Vascular Abnormalities of the Liver
Portal Hypertension
Overview: Portal hypertension is defined as a rise in pressure within the splanchnic venous system above 10 mm Hg. This increase in pressure may result from either increased resistance to hepatic venous drainage (presinusoidal and postsinusoidal) or increase in inflow pressure, such as is seen in persons with an arterioportal fistula.1 The pathophysiology and clinical presentation of portal hypertension depend on the underlying hepatic disorder.
Etiology: Cirrhosis, the most common cause of portal hypertension, is due to hepatic scarring resulting from chronic liver injury and is associated with deterioration of liver function. In pediatric patients, cirrhosis can result from a variety of conditions, including biliary atresia, cystic fibrosis, hemochromatosis, and Wilson disease (Box 93-1).2 The Child-Turcotte-Pugh classification system provides a severity score that plays a part in treatment decisions.3 Patients are stratified into grades A through C based on bilirubin elevation, albumin level, prothrombin time, ascites, and severity of encephalopathy.3,4
Patients with presinusoidal portal hypertension, such as extrahepatic portal vein occlusion, do not have intrinsic hepatic dysfunction. Extrahepatic portal vein occlusion is a relatively frequent cause of portal hypertension in children. It is increased in incidence in cases of complicated umbilical vein catheterization, sepsis, dehydration, hyperviscosity, shock, coagulopathy, and portal vein thrombosis, in persons with hypercoagulable syndromes (such as antithrombin III deficiency), and in persons with congenital portal venous webs, but it may occur without an identifiable cause.2,5 Cavernous transformation of the portal vein is a result of portoportal collaterals that develop along the thrombosed portal vein and that have been observed to occur in as little as 1 to 3 weeks after acute thrombus.6
Postsinusoidal obstruction includes primary liver disease with cirrhosis or hepatic vein obstruction, such as Budd-Chiari syndrome or hepatic venoocclusive disease, as seen in patients who have had bone marrow transplantation.2
Clinical Presentation: Children may present with the consequences of portal hypertension, such as gastrointestinal bleeding, unexplained splenomegaly, and hypersplenism without jaundice, ascites, or cholestasis.7,8
Imaging: Splenic enlargement may be the earliest imaging finding of portal hypertension. As resistance to portal flow increases, portal venous flow slows down, portal vein diameter decreases, and, with high resistance, portal venous flow may become reversed or even arterialized. In persons with a normal liver, the portal vein has a larger cross-sectional area than the splenic vein.8,9 If instead the portal vein is smaller than the splenic vein, the presence of collaterals diverting portal flow away from the liver must be assumed (Fig. 93-1). Reversal of venous flow in the superior mesenteric and splenic veins also is suggestive of collaterals and spontaneous portosystemic shunting. Hepatopetal flow in the main portal vein does not exclude severe portal hypertension when collaterals are present or when lobe-to-lobe shunting occurs; hepatofugal flow is a late finding in persons with portal hypertension.8,9
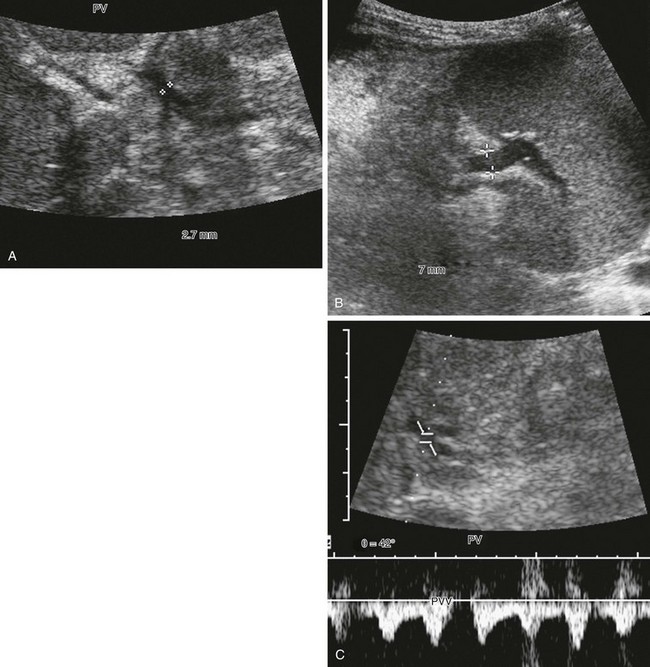
Figure 93-1 Severe portal hypertension following a Kasai portoenterostomy in a 2-year-old girl with biliary atresia. Ultrasound imaging was performed in preparation for liver transplantation.
A, The main portal vein is very small, measuring 2.7 mm in diameter (calipers). Note the heterogeneous echotexture of the liver. B, The splenic vein is much larger, measuring 7 mm in diameter (calipers). This combination alerts one to the presence of collateral veins and varices. C, Spectral Doppler imaging of the main portal vein (PV), which is reversed and arterialized, reflects severe liver disease with high resistance to flow, causing the portal vein to become a draining vein to the hepatic artery through arterioportal communications.
When portal venous pressure increases in the setting of portal hypertension, splanchnic venous return finds alternative drainage pathways connecting the portal circulation to the systemic circulation. Left gastric and splenic vein branches drain into the azygos system through esophageal and gastric varices, which in turn drain into the inferior vena cava (IVC) via the left renal vein. Paraumbilical veins (Fig. 93-2) communicate with inferior epigastric and internal mammary abdominal wall venous networks to drain into the inferior and superior vena cava, respectively. These collateral pathways can be visualized with cross-sectional imaging.6
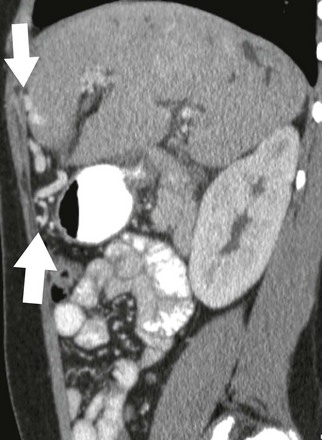
Figure 93-2 Paraumbilical collaterals in a 12-year-old girl with hepatic fibrosis, cirrhosis, and portal hypertension.
A sagittal contrast-enhanced computed tomography image shows enhancing paraumbilical venous collaterals and enhancing internal mammary collateral vessels anterior to the liver (arrows).
Additional collateral pathways develop with portal hypertension. Retroperitoneal and peripancreatic collaterals drain through renal and gonadal veins into the IVC and into paraspinal veins, which drain into the azygos system (Fig. 93-3). Inferior mesenteric branches drain into superior, middle, and inferior hemorrhoidal veins that lead to the iliac veins. Portosystemic collaterals also may form at enterocutaneous junctions in fistulas and enterostomies. Surgical anastomoses, such as a Roux-en-Y biliary-enteric anastomosis, may serve as sites of portoportal collaterals (Fig. 93-4). Finally, intercostal and phrenic veins also may serve as a means of portosystemic communication across the diaphragm.6
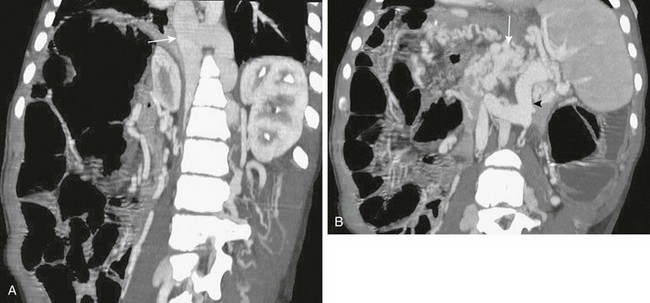
Figure 93-3 A liver transplant patient with an occluded portal vein and inferior vena cava.
