Chapter 245 Varicella-Zoster Virus Infections
Clinical Manifestations
Varicella
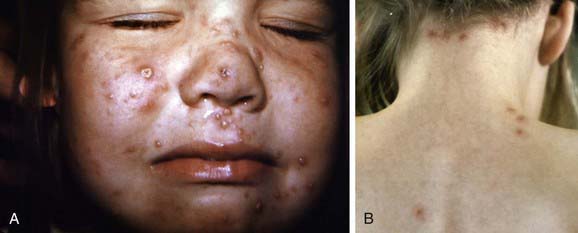
Varicella lesions often appear first on the scalp, face, or trunk. The initial exanthem consists of intensely pruritic erythematous macules that evolve through the papular stage to form clear, fluid-filled vesicles. Clouding and umbilication of the lesions begin in 24-48 hr. While the initial lesions are crusting, new crops form on the trunk and then the extremities; the simultaneous presence of lesions in various stages of evolution is characteristic of varicella (Fig. 245-1). The distribution of the rash is predominantly central or centripetal, in contrast to that in smallpox, which is more prominent on the face and distal extremities. Ulcerative lesions involving the mucosa of oropharynx and vagina are also common; many children have vesicular lesions on the eyelids and conjunctivae, but corneal involvement and serious ocular disease are rare. The average number of varicella lesions is about 300, but healthy children may have fewer than 10 to more than 1,500 lesions. In cases resulting from secondary household spread and in older children, more lesions usually occur, and new crops of lesions may continue to develop for a longer time. The exanthem may be much more extensive in children with skin disorders, such as eczema or recent sunburn. Hypopigmentation or hyperpigmentation of lesion sites persists for days to weeks in some children, but severe scarring is unusual unless the lesions were secondarily infected.
Congenital Varicella Syndrome
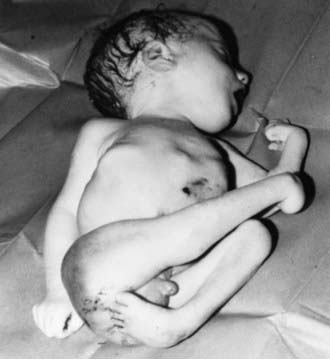
In utero transmission of VZV can occur; however, because most adults in temperate climates are immune, varicella complicating pregnancy is unusual. When pregnant women do contract varicella early in pregnancy, experts estimate that as many as 25% of the fetuses may become infected. Fortunately, clinically apparent disease in the infant is uncommon: the congenital varicella syndrome occurs in approximately 0.4% of infants born to women who have varicella during pregnancy before 13 wk of gestation and approximately 2% of infants born to women with varicella between 13 and 20 wk of gestation. Before availability of varicella vaccine in the USA, 44 cases of congenital varicella syndrome were estimated to occur each year. The congenital varicella syndrome is characterized by cicatricial skin scarring in a zoster-like distribution, limb hypoplasia, and neurologic (e.g., microcephaly, cortical atrophy, seizures, and mental retardation), eye (e.g., chorioretinitis, microphthalmia, and cataracts), renal (e.g., hydroureter and hydronephrosis) and autonomic nervous system abnormalities (neurogenic bladder, swallowing dysfunction, and aspiration pneumonia). Most of the stigmata can be attributed to virus-induced injury to the nervous system, although there is no obvious explanation why certain regions of the body are preferentially infected during fetal VZV infection. The characteristic cutaneous lesion has been called a cicatrix, a zigzag scarring, in a dermatomal distribution, often associated with atrophy of the affected limb (Fig. 245-2). Many infants with severe manifestations of congenital varicella syndrome (atrophy and scarring of a limb) have significant neurologic deficiencies.
Herpes Zoster
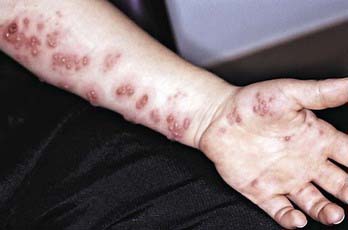
Herpes zoster manifests as vesicular lesions clustered within 1 or, less commonly, 2 adjacent dermatomes (Fig. 245-3). In the elderly, herpes zoster typically begins with burning pain followed by clusters of skin lesions in a dermatomal pattern. Almost half of the elderly with herpes zoster experience complications; the most frequent complication is postherpetic neuralgia, a painful condition that affects the nerves despite resolution of the shingles skin lesions. Unlike herpes zoster in adults, zoster in children is infrequently associated with localized pain, hyperesthesia, pruritus, and low-grade fever. In children, the rash is mild, with new lesions appearing for a few days; symptoms of acute neuritis are minimal; and complete resolution usually occurs within 1-2 wk. Unlike in adults, postherpetic neuralgia is very unusual in children. Approximately 4% of patients suffer a 2nd episode of herpes zoster; 3 or more episodes are rare. Transverse myelitis with transient paralysis is a rare complication of herpes zoster. An increased risk for herpes zoster early in childhood has been described in children who acquire infection with VZV in utero or in the 1st year of life (Fig. 245-4).
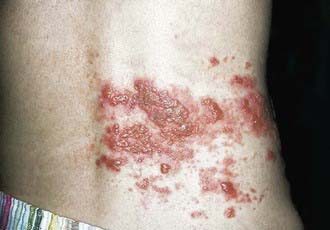
Figure 245-3 Herpes zoster involving the lumbar dermatome.
(From Mandell GL, Bennett JE, Dolin R, editors: Principles and practice of infectious diseases, ed 6, vol 2, Philadelphia, 2005, Elsevier, p 1783.)
