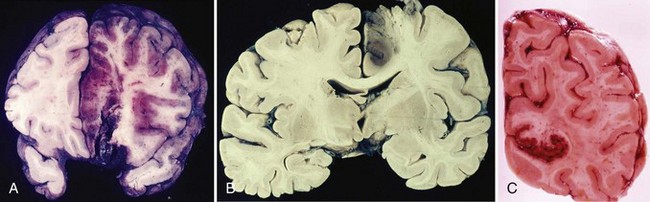
Hemorrhagic infarction is pathologically and pathogenically different from ICH in that it results from arterial or venous occlusion rather than from the vascular rupture that causes ICH. As a result, its pathological aspect is one of multifocal petechial hemorrhagic staining of an area of the brain primarily affected by ischemic necrosis (i.e., infarction) (Fig. 51B.7). Hemorrhagic infarction characteristically occurs in the setting of cerebral embolism or, less frequently, cerebral infarction secondary to venous occlusion (e.g., superior sagittal sinus thrombosis); in both, the bleeding reflects the mechanism of the infarct and is not due to therapeutic measures such as use of anticoagulant drugs.
Clinical differences between hemorrhagic infarction and ICH usually permit their clear distinction (Table 51B.1), but severe and confluent foci of hemorrhagic infarction may at times be difficult to distinguish from foci of primary ICH.
Table 51B.1 Differences Between Intracerebral Hemorrhage and Hemorrhagic Infarction
| Intracerebral Hemorrhage | Hemorrhagic Infarction (Embolic) | |
|---|---|---|
| CLINICAL | ||
| Onset | Sudden, followed by progression | Maximal from onset |
| Raised intracranial pressure | Prominent | Absent |
| Embolic source | No | Yes |
| COMPUTED TOMOGRAPHY | ||
| High attenuation | Dense, homogeneous | Spotted, mottled |
| Mass effect | Prominent | Absent or mild |
| Location | Subcortical, deep (gray nuclei) | Cortex more than subcortical white matter |
| Distribution | Beyond arterial territories | Along branch distribution |
| Late enhancement | Ring-type | Gyral-type |
| Ventricular blood | Yes | No |
| MAGNETIC RESONANCE IMAGING* | ||
| Hypointense blood (T2) | Homogeneous | Patchy, mottled |
| Hyperintense edema (T2) | Thin peripheral halo | Extensive, in vascular territory |
| ANGIOGRAM/MAGNETIC RESONANCE ANGIOGRAPHY | ||
| Characteristics | Mass effect (avascular) | Branch occlusion |
* Magnetic resonance imaging (MRI) depicts the same features as computed tomography (CT) in regard to mass effect, location, distribution, late enhancement, and ventricular blood. This table lists only the features MRI adds to those of CT.
Reprinted with permission from Kase, C.S., Mohr, J.P., Caplan, L.R., 2004. Intracerebral hemorrhage. In: Mohr, J.P., Choi, D.W., Grotta, J.C., et al. (Eds.), Stroke: Pathophysiology, Diagnosis, and Management, fourth ed. Churchill Livingstone, Philadelphia.
Head Trauma
ICH caused by cerebral contusion characteristically occurs in the surface of the brain, because its mechanism is one of direct brain trauma against its bony covering at the time of an acceleration-deceleration head injury (see Chapter 50B). This explains the sites of predilection for traumatic brain hemorrhages in the basal frontal, anterior temporal, and occipital areas, resulting from the coup and contrecoup mechanisms of injury. Thus traumatic brain hemorrhages are frequently multiple.
