64 Uveal Melanoma
Epidemiology, Genetics, and Environmental Factors
Uveal melanoma is the most common primary intraocular malignancy of adults. The annual incidence is estimated to be between four and seven cases per million people in the United States, Canada, and Europe.1–4 Ocular melanomas in the United States represent approximately 3% to 5% of all melanomas, with approximately 1500 to 2000 new cases diagnosed each year in the United States.3–6 In contrast to cutaneous melanoma, which has shown a fivefold to sixfold increase in incidence over the past several decades,7 the frequency of ocular melanoma appears not to have changed.8,9 Of ocular melanomas, 85% are reported to be uveal, 5% conjunctival, and 10% occur at other sites. Uveal melanoma occurs more frequently in light-skinned people with light eyes. The incidence rate among African Americans is reported nationally to be less than one eighth that found in whites; it is also rare among Americans of Asian extraction.3 A steady increase in incidence with age is seen, with peak incidence between 60 and 79 years of age, whereas less than 2% are younger than 20 years old at the time of diagnosis.5,10 Incidence rates are generally higher for males (RR = 1.3).3
The cause of uveal melanoma is largely unknown, and there is mixed evidence regarding genetic susceptibility and host-factor patterns. It is extremely rare to have bilateral tumors, multiple uveal melanomas, or familial uveal melanomas.11 Uveal melanoma is more prevalent in white patients in an eye with ocular or oculodermal melanocytosis (nevus of Ota).12,13 The lifetime risk of uveal melanoma in patients with oculodermal melanocytosis is estimated to be 1 in 400.14 Reports of cutaneous and uveal melanoma occurring as double primary malignancies, some in the presence of dysplastic nevi or atypical mole syndrome, as well as familial clustering of both melanomas have led to speculation that cutaneous and uveal melanoma may have a common inheritable variant.15,16 However, others have been unable to detect an association between intraocular melanoma and cutaneous dysplastic nevi.17,18
Various molecular and genetic markers are currently under study.19 Chromosomal alterations most often observed in uveal melanoma include deletion of chromosome 3 (a finding associated with poor prognosis), over-representation of 6p, loss of 6q, and multiplication of 8q.20–24 Loss of chromosome 3, loss of 6q, and gains of 8q are significant predictors of survival.25,26 Abnormalities of chromosome 3 and 8 are present in approximately 50% of all cases.27 Tumors with monosomy 3 also exhibit amplification of c-myc (encoded on chromosome 8q24.1), suggesting that both play a role in the pathophysiology of uveal melanomas.28 In contrast, monosomy 3 and amplification of 8q are rare in cutaneous melanomas. Other common genetic alterations in cutaneous lesions are otherwise rare in uveal melanomas; these include deletion and translocation of 1p, and mutations in TP53, BRAF, and CDKN2.20,29,30 Further, c-myc amplification, described in 70% of uveal melanomas, is associated with improved survival.31,32 The opposite is found in cutaneous melanoma, in which high levels of c-myc are associated with worse outcomes. The distinct cytogenetic characteristics of cutaneous and uveal melanomas support the hypothesis that different mechanisms are involved in the genesis and progression of these neoplasms.21 The role of other molecular targets remain under investigation, including Bcl-2, ubiquitin-proteasome, histone deactylase, mitogen-activated protein kinase, AKT, receptor tyrosine kinases, intercellular adhesion molecules, matrix metalloproteinase, and angiogenic factors.19,30
More recently, gene expression profiling data shows that a more accurate assessment of prognosis is possible with a transcriptomic classification of uveal melanomas based on ribonucleic acid analysis of the primary tumor. Tumor samples can be processed from enucleation tissue or fine-needle biopsy before institution of eye-sparing therapies.33,34 A “class 1” signature is associated with an excellent prognosis, whereas a “class 2” signature portends a high risk of metastatic death. The latter class is associated with other predictors of poor prognosis, including epithelioid cytology, looping extracellular matrix patterns, and monosomy 3. This molecular classification represents a valuable prognostic tool to identify high-risk patients.
The impact of other environmental factors such as exposure to sunlight and ultraviolet (UV) light have been studied in uveal melanoma because of the known association with cutaneous melanoma. However, unlike in cutaneous melanoma, intermittent outdoor leisure, chronic occupational sunlight exposure, and birth latitude are reported to be nonsignificant factors in a recent meta-analysis.35 Perhaps most problematic with the putative link between sunlight exposure and uveal melanoma is that virtually no UV-A or UV-B is transmitted through the lens and cornea in adults. The juvenile lens may allow small amounts of UV radiation to reach the retina, but tissue overlying the uveal tract would still likely filter out the residual UV radiation. Arc welding exposure is a significant risk factor for development of uveal melanoma.35,36 Other causal factors, including cell phone usage, have not shown convincing evidence of correlation to uveal melanoma.6
Anatomy
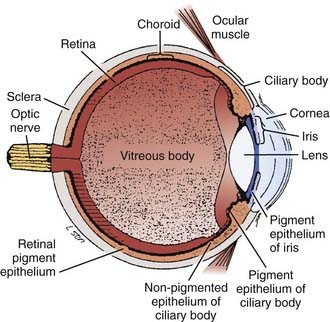
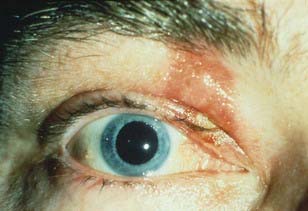
Melanomas develop in the uveal tract, the vascular support layer of the eye. Approximately 80% of uveal melanomas involve the choroid, which is the posterior portion of the uveal tract located under the retina. Less than 10% involve the iris; and approximately 10% to 15% involve the ciliary body, which contains the muscle responsible for accommodation and movement of the lens (Fig. 64-1).37 The Bruch membrane, the transparent inner membrane of the choroid, is significantly weaker than the sclera (which is the tough fibrous outer layer surrounding the choroid). As choroidal lesions grow, the Bruch membrane can stretch and eventually rupture, allowing the tumor to penetrate through and create the characteristic “collar button” or mushroom-shaped deformity by protrusion into the retina. This geometry is virtually pathognomonic for a uveal melanoma. Scleral penetration is significantly less common, and is often associated with tumor tracking along a vessel.
Pathologic Conditions and Prognostic Factors
Uveal melanoma cells are characteristically spindle-shaped or epithelioid; these latter, plumper cells are associated with a worse prognosis. In addition to cellular characteristics, pathologists have quantitated pigment content, argyrophil fiber content, and the presence of tumor necrosis. This is the basis for the Callender classification initially described in 1931.38 It has been modified to describe several pathologic patterns including spindle, mixed, necrotic, and epithelioid types. The American Joint Committee on Cancer (AJCC) 2002 histopathologic staging is shown in Table 64-1. It is likely that benign choroidal nevi were included in the “malignant” category in early reported series.39 Virtually no tumor-related mortality is observed with the spindle A cell type,40 although the spindle B variant is clearly malignant. In patients undergoing enucleation, eye wall resection, or irradiation, several factors are described as having poor prognostic significance. These include (1) gross tumor features, such as large tumor size (tumor diameter and tumor thickness), anterior location (ciliary body invasion), proximity of tumor to the foveal avascular zone, scleral penetration, and optic nerve invasion; (2) pathologic features, such as epithelioid cell type, high mitotic rate, large nucleolar size, lymphocytic infiltration, vascular pattern (microvascular loops and networks); (3) genetic features, including alterations in deoxyribonucleic acid content, gene expression profiling, and monosomy of chromosome 3; and (4) patient factors such as older age, and in some series male gender linked with impaired tumor control27,41–66 (Table 64-2 and Fig. 64-2).
Table 64-1 Histopathologic Staging of Uveal Melanomas
| Tumor |
|---|
| Pathologic |
|---|
| Genetic/Patient |
|---|
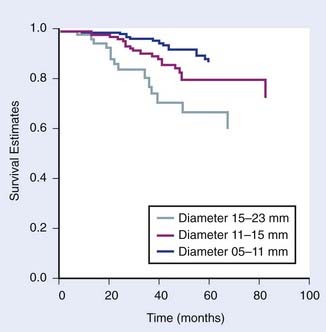
FIGURE 64-2 • Kaplan-Meier survival estimate for time until metastasis following helium ion or iodine-125 plaque by largest tumor diameter.
(From Char DH, Castro JR, Quivey JM, et al: Uveal melanoma radiation: 125-I brachytherapy versus helium ion irradiation, Ophthalmology 96:1708, 1989.)
In most studies, several additional factors have no consistent effect on survival with univariate or multivariate analysis, including duration of signs and symptoms, reticulin fiber content, pigmentation, necrosis, location of the posterior tumor margin, or penetration through the Bruch membrane.41,44 In most historic series, cell type is the most important single predictor of death following enucleation. The relative importance and relationship between various prognostic factors is uncertain, with current series showing gene expression profile of the tumor to be most tightly correlated with prognosis.33,34,67
The predictive value of melanoma genome studies are under study as a more quantifiable and reproducible system of tumor classification. Currently some groups report testing for monosomy 3 on tumor specimens for prognostic purpose. One investigator found that the relapse-free survival in 30 patients with monosomy 3 was 43% in contrast to 100% in 24 patients without this chromosomal loss.27 Our current practice has been to use a more sensitive technique of measuring intratumor melanoma gene alterations, which shows superior results in terms of accuracy of prognostic information.33,67 These gene studies are performed on patients who have either fine-needle biopsies or surgical resections, and the assays are better correlated with tumor-related mortality and patient survival than monosomy 3. Tumors in a class 1 pattern have less than 15% tumor-related mortality, whereas class 2 tumors have more than 85% metastatic deaths within 5 years. This tumor genomic classification provides a risk assessment of patients and potentially may help delineate which patients benefit from specific adjuvant management or prophylactic systemic treatment.
