CHAPTER 67 Vagus Nerve Stimulation for Intractable Epilepsy
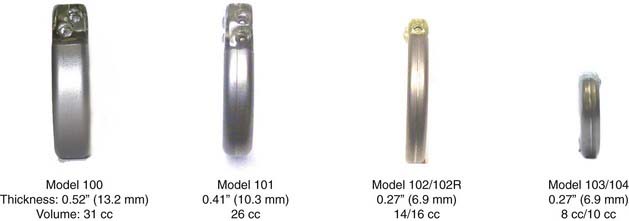
Since receiving Food and Drug Administration (FDA) approval in 1997, vagus nerve stimulation (VNS) delivered via the implantable Neurocybernetic Prosthesis (NCP) from Cyberonics, Inc. (Houston, Tex) has become an established method for treating patients with medically refractory seizures. The NCP delivers intermittent afferent electrical stimulation to the left cervical vagus nerve trunk, which secondarily transmits impulses that exert widespread effects on neuronal excitability throughout the central nervous system.1 More than 46,000 NCP devices have been implanted to treat epilepsy worldwide. Since introduction of the original model 100 generator, the device has been made progressively smaller and easier to implant and program (Figs. 67-1 and 67-2).

FIGURE 67-2 A model 103 generator (left) next to a model 102 generator (right) demonstrating decreased size.
Brief History
Experimental use of VNS to treat epilepsy can be traced to the 1880s.2 In 1938, Bailey and Bremmer demonstrated desynchronization of orbital cortex activity with the use of VNS in a cat model.3 Zanchetti and colleagues showed that intermittent VNS reduced or eliminated interictal epileptic events that were chemically induced in the frontal cortex of cats.4 In 1980, Radna and MacLean found that VNS caused changes in single-unit activity within the basal limbic structures of squirrel monkeys.5 Based on these experiments, Zabara in 1985 proposed that if VNS could desynchronize electroencephalographic activity, it might be effective in attenuating epileptic seizures.6 Subsequent animal work by Zabara7 and others8–10 supported Zabara’s hypothesis and allowed clinical trials to be performed in humans.
In 1987, a company—Cyberonics, Inc. (Houston, Tex)—was founded to develop VNS therapy in humans. In 1988, the first epileptic patient to undergo implantation of a VNS therapy device became seizure free.11 Five acute-phase clinical studies analyzing the safety and effectiveness of VNS therapy followed12–16 and culminated in FDA approval of VNS therapy “for use as an adjunctive therapy in reducing the frequency of seizures in adults and adolescents over 12 years of age with partial onset seizures that are refractory to antiepileptic medications.”17
Cyberonics, Inc., created a long-term outcome registry that compiled information on patients receiving VNS therapy. The registry opened on November 7, 1997, and closed on April 1, 2003. Participation was voluntary and data were provided by participating physicians. Data were not available for all patients at all time intervals. Median reductions in seizure activity were 46% (n = 4448) at 3 months, 57% (n = 2696) at 1 year, and 63% (n = 1114) after 2 years of VNS therapy.18 Overall, studies report that in responding patients, the effectiveness of VNS steadily improves over the first 3 to 12 months of stimulation. Many subsequent long-term studies have been published supporting the efficacy, durability, and cost-effectiveness of VNS therapy for epilepsy.19–33 In addition to refractory partial-onset seizures, VNS is used, off-label, to treat children younger than 12 years with generalized epilepsy and as an adjunct to other surgical procedures (when they are insufficient to control seizure activity).34–37
Anatomic Considerations
The majority of vagal nerve fibers are general somatic and special visceral afferents projecting to the brain, along with efferent projections to the larynx and parasympathetic projections to the heart, lungs, and gastrointestinal tract. The VNS electrode is applied to the midcervical portion of the vagal nerve, which is relatively free of branches. The upper cervical vagal nerve gives off branches to the pharynx, carotid sinus, and superior and inferior cardiac branches leading to the cardiac plexus. Studies in dogs suggest that the right vagal nerve preferentially innervates the sinoatrial node of the heart whereas the left vagal nerve projects to the atrioventricular node. Accordingly, the NCP electrode is usually applied to the left vagal nerve to avoid stimulation-related asystole or bradycardia.38 A small series of right-sided VNS implants in children, which included Holter monitoring of patients after surgery, failed to demonstrate any changes in heart rate with stimulation.39