Clinical Features of Intracerebral Hemorrhage
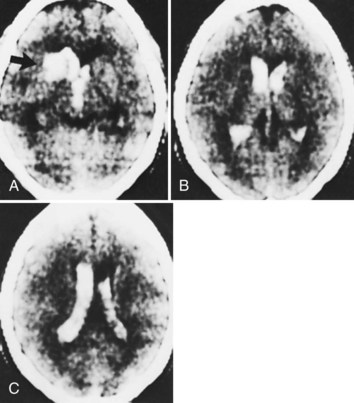
A characteristic of ICH at presentation is the frequent progression of focal neurological deficits over periods of hours. This early course reflects progressive enlargement of the hematoma (Fig. 51B.8), which at times amounts to volume increments of more than 300% as measured by serial CT scans (Brott et al., 1997). The presence of small foci of contrast extravasation, referred to as the spot sign, during CT angiography (CTA) in patients with acute ICH is predictive of hematoma enlargement (Goldstein et al., 2007; Wada et al., 2007). When CTA is performed within the first few hours post ICH onset, the presence of the spot sign (Fig. 51B.9) correlates with a frequency of hematoma enlargement in up to 77% of patients, compared to only 4% in patients without the sign (Wada et al., 2007). Further data have shown that features such as number of spot signs (>3), maximal diameter (>5 mm), and maximal attenuation (>180 Hounsfield units), are independent predictors of hematoma expansion (Thompson et al., 2009).
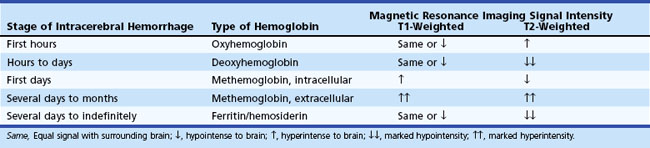
MRI adds further precision to the diagnosis of ICH, especially in determining the time elapsed between onset and time of MRI examination. The type of signal intensity change depicted by T1- and T2-weighted MRI sequences can be correlated with the hyperacute, acute, subacute, and chronic stages of evolution of an intracerebral hematoma (Table 51B.2).
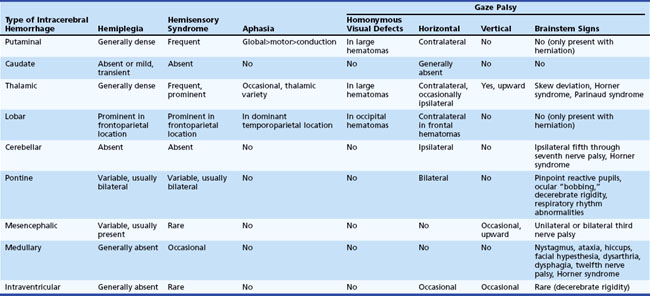
Physical examination findings that relate to the different anatomical locations of ICH are summarized in Table 51B.3.
Putaminal Hemorrhage
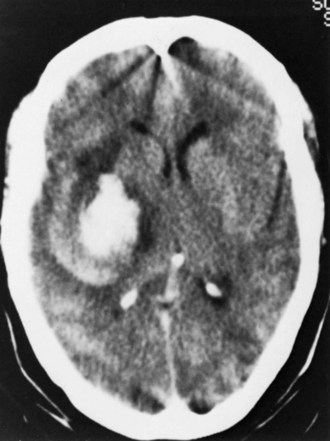
The most common variety of ICH, putaminal hemorrhage, represents approximately 35% of cases (Kase et al., 2004) (Fig. 51B.10). A wide spectrum of clinical severity relates to hematoma size, from minimally symptomatic cases presenting with pure motor hemiparesis or slight hemiparesis and dysarthria, to the extreme of coma with decerebrate rigidity in instances of massive hematomas with rupture into the ventricles. Modern CT series of putaminal hemorrhage document a mortality rate of 37%, in contrast to 65% to 75% from pre-CT data. This difference reflects the description of the full spectrum of hematoma size in recent reports, including smaller hematomas with benign outcomes, which were misdiagnosed as infarcts in the pre-CT era. Ventricular extension carries an invariably poor prognosis in putaminal hemorrhage.