A, Retroperitoneal, paraspinal, and intraspinal venous collaterals are present. The azygos and hemiazygos veins are massively enlarged (arrow). B, Extensive pancreatic (arrow) and perisplenic collaterals are present, along with a splenorenal shunt (arrowhead).
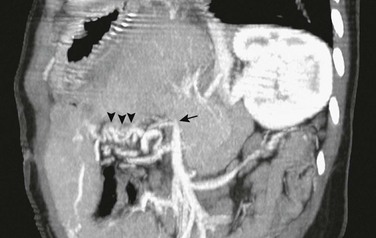
Figure 93-4 Nonacute portal vein thrombosis in an 11-year-old boy with a left lateral segment liver transplant.
An oblique sagittal reformat of contrast-enhanced computed tomography shows that the extrahepatic portal vein is occluded (arrow), and multiple corkscrew collaterals from jejunal branches of the superior mesenteric vein are seen at the Roux-en-Y loop (arrowheads) used for the biliary anastomosis. The reconstituted central portal vein was identified on other images. Capsular collaterals are seen at the inferior edge of the liver.
In patients with cavernous transformation of the portal vein, key cross-sectional imaging features include a tangle of venous channels in the liver hilum with no identifiable normal portal vein (Fig. 93-5). In most patients (76%), portoportal collaterals extend over a variable distance along the course of the intrahepatic portal branches. In extreme cases, no portal vein branches are demonstrated (Fig. 93-6). Preserved intrahepatic portal vein branches may be identified, some of which may demonstrate hepatofugal flow toward the cavernous vessels. Collateral veins may traverse the liver parenchyma to enter hepatic and capsular veins (Fig. 93-7). Doppler ultrasound imaging can be used to interrogate flow characteristics within the cavernous collaterals, revealing abnormal flow.10
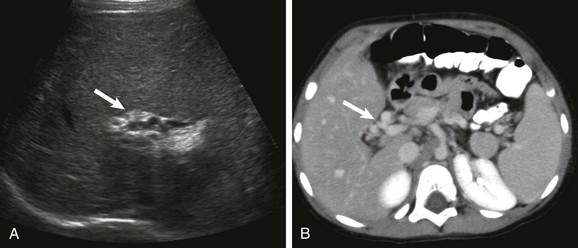
Figure 93-5 Cavernous transformation of the portal vein in a 3- year-old boy with cirrhosis.
A, A transverse sonogram shows a tangle of periportal vessels without an identifiable normal portal vein (arrow). B, Axial contrast-enhanced computed tomography confirms multiple collateral vessels in the porta hepatis (arrow).
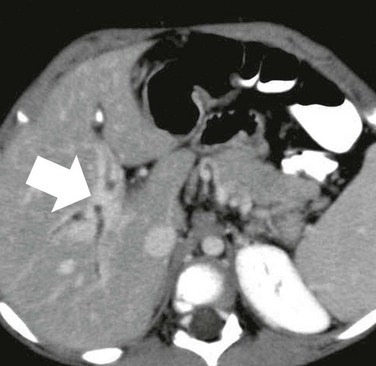
Figure 93-6 Cavernous transformation of the portal vein in a 3-year-old girl with biliary atresia and cirrhosis.
No intrahepatic portal vein branches are seen anywhere in the liver. Tangles of intrahepatic collaterals (arrow) follow the expected course of the entire intrahepatic portal system.
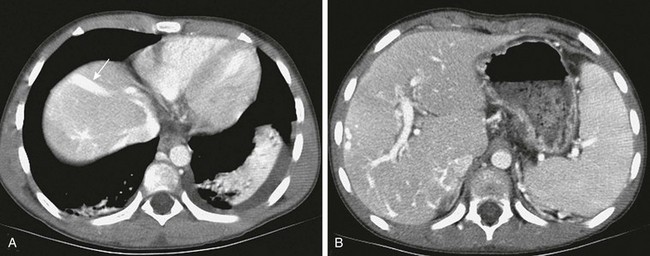
Figure 93-7 Portovenous and transcapsular collaterals in a patient with extrahepatic portal vein obstruction. Portovenous and transcapsular connections are present across the liver parenchyma.
A, A high axial slice at the liver dome shows a peripheral large collateral vein (arrow) communicating with the left hepatic vein. B, A lower axial computed tomography slice shows numerous transcapsular venous collaterals that communicate with the intrahepatic portal vein branches.
Treatment and Prognosis: A wide range of therapeutic options exist for children with portal hypertension, depending on the underlying cause and severity of liver disease. Treatments range from percutaneous transjugular intrahepatic portosystemic shunts to sclerotherapy and variceal ligation to surgical portosystemic shunts.2,11 Endovascular therapies have become more refined and are used with increasing frequency.12,13
Surgical portosystemic shunts include splenorenal shunts, mesocaval shunts, and the mesoportal (Rex) bypass. In the splenorenal shunt, the distal splenic vein is connected end-to-side to the left renal vein (Fig. 93-8), leaving the superior mesenteric vein connected to the liver. The Rex bypass was first described in 1992 by de Ville de Goyet and coworkers14 and is used in patients with extrahepatic portal vein obstruction.15 In this bypass procedure, a venous graft is interposed between the superior mesenteric vein (inferior to the pancreas) and the left portal vein, restoring portal venous flow into the liver (Fig. 93-9). The Rex shunt may be definitive therapy for children with extrahepatic portal vein obstruction.16 The collapsed intrahepatic portal system, which may be difficult to image before surgery because of exuberant intrahepatic collaterals that dominate portal flow, has been shown to distend rapidly and accommodate the large volume of flow from the shunt. The shunt can be seen by all vascular imaging modalities and should demonstrate hepatopetal flow.17
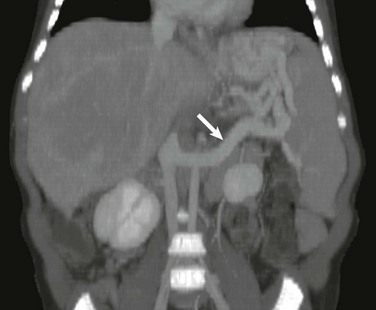
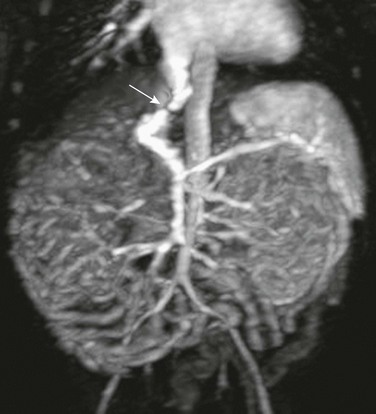
Figure 93-8 A distal splenorenal shunt in an 8-year-old boy.
Coronal reconstruction from a computed tomography angiogram shows the splenic vein connected end to side (arrow) to the left renal vein draining into the inferior vena cava.