Clinical Presentation
The appearance of fundus lesions on indirect ophthalmoscopy is often diagnostic. Examination includes measurement of the tumor diameter and thickness, delineation of tumor location, geometry, and tumor coloration. Patients are also evaluated for associated subretinal fluid or exudative detachment. The pigmentation of uveal melanomas is variable, with as many as 25% of cases being amelanotic; most uveal melanomas are light to medium in pigmentation. Lesions that most commonly simulate uveal melanoma include nevi, choroidal hemangiomas, rhegmatogenous retinal detachments, age-related disciform lesions, scleritis, and choroidal metastases (most commonly from breast, lung, and gastrointestinal cancer).68–70 Making the correct diagnosis in suspected uveal melanomas is a function of observer experience, media clarity, and tumor size.71 Most ocular oncology units currently report a 99% accuracy,72 although a generation ago, 20% of eyes enucleated with a clinical diagnosis of this malignancy had a simulating lesion on histopathologic examination.39,73–77
Diagnostic and Staging Studies
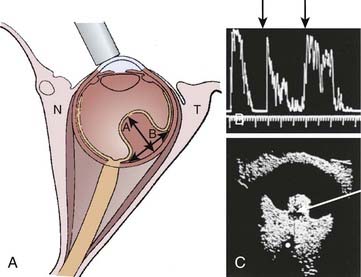
The evaluation of patients with a possible uveal melanoma includes clinical examination, fundus photography, and ocular ultrasonography with immersion B-scan and Kretz A-scan (Fig. 64-3). Other diagnostic tests include visual field testing, indocyanine green angiography, optical coherence tomography, and magnetic resonance imaging (MRI). Clinical findings more frequent in larger tumors include the presence of subretinal fluid and a collar button configuration. Fluorescein angiography, which can identify tumor vessel leakage, is often not diagnostically useful except in differentiating uveal melanomas from hemorrhagic choroidal simulating lesions; in a masked study without other ancillary tests, this technique has a diagnostic accuracy of only 63%.75 In contrast, experienced ultrasonographers can differentiate uveal melanomas from simulating lesions in approximately 89% to 96% of cases that require therapeutic intervention.78,79 Ultrasound factors useful for diagnosing melanoma include choroidal excavation, orbital shadowing, acoustic quiet zone, and homogeneity on A-scan (Fig. 64-4). Ultrasound is also useful and reproducible in measuring tumor height, although it is less reliable in tumors located anterior to the equator because of the difficulty in positioning of the A-scan probe. Extensive subretinal fluid over the lesion can also lead to inaccuracy of A-scan with an overestimate of tumor height. Some observers have used ultrasound to estimate tumor diameter, although we note this to be less accurate in lesions that have a large area of relatively flat tumor extension.
MRI has been evaluated in this neoplasm.80–83 Although the T1 and T2 pattern of pigmented uveal melanomas is relatively typical, a number of false-negative and false-positive cases have been reported. This is a relatively expensive test that usually does not increase diagnostic accuracy afforded by ultrasound. MRI can be diagnostically useful (although inaccuracies have been noted) in the detection of localized extraocular extension, and in the differentiation between a uveal melanoma and a peripheral choroidal hemorrhage resulting from an extramacular disciform lesion. MRI is potentially useful in providing anatomic information that can improve tumor volume delineation for treatment planning purposes.84,85
For smaller pigmented lesions, noninvasive diagnostic tests are less accurate and it is often difficult to distinguish an atypical benign melanocytic proliferation from a small melanoma less than 3 mm thick.74 We have labeled patients who have indeterminate pigmented choroidal lesions with the neologism nevoma. This includes lesions that are too large to be clinically thought of as nevi (generally >7 mm in diameter and >1.5 mm thick), but that appear to be relatively inactive. The clinical approach to these lesions is usually close observation with serial fundus photography to assess changes in tumor borders, and ultrasound A-scan to assess changes in tumor thickness (height).
It is unusual (approximately 1% in contemporary series) to simultaneously present with metastatic disease and a primary uveal melanoma; the markedly shortened survival in this setting significantly influences the treatment approach. The most common sites of metastatic spread are liver, lung, and bone.86 The metastatic evaluation includes a thorough physical examination with liver function studies and a computed tomography (CT) scan of the abdomen if serum abnormalities are detected. Chest x-rays have a very low yield for detecting metastasis, but are a useful baseline study.87,88 Whole-body positron emission tomography (PET)-CT scans have been increasingly used for detection of uveal melanoma metastases. The clinician must be mindful of other primary neoplasms, including breast, lung, and genitourinary cancers, which may produce choroidal metastases that simulate uveal melanoma.
Fine-needle aspiration and gene profiling of tumor samples can be used for diagnostic and prognostic purpose. Gene expression separated into class I and II patterns is shown to correlate tightly with tumor-related mortality and provides risk assessment that can be useful for clinical decision-making.33,34,67
Staging System
There are two staging systems commonly used in uveal melanomas, AJCC and Collaborative Ocular Melanoma Study (COMS).89–92 The AJCC tumor (T) classification is defined according to the tumor dimensions, including the largest basal diameter and the tumor thickness or height. The AJCC staging also subgroups tumors with microscopic or macroscopic extraocular extension within the T1 through T2 categories. Stage grouping is as follows: stage I includes T1-N0-M0; stage II is T2-N0-M0; and stage III is T3– and T4-N0-M0 disease. Any nodal or metastatic spread is considered stage IV disease. Of note, ciliary body and choroidal melanomas are staged differently from iris melanomas.89 In general, iris melanomas are often detected earlier and are smaller in size than posterior melanomas.
A second staging system is recommended by the COMS. The two systems, presented in Table 64-3, differ in the definition of stages, although the newer AJCC staging system is modified based on clinical observations in the COMS trials. Size criteria definitions vary across studies and must be reviewed carefully when comparing patient series. Generally, COMS small, medium, and large tumors now roughly correspond, though not exactly, with AJCC T1, T2, and T3 or greater tumors, respectively. Unfortunately, neither staging system takes primary tumor location or visual acuity into consideration. These factors greatly influence the optimization of treatment modalities. Most authors with large series have reported these factors and attempted to correlate them with tumor control and treatment complications.
Standard Therapeutic Approaches
The optimum management of uveal melanomas is controversial. In small, indeterminate, pigmented tumors (≤3 mm thick and ≤10 mm in diameter), data reported by our group and others support the concept that serial observation of these lesions is reasonable unless growth is documented.93 Approximately two thirds of these lesions do not grow. The survival in patients whose lesions subsequently grow and require treatment is no worse than has been reported with prompt, early therapy of small lesions. In addition, many such patients are allowed to maintain good vision in an eye that would have had marked treatment sequelae.
For medium-sized, T2 or growing T1 tumors, various surgical and radiation-based therapeutic alternatives are reported in the literature. Surgical intervention includes local resection with or without adjuvant radiation, enucleation, or exenteration. Radiation modalities include plaque brachytherapy, charged particles (i.e., protons or helium), and radiosurgery. Both prospective COMS and retrospective studies demonstrate comparable survival rates between enucleation and irradiation.94–96 Selecting the optimal radiation modality depends on tumor and patient parameters as well as accessibility to specialized treatment facilities.
Photocoagulation, Laser Treatment, and Thermotherapy
Although photocoagulation and laser treatment have been used to treat small choroidal melanomas and high-risk nevomas for approximately 40 years, the efficacy of these treatment modalities is limited. Newer laser approaches have used an 810 nm laser with long duration treatments. This treatment, termed transpupillary thermal therapy, involves delivering a beam of infrared radiation through a dilated pupil into an intraocular tumor. The exact cytotoxic mechanism of this infrared diode laser is uncertain; however, it is thought to result in tumor cell necrosis by cell membrane damage, protein denaturation, chromosomal damage, and breakdown of mitochondria.97 The maximum depth of penetration of this modality is estimated to be about 4 mm. In some centers, the use of transscleral (rather than transpupillary) infrared diode laser is being investigated.
Most centers use thermotherapy to treat small posterior pole choroidal tumors, such as high-risk choroidal nevomas (based on clinical, ultrasound, and fluorescein angiographic criteria); small, growing melanomas in close proximity to the optic nerve or fovea; or small, growing tumors in the posterior pole that have produced sufficient subretinal fluid to decrease vision.98 Some investigators have combined infrared diode laser with radiation, either to more promptly reduce exudative retinal detachments99 or for adjunctive therapy in posterior pole choroidal melanomas to improve the efficacy of brachytherapy.100
Melanomas contiguous to the disc should not be treated by this technique. There is as much as a 60% late recurrence rate, and several centers report localized extraocular recurrences from the intrascleral extension of the tumor not effectively treated by this modality.100 Retinal complications are described in approximately 30% to 75% of treated eyes, including retinal detachment, retinal vascular occlusions, hemorrhage, or internal limiting membrane contracture and associated decreased vision.
Eyewall Resection
Surgical resection of uveal melanoma has been described in several series.101–104 The rationale for eyewall resection is to attempt to preserve the eye and useful vision. Adjunctive radiation is an important part of this approach, using either protons or radioactive episcleral plaques. Eyewall resection with or without radiation has been recommended for patients in whom radiation alone may carry increased complication risk (i.e., those with thicker tumors) or decreased expected local control. A secondary benefit of eyewall resection is the availability of tumor specimen for diagnostic and prognostic research purpose.
The major problem with a local tumor resection is lack of predictability. In our experience we have treated tumors up to 18 mm in diameter and 14 mm thick with tumor resection. Although some of the eyes were retained with 20/20 vision, others have been lost at the time of surgery and others have had what appeared to be good tumor resection but with major ocular complications. In a review of 125 patients followed for a mean of 6 years, 76% of eyes were successfully retained and 53% had a final visual acuity of at least 20/40.105
A recent analysis reports on eyewall resection of 344 cases with median tumor diameter 13 mm (range 4-21 mm) and median tumor thickness 8 mm (range 1.5-16.5 mm).104 Adjunctive ruthenium plaque brachytherapy was used in 37.5% of patients. Local tumor control at 8 years was 47% to 75%, depending on the number of risk factors (higher local failure rate with diameter >15 mm, epithelioid cells, posterior extension < 3 mm from disc or fovea). Ocular conservation rate at 8 years ranged from 57% to 81%. Visual acuity of at least 20/40 was maintained in 38% to 43% of patients; and if only conserved eyes were analyzed, then 50% of eyes retained this level of vision. Others have retrospectively reported on large tumors with increased local tumor relapse and enucleation rate with local resection (with or without radiation) versus plaque therapy alone.106
The exact role of eyewall resection remains to be defined. Generally, eyewall resection is not considered ideal in cases of very large tumor size, optic disc involvement, extensive ciliary body or retinal invasion, extrascleral extension, diffuse or multifocal disease, ring melanomas, and if it is unlikely that useful vision will be conserved in the affected eye.103,106 Eyewall resection is a technically difficult surgery, can require hypotensive anesthesia, and is not readily available at many centers. Complications for eyewall resection can include hemorrhage, retinal detachment, ocular hypotony, and anesthetic effects. Postoperative brachytherapy can cause maculopathy, optic neuropathy, glaucoma, and wound dehiscence.