Caudate Hemorrhage
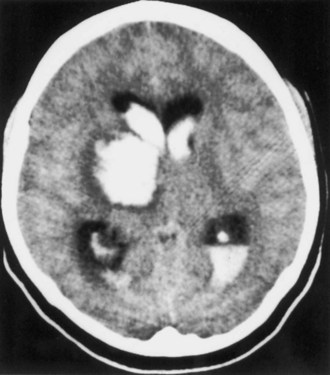
Caudate hemorrhage is a rare variety of ICH that accounts for only approximately 5% of cases (Kase et al., 2004) (Fig. 51B.11). It results from rupture of penetrating arteries from the anterior and middle cerebral arteries, and its most common cause is hypertension. Presentation is similar to that of subarachnoid hemorrhage in that the clinical picture is dominated by signs of intracranial hypertension and meningeal irritation, with focal neurological deficits (hemiparesis, horizontal gaze palsy, Horner syndrome) being minimal and transient or altogether absent. At times, the main manifestations of caudate ICH are neuropsychological deficits including abulia, disorientation, and memory disturbances, occasionally accompanied by language disturbances (Kumral et al., 1999). The main differential diagnosis of caudate ICH is ruptured anterior communicating artery aneurysm with bleeding through the septum pellucidum into the ventricular system. In this instance, CT shows blood in the interhemispheric fissure and in the lowermost frontal cuts, as opposed to the higher location of the unilateral clot in the head of one caudate nucleus in primary caudate ICH. Ventricular extension of the hemorrhage is a regular feature in caudate ICH, and hydrocephalus is usually present. Nevertheless, the outcome is generally good. The majority of patients recover without neurological sequelae, although at times neuropsychological deficits persist.
Thalamic Hemorrhage
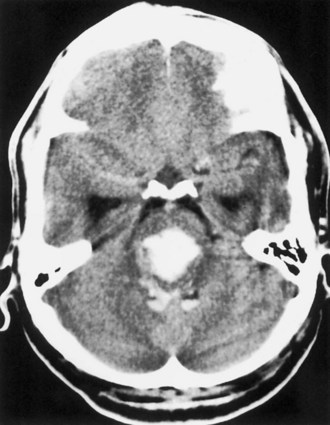
Thalamic hemorrhage represents 10% to 15% of ICH cases (Kase et al., 2004) (Fig. 51B.12). Its onset tends to be more abrupt than that of putaminal hemorrhage, and slow progression of deficits is less common. These features may reflect early communication of the medially located hematoma with the third ventricle. The prognosis in thalamic hemorrhage relates to hematoma size and level of consciousness at presentation (Kumral et al., 1995). Another reliable sign of poor prognosis in thalamic ICH is the presence of hydrocephalus, an occasional complication that occurs abruptly secondary to aqueductal obstruction by an intraventricular clot; here, however, ventriculostomy may result in a reversal of symptoms.
Lobar Hemorrhage
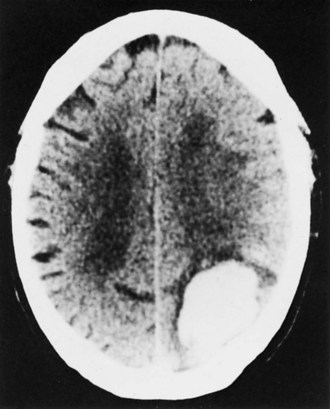
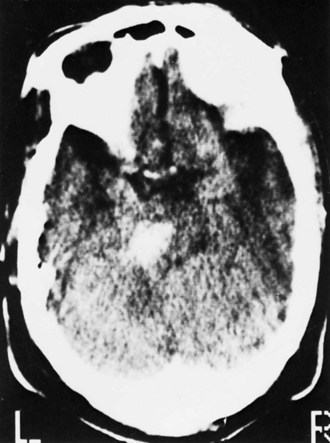
Lobar hemorrhage is second to putaminal hemorrhage in frequency, accounting for approximately 25% of ICH cases (Kase et al., 2004) (Fig. 51B.13). Nonhypertensive mechanisms including AVMs, sympathomimetic agents (in young patients), and CAA (in elderly patients) are frequent causes. The peripheral (subcortical) location of these hematomas explains the lower frequency of coma at onset, as compared with the deep ganglionic forms of supratentorial ICH. Although seizures at the time of presentation of ICH are rare, they occur in as many as 28% of patients with lobar ICH. The clinical features reflect location: hemiparesis of upper limb predominance in frontal hematomas, sensorimotor deficit and hemianopia in parietal hemorrhages, fluent aphasia with relatively preserved repetition in dominant temporal hematomas, and homonymous hemianopia in occipital lobe hemorrhages. The mortality rate in individuals with lobar ICH is lower than in those with hematomas in other locations, and the long-term functional outcome may also be better.