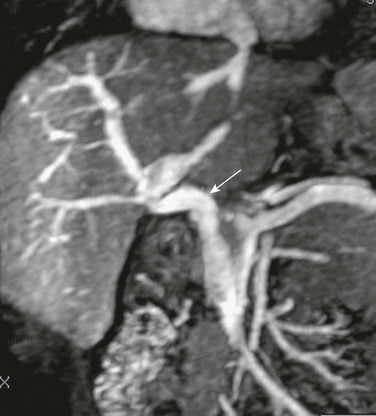
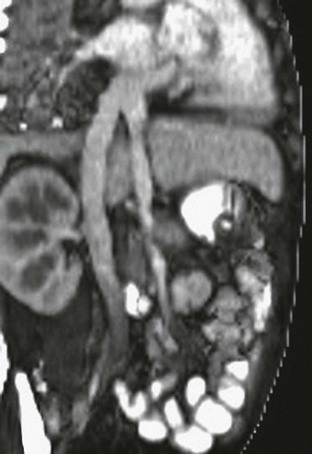
Figure 93-9 Rex (superior mesenteric to left portal vein) bypass.
Angled coronal magnetic resonance angiography shows the harvested jugular vein (arrow) connecting the superior mesenteric vein below the pancreas to the left portal vein.
Because the Rex shunt is a bypass graft, portal flow is hepatopetal. Portal vein flow is hepatofugal in patent mesocaval and proximal splenorenal shunts; it may be hepatopetal in the more selective distal splenorenal shunts, which are designed to decompress esophageal varices and preserve some portal venous flow. These shunts provide short- and long-term palliation in children with portal hypertension to prevent gastrointestinal hemorrhages and improve hypersplenism. In children with severe underlying liver disease, these shunts are temporizing procedures before liver transplantation.18
Hepatopulmonary Syndrome
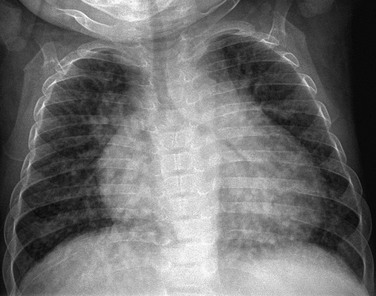
Overview: Hepatopulmonary syndrome is defined as an elevated age-adjusted alveolar-arterial oxygen gradient that often leads to hypoxemia and pulmonary vascular dilation in patients with chronic liver disease.19
Etiology: Hepatopulmonary syndrome is associated with hepatic vascular abnormalities (such as portal hypertension) that alter the normal portosystemic circulation and normal delivery of hepatic venous blood to the lungs, leading to right to left intrapulmonary shunting and pulmonary hypertension. The pathophysiology of hepatopulmonary syndrome has not been fully elucidated, but most hypotheses agree on the role of increased pulmonary expression of endothelin B receptor, leading to nitric oxide overproduction mediated by endothelin-1 and nitric oxide synthetase. Endothelial and arterial wall changes produce either (1) ventilation-perfusion mismatch through vasodilatation and arteriovenous shunting or (2) pulmonary hypertension.20
Clinical Presentation: The clinical presentation can range from relatively asymptomatic to the presence of cyanosis and clubbing.19 Pulmonary manifestations may precede the clinical presentation of the liver disease and may progress rapidly within months of the initial presentation.
Imaging: Plain radiographs may demonstrate increased vascular markings and cardiomegaly (Fig. 93-10). Computed tomography (CT) may outline enlarged vessels, predominantly in the lung bases. Echocardiography with contrast material injected into the antecubital vein will demonstrate echogenic bubbles in the pulmonary veins and in the left atrium as a result of intrapulmonary arteriovenous shunting.
Treatment and Prognosis: The treatment of hepatopulmonary syndrome is aimed at the underlying disease, which affects prognosis. In patients in whom the syndrome is a consequence of a portovenous shunt, occlusion of the shunt can lead to regression of the intrapulmonary shunts and resolution of symptoms.13,19,21
Budd-Chiari Syndrome
Overview: In persons with Budd-Chiari syndrome, obstruction of the hepatic veins and IVC at their confluence results in severe liver congestion, ascites, and portal hypertension.22 As sinusoidal pressure increases, the pressure gradient between the portal venous system and the sinusoidal system is reversed, causing the portal vein to become a draining system for the hepatic artery. In complete Budd-Chiari syndrome, blood supply to the liver is solely from the hepatic artery. Because the caudate lobe has separate venous drainage, it is spared in most patients with Budd-Chiari syndrome.
Etiology: The causes of Budd-Chiari syndrome include a caval web, thrombosis in hypercoagulable states such as nephrotic syndrome, tumor extension into the IVC as in patients with hepatoblastoma or Wilms tumor, external compression, or liver transplantation; the syndrome also may be idiopathic.22–24
Clinical Presentation: Clinically, collateral venous drainage develops in some patients, and they may be relatively asymptomatic. Other patients present with intractable ascites, liver failure, or gastrointestinal hemorrhage. Children who experience hepatic vein or IVC obstruction after liver transplantation may experience recurrence of portal hypertension.25
Imaging: On cross-sectional imaging, hepatomegaly with ascites and poorly or nonvisualized hepatic veins are characteristic.24,26 Portal venous flow usually is reversed in complete cases; however, in patients with partial hepatic vein occlusion, the flow may shunt from higher resistance segments into lower resistance segments, such as the caudate lobe. Intrahepatic portal-to-systemic collaterals may occur via transcapsular and paraumbilical veins, and extrahepatic collaterals occur via esophageal and gastric varices. These hemodynamic changes can be well demonstrated by Doppler ultrasound, CT angiography, and magnetic resonance (MR) imaging.9,24,26
On CT and CTA (Fig. 93-11), the liver parenchyma may demonstrate patchy, abnormal, wedge-shaped areas of attenuation with the apex pointing to the IVC. A heterogeneous, reticular, or mosaic pattern in the hepatic arterial phase that persists during the portal phase also may be observed. The caudate lobe retains a normal appearance, and the normal hepatic veins are not visualized. The portal vein may fill before its tributaries as a result of intrahepatic flow reversal. Similar findings are found on MRI and MR arteriography.
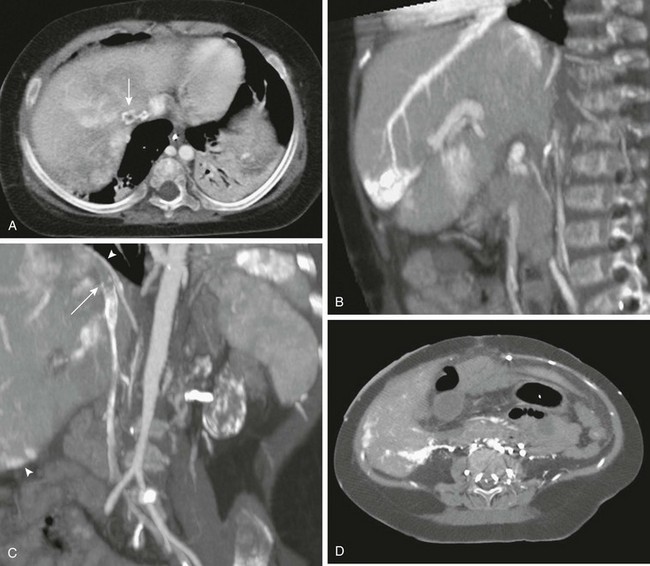
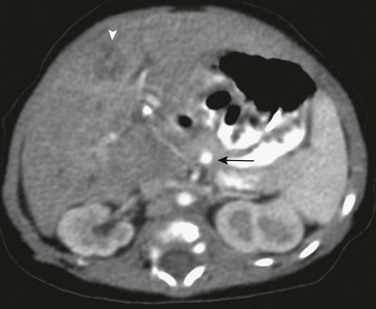
Figure 93-11 Budd-Chiari syndrome due to inferior vena cava (IVC) thrombosis.