Other nerves in the region of the vagal nerve can be affected at surgery. The phrenic nerve lies deep to the carotid sheath, and unilateral paralysis of the left hemidiaphragm has been reported during periods of VNS. Hypoglossal and facial nerve fibers are found well above the midcervical trunk, but injuries to both have been reported after VNS implantation. The sympathetic trunk runs deep to the common carotid artery and provides fibers that ascend with the internal carotid artery. There is a report of Horner’s syndrome developing after VNS implantation, presumably caused by injury to the sympathetic plexus or the fibers along the internal carotid.40
Neurocybernetic Prosthesis
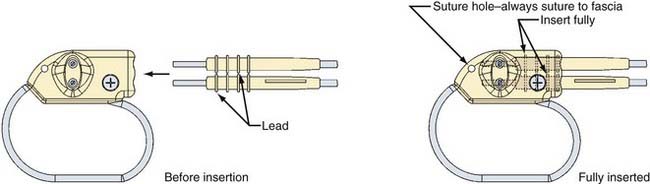
The NCP has two implantable components: a generator and a stimulating electrode (Figs. 67-3 to 67-6; also see Figs. 67-1 and 67-2). The generator consists of an epoxy resin header with a receptacle for the connector pin or pins from the electrode and a titanium module containing a lithium battery and the generator. The electrode is secured to the connector pin receptacle with a set screw or screws tightened with a hexagonal torque wrench included with the generator packaging. The generator contains an antenna that receives radiofrequency signals from the programming telemetry wand and transfers them to a microprocessor that regulates the electrical output of the pulse generator. The generator delivers a charge-balanced waveform characterized by five programmable parameters: output current, signal frequency, pulse width, signal-on time, and signal-off time. Higher stimulation frequencies and longer signal-on times result in a shorter duration of battery service life. The NCP electrode is insulated with a silicone elastomer and can be implanted safely in patients with latex allergies. One end of the lead has a connector pin or pins that insert directly into the generator (see Figs. 67-3 and 67-4); the other end has an electrode array consisting of three discrete helical coils that are placed around the vagal nerve (see Figs. 67-5 and 67-6). The middle and distal coils are the positive and negative electrodes, respectively, and the most proximal coil serves as an anchoring tether to prevent excessive force from being transmitted to the electrodes when patients turn their neck. Each electrode helix contains three loops. Embedded inside the middle turn is a platinum coil that is welded to the lead wire. Suture tails extending from either end of the helix allow manipulation of the coils without injuring the platinum contacts. A silicone electrode collar is included with the electrode and is used to anchor the electrode to the soft tissue of the neck, proximal to the helical coils. The portion of the electrode between the electrode collar and the inferior helix creates a “strain release loop” that further protects the vagal nerve from unwanted traction. A handheld NCP magnet performs several functions. When passed over the chest wall overlying the generator, it triggers stimulation superimposed on the baseline output. This on-demand stimulation can be performed by a patient or caregiver at the onset of an aura and can sometimes diminish or abort an impending seizure. In addition, if the NCP appears to be malfunctioning or if the patient wishes to terminate stimulation for any other reason, the system can be turned off by placing the magnet over the generator site continuously.
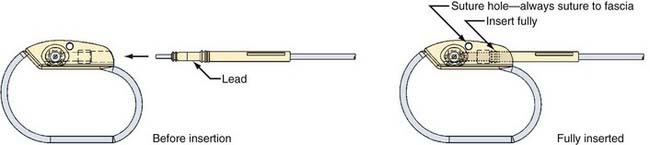
FIGURE 67-3 Diagram demonstrating connection of a monopolar electrode to a monopolar vagal nerve stimulation generator.