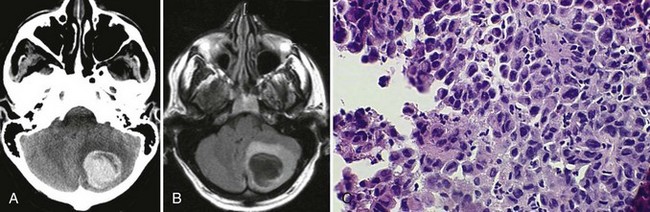
Cerebellar Hemorrhage
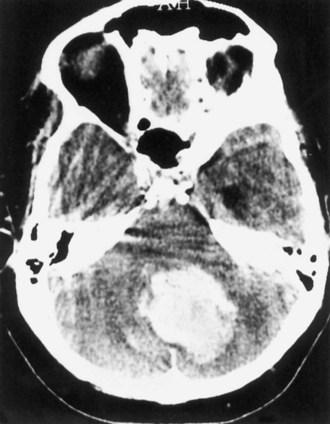
Cerebellar hemorrhage represents approximately 5% to 10% of ICH cases (Kase et al., 2004) (Fig. 51B.14). Its clinical presentation is characteristic, with abrupt onset of vertigo, headache, vomiting, and inability to stand and walk, but absence of hemiparesis or hemiplegia. The physical findings that allow its clinical diagnosis are the triad of appendicular ataxia, horizontal gaze palsy, and peripheral facial palsy, all ipsilateral to the hemorrhage.
Pontine Hemorrhage
Pontine hemorrhage represents approximately 5% of ICH cases (Kase et al., 2004) (Fig. 51B.15). The massive bilateral basal-tegmental variety produces the classic picture of coma, quadriplegia, decerebrate posturing, horizontal ophthalmoplegia, ocular bobbing, pinpoint reactive pupils, abnormalities of respiratory rhythm, and preterminal hyperthermia. Since the introduction of CT and MRI, less severe forms of pontine hemorrhage are recognized that are compatible with survival. These hemorrhages are frequently located in the tegmentum, lateral to the midline (Fig. 51B.16), and thus produce syndromes of predominantly unilateral dorsal pontine involvement (“one-and-a-half” syndrome, internuclear ophthalmoplegia, fifth and seventh nerve palsies), with variable degrees of long-tract interruption. These hematomas result from rupture of distal tegmental branches of a long circumferential artery originating from the basilar trunk.