Treatment
Complications
Encephalitis and Cerebellar Ataxia
Encephalitis (1/50,000 cases of varicella in unvaccinated children) and acute cerebellar ataxia (1/4,000 cases of varicella in unvaccinated children) are well-described neurologic complications of varicella; morbidity from CNS complications is highest among patients younger than 5 yr or older than 20 yr. Nuchal rigidity, altered consciousness, and seizures characterize meningoencephalitis. Patients with cerebellar ataxia have a gradual onset of gait disturbance, nystagmus, and slurred speech. Neurologic symptoms usually begin 2-6 days after the onset of the rash but may occur during the incubation period or after resolution of the rash. Clinical recovery is typically rapid, occurring within 24-72 hr, and is usually complete. Although severe hemorrhagic encephalitis, analogous to that caused by herpes simplex virus, is very rare in children with varicella, the consequences are similar to those of herpes encephalitis. Reye syndrome (hepatic dysfunction with hypoglycemia and encephalopathy) associated with varicella and other viral illnesses such as influenza has become rare now that salicylates are no longer used as antipyretics in these situations (Chapter 353).
Prevention
VZV transmission is difficult to prevent because an infected person is contagious for 24-48 hr before the rash appears. Infection control practices, including caring for infected patients in isolation rooms with filtered air systems, are essential. All health care workers should have evidence of varicella immunity (Table 245-1). Unvaccinated health care workers without other evidence of immunity who have had a close exposure to VZV should be furloughed for days 8-21 after exposure because they are potentially infectious during this period.
Table 245-1 EVIDENCE OF IMMUNITY TO VARICELLA
Evidence of immunity to varicella consists of any of the following:
* For children who received their first dose at age <13 yr and for whom the interval between the 2 doses was ≥28 days, the second dose is considered valid.
† Commercial assays can be used to assess disease-induced immunity, but they lack sensitivity to always detect vaccine-induced immunity (i.e., they might yield false-negative results).
§ For health-care personnel, pregnant women, and immunocompromised persons, birth before 1980 should not be considered evidence of immunity.
¶ Verification of history or diagnosis of typical disease can be provided by any health care provider (e.g., school or occupational clinic nurse, nurse practitioner, physician assistant, or physician). For persons reporting a history of, or reporting with, atypical or mild cases, assessment by a physician or his/her designee is recommended, and one of the following should be sought: (1) an epidemiologic link to a typical varicella case or to a laboratory-confirmed case or (2) evidence of laboratory confirmation if it was performed at the time of acute disease. When such documentation is lacking, persons should not be considered as having a valid history of disease, because other diseases might mimic mild atypical varicella.
Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361-368.
Brunell PA, Miller LH, Lovejoy F. Zoster in children. Amer J Dis Child. 1968;115:432-437.
Centers for Disease Control and Prevention. A new product (VariZIG) for postexposure prophylaxis of varicella available under an investigational new drug application expanded access protocol. MMWR Morb Mortal Wkly Rep. 2006;55:1-2.
Chaves SS, Zhang J, Civen R, et al. Varicella disease among vaccinated persons: clinical and epidemiological characteristics, 1997–2005. J Infect Dis. 2008;197:S127-S131.
Choo PW, Donahue JG, Manson JE, et al. The epidemiology of varicella and its complications. J Infect Dis. 1995;172:706-712.
Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
Feder HMJr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457.
Gershon AA. Live-attenuated varicella vaccine. Infect Dis Clin North Am. 2001;15:65-81.
Gershon AA, LaRussa P. Varicella vaccine. Pediatr Infect Dis J. 1998;17:248-249.
Gershon AA, Mervish N, LaRussa P, et al. Varicella-zoster virus infection in children with underlying human immunodeficiency virus infection. J Infect Dis. 1997;176:1496-1500.
Guess HA, Broughton DD, Melton LJII, et al. Population-based studies of varicella complications. Pediatrics. 1986;78:723-727.
Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J Infect Dis. 2008;197:S71-S75.
Hardy I, Gershon AA, Steinberg S, et al. The incidence of zoster after immunization with live attenuated varicella vaccine: a study in children with leukemia. N Engl J Med. 1991;325:1545-1550.
Harris D, Redhead J. Should acyclovir be prescribed for immunocompetent children presenting with chickenpox? Arch Dis Child. 2005;90:648-650.
Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-1376.
Ji G, Niu J, Shi Y, et al. The effectiveness of repetitive paravertebral injections with local anesthetics and steroids for the prevention of postherpetic neuralgia in patients with acute herpes zoster. Anesth Analg. 2009;109:1651-1655.
Kurlan JG, Connelly BL, Lucky AW. Herpes zoster in the first year of life following postnatal exposure to varicella-zoster virus. Arch Dermatol. 2004;140:1268-1272.
Kustermann A, Zoppini C, Tassis B, et al. Prenatal diagnosis of congenital varicella infection. Prenat Diagn. 1996;16:71-74.
Lopez AS, Zhang J, Brown C, et al. Varicella-related hospitalizations in the United States, 2000-2006: the 1-dose varicella vaccination era. Pediatrics. 2011;127:238-245.
Marin M, Guris D, Chaves S, et al. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Recomm Rep. 2007;56(No. RR-4):1-40.
Nikkels AF, Piérard GE. Occult varicella. Pediatr Infect Dis J. 2009;28:1073-1075.
Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med. 2005;352:450-458.
Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
Patel RA, Binns HJ, Shulman ST. Reduction in pediatric hospitalizations for varicella-related invasive group A streptococcal infections in the varicella vaccine era. J Pediatr. 2004;144:68-74.
Seward JF, Marin M, Vasquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis. 2008;197:S82-S89.