The NCP has undergone a series of revisions since introduction of the model 100 (see Fig. 67-1). The original model 100 and the second-generation model 101 were used with a bipolar helical lead. The third- and fourth-generation models (102 and 103) incorporated a monopolar lead. Generators 102R and 104 have bipolar lead acceptors, so revision of models 100 and 101 (with bipolar electrodes) can be performed without replacing the electrodes (see Figs. 67-3 and 67-4). The original programming hardware included a programming wand attached to a laptop computer. The laptop has been replaced with a personal digital assistant (PDA) and a similar programming wand (Fig. 67-7). Typically, we turn the generator on at low stimulation settings in the operating room at the time of implantation. The device is turned up sequentially over a period of several weeks until the desired stimulation parameters are reached, and then adjustments can be made every few months as needed.
Operative Procedure
Operative Technique
Attention is then directed to the chest wall overlying the lateral border of the pectoralis major (PM). An incision overlying and running parallel to the PM and measuring between 2 and 1.5 cm (models 103 and 104) to 6 cm (models 100 to 102) is created sharply. Dissection is then continued through soft tissue to the lateral border of the PM. A plane is developed between the PM and pectoralis minor with a blunt technique. Once a pocket large enough to accommodate the generator has been created between the two muscles, a NCP tunneling device is used to create a track from the chest wall pocket to the neck incision (Fig. 67-8). Tunneling can be performed in either direction (chest to neck or neck to chest). Care must be taken to avoid injuring the soft tissues of the neck with the tunneling device. The tunneling device has a bullet tip that screws onto the end of the metal shaft of the tunneling device and holds a clear hollow sheath around the tunneler. Once the track has been created, the bullet tip is removed and the shaft withdrawn from the clear sheath. The free end of the electrode is then placed inside the sheath and drawn, with the sheath, from the neck incision to the chest wall incision. The electrode is then removed from the sheath. The generator is next brought into the field and attached to the electrode with the set screw or screws and torque wrench. The generator is then introduced into the chest wall pocket while keeping the electrode deep to the generator. An anchoring stitch can be passed through the generator header and pectoralis to secure the generator to the chest wall. The platysma and subcutaneous structures of the neck, as well as the pectoralis fascia and soft tissues of the chest, are closed in layers. Next, the programming wand is introduced into the operative field within a sterile drape. Electrodiagnostic testing is performed by the neurologist with the PDA. The draped programming wand is held over the generator during this process. Anesthesia personnel are alerted before performing the lead test portion of the electrodiagnostics and asked to carefully monitor the patient’s vital signs during this test. Rarely, profound bradycardia/asystole necessitating the use of atropine has been reported during the lead test. If the diagnostic parameters are unsatisfactory, the neck wound is reopened to confirm or adjust electrode placement, and the chest wall wound is reopened to confirm good contact between the electrode and generator. The diagnostics are repeated until satisfactory data are obtained. The NCP is then programmed by the neurologist to the initial stimulating parameters with the PDA. Closure of the neck and chest wall incisions is completed with Dermabond.
Lead Revision
If preoperative or intraoperative electrodiagnostics suggest lead failure, the neck incision is reopened and blunt dissection is used to follow the electrode to the helical coils. Typically, the electrodes and vagal nerve are engulfed in a dense field of fibrosis. The electrodes can, however, be safely removed from around the nerve.41
Avoidance and Management of Complications
Infection
In a meta-analysis of 454 patients enrolled in five controlled clinical trials, the most frequent surgical complication was generator or lead implant site infection. The overall infection rate was 2.86%, but most were successfully treated with antibiotic therapy alone. Only 1.1% required explantation of the device for infection.16 Smyth and coauthors reported a higher rate of deep infection of 3.5% that required removal of the device in children.42
Vocal Cord Abnormalities
Transient vocal cord paralysis was reported in 0.7% of patients in the meta-analysis.16 Because no preoperative or postoperative vocal cord examination was performed, this is probably an underestimate of postimplant vocal cord dysfunction. Happily, most clinically significant cases are self-limited. Smyth and associates reported one case of vocal cord paralysis and one case of fatal aspiration pneumonia in a series of 74 children after VNS implantation.42 A prospective study in which 13 patients underwent preimplantation and postimplantation laryngeal electromyography, videolaryngoscopy, measurement of maximal phonation time, determination of the Voice Handicap Index, and Consensus Auditory-Perceptual Evaluation of Voice was published in 2006. Six of the patients had significant abnormalities in vocal fold mobility 2 weeks after surgery. Five patients had significant electromyographic abnormalities before implantation, and all 5 experienced vocal cord paresis 3 months after implantation. The data suggest that patients with preexisting vocal cord abnormalities are at greater risk for long-term vocal cord paresis after VNS implantation than those with normal preimplant vocal cord function.43 It is our practice to refer patients with hypotonia for preoperative ear, nose, and throat evaluation to assess their vocal cord function. If vocal cord dysfunction is detected, VNS therapy is not offered to the affected patient.