A, An axial image from computed tomographic angiography (CTA) shows a filling defect within the intrahepatic IVC (arrow). Note the patchy, inhomogeneous enhancement of the liver. B, Sagittal reformatting from CTA shows intense contrast retention peripherally in the territory of the obstructed hepatic vein and transcapsular collateral. C, Thrombus is seen in the IVC (arrow) in this coronal reconstruction. Transcapsular collaterals are seen at the liver dome and lower lateral edge (arrowheads). D, An axial lower CT slice shows extensive retroperitoneal, paraspinal, and transcapsular collaterals enhancing brightly.
Treatment and Prognosis: Treatment consists of medical control of ascites, percutaneous angioplasty when feasible, surgical creation of a diverting shunt, and liver transplantation in severe cases.23,27 Prognosis of Budd-Chiari syndrome is highly variable depending on the timeliness of diagnosis and the ability to treat the underlying causes.22
Hepatic Venoocclusive Disease
Overview: Hepatic venoocclusive disease (VOD) refers to severe endothelial and perivascular hepatocyte damage that may be followed by fibrosis and obliteration of the terminal hepatic venules.
Etiology: Hepatic VOD develops most frequently in patients undergoing myeloablative therapy for bone marrow or stem cell transplantation. The reported frequency of VOD in patients who undergo bone marrow transplantation varies from 11% to 31% and is higher in patients who undergo bone marrow transplantation for malignant rather than nonmalignant indications. Risk factors include the presence of previous liver disease or other preexistent conditions such as osteopetrosis, repeat myeloablative procedure beyond second relapse, and specific chemotherapeutic regimens, such as the use of busulfan.28 In patients who have not undergone bone marrow transplantation, the entity can occur when the patient has received hepatic radiation or actinomycin D, and it can also occur after liver transplantation. Because the damage occurs in the hepatic sinusoidal endothelial cells of the hepatic venules, the term “sinusoidal obstructive disease” has been suggested.29
Clinical Presentation: Patients present with fluid retention, abdominal pain, and hepatomegaly. The clinical diagnosis of hepatic VOD requires the presence of two of the following clinical criteria: jaundice, ascites or fluid retention, and painful hepatomegaly within the first 20 days after bone marrow transplantation.30
Imaging: A constellation of findings may be identified on ultrasound imaging, including hepatomegaly, ascites, and thickening of the gallbladder wall. On Doppler imaging, decreased or reversed flow in the portal vein, increased resistive index of the hepatic artery, and the appearance of hepatofugal flow in the paraumbilical veins have been described in patients with VOD31 (Fig. 93-12). In some early cases, partial hemodynamic involvement may be present, resulting in flow reversal in only one segmental or lobar portal vein branch.32 The entire constellation of findings is seldom present, and in many patients, no specific ultrasound findings may be observed.31
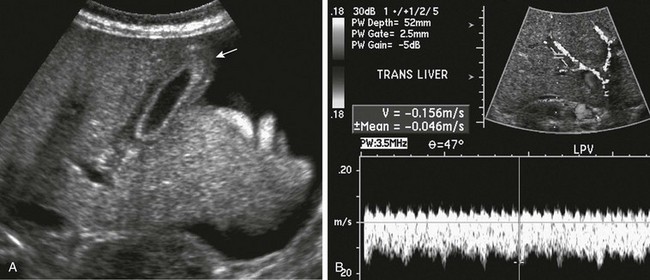
Figure 93-12 Hepatic venoocclusive disease.
A, A longitudinal ultrasound image shows thickening of the gallbladder wall (arrow) and ascites. B, Doppler imaging shows reversal of portal venous flow.
MRI findings include hepatomegaly, abnormal periportal cuffing, gallbladder wall thickening with intense wall signal on T2-weighted sequences, ascites, and pleural effusion.33
Treatment: Treatment consists of supportive care and controlling the inflammation and the deposition of fibrin. Agents that have been used include antithrombotic agents such as prostaglandin E1 and heparin, which are of limited value. More promising is defibrotide, which is derived from porcine tissue and has antithrombotic and antiischemic properties, with complete resolution seen in 35% to 55% of patients in reported uncontrolled clinical trials.28,29,34
Congenital Anomalies of Liver Vasculature
Preduodenal Portal Vein
Overview: Preduodenal portal vein is a rare anomaly in which the portal vein courses ventral to the duodenum and pancreatic head.
Etiology: The proposed embryology is nonregression of the caudal anastomotic vein that connects the two vitelline veins ventral to the duodenum, where it then contributes to the formation of the portal vein.35,36
Clinical Presentation: The anomaly is closely associated with the heterotaxy syndromes, particularly polysplenia37 and malrotation, as well as splenic, pancreatic, cardiac, and duodenal anomalies. In approximately 50% of patients, an associated duodenal obstruction is present, such as intrinsic duodenal stenosis/membrane or malrotation with Ladd bands; however, the anomaly may be asymptomatic and discovered incidentally, particularly during workup of patients with heterotaxy or biliary atresia, because approximately 10% of patients with biliary atresia have associated heterotaxy/polysplenia.36–39
Imaging: The preduodenal portal vein is seen as a vein anterior to the duodenum and pancreas, which can be visualized on cross-section imaging including ultrasound, CT, and MRI; it is particularly well visualized on sagittal images (Fig. 93-13).36,37
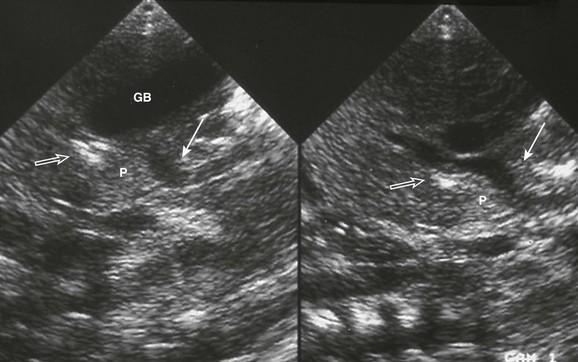
Figure 93-13 Preduodenal portal vein. Midline sagittal ultrasound imaging in an infant with polysplenia.
The superior mesenteric vein (solid arrow) courses anterior to pancreas (P) and duodenum (open arrow) to enter the liver. GB, Midline gallbladder. (Courtesy Marta Hernanz-Schulman, MD, Nashville, TN.)