Enucleation
Although enucleation was the standard method for treating uveal melanomas as early as 1882,107 the effect of this treatment on the natural history of uveal melanomas was called into question by Zimmerman,108 who proposed that surgical manipulation at the time of enucleation might cause tumor dissemination. Several subsequent analyses pointed out the pitfalls inherent to his retrospective review and challenged this conclusion.109 Nonetheless, the role of enucleation in the treatment of uveal melanoma has diminished with the availability of successful alternative therapies. Several indications still exist for enucleation, including patient request after informed consent, failure of alternative therapy, tumor involving more than 40% of the intraocular volume, tumor with large extrascleral extension, tumor in a blind or painful eye, and a melanoma in an eye with marked neovascularization. In multiple studies, patients who have known visceral metastases from uveal melanoma have a median survival of less than 6 to 7 months.88,109 Patients with metastatic disease do not benefit from enucleation unless the eye is painful.
Adjunctive pre-enucleation radiation therapy or postoperative radiation has been used in an attempt to minimize local recurrence and tumor-related mortality.110–113 Preoperative irradiation was given to 42 patients in our institution in the hopes of exploiting the theoretic advantage of this approach (Fig. 64-5). Unfortunately, when compared with 33 patients with similar tumors enucleated by the same surgeon without preoperative irradiation, no benefit could be measured.110 The dose of radiation was 20 Gy given in 5 fractions of 4 Gy in this small series. A subsequent study by the COMS group using the same daily fractionation and total dose but done in a multi-institutional, prospective randomized setting, also found no survival advantage with pre-enucleation radiation.92,114
Exenteration
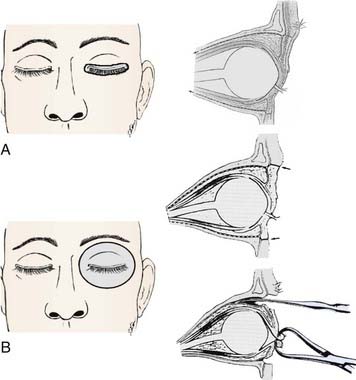
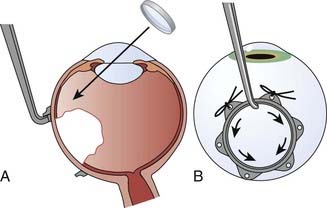
If there is diffuse extraocular extension, exenteration is indicated provided there are no metastases.115 Postoperative external-beam radiation therapy may be employed. Exenteration can be performed using either lid-sparing, or non–lid-sparing techniques (Fig. 64-6A, B). An incision is made through the periosteum at the orbital rim to free orbital contents from the orbital bones. A temporalis muscle flap is often used to minimize the surgical defect and a partial thickness skin graft can cover the defect left by the eyelid resection.
Charged-Particle Radiotherapy
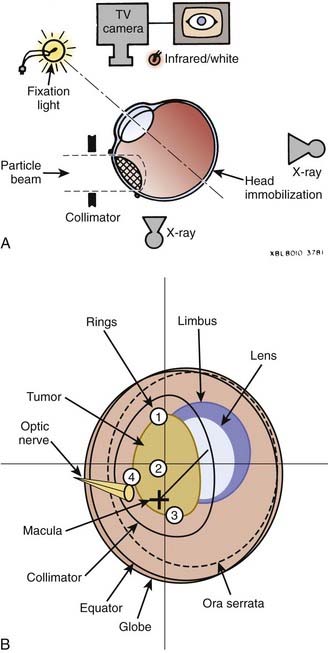
The development of uveal melanoma treatment with the expanded Bragg peak of charged particle beams was described by Constable and others working at the Harvard cyclotron with a 160-MeV proton beam.116,117 Accelerated charged particles, including protons and helium ions, can treat small targets with minimal lateral scatter and radiation dose beyond the end of the Bragg peak. Studies with modulated and collimated proton beams demonstrate a relative biologic effectiveness (RBE) of close to unity when compared to 2-meV photon beams. The adaptation of this technique for humans required the development of sophisticated tumor mapping using the surgical placement of tantalum rings (Fig. 64-7A), immobilization devices controlling for both head position and gaze angle, and a computerized treatment planning program.118,119 A similar technique using helium ions was developed at the Lawrence Berkeley National Laboratory (LBNL) in collaboration with the Department of Radiation Oncology and the Ocular Oncology Unit at the University of California, San Francisco (UCSF). The RBE of the helium ion beam is 1.3, slightly greater than the proton beam, and accordingly dose adjustments were made.120 Because of decommissioning of the heavy ion synchrotron at LBNL, the program was moved to a 76-inch cyclotron at the Crocker Nuclear Laboratory (CNL) on the University of California, Davis, campus.121
More than 3000 patients with uveal melanoma have been treated with protons at the Harvard cyclotron to date. The prescribed dose in their series is 70 cobalt gray equivalent (CGE) in five fractions over 7 to 10 days (assuming CGE = proton Gy × RBE 1.1). Reporting on more than 2000 of their patients, their local control at 5 years is 97% and at 15 years is 95%.122–125 The 5-year actuarial survival for the patients treated with protons is 78.5% for the entire group, with 97.5%, 85.8%, and 58.1% survival for patients with small, intermediate, and large melanomas, respectively. The 5-, 10-, and 15-year tumor-specific survival rates are 86%, 77%, and 73%, respectively. Using the Cox proportional hazards model, the factors that predict decreased survival on multivariate analysis include maximum tumor diameter greater than 15 mm, ciliary body involvement, age older than 59 years, and the presence of extrascleral tumor extension.52
These results have been confirmed at the Paul Sherrer Institute by Egger and colleagues,43 who reported on 2435 patients treated between 1984 and 1998 with 54.5 Gy in four fractions (corresponding to 60 CGE). The local control rate is estimated to be 95.8% at 5 years and 94.8% at 10 years (by Kaplan-Meier). Patients treated with a reduced safety margin because of proximity of the tumor to the fovea have a much higher local failure rate at 5 years of 28.7%. Local tumor control is correlated with survival rates; the overall 10-year CSS is 72.6% for patients with controlled tumors versus 47.5% for those with local recurrence. In their series, with greater experience including updated planning software and techniques, patients treated after 1993 have higher control rates than those treated before (5-year local control 99% versus 90%-96% and 5-year CSS 88% versus 82%-83%, respectively). Additional factors adversely affecting prognosis include older age, increased amount of retinal detachment, ocular melanosis, large tumor diameter, increased tumor height, and extrascleral extension.
A randomized dose trial for proton-beam therapy was conducted on 188 patients comparing doses of 50 CGE and 70 CGE in five fractions and found no significant difference between dose regimens in terms of ocular toxicity or local control at 5 years.126 As described, Egger and colleagues reports 99% control with a contemporary subgroup of patients treated with proton therapy to a dose of 54.5 Gy in four fractions (60 CGE).
Numerous single-institutional reports on proton-beam therapy similarly describe excellent 5-year local control in the range of 95% or greater (range 90.5%-99%).43,122–130 With 15-year follow-up, authors report local control to be maintained at 95%.124–125 At the UCSF–CNL treatment facility, more than 1000 uveal melanoma patients have been treated since 1994, and the dose we currently use is 56 Gy equivalent in four fractions over 4 consecutive days with comparable high local control rates. Overall survival at 5 years is approximately 80% across series (range 70%-88%), with small tumors ranging from 95% to 98%, medium from 80% to 86%, and large at 60%. Enucleation rate after proton therapy at 5 years is approximately 10% (range 0%-25%), with small tumors ranging from 2% to 3%, medium from 7% to 8%, and large from 22% to 25%.122–132 One author reports on a contemporary series of patients treated with current proton-beam technique who achieved enucleation rates of 0% for small, 0.3% for medium, and 10.5% for large tumors.132 The overall enucleation rate at 15 years is approximately 15%.125
Approximately 60% of enucleations following proton-beam therapy are performed for complications related to neovascular glaucoma (NVG). The remainder of enucleations are done for local salvage after recurrence and treatment of other complications, including retinal detachment, severe inflammation, painful eye, and functional loss. The enucleation rate is shown to vary based on risk factors including tumor height and diameter, volume of lens irradiated, tumor distance from optic disc or macula, retinal detachment, ciliary body involvement, patient gender, intraocular pressure, and baseline visual acuity. The majority of enucleations are done between 0 and 3 years after treatment, but can be required at longer follow-up as well. Useful vision preservation (>20/200) with proton irradiation is reported at 3 years to be variable in the range of 40% to 65%.127,129,133,134
The results with helium ion therapy are similar to those described for protons. A series of 347 patients were treated with a dose of 50, 60, 70, or 80 Gy equivalents (GyE) in five fractions (CGE = helium ion dose Gy × RBE 1.3) in a dose-searching effort to establish the minimum dose necessary for optimal tumor control. The 5-year local control is 96% for doses between 50 and 80 GyE and the 5-year survival is 80%.135–137 The Kaplan-Meier survival is 76% at 10 years and 72% at 15 years.138 The enucleation rate is reported at 19%; the rate is 3% for local failure and 16% for complications, particularly NVG.
A series of 59 patients were treated with carbon-ion radiotherapy at the National Institute of Radiological Sciences in Chiba, Japan, for locally advanced or unfavorably located choroidal melanoma.139 They report 3-year local control, disease-free survival, and overall survival, as 97.4%, 84.8%, and 88.2%, respectively. Of 57 patients, 23 (40%) developed NVG and 4 received enucleation, 3 because of intense pain and 1 for recurrence. Six patients developed distant metastases.