Bradycardia/Asystole
Ventricular asystole occurring intraoperatively during the lead test portion of VNS electrodiagnostic testing has been observed rarely in adult patients. The estimated incidence is 1 in 800 to 1000 patients. The asystole is treated with atropine and the VNS is turned off. Some affected patients are able to tolerate VNS at very low settings initially, which are slowly turned up to therapeutic levels.44,45
Sleep-Related Breathing Disorder
Some children evaluated after VNS implantation are found to have decreased respiratory airflow during sleep. In one patient, obstructive sleep apnea on polysomnography had been reported to have developed but resolved with cessation of VNS stimulation.46 This can be managed with positive pressure treatment or by varying VNS stimulation parameters. Patients with known sleep apnea should be monitored carefully after VNS implantation. If the sleep apnea worsens, positive pressure treatment or adjustment of the VNS stimulation should be pursued.
Ali II, Pirzada NA, Kanjwal Y, et al. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav. 2004;5:768-771.
Amar AP, Apuzzo MI, Liu CY. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the Vagus Nerve Stimulation Therapy Outcome Registry. Neurosurgery. 2004;55:1086-1093.
, 1995 A randomized controlled trial of chronic vagal nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995;45:224-230.
DeGiorgio CM, Amar AP, Apuzzo MLJ. Vagus nerve stimulation: surgical anatomy, technique and operative complications. In: Schacter S, Schmidt D, editors. Vagal Nerve Stimulation. London: Dunitz; 2001:31-50.
Handforth A, Degiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial onset seizures: a randomized active control trial. Neurology. 1998;51:48-55.
Helmers SL, Griesemer DA, Dean JC, et al. Observations on the use of vagal nerve stimulation earlier in the course of pharmacoresistant epilepsy: patients with seizures for six years or less. Neurologist. 2002;9:160-164.
Hsieh T, Chen M, McAfee A, et al. Sleep-related breathing disorder in children with vagal nerve stimulators. Pediatr Neurol. 2008;38:99-103.
MacLachlan RS. Suppression of interictal spikes and seizures by stimulation of the vagus nerve. Epilepsia. 1993;34:918-923.
Morris GL, Mueller WM. Vagus nerve stimulation study group E01-E05: long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology. 1999;53:1731-1735.
Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal nerve stimulation in humans: preliminary results. Epilepsia. 1990;31(suppl 2):S40-S43.
Renfro JB, Wheless JB. Earlier use of adjunctive vagus nerve stimulation for refractory epilepsy. Neurology. 2002;59(suppl 4):S26-S30.
Shaw GY, Sechtem P, Searl J, et al. Predictors of laryngeal complications in patients implanted with the Cyberonics vagal nerve stimulator. Ann Otol Rhinol Laryngol. 2006;115:260-267.
Smyth MD, Tubbs RS, Bebin EM, et al. Complications of chronic vagus nerve stimulation for epilepsy in children. J Neurosurg. 2003;99:500-503.
Tatum WO, Moore DB, Stecker MM, et al. Ventricular asystole during vagal nerve stimulation for epilepsy in humans. Neurology. 1999;52:1267.
Uthman BM, Reichl AM, Dean JC, et al. Effectiveness of vagal nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124-1126.
Uthman BM, Wilder BJ, Hammond EJ, et al. Efficacy and safety of vagus nerve stimulation in patients with complex partial seizures. Epilepsia. 1990;31(suppl 2):S44-S50.
Uthman BM, Wilder BJ, Penry JK, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 1993;43:1338-1345.
Zabara J. Inhibition of experimental seizures in canines by repetitive vagal nerve stimulation. Epilepsia. 1992;33:1005-1012.