Treatment: Treatment addresses correction of the underlying duodenal obstruction, if present. Awareness of this anatomic abnormality is necessary because it can pose a surgical hazard during other hepatobiliary or proximal intestinal surgical procedures, particularly in patients with heterotaxy/polysplenia who have biliary atresia.35,39
Extrahepatic Congenital Portosystemic Shunts
Overview: Abernethy40 first described an extrahepatic portosystemic connection in 1793, and this malformation is now known as the Abernethy malformation. According to Morgan and Superina,41 these malformations can be classified as type 1 or 2. Type 1 involves a complete shunt; no portal blood reaches the liver, as in congenital absence of the portal vein. In type 1a, the superior mesenteric and splenic veins do not join, and in type 1b, the superior mesenteric and splenic veins join before draining into the systemic circulation. Type 2 involves a partial shunt to the hepatic vein or IVC.41,42
Etiology: Extrahepatic portosystemic shunts result from maldevelopment of the coordinated preservation and atresia of the left and right vitelline veins in the abdomen. Such maldevelopment could arise de novo, such as in patients with heterotaxy in whom orderly right-left processes are affected, with secondary hypoplasia of the portal venous system, or they may be a result of hypoplasia of the portal venous system with redirection of portal venous flow through extrahepatic channels.43
Clinical Presentation: Extrahepatic portosystemic shunts are associated with other anomalies, such as polysplenia, biliary atresia, and cardiac and renal anomalies. They also are associated with the development of liver lesions, including focal nodular hyperplasia. Malignancies, including hepatoblastoma, hepatocellular carcinoma, and sarcoma, also have been reported in these patients. With the bypass of the hepatic circulation, systemic hyperammonemia also can be present, although hyperammonemic encephalopathy is more typically seen in older and adult patients, dependent in part on the volume of the shunt; increased circulation of galactose and bile acids also occurs.43–46
Imaging: Absence of the portal vein may be noted prenatally, with congenital agenesis of the ductus venosus and umbilical vein drainage into the IVC or directly into the heart.47
When the portal vein is absent, the hepatic artery is larger than expected relative to the size of the liver and the patient’s age.44 When the portal vein is present, it is small (Fig. 93-14, A). The shunt’s vascular connection may be demonstrated by ultrasound imaging, particularly with the aid of color Doppler imaging (Fig 93-14, B), but CTA and MRA are more likely to demonstrate its complete course and anatomy (Fig. 93-15). Associated hepatic parenchymal nodules may be demonstrated by all cross-sectional imaging modalities (Fig. 93-16).
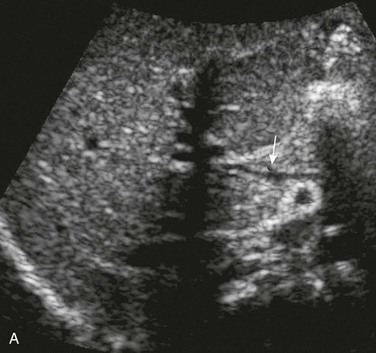
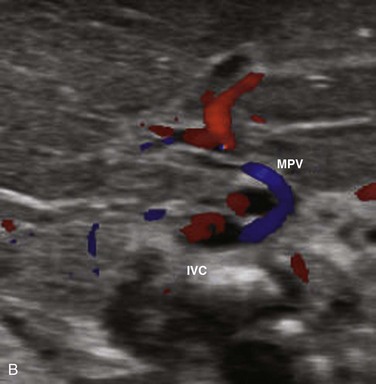
Figure 93-14 A congenital extrahepatic portosystemic shunt.
A, A diminutive main portal vein is present (arrow). B, Transverse ultrasound Doppler imaging in another infant shows main portal vein (MPV) draining into the inferior vena cava (IVC).
Treatment and Prognosis: Treatment, particularly in symptomatic patients, is aimed at surgical or endovascular occlusion of the abnormal connection if evidence exists that intrahepatic flow can be restored; otherwise, extrahepatic portal hypertension will ensue. Surgical or endovascular occlusion may be accomplished with a staged procedure, with narrowing of the shunt before complete occlusion is undertaken. Liver transplantation may be needed in persons with symptomatic extrahepatic shunts with agenesis of the portal vein and its branches.44,46,48
Intrahepatic Congenital Portosystemic Shunts
Overview: Intrahepatic congenital portosystemic shunts also are relatively rare anomalies, in which one or more intrahepatic portosystemic venous shunts may be present. Park et al.49 have classified intrahepatic portosystemic shunts into four types. Type I is a single large vein connecting the right portal and hepatic veins. Type II features peripheral single or multiple communications in one segment. Type III consists of an aneurysm connecting a peripheral portal vein and a hepatic vein branch. Type IV represents diffuse communications in multiple lobes. Type I is the most common.
Etiology: Intrahepatic congenital portosystemic shunts represent persistent communications between the portal and hepatic venous derivatives of the embryologic vitelline veins, or between the vitelline and subcardinal veins.44,50 A patent ductus venous represents a form of portosystemic shunt from the left portal vein into the IVC.
Clinical Presentation: These shunts may be asymptomatic and diagnosed incidentally. When encountered in neonates, some of these shunts may resolve spontaneously within the first 12 to 24 months of life; however, the proportion of patients in whom this occurs is not known.44
When these shunts are clinically apparent, presentation can include jaundice, hyperammonemia, hypergalactosemia, encephalopathy, and pulmonary hypertension.44,46 Type IV shunts may be associated with massive shunting and heart failure. Cutaneous hemangiomas have been described in neonates with intrahepatic portosystemic shunts.
Imaging: Intrahepatic congenital portosystemic shunts often are demonstrated by ultrasound imaging, sometimes when evaluating a child with a cutaneous hemangioma. The nonuniform size of the portal vein and hepatic vein branches may locate additional communications. The flow pattern in the portal veins, and at times also in the splenic vein, may demonstrate a biphasic or triphasic pattern, reflecting bypass of the hepatic parenchyma. Intrahepatic shunts are well demonstrated by CTA and MRA (Fig. 93-17).
Treatment: Asymptomatic children with mild metabolic abnormalities can be treated conservatively by dietary means and Doppler imaging follow-up, because some of these shunts may close spontaneously.51 Contemporary literature has described endovascular treatment with increasing frequency using a variety of surgical techniques and devices.48,50
Subdiaphragmatic Anomalous Pulmonary Venous Connections
Overview: Although anomalous pulmonary venous connections usually occur above the diaphragms, multiple subdiaphragmatic embryonal connections may persist, with subdiaphragmatic partial or total pulmonary venous drainage.
Etiology: Pulmonary veins develop in the fourth week of gestation as a plexus that initially drains into both the cardinal veins and the umbilicovitelline venous system. As lung development proceeds, the common pulmonary vein is formed by the confluence of the four pulmonary veins and fuses with the posterior wall of the left atrium. If this normal fusion and drainage into the left atrium does not occur, embryonic connections to the splanchnic and umbilicovitelline systems persist.38,52 Total anomalous pulmonary venous return below the diaphragm drains most frequently into the portal vein or ductus venosus.
Clinical Presentation: Patients with infradiaphragmatic venous return are invariably obstructed and present with severe pulmonary edema. The obstruction may be at the level of the diaphragm, through intrinsic stenosis of the anomalous vessel, through closure of the ductus venosus, or through passage across the hepatic vascular bed in cases of drainage into the portal vein.52,53
Imaging: On sonography, the anomalous common pulmonary vein is seen as a large vascular channel that enters the diaphragmatic hiatus anterior to the esophagus and inserts into the left portal vein or ductus venosus. CT and MRI show the same findings (Fig. 93-18).