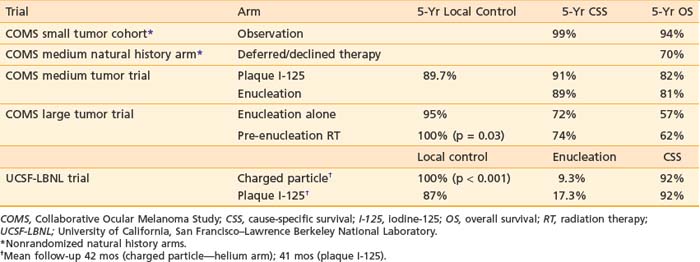
Based on current literature, short- and long-term local control rates are generally higher for charged particles compared with plaque brachytherapy, discussed in the following text.135,140 Survival rates across studies are comparable between enucleation, plaque, and particle therapies. Many patients are able to preserve the eye and useful vision with irradiation.
Particle Therapy Technique
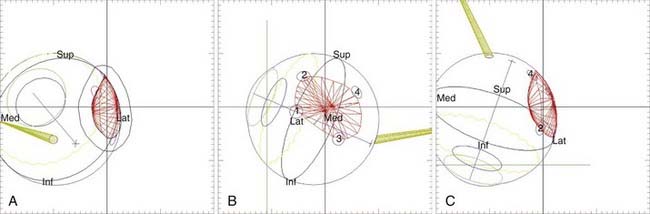
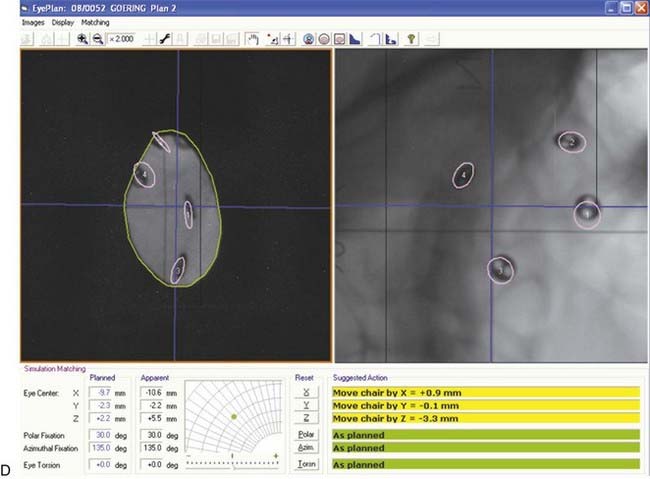
The EYEPLAN software used for treatment planning was developed by Goitein and Miller118 at Massachusetts General Hospital and has since been modified.141,142 The input data to the planning program includes (1) the spatial coordinates of the rings relative to the axis of the eye, obtained from orthogonal x-ray films; (2) the axial length and tumor height as measured on ultrasound; and (3) the shape of the tumor as drawn manually on the computer screen. The tumor relation to the rings is obtained from the surgeon’s mapping, fundus drawing, and fundus photography, as well as three-dimensional (3-D) MRI images.84 The program schematically displays a line drawing of the patient’s eye, including such anatomic structures as the globe, lens, optic nerve, and macula. Fig. 64-7B shows the display of the eye contours (globe, lens, limbus, disc, and macula), rings, tumor, and aperture.
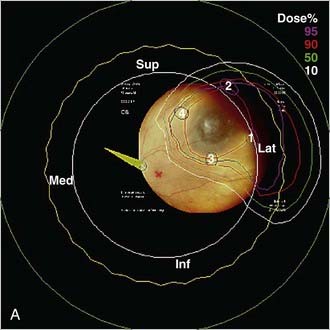
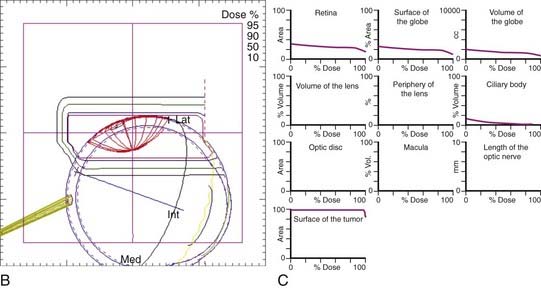
Figure 64-8 illustrates the treatment plan of a uveal melanoma of the eye and Fig. 64-9 shows the dose distribution in polar and in horizontal plane and the associated DVHs. The polar and horizontal view of the dose distribution example shows the 95% isodose line to cover the tumor volume; critical structures, including lens, optic disc, macula, and optic nerve, receive 0% of the prescribed dose; and 3% of the cilary body volume receives 50% of the prescribed dose as indicated by DVHs.
Stereotactic Radiosurgery
Radiosurgical techniques using gamma knife, cyber knife, and other linear accelerator–based stereotactic photon therapy have been described.143–153 These are often more readily available than other radiation techniques and hence may provide an opportunity for treatment of uveal melanoma outside of specialized particle or brachytherapy centers. However, issues of tumor dose inhomogeneity, fractionation of dosing, extended treatment time, monitoring, and fixation of head position and gaze angle remain a challenge for radiosurgical therapy. Invasive techniques, including application of stereotactic frames and suturing of the extraocular muscles to achieve targeting, generally limit the number of treatment fractions. In addition we find that peripheral doses to the contralateral eye and thyroid as well as whole-body doses tend to be higher with radiosurgery compared with proton therapy.154 Similarly within the eye, radiosurgical techniques tend to deliver higher doses to critical ocular structures, except to anterior tissues such as ciliary body and lens, which tend to have higher doses with the proton beam. Prospective randomized trials and longer follow-up are needed to evaluate radiosurgical therapy for control and complication rates.
Plaque Brachytherapy
Brachytherapy with various radioactive sources has been employed for treatment of uveal melanoma. The American Brachytherapy Society (ABS) has published guidelines for uveal melanoma plaque brachytherapy with the goal of standardizing both treatment and dose reporting policies.155 The recommendation for small T1 tumors is to observe unless there is growth. For medium-sized T2 tumors and select large tumors, episcleral plaques can be used. Patients with large tumors or with tumors at peripapillary or macular locations have a poorer visual outcome and lower local control with plaque therapy. The ABS guidelines advise that patients with gross extrascleral extension, ring melanoma, and tumor involving more than half of the ciliary body are not suitable for plaque therapy. Iodine-125 (I-125) is the most commonly used isotope in the United States. The ABS recommends a minimum tumor I-125 dose of 85 Gy at a dose rate of 0.60 to 1.05 Gy per hour. The technique for plaque placement and dosimetry is detailed later in the chapter.
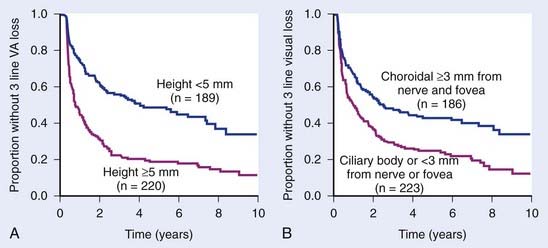
Analysis of high-intensity I-125 plaque treatment of uveal melanoma at UCSF included 449 patients treated between 1983 and 2000.45,140 Patients received 70 Gy to 1 mm above the apex of the tumor with a horizontal margin of at least 1.5 mm during a 4-day treatment course. Local control at 5 and 10 years is 88.5% and 84.2%, respectively, with a median interval between treatment and detection of recurrence of 2.9 years (range 0.2 to 15.3 years). Of those who fail locally during the follow-up period, approximately 20% have “late” intraocular tumor failures detected from 5.5 to 15.3 years after treatment. The 5-year metastasis rate is estimated at 12%, with a higher rate for epithelioid and mixed-cell types versus spindle-cell type. Four factors were associated with increased local failure: smaller tumor height, closer proximity to the fovea and disc, larger tumor diameter, and lower radiation dose. Interestingly, these factors were not observed in the late treatment failures. The incidence of distant metastatic disease was associated with larger tumor diameter. In our experience with I-125 plaques and charged particle therapy (helium ion or proton), there is an increased local recurrence after plaque irradiation (Fig. 64-10).140 The details of the prospective randomized trial of helium ions versus I-125 brachytherapy conducted at UCSF and the LBNL are discussed later in the text.
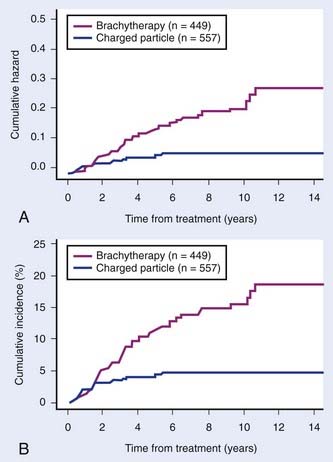
FIGURE 64-10 • A, Cumulative hazard of local recurrence after radiation. B, Probability of local recurrence after radiation.
(From Char DH, et al: Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle [proton or helium ion] therapy, Ophthalmology 109:1850, 2002.)
Numerous series confirm a 5-year local control rate in the range of 81% to 96% with contemporary plaque therapy.42,45,155–168 The COMS medium tumor trial compared enucleation with I-125 plaque therapy.42,91 The 5-year local control rate is reported at 89.7% with I-125. Survival rates are comparable between plaque therapy and enucleation; with 5-year overall survival at 82% and 81%, respectively. The COMS study shows I-125 therapy to result in a 5-year enucleation rate of 12%; other recent series show a range of enucleation rates between 4% and 20%.106,155–168 The 5-year useful visual acuity preservation (>20/200) is 37% on the I-125 arm of the COMS study; others report widely variable useful vision retention outcomes, ranging from 23% to 73%.155 Dose, dose rate, maximum tumor diameter, tumor location, and older age are important prognostic factors.42,169 Higher recurrence rates are noted with larger tumors and with tumors in close proximity to the fovea, optic nerve, and ciliary body.