1 Henry TR. The antiseizure effect of VNS is mediated by ascending pathways. In: Miller JW, Silbergeld DL, editors. Epilepsy Surgery: Principles and Controversies. New York: Taylor & Francis; 2006:624-629.
2 Lanska DJ. J.L. Corning and vagal nerve stimulation for seizures in the 1880’s. Neurology. 2002;58:452-459.
3 Bailey P, Bremer F. A sensory cortical representation of the vagus nerve with a note on the effects of low pressure on the cortical electro gram. J Neurophysiol. 1938;1:405-412.
4 Zanchetti A, Wang SC, Moruzzi G. The effect of vagal afferent stimulation on EEG pattern of the cat. Electroencephaolgr Clin Neurophysiol. 1952;213:45-61.
5 Radna RJ, MacLean PD. Vagal elicitation of respiratory-type and other unit responses in basal limbic structures of squirrel monkeys. Brain Res. 1980;213:45-61.
6 Zabara J. Peripheral control of hypersynchronous discharge in epilepsy. Electroencephalogr Clin Neurophysiol. 1985;S61:162.
7 Zabara J. Inhibition of experimental seizures in canines by repetitive vagal nerve stimulation. Epilepsia. 1992;33:1005-1012.
8 MacLachlan RS. Suppression of interictal spikes and seizures by stimulation of the vagus nerve. Epilepsia. 1993;34:918-923.
9 Takaya M, Terry WJ, Naritoku DK. Vagus nerve stimulation induces a sustained anticonvulsant effect. Epilepsia. 1996;37:1111-1116.
10 Lockard JS, Congdon WC, DuCharme LL. Feasibility and safety of vagal stimulation in a monkey model. Epilepsia. 1990;31(suppl 2):S20-S26.
11 Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal nerve stimulation in humans: preliminary results. Epilepsia. 1990;31(suppl 2):S40-S43.
12 Uthman BM, Wilder BJ, Hammond EJ, et al. Efficacy and safety of vagus nerve stimulation in patients with complex partial seizures. Epilepsia. 1990;31(suppl 2):S44-S50.
13 Uthman BM, Wilder BJ, Penry JK, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 1993;43:1338-1345.
14 A randomized controlled trial of chronic vagal nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995;45:224-230.
15 Handforth A, Degiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial onset seizures: a randomized active control trial. Neurology. 1998;51:48-55.
16 Morris GL, Mueller WM. Vagus nerve stimulation study group E01-E05: long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology. 1999;53:1731-1735.
17 Cyberonics, Inc. Physicians manual for the VNS therapy pulse model 102 Generator. Houston, TX: Cyberonics; 2002.
18 Renfro JB, Wheless JB. Earlier use of adjunctive vagus nerve stimulation for refractory epilepsy. Neurology. 2002;59(suppl 4):S26-S30.
19 Helmers SL, Griesemer DA, Dean JC, et al. Observations on the use of vagal nerve stimulation earlier in the course of pharmacoresistant epilepsy: patients with seizures for six years or less. Neurologist. 2002;9:160-164.
20 Wheless JW, Maggio V. Vagus nerve stimulation therapy in patients younger than 18 years. Neurology. 2002;59(suppl 4):S21-S25.
21 Cramer JA. Exploration of changes in health-related quality of life after 3 months of vagal nerve stimulation. Epilepsy Behav. 2001;2:563-567.
22 Labar DR. Antiepileptic drug use during the first 12 months of vagus nerve stimulation therapy: a registry study. Neurology. 2002;59(suppl 4):S38-S43.
23 Amar AP, Apuzzo MI, Liu CY. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the Vagus Nerve Stimulation Therapy Outcome Registry. Neurosurgery. 2004;55:1086-1093.
24 Karceski S. Vagus nerve stimulation and Lennox-Gasteaux syndrome: a review of the literature and data from the VNS patient registry. CNS Spectr. 2001;6:766-770.
25 Spanaki MV, Allen LS, Mueller WM, et al. Vagus nerve stimulation therapy: 5-year or greater outcome at a university-based epilepsy center. Seizure. 2004;13:587-590.