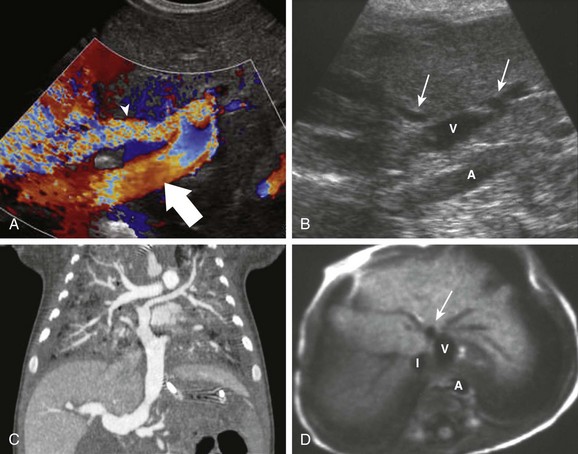
Figure 93-18 Total anomalous pulmonary venous return.
A, A 2-week-old boy with pulmonary edema since birth. A transverse color Doppler ultrasound image shows a large common pulmonary vein (arrow) coursing caudally toward the portal vein (arrowhead). B, A sagittal gray-scale ultrasound image in a different child with asplenia shows the anomalous vein (V) entering the abdomen and joining the patent ductus venosus (arrows), partially outside the field of view. Note the stenosis at the junction between the anomalous vein and the ductus. C, Coronal reformatting from a computed tomography angiogram in another patient shows convergence of individual pulmonary veins into the common pulmonary vein, which in this case drains into the portal vein. Note the severe pulmonary edema. D, T1-weighted axial magnetic resonance in the same infant depicted in B. The anomalous vein (V) is seen transversely, with the dilated ductus venosus into which it drains (arrow) anteriorly. A, Aorta; I, IVC. (B and D, Courtesy Marta Hernanz-Schulman, MD, Nashville, TN.)
Hepatic Arteriovenous Malformations
Overview: Arteriovenous malformations refer to a tangled collection of nonneoplastic vessels; they are much less common than infantile hemangiomas, although occasionally the two may be difficult to differentiate. Unlike hemangiomas, they do not regress and do not respond to medical therapy.38,55
Arterioportal fistulas refer to direct connections between the hepatic arterial and portal venous system, which can be intrahepatic or extrahepatic. Norton et al.56 have classified these lesions according to their afferent supply: Type 1 is supplied by either the right or left hepatic artery; type 2 is supplied by both right and left hepatic arteries or their braches; and type 3 refers to a complex lesion, with vascular supply including extrahepatic arteries. Fistulas between the arterial and the systemic hepatic venous system are exceedingly rare, and when congenital, they are seen most often in the setting of other anomalies, such as hemorrhagic telangiectasia, hepatic carcinoma, and hemangioma.10
Etiology: Congenital arteriovenous fistulas may be single or multiple and may be isolated lesions or part of the spectrum of Rendu-Osler-Weber syndrome (hereditary hemorrhagic telangiectasia) and Ehlers-Danlos syndrome. Most lesions are not congenital but are acquired in patients with biliary cirrhosis (such as children with biliary atresia) and after blunt or penetrating trauma (including after a liver biopsy).55,57
Clinical Presentation: Arteriovenous malformations may present with congestive heart failure, hepatic ischemia, or portal hypertension.55 Clinical manifestations of arterioportal fistulas are presinusoidal portal hypertension, with consequent ascites, splenomegaly, and gastrointestinal bleeding, as well as malabsorption and failure to thrive.55,56 A murmur and thrill may be detectable over the right upper quadrant in approximately 50% of cases. 56 High-output heart failure may be seen in infants, mainly when the ductus venosus is patent; acute closure of the ductus venosus can result in fatal gastrointestinal bleeding.55 If left untreated, further liver damage with hepatoportal sclerosis may aggravate the portal hypertension.10 Acquired postbiopsy fistulas that are small and peripheral and produce no symptoms usually are self-limited.
Imaging: Hepatic congenital arteriovenous malformations and arteriovenous fistulas can be diagnosed with ultrasound, CT, and MRI. In congenital arteriovenous malformations, ultrasound demonstrates a tangle of vessels of variable size.55 The size of the hepatic artery is proportional to the size of the shunt; the draining hepatic veins are distended, and the aorta may taper after the takeoff of the hepatic artery. The hepatic artery demonstrates very high systolic Doppler shifts and high diastolic flow, whereas the hepatic veins demonstrate pulsatile or arterialized high-velocity flow.
In patients with arteriovenous fistulas, ultrasound demonstrates enlargement of the hepatic artery as well as dilatation of the portal vein at the site of the fistulous connection.38 Portal venous flow is reversed on Doppler sonography in the draining vein of an arterioportal fistula and may be reversed in the main portal vein, depending on shunt size, as well as in the splenic and superior mesenteric veins.1 The reversed portal venous flow usually is arterialized on spectral Doppler evaluation, with high flow velocity. The hepatic artery, or the branch that leads into the fistula, demonstrates high velocity and a decreased resistive index (Fig. 93-19). Ascites and bowel wall thickening may be present. The fistula is intensely visible by color and power Doppler imaging, with vibration artifact in the hepatic vein. CT and CTA demonstrate intense enhancement and rapid washout in malformations and fistulas; rapid appearance of the portal vein occurs during the arterial phase, with enhancement intensity similar to that of the aorta.38 The enlarged arterial supply and venous drainage are well demonstrated by CTA, with early appearance of the draining veins.38 The excellent temporal resolution of fast-sequence MRA allows visualization of the vascular nidus and identification of fast clearance in arteriovenous malformations and fistulas. Angiography shows similar findings (Fig. 93-20) and is reserved for imaging prior to therapeutic embolization.55
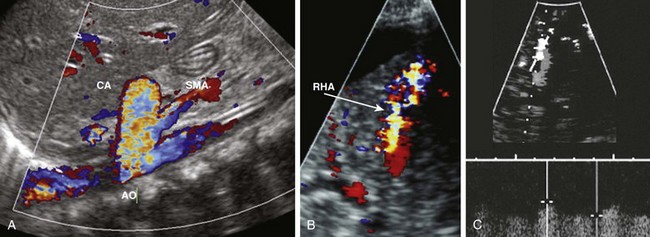
Figure 93-19 Congenital arterioportal shunts in two patients.
A, A color Doppler image in a 4-month-girl with heart failure shows an enlarged celiac artery (CA) relative to the adjacent aorta (AO) and superior mesenteric artery (SMA), with color aliasing indicative of high flow velocity. B, Postbiopsy arterioportal fistula in an 8-year-old boy with a liver transplant. A color Doppler ultrasound image shows an intense high-velocity aliasing Doppler shift in a peripheral, small arteriovenous fistula arising from a right hepatic artery (RHA) branch (arrow) at the site of a previous liver biopsy. Ascites also is seen outlining the liver. C, Color (depicted in gray scale) and spectral Doppler images show a very high diastolic flow and a low resistive index in the hepatic artery branch leading into the fistula.
