Vaporizers
The vapor pressures of most modern inhalation anesthetic agents at room temperature are much greater than the partial pressure required to produce anesthesia (Table 6-1). To produce clinically useful concentrations, a vaporizer must bring about dilution of the saturated vapor. The total gas flow from the flowmeters goes through the vaporizer, picks up a predictable amount of vapor, and then flows to the common gas outlet. A single calibrated knob or dial is used to control the concentration of the agent.
Table 6-1
Vapor Pressure of Inhaled Anesthetic Agents at 20° C
| Anesthetic Agent | Vapor Pressure, mm Hg |
| Isoflurane | 239 |
| Sevoflurane | 160 |
| Desflurane | 664 |
Concentration-calibrated vaporizers
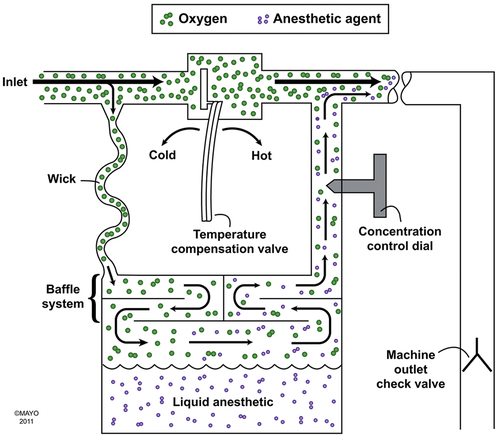
Concentration calibration may be accomplished by splitting the flow of gas that passes through the vaporizer. Some gas passes through the vaporizing chamber (the part of the vaporizer containing the liquid anesthetic agent), and the remainder goes through a bypass to the vaporizer outlet (Figure 6-1). The ratio of bypass gas to the gas flowing to the vaporizing chamber is called the splitting ratio and depends on the resistances in the two pathways. It also depends on the setting of the concentration dial that allows more gas to pass through the vaporizing chamber as higher output concentrations are set. The splitting ratio may also depend on the total gas flow through the vaporizer. Another method of controlling the outlet concentration is to direct enough carrier gas to flow through the vaporizing chamber to achieve the concentration set on the vaporizer. This is determined by a computer.