Plaque Therapy Technique
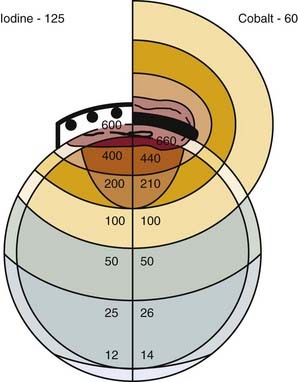
Brachytherapy has been used in uveal melanoma management since 1929,170 and Stallard has been credited with initially popularizing the use of cobalt-60 (Co-60) episcleral plaques.171 Sealy described an adaptation of the episcleral technique using the lower-energy isotope I-125 with intrinsic shielding in 1976.172 The ability to shield the ocular adnexa, including the eyelids and lacrimal apparatus, and markedly decrease the radiation dose to the surgical team was a major advance. A comparison of the dosimetry of plaques using different isotopes was described by Luxton, who noted that for tumors up to about 10 mm in thickness, the inverse square law effect is preponderant, which is independent of the photon emission energy.173 Although it may be counterintuitive, for a given dose to the tumor apex, the tumor base dose is similar for photon emitters from Co-60 (1.2 meV) to I-125 (27 keV) (Fig. 64-11).174 In contrast, beta emitters including ruthenium-106 deliver a very high dose to the sclera and are less suitable for the treatment of tumors thicker than 3 mm, although these applicators may be used to minimize the dose to other visually sensitive structures.175,176 A number of other radioisotopes are reported, including gold-198, iridium-192, and palladium-103.155
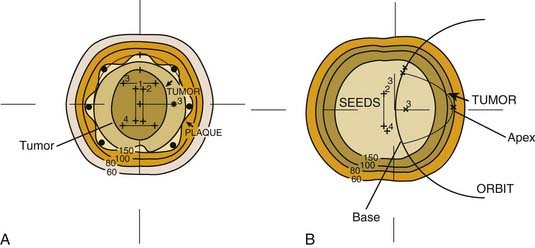
The most commonly used isotope for uveal melanoma therapy in the United States is I-125 because of its ease of shielding, availability in a wide range of seed strengths, and favorable half-life (59.6 days), which allows multiple usage. I-125 seeds exhibit considerable anisotropy, which requires that dose calculations be done with linear source modeling. Published computer algorithms have good agreement with measured values for depths from 0.5 to 1.5 cm along the central axis at angles near 90 degrees.173,177 The gold backing of the applicator reduces the measured dose in the first 1.5 cm depth by 8%.178 Further, Monte Carlo evaluations suggest a specific dose constant 14% lower than previously recommended because of disparities between the dose measured in air and in water.179 Although the uncertainties of dose with I-125 dosimetry have frustrated attempts to standardize the clinical implementation of this technique, it is clear that the optimal seed array and activity depends on the tumor configuration and location. A sample dose distribution for a plaque is shown in Fig. 64-12A, B.
Several computerized treatment planning programs have been developed to include dose distribution to the tumor and the visually critical structures such as the fovea, optic nerve, and the lens. Although treatment planning programs using CT or MRI data have been proposed,180 the lack of precision of these techniques to define the target volume, as well as the additional cost, has led most clinicians to rely on more pragmatic approaches. Some of these programs include fixed-source positions into which seeds may be secured and may also include an algorithm to estimate the effect of the gold shielding applicator. The applicator is designed to follow the external curvature of the globe, perhaps with a notch or a cut-out to accommodate the optic nerve, or with a rim or a lip around the edge to “collimate” the beam (Fig. 64-13A, B). If a rimmed plaque is used, it must be 4 mm larger than the maximum tumor diameter to ensure adequate dose at the tumor base margin (this allows a 2-mm circumferential margin to account for tumor extension and plaque placement uncertainties). It is important to note that although the gold plaque is clearly able to minimize the dose of radiation to the medical team and the periorbital tissues, it has not been demonstrated that the rim can limit radiation-induced visual loss, while achieving similar local control.
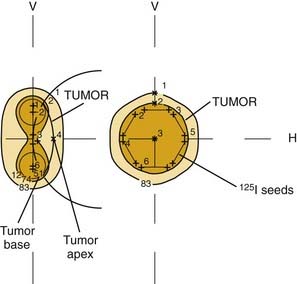
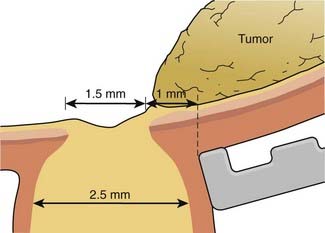
The brachytherapy treatment plan delivers a “tumor dose” of 70 to 100 Gy to the tumor apex with a margin to include the scleral thickness (1 mm), an additional millimeter of tumor thickness or height, and a 2-mm surround of the tumor diameter as defined by the ophthalmologist. Most authors plan for an overall treatment time between 4 and 7 days. Guidelines recently published recommend a minimum tumor I-125 dose of 85 Gy at a dose rate of 0.60 to 1.05 Gy per hour.155,169 The dose rate at the scleral surface depends on the seed activity and the tumor thickness. A 1-mm spacer (or contact lens) is recommended to minimize hot spots over the individual seeds. In spite of very high doses to the sclera, scleral necrosis is a rare event, and often correlates with an anteriorly located tumor where the sclera is known to be thinner and inflammation more common from eroded or retracted conjunctiva. In general, radioactive seed selection and placement is dictated by the tumor geometry. Relatively flat or thin tumors are best treated with the seeds on the tumor perimeter, whereas thick tumors require hot central sources to ensure a high central-axis dose (Fig. 64-14).
Patient selection is critical for the successful application of brachytherapy techniques. Although tumors as thick as 10 or 12 mm have been treated using episcleral plaques, the optimal long-term visual results are for tumors with ultrasound height measurements less than 6 mm. The maximum basal tumor diameter is highly correlated with both local control and metastatic disease. Finally, the location of the tumor relative to the optic nerve, fovea, lens, and attachment of extraocular muscles must be understood if one is to plan the plaque design. Although the plaque can theoretically be placed anywhere on the globe, the attachment of extraocular muscles or the optic nerve can displace the applicator (Fig. 64-15). To allow for the thickness of the optic nerve and its sheath, peripapillary tumors often require an eccentric seed array and a cutout or notched plaque.45 Further, if the tumor is located close to a rectus muscle insertion, one can avoid the surgical detachment of the muscle by using a similar approach. The design of the plaque must be thoroughly understood by the ophthalmologist, as accurate placement is critical to tumor control. Great care must be taken to ensure that the surgeon understands the dose distribution relative to the physical plaque edge. Because this is not always symmetric, the orientation of the seed array can be indicated with a hatch mark on the plaque as well as the dummy plaque.
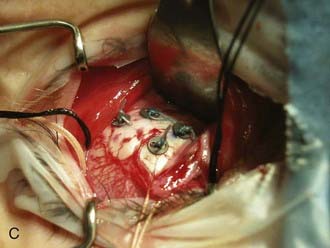
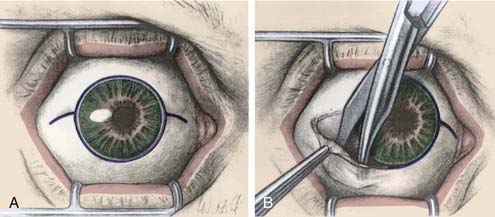
The surgical placement of the plaque is done with either general or local anesthesia. The eye is dilated before the procedure to ensure adequate tumor localization. A 360-degree perilimbal incision is made and the Tenon capsule is entered (Fig. 64-16A, B). The rectus muscles are isolated and suture slings are placed to allow rotation of the globe. The melanoma is localized with diffuse corneal transillumination using diathermy marks to outline the scleral shadow cast by the tumor; the position is confirmed using point-source transillumination with indirect ophthalmoscopy (Fig. 64-17A, B). This latter step is crucial to ensure that “shadowing” from the anteriorly placed diffuse transilluminator has not led to a misinterpretation of the tumor location. If necessary for the plaque placement, extraocular muscles can be detached. Intraoperative needle aspiration biopsy can be done for diagnosis and gene expression profile studies, which provide risk assessment for survival and potential need for adjunctive therapy. A dummy plaque is sutured into place and the position verified before the placement of the radioactive plaque. The radiation oncologist should be present in the operating room with the isodose curves to ensure optimal plaque placement. After the radioactive plaque is secured, the eye is irrigated with antibiotic solution and the conjunctiva closed; the dressing is covered with a lead eye shield, which is left in place during the application. Verification of plaque placement can be accomplished with the use of intraoperative ultrasound. The patient can usually be discharged within 24 hours, depending on state regulations, and return for plaque removal within 4 to 7 days, with reattachment of rectus muscles, if necessary.
Of note, plaque tilt after initial accurate placement can occur frequently during the course of treatment, particularly in posterior lesions near the fovea or disc, which are well known to have lower local control rates. This tilt movement affects the radiation dose delivered and may represent an important factor in local failure with plaque therapy.156
Management and Prospective Trials
Small Tumors
Small (≤3 mm thick and ≤10 mm in diameter without detachment), apparently inactive lesions in patients with good vision are suitable for observation. The COMS study of small tumors was a prospective observational study that supports the safety and appropriateness of observation for small, nongrowing tumors. Patients with tumors 1 to 3 mm in apical height and 5 to 16 mm in basal diameter were included. Initial results were published on 204 patients enrolled and monitored with annual follow-up (mean follow-up was 92 months).90,181 The Kaplan-Meier estimate of 2-year and 5-year tumor growth is 21% and 31%, respectively. Greater initial tumor thickness (2.5 to 3 mm) and larger diameter (12.1 to 16 mm) are associated with higher recurrence and formed the basis for the modifications in the most recent AJCC staging system. The presence of orange pigment on color photography is also significantly associated with tumor growth on multivariate analysis, whereas the presence of drusen (hyaline or colloid bodies within the lesion) and area of change in the retinal pigment epithelium adjacent to tumor are significantly associated with nongrowing tumors. Of 27 deaths in this study, only 6 are reported as melanoma-related. The Kaplan-Meier estimates for 5-year and 8-year melanoma-specific mortality are 1% and 3.7%, respectively.
Medium Tumors
Prospective randomized trials give some comparative information regarding optimal treatment of medium-sized uveal melanomas. Despite this, there remains a wide variety of options, including radiation with brachytherapy, charged particles, and radiosurgery, as well as surgical techniques of enucleation or local resection with or without adjuvant treatment. Further research and follow-up is required to determine the relative appropriateness of various options (Table 64-4).