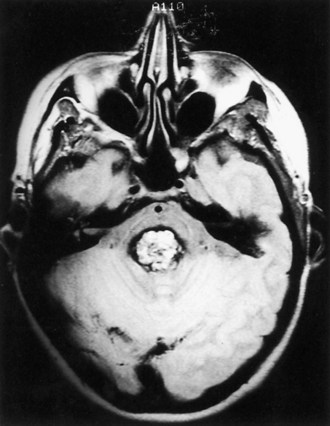
Mesencephalic Hemorrhage
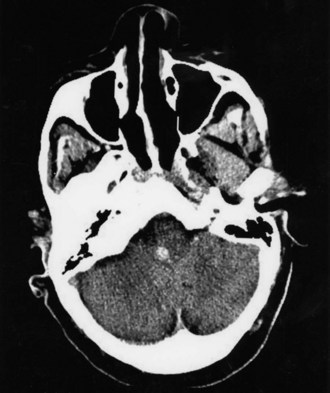
Mesencephalic hemorrhage is exceptionally rare (Kase et al., 2004). The causal mechanism was hypertension or ruptured AVM in half of the reported cases, the others being of undetermined cause. Occasional unilateral hematomas (Fig. 51B.17) can present with ipsilateral third nerve palsy, cerebellar ataxia, and contralateral hemiparesis. Bilateral cases frequently have prominent tectal-tegmental signs, with bilateral ptosis, paralysis of upward gaze, and small pupils with light-near dissociation (see Chapter 19). Often patients survive without surgical treatment, but with persistent sequelae.
Medullary Hemorrhage
Examples of pure primary ICH involving the medulla alone (Fig. 51B.18) are rare, with most reported cases representing medullary extension of caudal pontine hematomas. The clinical presentation of primary medullary hemorrhage reflects the location of the lesion on one-half of the medulla, generally extending beyond the dorsolateral region, both medially (resulting in ipsilateral hypoglossal nerve palsy) and ventrally (resulting in contralateral hemiparesis). These two features distinguish most examples of medullary hemorrhage from the classical presentations of Wallenberg lateral medullary syndrome, caused by infarction rather than hemorrhage (see Chapter 19).
Intraventricular Hemorrhage
The clinical presentation of intraventricular hemorrhage is with acute onset of headache, nausea, vomiting, and decreased level of consciousness, with focal neurological deficits either minimal or altogether absent (Martí-Fàbregas et al., 1999). This presentation is identical to that of subarachnoid hemorrhage from ruptured aneurysm or AVM. If focal deficits such as hemiparesis or ocular motor disturbances are prominent, the picture is not strictly that of a pure intraventricular hemorrhage but rather one of primary ICH with ventricular extension.
The prognosis of intraventricular hemorrhage is strongly dependent on the severity of the initial manifestation and its mechanism. Patients who are comatose as a result of the initial hemorrhage generally succumb, especially if they have early signs of brainstem involvement (ophthalmoparesis, loss of pupillary reflexes, decerebrate rigidity). Those who remain alert or obtunded without signs of parenchymal involvement tend to recover without neurological sequelae, although memory disturbances may be a relatively frequent residual deficit (Martí-Fàbregas et al., 1999). Patients with the idiopathic form of intraventricular hemorrhage have the best prognosis.
Treatment of Intracerebral Hemorrhage
General Management of Intracerebral Hemorrhage
Initial Evaluation
Laboratory testing in cases suggestive of ICH should include coagulation studies, especially in instances of hemorrhage in patients receiving anticoagulants or those previously treated with thrombolytic agents. Coagulation abnormalities in patients receiving anticoagulants should be treated emergently because if anticoagulation is not reversed, it can lead to progressive enlargement of the hematoma. Patients with ICH in the setting of heparin anticoagulation should be treated with protamine sulfate, 1 mg per 100 units of heparin estimated in plasma, whereas those on warfarin should receive 5 to 25 mg of IV vitamin K1 and, most important, fresh frozen plasma (10-20 mL/kg) or prothrombin complex concentrate. In view of the expected delays in having fresh frozen plasma immediately ready in these instances, the current availability of recombinant factor VIIa for IV injection offers the option of a more rapid reversal of the abnormally prolonged INR in cases of warfarin-related ICH (Deveras and Kessler, 2002). Instances of ICH after thrombolytic therapy are best treated with 4 to 6 units of cryoprecipitate or fresh frozen plasma, as well as single-donor platelets.