26 Uthman BM, Reichl AM, Dean JC, et al. Effectiveness of vagal nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124-1126.
27 Zamponi N, Rychlicki F, Corpaci L, et al. Vagus nerve stimulation is effective in treating catastrophic epilepsy in very young children. Neurosurg Rev. 2008;31:291-297.
28 Ardesch JJ, Buschman HP, Wagener-Schimmel LJ, et al. Vagus nerve stimulation for medically refractory epilepsy: a long-term follow-up study. Seizure. 2007;16:579-585.
29 Montavont A, Demarquay G, Ryvlin P, et al. Long-term efficiency of vagus nerve stimulation (VNS) in non-surgical refractory epilepsies in adolescents and adults. Rev Neurol. 2007;163:1169-1177.
30 Alexopoulos AV, Kotagel P, Loddenkemper T, et al. Long-term results with vagus nerve stimulation in children with pharmacoresistant epilepsy. Seizure. 2006;15:491-503.
31 Rychlicke F, Zamponi N, Trignani R, et al. Vagus nerve stimulation: clinical experience in drug-resistant pediatric patients. Seizure. 2006;15:483-490.
32 McHugh JC, Singh HW, Phillips J, et al. Outcome measurement after vagal nerve stimulation therapy: proposal of a new classification. Epilepsia. 2007;48:375-378.
33 De Herdt V, Boon P, Ceulemans B, et al. Vagus nerve stimulation for refractory epilepsy: a Belgian multi-center study. Eur J Paediatr Neurol. 2007;11:261-269.
34 Wheless JW. Vagus nerve stimulation therapy. In: Wylie E, editor. The Treatment of Epilepsy: Principles and Practice. Philadelphia: Lippincott Williams & Wilkins; 2006:969-980.
35 Holmes MD. Is vagus nerve stimulation therapy effective for generalized epilepsy? In: Miller JW, Silbergeld DL, editors. Epilepsy Surgery: Principles and Controversies. New York: Taylor & Francis; 2006:620-623.
36 Salinsky MC. The efficacy of vagal nerve stimulation relative to other medical and surgical treatments. In: Miller JW, Silbergeld DL, editors. Epilepsy Surgery: Principles and Controversies. New York: Taylor & Francis; 2006:608-613.
37 Schmidt D. Should VNS be considered before corpus callosotomy? In: Miller JW, Silbergeld DL, editors. Epilepsy Surgery: Principles and Controversies. New York: Taylor & Francis; 2006:614-619.
38 DeGiorgio CM, Amar AP, Apuzzo MLJ. Vagus nerve stimulation: surgical anatomy, technique and operative complications. In: Schacter S, Schmidt D, editors. Vagal Nerve Stimulation. London: Dunitz; 2001:31-50.
39 McGregor A, Wheless J, Baumgartner J, et al. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005;46:91-96.
40 Amar AP, Levy ML, Appuzzo MLJ. Vagus nerve stimulation for intractable epilepsy. In: Winn HR, editor. Youmans Neurological Surgery. 5th ed. Philadelphia: WB Saunders; 2001:2643-2653.
41 Espinosa J, Aiello MT, Naritoku DK. Revision and removal of stimulating electrodes following long-term therapy with the vagus nerve stimulator. Surg Neurol. 1999;51:659-664.
42 Smyth MD, Tubbs RS, Bebin EM, et al. Complications of chronic vagus nerve stimulation for epilepsy in children. J Neurosurg. 2003;99:500-503.
43 Shaw GY, Sechtem P, Searl J, et al. Predictors of laryngeal complications in patients implanted with the Cyberonics vagal nerve stimulator. Ann Otol Rhinol Laryngol. 2006;115:260-267.
44 Tatum WO, Moore DB, Stecker MM, et al. Ventricular asystole during vagal nerve stimulation for epilepsy in humans. Neurology. 1999;52:1267-1269.
45 Ali II, Pirzada NA, Kanjwal Y, et al. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav. 2004;5:768-771.
46 Hsieh T, Chen M, McAfee A, et al. Sleep-related breathing disorder in children with vagal nerve stimulators. Pediatr Neurol. 2008;38:99-103.