Collaborative Ocular Melanoma Study: Enucleation versus Iodine-125 Brachytherapy
In the medium choroidal melanoma study, COMS compared brachytherapy with I-125 and enucleation with respect to mortality.42,91 This study addresses a long-time controversy regarding the efficacy of definitive irradiation. Patients with tumors measuring 2.5 to 10 mm in apical height and 16 mm or less in diameter were randomized to receive I-125 plaques or enucleation. Brachytherapy was delivered using plaques with a 2- to 3-mm margin beyond the tumor base, delivering a dose of 85 Gy to the prescription point (the apex for tumors 5 mm or greater in height, or at 5 mm for tumors between 2.5 and 4.9 mm) using Task Group 43 guidelines.182 Patient selection was similar to the UCSF randomized trial between I-125 plaques and helium ions discussed later in the chapter. However, patients at highest risk for local recurrence following plaque therapy were excluded from the COMS analysis. Thus, patients whose tumors were contiguous with the optic disc were ineligible, and those with peripapillary tumors (2 mm or closer to the optic disc) were eligible only when the tumor was limited within 90 degrees. Tumors located predominantly in the ciliary body; tumors with extrascleral extension 2 mm or thicker; and diffuse, ring, or multifocal tumors were also excluded.
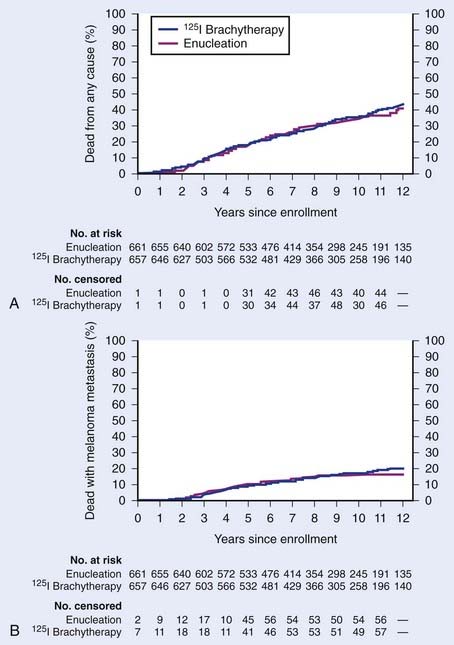
The 5-year overall survival rates are 81% and 82% for the enucleation and brachytherapy arms, respectively, firmly establishing the equivalency of these techniques. The 5-year tumor-related mortality rates are 11% and 9% for the enucleation and brachytherapy arms. Only patient age, maximum tumor basal diameter, and location of the tumor relative to the optic disc are independent and statistically significant predictors of death with melanoma. The 5-year local control rate is 89.7% with I-125 and enucleation rate is 12.5%. Treatment failure is the most common reason for enucleation within 3 years of plaque treatment; ocular pain is the most common reason after 3 years. Multivariate analysis indicates that older patient age, greater tumor height, and proximity to the foveal avascular zone are associated with treatment failure. The risk of failure in persons older than age 50 is nearly three times as high as in those younger than age 50.152 The 12-year update confirms no difference in survival between the radiation and enucleation arms (12-yr overall survival 57%-59%, 12-yr CSS 79%-83%; Fig. 64-18).183
Quality-of-life outcomes from this COMS study reveal that patients treated with radiation report significantly better visual function than those in the enucleation arm, with respect to driving and peripheral vision for up to 2 years following treatment. These initial differences diminish on longer follow-up, because of eventual decline in visual acuity in brachytherapy-treated eyes. Patients are more likely to experience anxiety in the brachytherapy arm, likely related to concern for cancer recurrence.184 Of note, 42 patients with medium-sized tumors eligible for the COMS trial who either declined therapy (20 patients) or deferred treatment until a later date (22 patients) were followed for their natural history. This cohort experienced a 5-year overall survival of 70%, suggesting that observation is not as effective as upfront treatment for medium-sized tumors.185
University of California, San Francisco–Lawrence Berkeley National Laboratory: Charged Particles versus Iodine-125 Brachytherapy
A prospective randomized trial of helium ions versus I-125 brachytherapy was conducted at UCSF and the LBNL.135 This trial included a total of 184 patients with tumors less than 15 mm in diameter and less than 10 mm in thickness. The average tumor diameter for enrolled patients was 10.6 mm, and average height was 5.5 mm. A minimum tumor dose of 70 GyE was delivered to the tumor apex, with a dose rate in the particle arm of 5 fractions over 5 to 7 days delivered in less than 2-minute fractions versus continuous low-dose-rate radiation in the I-125 arm (0.7 to 0.75 Gy per hour at the tumor apex).
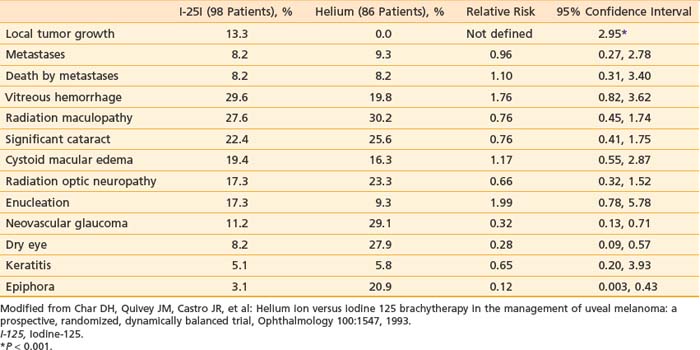
Local control was higher in the particle arm versus the I-125 arm (100% versus 87%, respectively, at mean follow-up 41 months). Enucleation rate was also improved in the particle arm, 9.3% versus 17.3% in the I-125 arm. The higher rate of local failure in the plaque-treated patients led to an increase in the dose and dose rate of subsequent patients treated with plaques. There were clear differences in ocular morbidity, with dry eye, epiphora, and NVG more common following helium ion therapy; and temporary strabismus unique to I-125–treated patients resulting from the need to temporally disinsert a muscle to facilitate plaque placement (Table 64-5). Long-term follow-up of the patients in this series shows no differences in tumor-related mortality between the two groups.
Concerns about the development of NVG after helium ion irradiation has led to an in-depth analysis of factors that correlate with this complication.186 Currently, we are using proton beams, with careful attention to minimize the radiation dose to the lens in an effort to maximize local control and minimize late visual complications. Modification of our proton treatment protocol has led to a significant decrease in NVG. In our experience, the incidence of NVG with these recent technical modifications is less than that observed with I-125 brachytherapy.
The equivalency of enucleation and definitive irradiation for medium-sized tumors is supported by the COMS study. If the patient desires organ preservation, the selection of technique (brachytherapy or proton therapy) depends on the tumor location vis-à-vis the fovea, optic nerve, and ciliary body. Substantial retrospective data from many institutions show a significantly higher local failure rate when tumors in these latter areas are treated with episcleral plaques. In fact, these lesions were specifically excluded from the COMS “medium” melanoma trial. The available data from contemporary randomized and nonrandomized series show a higher rate of local control with charged particle beams of helium ions or protons (95% to 99%) than has been observed with brachytherapy techniques. “Late” radiation failures (beyond 5 years after treatment) are also more frequently observed after plaque therapy.140 Because of the limited number of proton facilities, many centers prefer to use episcleral plaques, citing the equivalence of melanoma mortality across series. Certainly, for frail or elderly patients who are unable to travel for therapy, this is clinically appropriate. Visual outcome analysis remains somewhat disappointing, as the COMS observations regarding visual function closely parallels our own observations.
Large Tumors
Enucleation has been the historical standard, although eye-conserving approaches are now successfully applied even in patients with large tumors. Patients must be adequately advised regarding the issues of local control, subsequent enucleation risk, and vision loss with eye-conserving procedures. There is some data to suggest that increased local relapse, such as with eye-conserving therapy, may be correlated with decreased survival.42,43,164 However, to date, survival with proton therapy for selected patients with large tumors has been comparable to enucleation (∼60% at 5-years).92,114,122,123
Collaborative Ocular Melanoma Study: Enucleation With or Without Preoperative Radiation Therapy
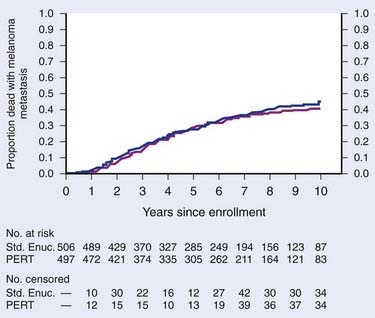
A COMS randomized prospective trial including patients with large tumors (>10-mm apical height or >16-mm base diameter) was conducted to evaluate the benefit of pre-enucleation radiation. Between 1986 and 1994, 1003 patients with large-size melanoma were randomized to either receive enucleation alone or preceded by irradiation. Analysis of the data at 5 years showed no statistically significant difference in overall survival between the two treatment arms (57% for enucleation versus 62% for pre-enucleation radiation, p = 0.32).92,114 Tumor-related mortality at 5 years was 27% (28% for enucleation versus 26% for pre-enucleation radiation). There was a lower incidence of orbital recurrence in the irradiated patients (0% versus 5%, p = 0.03). The 10-year update confirms no survival advantage between arms (10-year overall survival and CSS of 39% and 55%-60%, respectively; Fig. 64-19).187 Older age and larger tumor diameter are primary negative predictors of survival.
Local Recurrence
Recurrent tumor without signs of metastatic disease most often requires surgical salvage. A recent series reports on the use of proton reirradiation for local relapse. A second course of proton therapy between 48 and 70 CGE (for a total lifetime dose of 118-140 CGE) was delivered to 31 patients with resultant 5-year rates for local control of 69%, overall survival in 64%, eye-retention in 55%, and useful vision preservation in 27%.133
Of note, a recent COMS article on secondary enucleation after failed plaque treatment notes that in 40% of eyes enucleated because of suspected local failure, increased tumor size could not be histologically confirmed.188 Eyes enucleated for local control failure did have a greater proportion of viable tumor and more had retinal detachment compared with eyes enucleated for pain or poor vision.