General Measures for Prevention of Further Elevation of Intracranial Pressure
General measures include control of hypertension and treatment of seizures. The former can be necessary because persistent hypertension, by causing increased cerebral perfusion pressure, may produce an increase in cerebral edema around the ICH, with further elevation of ICP. However, this potential benefit of antihypertensive therapy must be balanced against the possible harmful effects of drug-induced hypotension, with resulting cerebral ischemia and further neurological deterioration. This difficult clinical problem is compounded by the lack of knowledge concerning optimal balance between adequate cerebral perfusion and control of ICP. Pharmacological correction of severe hypertension (blood pressure >180/105 mm Hg) is recommended in the acute phases of ICH, with the goal being maintenance of normal cerebral perfusion pressure levels on the order of 60 to 70 mm Hg, aiming at a blood pressure of 160/90 mm Hg (Morgenstern et al., 2010). The antihypertensive agent of choice in this setting is the IV beta- and alpha-blocking agent, labetalol, often used in combination with loop diuretics. The use of the IV calcium channel blocker, nicardipine, is an equally appropriate choice in this setting in view of its lack of cerebral vasodilatory effect. These IV agents have the advantage of being rapidly effective and easy to titrate.
Choice Between Medical and Surgical Therapy in Intracerebral Hemorrhage
Six randomized clinical trials compared surgical with nonsurgical treatment of ICH, and the results were generally inconclusive, mostly because of methodological issues (Fernandes et al., 2000). Mendelow and associates (2005) reported the results of a prospective international multicenter clinical trial comparing surgical and nonsurgical treatment of ICH. The international STICH (Surgical Trial of Intracerebral Haemorrhage) trial randomized 1033 patients into each treatment arm, with the surgery for hematoma evacuation being performed within 4 days of ICH onset. The primary trial outcome, death or disability (measured with the extended Glasgow Outcome Scale) at 6 months, was virtually identical in the two groups: 74% in the surgical group and 76% in the nonsurgical group. Similarly, mortality at 6 months was 36% and 37%, respectively. Prespecified subgroup analyses showed no superiority of one treatment modality over the other, with the only exception being that hematomas located at a depth of less than 1 cm from the cortical surface fared better with surgical treatment. This well-conducted prospective study has added to the mounting evidence of a lack of benefit of surgical treatment for most varieties of supratentorial ICH (Juvela and Kase, 2005).
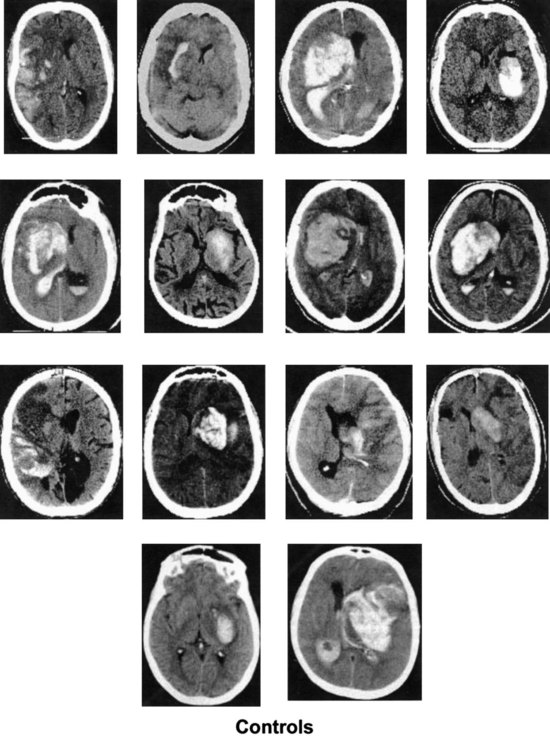
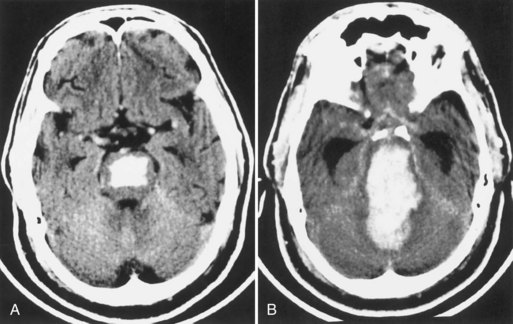
The other group for whom surgery is frequently considered includes patients with cerebellar hemorrhage. Although a benign outcome without surgical evacuation is well documented in small cerebellar hemorrhages, the potential for sudden deterioration to coma and death, not infrequently after a clinically stable course under hospital observation, is well recognized. CT criteria for early selection of candidates for surgical therapy are large hematomas (diameter of 3 cm or more), presence of hydrocephalus, and obliteration of the quadrigeminal cistern (Fig. 51B.19). In addition to these CT features, early signs of pontine tegmental compression and development of obtundation and extensor plantar responses constitute indications for emergency surgical therapy, because otherwise the outcome is often fatal.