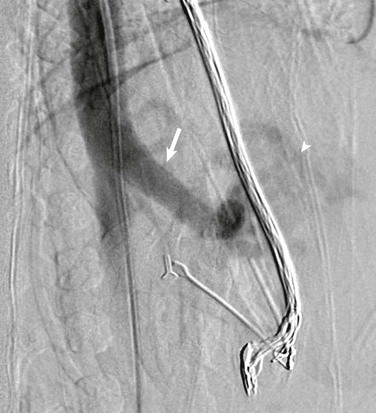
Figure 93-20 A lateral aortogram in same patient as depicted in Figure 93-19, A, confirms an enlarged celiac trunk (arrow) and decreased caliber of the more distal aorta. Early filling of the shunt vessels (arrowhead) is seen over the liver.
Treatment and Prognosis: Congenital hepatic arteriovenous malformations and fistulas do not respond to medical management. Treatment is aimed at obliteration of the abnormal arteriovenous connection and consists of embolization, surgical ligation, hepatic lobectomy, or liver transplantation. Embolization of feeding vessels may lead to dilatation of subclinical channels and recurrence of the abnormal connection and may require further embolization of escalation of therapy.1,38 Heparinization may be needed after embolization to prevent postembolization portal vein thrombosis.55 As noted earlier, infants with biliary atresia and arterioportal shunt present a special problem; they are unlikely to tolerate interruption in hepatic arterial flow, and early liver transplantation may be the treatment of choice.38,58,59 Prognosis depends on the severity of the underlying disease and the success of various treatments.
Alonso-Gamarra, E, Parron, M, Perez, A, et al. Clinical and radiologic manifestations of congenital extrahepatic portosystemic shunts: a comprehensive review. Radiographics. 2011;31:707–731.
Altuntas, B, Erden, A, Karakurt, C, et al. Severe portal hypertension due to congenital hepatoportal arteriovenous fistula associated with intrahepatic portal vein aneurysm. J Clin Ultrasound. 1998;26(7):357–360.
Biyyam, DR, Chapman, T, Ferguson, MR, et al. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics. 2010;30(6):1721–1738.
Cool, CD, Deutsch, G. Pulmonary arterial hypertension from a pediatric perspective. Pediatr Dev Pathol. 2008;11(3):169–177.
Dehghani, SM, Haghighat, M, Imanieh, MH, et al. Tacrolimus related hypertrophic cardiomyopathy in liver transplant recipients. Arch Iran Med. 2010;13(2):116–119.
Fink, MA, Berry, SR, Gow, PJ, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22(1):119–124.
Glassman, MS, Klein, SA, Spivak, W. Evaluation of cavernous transformation of the portal vein by magnetic resonance imaging. Clin Pediatr (Phila). 1993;32(2):77–80.
Hammon, JW, Jr., Bender, HW, Jr., Graham, TP, Jr., et al. Total anomalous pulmonary venous connection in infancy. Ten years’ experience including studies of postoperative ventricular function. J Thorac Cardiovasc Surg. 1980;80(4):544–551.
Hammon, JW, Jr. Total anomalous pulmonary connection: then and now. Ann Thorac Surg. 1993;55(4):1030–1032.
Lassau, N, Leclère, J, Auperin, A, et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: value of gray-scale and Doppler US in 100 patients. Radiology. 1997;204(2):545–552.
Lee, W, Chang, S, Duddalwar, VA, et al. Imaging assessment of congenital and acquired abnormalities of the portal venous system. Radiographics. 2011;31:905–926.
Mathieu, D, Vasile, N, Dibie, C, et al. Portal cavernoma: dynamic CT features and transient differences in hepatic attenuation. Radiology. 1985;154(3):743–748.
Park, JH, Cha, SH, Han, JK, et al. Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol. 1990;155(3):527–528.
References
1. Tannuri, AC, Tannuri, U, Lima, FR, et al. Congenital intrahepatic arterioportal fistula presenting as severe undernutrition and chronic watery diarrhea in a 2-year-old girl. J Pediatr Surg. 2009;44(10):e19–e22.
2. Ryckman, FC, Alonso, MH. Causes and management of portal hypertension in the pediatric population. Clin Liver Dis. 2001;5(3):789–818.
3. Dehghani, SM, Gholami, S, Bahador, A, et al. Comparison of Child-Turcotte-Pugh and pediatric end-stage liver disease scoring systems to predict morbidity and mortality of children awaiting liver transplantation. Transplant Proc. 2007;39(10):3175–3177.
4. Pugh, RN, Murray-Lyon, IM, Dawson, JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649.
5. Weiss, B, Shteyer, E, Vivante, A, et al. Etiology and long-term outcome of extrahepatic portal vein obstruction in children. World J Gastroenterol. 2010;16(39):4968–4972.
6. De Gaetano, AM, Lafortune, M, Patriquin, H, et al. Cavernous transformation of the portal vein: patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol. 1995;165(5):1151–1155.
7. Morag, I, Shah, PS, Epelman, M, et al. Childhood outcomes of neonates diagnosed with portal vein thrombosis. J Paediatr Child Health. 2011;47(6):356–360.
8. Schettino, GC, Fagundes, ED, Roquete, ML, et al. Portal vein thrombosis in children and adolescents. J Pediatr (Rio J). 2006;82(3):171–178.
9. Kok, T, van der Jagt, EJ, Haagsma, EB, et al. The value of Doppler ultrasound in cirrhosis and portal hypertension. Scand J Gastroenterol Suppl. 1999;230:82–88.
10. Gallego, C, Velasco, M, Marcuello, P, et al. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22(1):141–159.
11. Zargar, SA, Javid, G, Khan, BA, et al. Endoscopic ligation compared with sclerotherapy for bleeding esophageal varices in children with extrahepatic portal venous obstruction. Hepatology. 2002;36(3):666–672.
12. Shigeta, T, Kasahara, M, Sakamoto, S, et al. Balloon-occluded retrograde transvenous obliteration for a portosystemic shunt after pediatric living-donor liver transplantation. J Pediatr Surg. 2011;46(6):e19–e22.
13. Alonso, J, Sierre, S, Lipsich, J, et al. Endovascular treatment of congenital portal vein fistulas with the Amplatzer occlusion device. J Vasc Interv Radiol. 2004;15(9):989–993.
14. De Ville de Goyet, J, Alberti, D, Clapuyt, P, et al. Direct bypassing of extrahepatic portal venous obstruction in children: a new technique for combined hepatic portal revascularization and treatment of extrahepatic portal hypertension. J Pediatr Surg. 1998;33(4):597–601.
15. Emre, S, Dugan, C, Frankenberg, T, et al. Surgical portosystemic shunts and the Rex bypass in children: a single-centre experience. HPB (Oxford). 2009;11(3):252–257.
16. Krebs-Schmitt, D, Briem-Richter, A, Grabhorn, E, et al. Effectiveness of Rex shunt in children with portal hypertension following liver transplantation or with primary portal hypertension. Pediatr Transplant. 2009;13(5):540–544.
17. Chaves, IJ, Rigsby, CK, Schoeneman, SE, et al. Pre- and postoperative imaging and interventions for the meso-Rex bypass in children and young adults. Pediatr Radiol. 2012;42(2):220–232. [quiz 271-272].
18. Van Ha, TG, Funaki, BS, Ehrhardt, J, et al. Transjugular intrahepatic portosystemic shunt placement in liver transplant recipients: experiences with pediatric and adult patients. AJR Am J Roentgenol. 2005;184(3):920–925.