Metastatic Disease
Uveal melanoma is known to have an unpredictable clinical course in which metastatic disease may develop after a prolonged disease-free interval (more than 40 years).189,190 In a study from Finland of 289 patients with uveal melanoma, a histopathologic audit of reported cause of death revealed that previous reports frequently underestimated late melanoma-related deaths.191 Their long-term follow-up shows that metastatic disease was the leading cause of death in melanoma patients, with uveal melanoma–related mortality rates of 31% at 5 years, 45% at 15 years, and 52% at 35 years. Of those that died of melanoma, 62% died within 5 years and 90% died within 15 years. The COMS Group reported on 7541 patients screened for COMS trials. Although less than 1% had metastatic disease detected at time of screening, the majority of eventual deaths on trial were associated with metastatic melanoma.88
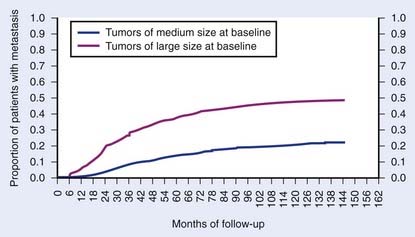
The COMS group reported their results concerning metastatic progression in 2320 patients enrolled on the two randomized trials for patients with medium and large sized choroidal melanomas.88,192 Patients were followed with semiannual to annual clinical examinations, liver function tests, chest x-ray, and further diagnostic tests as warranted. The 5- and 10-year metastatic melanoma rates were 25% and 34%, respectively. Increased tumor size was a poor prognostic feature with 5-year metastatic rates of 38% versus 14% for large- and medium-sized melanomas, respectively (Fig. 64-20). The median survival from time of diagnosis of metastasis to death was approximately 6 months. The mortality rate after diagnosis of metastatic disease at 1 year was 80% and at 2 years was 92%.
Location of metastatic spread was similar for large- and medium-sized tumors, with the most common sites being liver, lung, and bone. Of those diagnosed with metastatic disease, 91% had liver involvement, 28% lung, 18% bone, 12% skin and subcutaneous tissue, 11% lymph nodes, 5% brain, 1% spinal cord, and 1% fellow eye or orbit of either eye. A majority of patients (52%) had one site of metastatic involvement, 25% had two sites, and 24% had three or more sites of metastatic spread. Active therapeutic options are limited in the setting of metastatic disease; new combined systemic therapies and molecular targeted agents are currently being pursued.19,30,88
Radiation and Critical Normal Tissues
Episcleral radioactive plaques and charged particle beams are used to treat uveal melanomas to control tumor while salvaging the globe and potentially preserving vision. Episcleral I-125 plaques can deliver a high tumor dose while usually shielding the ocular adnexa, including the lids and the lacrimal apparatus. These structures are not as easily shielded using charged-particle techniques. Thus, acute epitheliitis of the eyelid, lash loss, dry eye, and epiphora (abnormal overflow of tears caused by excess secretion of tears or obstruction of the lacrimal duct) are more common with charged-particle therapy (Fig. 64-21). Careful attention to lid retraction can remove the lids from the beam in two thirds of the patients. The incidence of NVG (caused by abnormal new blood vessel formation in and on the iris, with closure of angle drainage structures) was historically higher in this latter group, presumably because of the higher radiation dose to the anterior filtration apparatus. However, with recent technical modifications in our proton-treatment protocol, our incidence of NVG is lower than that seen with plaques. An analysis of the development of NVG following helium-ion radiation shows a strong correlation with the percent of lens and anterior chamber in the treatment field, increasing tumor height, proximity of tumor to the fovea, a history of diabetes, and the development of vitreous hemorrhage.186
Significant cataract, radiation maculopathy, and cystic macular edema are seen with equal frequency with plaques and particle techniques. Incidence and time to development of cataracts depends on radiation dose; overall cataract incidence rates may be in the range of 25% to 40%, although reports range from 6% to 83% at 5 years.193 Vitreous hemorrhage and secondary strabismus are more frequently observed after I-125 plaques.
The long-term visual outcome is important to consider when evaluating irradiation techniques. The 1993 report of a randomized trial at UCSF comparing plaques with helium ions confirmed that visual acuities decreased by four or more Snellen lines in 69% of the patients in each group.135 Visual acuity analysis shows the effect of tumor location (>3 mm from the nerve or fovea, no ciliary body involvement) and tumor height (<6 mm) on good visual outcome. Nearly 60% of these more favorable patients had visual acuities of 20/50 or better at last follow-up. An update of the visual outcome analysis identified several factors associated with the rate of visual loss and the post-treatment visual acuity. These include pretreatment visual acuity; tumor thickness; tumor location vis-à-vis the nerve, fovea, and ciliary body; patient age; and the degree of retinal detachment at the time of therapy.194 Groups of patients with low and high probability of good visual outcome can be identified before treatment. The rate of visual loss can be shown using cumulative hazard plots (Fig. 64-22A, B).
The COMS randomized trial of I-125 brachytherapy for medium-sized choroidal melanomas shows decreasing visual acuity to 20/200 or worse in 17% of patients by 1 year, 33% by 2 years, and 43% by 3 years.195 The loss of six lines of visual acuity after treatment was statistically associated with greater baseline tumor apical height and shorter distance between the tumor and the foveal avascular zone. Patient history of diabetes, presence of tumor-associated retinal detachment, and tumors that were not dome-shaped also were associated with greater risk for poor vision outcomes.
Vision preservation with proton irradiation is comparable, with the rate of useful vision (>20/200) in the treated eye at 3 years in the range of approximately 40% to 65%.127,129,134 Important factors similarly include tumor height, distance from optic disc or fovea, retinal detachment, and initial visual acuity.
A 10-year follow-up study of the COMS trials shows no evidence that fellow eyes of patients who underwent radiation (plaque or pre-enucleation radiation therapy) were at greater risk of visual acuity loss or new ophthalmic diagnoses compared with the enucleation arms of both trials.196
Follow-Up
During the first months after treatment, some tumors may continue to enlarge before shrinking. Historically these tumors were often enucleated immediately. Similarly, an exudative retinal detachment or bleeding can cause the tumors to appear large at follow-up examinations and previously would have been enucleated immediately. These tumors may instead be followed for a finite amount of time (e.g., 3 months) and enucleated if continuous growth is measured or if other high-risk features are noted during the observation period.132
1 Egan KM, et al. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988;32:239.
2 Virgili G, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114:2309.
3 McLaughlin CC, et al. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103(5):1000.
4 Singh AD, Topham A. Incidence of uveal melanoma in the United States. Ophthalmology. 2003;110(5):1973-1997.
5 Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664.
6 Inskip PD, Devesa SS, Fraumeni JFJr. Trends in the incidence of ocular melanoma in the United States, 1974-1998. Cancer Causes Control. 2003;14:251.
7 Armstrong BK, Kricker A. Cutaneous melanoma. Cancer Surv. 1994;19:219.
8 Strickland D, Lee JAH. Melanomas of the eye: stability of rates. Am J Epidemiol. 1981;113:700.
9 Osterlind A. Trends in incidence of ocular malignant melanoma in Denmark: 1943-1982. Int J Cancer. 1987;40:161.
10 Barr CC, McLean IW, Zimmerman LE. Uveal melanoma in children and adolescents. Arch Ophthalmol. 1981;99:2133.
11 Singh AD, et al. Familial uveal melanoma. Clinical observations on 56 patients (see comments). Arch Ophthalmol. 1996;114:392.
12 Gonder JR, et al. Uveal malignant melanoma associated with ocular and oculodermal melanocytosis. Ophthalmology. 1982;89:953.
13 Dutton JJ, et al. Orbital malignant melanoma and oculodermal melanocytosis: report of two cases and review of the literature. Ophthalmology. 1984;91:497.
14 Singh AD, et al. Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis. Ophthalmology. 1998;105:195.
15 Abramson DH, Rodriguez-Sains RS, Rubman R. B-K mole syndrome. Cutaneous and ocular malignant melanoma. Arch Ophthalmol. 1980;98:1397.
16 Augsburger J, et al. Diffuse primary malignant melanoma of the choroid arising in a patient with primary cutaneous melanoma. Arch Ophthalmol. 1980;98:1261.
17 Greene MH, et al. The familial occurrence of cutaneous melanoma, intraocular melanoma, and the dysplastic nevus syndrome. Am J Ophthalmol. 1983;96:238.
18 Swerdlow AJ, Storm HH, Sasieni PD. Risks of second primary malignancy in patients with cutaneous and ocular melanoma in Denmark, 1943-1989. Int J Cancer. 1995;61:773.
19 Saraiva VS, Edelstein C, Burnier MN. New prognostic factors in uveal melanomas: Potential molecular targets for therapy. Can J Ophthalmol. 2004;39:422.
20 Kishore K, et al. P53 gene and cell cycling in uveal melanoma. Am J Ophthalmol. 1996;121:561.
21 Wiltshire RN, et al. Cytogenetic analysis of posterior uveal melanoma. Cancer Genet Cytogenet. 1993;66:47.
22 Gordon KB, et al. Comparative genomic hybridization in the detection of DNA copy number abnormalities in uveal melanoma. Cancer Res. 1994;54:4764.
23 Prescher G, et al. Cytogenetics of twelve cases of uveal melanoma and patterns of nonrandom anomalies and isochromosome formation. Cancer Genet Cytogenet. 1995;80:40.
24 Horsman DE, White VA. Cytogenetic analysis of uveal melanoma. Consistent occurrence of monosomy 3 and trisomy 8q. Cancer. 1993;71:811.
25 Patel KA, et al. Prediction of prognosis in patients with uveal melanoma using fluorescence in situ hybridization. Br J Ophthalmol. 2001;85:1440.
26 Sisley K, et al. Association of specific chromosome alterations with tumour phenotype in posterior uveal melanoma. Br J Cancer. 2000;82:330.
27 Prescher G, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222.
28 Parrella P, et al. Detection of c-myc amplification in uveal melanoma by fluorescent in situ hybridization. Invest Ophthalmol Vis Sci. 2001;42:1679.
29 Bale SJ, et al. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N Engl J Med. 1989;320:1367.
30 Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008;34:247.