In addition to direct evacuation of a hematoma, there is the option of ventricular drainage for the relief of hydrocephalus and increased ICP in cases of cerebellar, thalamic, and caudate ICH. In cerebellar hemorrhage, massive hydrocephalus can be a major cause of clinical deterioration, and ventriculostomy may provide dramatic improvement. Because ventricular drainage does not diminish compression of the brainstem, and because of the potential for upward transtentorial cerebellar herniation following decompression of the supratentorial ventricular system, this approach is only rarely the sole form of surgical treatment. It may, however, be used immediately before occipital craniectomy for decompression of the posterior fossa and hematoma drainage. Patients with thalamic hemorrhage occasionally show a dramatic reversal of oculomotor signs, coma, or both, after ventricular drainage. Patients with primary intraventricular hemorrhage and hydrocephalus benefit from ventricular drainage as well. Preliminary data suggest that ventricular drainage facilitated by local intraventricular instillation of tPA achieves a more rapid and efficient removal of the intraventricular blood, without an increase in the risk of rebleeding. This approach is currently being evaluated in a prospective randomized clinical trial that is correlating the removal of blood from the ventricular system with clinical outcomes (Morgan et al., 2008).
Hemostatic Therapy of Intracerebral Hemorrhage
The general scarcity of effective surgical therapies for ICH, plus the documented tendency of hematomas to enlarge after onset, have stimulated an interest in developing treatments aimed at retarding this process. Mayer and colleagues (2005) tested the procoagulant agent, recombinant activated factor VII (rFVIIa), in 399 patients with ICH within 4 hours from symptom onset and documented a significant reduction in hematoma growth with three dosages of rFVIIa (40, 80, 160 µg/kg) in comparison with placebo. This was also associated with a significant trend in favor of rFVIIa when clinical outcomes and mortality were compared at 90 days. Thromboembolic complications were more frequent in the rFVIIa groups (7%) than in the placebo group (2%). These encouraging preliminary results were tested in the phase III FAST (rFVIIa in Acute Haemorrhagic Stroke Treatment) trial. This study compared rFVIIa in two dosages (20 and 80 µg/kg) with placebo in patients with ICH treated within 4 hours from onset. Although the subjects treated with 80 µg/kg of rFVIIa had a significantly smaller increase in hematoma volume at 24 hours post treatment, this was not translated into clinical benefit: mortality and severe disability at 90 days occurred with essentially the same frequency in the three treatment groups (Mayer et al., 2008). In addition, the rate of arterial thromboembolic complications was significantly higher (10%) in the group that received rFVIIa at a dose of 80 µg/kg, in comparison with placebo (5%). Further testing of this agent is likely to be limited to specific patient subpopulations. One such group is that of patients at high risk of hematoma expansion, with a positive “spot sign” detected on CTA early after presentation with ICH. The NINDS-sponsored STOP-IT trial is testing rFVIIa against placebo in this setting.