19. Cheung, KM, Lee, CY, Wong, CT, et al. Congenital absence of portal vein presenting as hepatopulmonary syndrome. J Paediatr Child Health. 2005;41(1-2):72–75.
20. Tang, L, Luo, B, Patel, RP, et al. Modulation of pulmonary endothelial endothelin B receptor expression and signaling: implications for experimental hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1467–L1472.
21. Chiu, SN, Ni, YH, Wang, JK, et al. Resolution of secondary pulmonary arteriovenous malformations after embolization of a congenital superior-mesenteric-vein-to-left-renal-vein shunt. J Vasc Interv Radiol. 2002;13(3):333–336.
22. Plessier, A, Valla, DC. Budd-Chiari syndrome. Semin Liver Dis. 2008;28(3):259–269.
23. Darwish Murad, S, Plessier, A, Hernandez-Guerra, M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151(3):167–175.
24. Cura, M, Haskal, Z, Lopera, J. Diagnostic and interventional radiology for Budd-Chiari syndrome. Radiographics. 2009;29(3):669–681.
25. Bittencourt, PL, Couto, CA, Ribeiro, DD. Portal vein thrombosis and Budd-Chiari syndrome. Clin Liver Dis. 2009;13(1):127–144.
26. Pariente, D, Franchi-Abella, S. Paediatric chronic liver diseases: how to investigate and follow up? Role of imaging in the diagnosis of fibrosis. Pediatr Radiol. 2010;40(6):906–919.
27. Orloff, MJ, Isenberg, JI, Wheeler, HO, et al. Budd-Chiari syndrome revisited: 38 years’ experience with surgical portal decompression. J Gastrointest Surg. 2011;16(2):286–300.
28. Lee, SH, Yoo, KH, Sung, KW, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2010;45(8):1287–1293.
29. DeLeve, LD, Shulman, HM, McDonald, GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22(1):27–42.
30. McDonald, GB, Hinds, MS, Fisher, LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267.
31. McCarville, MB, Hoffer, FA, Howard, SC, et al. Hepatic veno-occlusive disease in children undergoing bone-marrow transplantation: usefulness of sonographic findings. Pediatr Radiol. 2001;31(2):102–105.
32. Lassau, N, Auperin, A, Leclere, J, et al. Prognostic value of Doppler-ultrasonography in hepatic veno-occlusive disease. Transplantation. 2002;74(1):60–66.
33. Mortelé, KJ, Van Vlierberghe, H, Wiesner, W, et al. Hepatic veno-occlusive disease: MRI findings. Abdom Imaging. 2002;27(5):523–526.
34. Chopra, R, Eaton, JD, Grassi, A, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111(4):1122–1129.
35. Mimatsu, K, Oida, T, Kano, H, et al. Preduodenal portal vein, intestinal malrotation, polysplenia, and interruption of the inferior vena cava: a review of anatomical anomalies associated with gastric cancer. Surg Radiol Anat. 2012;34(2):179–186.
36. Singal, AK, Ramu, C, Paul, S, et al. Preduodenal portal vein in association with midgut malrotation and duodenal web-triple anomaly? J Pediatr Surg. 2009;44(2):e5–e7.
37. Hernanz-Schulman, M, Ambrosino, MM, Genieser, MB, et al. Current evaluation of the patient with abnormal visceroatrial situs. AJR Am J Roentgenol. 1990;154(4):797–802.
38. Gallego, C, Miralles, M, Marin, C, et al. Congenital hepatic shunts. Radiographics. 2004;24(3):755–772.
39. Ohno, K, Nakamura, T, Azume, T, et al. Evaluation of the portal vein after duodenoduodenostomy for congenital duodenal stenosis associated with the preduodenal superior mesenteric vein, situs inversus, polysplenia, and malrotation. J Pediatr Surg. 2007;42(2):436–439.
40. Abernethy, J. Account of two instances of uncommon formation in the viscera of the human body. Philos Trans R Soc Lond. 1793;83:59–66.
41. Morgan, G, Superina, R. Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg. 1994;29(9):1239–1241.
42. Niwa, T, Aida, N, Tachibana, K, et al. Congenital absence of the portal vein: clinical and radiologic findings. J Comput Assist Tomogr. 2002;26(5):681–686.
43. Murray, CP, Yoo, SJ, Babyn, PS. Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33(9):614–620.
44. Stringer, MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21(2):147–157.
45. Botha, JF, Campos, BD, Grant, WJ, et al. Portosystemic shunts in children: a 15-year experience. J Am Coll Surg. 2004;199(2):179–185.
46. Kim, MJ, Ko, JS, Seo, JK, et al. Clinical features of congenital portosystemic shunt in children. Eur J Pediatr. 2012;171(2):395–400.
47. Shen, O, Valsky, DV, Messing, B, et al. Shunt diameter in agenesis of the ductus venosus with extrahepatic portosystemic shunt impacts on prognosis. Ultrasound Obstet Gynecol. 2011;37(2):184–190.
48. Stewart, JK, Kuo, WT, Hovsepian, DV, et al. Portal venous remodeling after endovascular reduction of pediatric autogenous portosystemic shunts. J Vasc Interv Radiol. 2011;22(8):1199–1205.
49. Park, JH, Cha, SH, Han, JK, et al. Intrahepatic portosystemic venous shunt. AJR Am J Roentgenol. 1990;155(3):527–528.
50. Tanoue, S, Kiyosue, H, Komatsu, E, et al. Symptomatic intrahepatic portosystemic venous shunt: embolization with an alternative approach. AJR Am J Roentgenol. 2003;181(1):71–78.
51. Kim, MJ, Ko, JS, Seo, JK, et al. Clinical features of congenital portosystemic shunt in children. Eur J Pediatr. 2012;171(2):395–400.
52. Hines, M, Hammon, JW. Anatomy of total anomalous pulmonary venous connection. Op Tech Cardiovasc Surg. 2001;6(1):2–7.
53. Lucas, RV, Jr., Lock, JE, Tandon, R, et al. Gross and histologic anatomy of total anomalous pulmonary venous connections. Am J Cardiol. 1988;62(4):292–300.
54. Seale, AN, Uemura, H, Webber, SA, et al. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation. 2010;122(25):2718–2726.
55. Burrows, PE, Dubois, J, Kassarjian, A. Pediatric hepatic vascular anomalies. Pediatr Radiol. 2001;31(8):533–545.
56. Norton, SP, Jacobson, K, Moroz, SP, et al. The congenital intrahepatic arterioportal fistula syndrome: elucidation and proposed classification. J Pediatr Gastroenterol Nutr. 2006;43(2):248–255.
57. Khalid, SK, Garcia-Tsao, G. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia. Semin Liver Dis. 2008;28(3):247–258.
58. Nishimori, A, Okajima, H, Okumura, K, et al. Living donor liver transplantation as a means of rescuing post-embolization hepatic failure in a patient with idiopathic intrahepatic arteriovenous malformation in the liver. J Hepatobiliary Pancreat Surg. 2009;16(3):382–385.
59. Tercier, S, Delarue, A, Rouault, F, et al. Congenital portocaval fistula associated with hepatopulmonary syndrome: ligation vs liver transplantation. J Pediatr Surg. 2006;41(2):e1–e3.