Brott T., Broderick J., Kothari R., et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
Bruno A., Levine S.R., Frankel M.R., et al. Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology. 2002;59:669-674.
Casetta I., Granieri E., Portaluppi F., et al. Circadian variability in hemorrhagic stroke. JAMA. 2002;287:1266-1267.
Deveras R.A.E., Kessler C.M. Reversal of warfarin-induced excessive anticoagulation with recombinant human factor VIIa concentrate. Ann Intern Med. 2002;137:884-888.
Fernandes H.M., Gregson B., Siddique S., et al. Surgery in intracerebral hemorrhage: The uncertainty continues. Stroke. 2000;31:2511-2516.
Furlan A., Higashida R., Wechsler L., et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA. 1999;282:2003-2011.
Goldstein J.N., Fazen L.E., Snider R., et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889-894.
Gould D.B., Phalan F.C., van Mil S.E., et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489-1496.
Halpin S.F., Britton J.A., Byrne J.V., et al. Prospective evaluation of cerebral angiography and computed tomography in cerebral haematoma. J Neurol Neurosurg Psychiatry. 1994;57:1180-1186.
Hart R.G., Boop B.S., Anderson D.C. Oral anticoagulants and intracranial hemorrhage: facts and hypotheses. Stroke. 1995;26:1471-1477.
Juvela S., Kase C.S. Advances in intracerebral hemorrhage management. Stroke. 2005;37:301-304.
Kakuda W., Thijs V.N., Lansberg M.G., et al. Clinical importance of microbleeds in patients receiving IV thrombolysis. Neurology. 2005;65:1175-1178.
Kase C.S., Furlan A.J., Wechsler L.R., et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603-1610.
Kase C.S., Mohr J.P., Caplan L.R. Intracerebral hemorrhage. Mohr J.P., Choi D.W., Grotta J.C., et al. Stroke: Pathophysiology, Diagnosis and Management, fourth ed, Philadelphia: Churchill Livingstone, 2004.
Kernan W.N., Viscoli C.M., Brass L.M., et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826-1832.
Kidwell C.S., Saver J.L., Villablanca P., et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke. 2002;33:95-98.
Koennecke H.-C. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165-171.
Kumral E., Evyapan D., Balkir K. Acute caudate vascular lesions. Stroke. 1999;30:100-108.
Kumral E., Kocaer T., Ertubey N.O., et al. Thalamic hemorrhage: a prospective study of 100 patients. Stroke. 1995;26:964-970.
Martí-Fàbregas J., Piles S., Guardia E., et al. Spontaneous primary intraventricular hemorrhage: clinical data, etiology and outcome. J Neurol. 1999;246:287-291.
Mayer S.A., Brun N.C., Begtrup K., et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777-785.
Mayer S.A., Brun N.C., Begtrup K., et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127-2137.
Mendelow A.D., Gregson B.A., Fernandes H.M., et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomized trial. Lancet. 2005;365:387-397.
Morgan T., Awad I., Keyl P., et al. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir. 2008;105:217-220.
Morgenstern L.B., Hemphill J.C., Anderson C., et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2001-2023.
National Institute of Neurological Diseases and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587.
National Institute of Neurological Diseases and Stroke t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109-2118.
O’Donnell H.C., Rosand J., Knudsen K.A., et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240-245.
Rosand J., Hylek E.M., O’Donnell H.C., et al. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947-951.
Smith E.E., Rosand J., Knudsen K.A., et al. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59:193-197.
Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group. A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin. Ann Neurol. 1997;42:857-865.
Thompson A.L., Kosior J.C., Gladstone D.J., et al. Defining the CT angiography ‘spot sign’ in primary intracerebral hemorrhage. Can J Neurol Sci. 2009;36:456-461.
Viswanathan A., Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37:550-555.
Wada R., Aviv R.I., Fox A.J., et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257-1262.
Woo D., Sauerbeck L.R., Kissela B.M., et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190-1196.