31 Chana JS, et al. c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br J Ophthalmol. 1999;83:110.
32 Chana JS, et al. The prognostic importance of c-myc oncogene expression in head and neck melanoma. Ann Plast Surg. 2001;47:172.
33 Onken MD, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205.
34 Onken MD, et al. Prognostic testing in uveal melanoma by transcriptomic profiling of fine needle biopsy specimens. J Mol Diagn. 2006;8(5):567.
35 Shah CP, et al. Intermittent and chronic ultraviolet light exposure and uveal melanoma: A meta-analysis. Ophthalmology. 2005;112(9):1599.
36 Holly EA, et al. Uveal melanoma in relation to ultraviolet light exposure and host factors. Cancer Research. 1990;50:5773.
37 Jensen OA. Malignant melanoma of the uvea in Denmark 1943-1952: a clinical, histopathological and prognostic study. Acta Ophthalmol Scand. 1963;65(suppl):17.
38 Callender G. Malignant melanotic tumors of the eye: a study of the histologic types on 111 cases. Trans Am Acad Ophthalmol Otolaryngol. 1931;36:131.
39 Gass DM. Problems in the differential diagnosis of choroidal nevi and malignant melanomas. Am J Ophthalmol. 1977;83:299.
40 Paul E, Parnell BI, Fraker M. Prognosis of malignant melanomas of the choroid and ciliary body. Int Ophthalmol Clin. 1962;2:387.
41 McLean IW, Saraiva VS, Burnier MN. Pathological and prognostic features of uveal melanomas. Can J Ophthalmol. 2004;39:343.
42 Jampol LM, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002;109:2197.
43 Egger E, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys. 2001;51:138.
44 McLean IW, Foster WD, Zimmerman LE. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977;95:48.
45 Quivey JM, et al. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys. 1993;26:613.
46 Zimmerman LE, McLean IW. An evaluation of enucleation in the management of uveal melanomas. Int Ophthalmol Clin. 1980;20:1.
47 Shammas HF, Blodi FC. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol. 1977;95:63.
48 Zimmerman LE, McLean IW, Foster WD. Statistical analysis of follow-up data concerning uveal melanomas, and the influence of enucleation. Ophthalmology. 1980;87:557.
49 Packard RBS. Pattern of mortality in choroidal malignant melanoma. Br J Ophthalmol. 1980;64:565.
50 Diener-West M, et al. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol. 1992;110:245.
51 Seddon JM, et al. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983;101:1894.
52 Gragoudas ES, et al. Prognostic factors for metastasis following proton beam irradiation of uveal melanomas. Ophthalmology. 1986;93:675.
53 Gragoudas ES, et al. Metastasis from uveal melanoma after proton beam irradiation. Ophthalmology. 1988;95:992.
54 Char DH, Kroll SM, Miller T, et al. Irradiated uveal melanomas: Cytopathologic correlation with prognosis. Am J Ophthalmol. 1996;122:509.
55 Char DH, et al. Uveal melanoma radiation. 125I brachytherapy versus helium ion irradiation. Ophthalmology. 1989;96:1708.
56 Karlsson M, Boeryd B, Carstensen J, et al. Correlations of Ki-67 and PCNA to DNA ploidy, S-phase fraction and survival in uveal melanoma. Eur J Cancer. 1996;32A:357.
57 Mooy CM, et al. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am J Pathol. 1995;147:1097.
58 Pe’er J, et al. Mean of the ten largest nucleoli, microcirculation architecture, and prognosis of ciliochoroidal melanomas. Ophthalmology. 1994;101:1227.
59 Coleman K, et al. Prognostic factors following enucleation of 111 uveal melanomas (see comments). Br J Ophthalmol. 1993;77:688.
60 Gamel JW, McLean IW. Computerized histopathologic assessment of malignant potential. III. Refinements of measurement and data analysis. Anal Quant Cytol. 1984;6:37.
61 Gamel JW, McLean IW, McCurdy JB. Biologic distinctions between cure and time to death in 2892 patients with intraocular melanoma. Cancer. 1993;71:2299.
62 Seddon JM, Gragoudas ES, Egan KM, et al. Relative survival rates after alternative therapies for uveal melanoma. Ophthalmology. 1990;97:769.
63 Barr CC, Sipperley JO, Nicholson DH. Small melanomas of the choroid. Arch Ophthalmol. 1978;96:1580.
64 Flocks M, Gerende J, Zimmerman LE. The size and shape of malignant melanomas of the choroid and ciliary body in relation to prognosis and histologic characteristics. Trans Am Acad Ophthamol Otolaryngol. 1955;Nov.-Dec.:740.
65 Folberg R, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389.
66 McLean IW, et al. Uveal melanoma: the importance of large nucleoli in predicting patient outcome—an automated image analysis study. Cancer. 1997;79:982.
67 Onken MD, et al. Loss of heterozygosity of chromosome 3 detected with single nucleotide polymorphisms is superior to monosomy 3 for predicting metastasis in uveal melanoma. Clin Cancer Res. 2007;13:2923.
68 Ferry AP. Lesions mistaken for malignant melanoma of the posterior uvea: a clinical pathologic analysis of 100 cases with ophthalmoscopically visible lesions. Arch Ophthalmol. 1964;72:463.
69 Shields JA. Lesions simulating malignant melanoma of the posterior uvea. Arch Ophthalmol. 1973;89:466.
70 Shields JA, McDonald PR. Improvements in the diagnosis of posterior uveal melanomas. Arch Ophthalmol. 1974;91:259.
71 Char DH, Miller T. Accuracy of presumed uveal melanoma diagnosis before alternative therapy. Br J Ophthalmol. 1995;79:692.
72 Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol. 1998;125:745.
73 Group COMS. Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS Report No. 1. Arch Ophthalmol. 1990;108:1268.
74 Char DH. The management of small choroidal melanomas. Surv Ophthalmol. 1978;22:377.
75 Char DH, et al. Diagnostic modalities in choroidal melanoma. Am J Ophthalmol. 1980;89:223.
76 Davidorf FH, et al. Incidence of misdiagnosed and unsuspected choroidal melanomas. A 50-year experience. Arch Ophthalmol. 1983;101:410.
77 Robertson DM, Campbell RJ. Errors in the diagnosis of malignant melanoma of the choroid. Am J Ophthalmol. 1979;87:269.
78 Coleman DJ. Reliability of ocular tumor diagnosis with ultrasound. Trans Am Acad Ophthalmol Otolaryngol. 1977;77:677.
79 Collaborative Ocular Melanoma Study Group. Baseline echographic characteristics of tumors in eyes of patients enrolled in the COMS: COMS report no. 29. Ophthalmology. 2008;115:1390.
80 Raymond WR, Char DH, Norman D, et al. Magnetic resonance imaging evaluation of uveal tumors. Am J Ophthalmol. 1991;111:633.
81 Mihara F, et al. Intraocular hemorrhage and mimicking lesions: role of gradient-echo and contrast-enhanced MRI. Clin Imaging. 1993;17:171.
82 De Potter P, et al. The role of fat-suppression technique and gadopentetate dimeglumine in magnetic resonance imaging evaluation of intraocular tumors and simulating lesions (see comments). Arch Ophthalmol. 1994;112:340.
83 Bloom PA, et al. Magnetic resonance imaging. Diverse appearances of uveal malignant melanomas. Arch Ophthalmol. 1992;110:1105.
84 Daftari I, et al. 3D MRI-based tumor delineation of ocular melanoma and its comparison with conventional techniques. Med Phys. 2005;32(11):3355.
85 Marnitz S, et al. Proton therapy of uveal melanomas: intercomparison of MRI-based and conventional treatment planning. Strahlenther Onkol. 2006;182(7):395.
86 Char DH. Metastatic choroidal melanoma. Am J Ophthalmol. 1978;86:76.
87 Eskelin S, et al. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85:1151.
88 Diener-West M, et al. Screening for metastasis from choroidal melanoma: the collaborative ocular melanoma study group report 23. J Clin Oncol. 2004;22:2438.
89 AJCC, AJCC Cancer Staging Manual. Edge SB, Byrd DR, Compton CC, et al, editors. ed 7. 2010; Springer, New York:5017.
90 The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. Arch Ophthalmol. 1997;115:1537.
91 Diener-West M, et al. The COMS randomized trial of iodine-125 brachytherapy for choroidal melanoma. III: Initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969.
92 The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma III: local complications and observations following enucleation. COMS report no. 11. Am J Ophthalmol. 1998;126:362.
93 Butler P, Char DH, Zarbin M, et al. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology. 1994;101:710.
94 Seddon JM, et al. Comparison of survival rates for patients with uveal melanoma after treatment with proton beam irradiation or enucleation. Am J Ophthalmol. 1985;99:282.
95 Augsburger JJ, et al. Enucleation vs cobalt plaque radiotherapy for malignant melanomas of the choroid and ciliary body. Arch Ophthalmol. 1986;104:655.
96 Adams KS, et al. Cobalt plaque versus enucleation for uveal melanoma: comparison of survival rates. Br J Ophthalmol. 1988;72:494.
97 Bell DJ, Wilson MW. Choroidal melanoma: natural history and management options. Cancer Control. 2004;11(5):296.
98 Shields CL, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: Outcomes and limitations. Ophthalmology. 2002;109:225.
99 Char DH, Bove RM, Phillips TL. Laser and proton radiation to reduce uveal melanoma associated exudative retinal detachments. Am J Ophthalmol. 2003;136:180.
100 Harbour JW, et al. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology. 2003;110:2207.
101 Shields JA, Shields CL. Surgical approach to lamellar sclero uvectomy for posterior uveal melanomas: The 1986 Schoenberg lecture. Ophthalmic Surg. 1988;19:774.
102 Shields JA, Shields CL, De Potter P. Local resection of posterior uveal tumors. Int Ophthalmol Clin. 1993;33:137.
103 Damato B, Jones AG. Uveal melanoma: resection techniques. Ophthalmol Clin N Am. 2005;18:119.
104 Damato B. The role of eyewall resection in uveal melanoma management. Int Ophthalmol Clin. 2006;46(1):81.
105 Char DH, Miller T, Crawford JB. Uveal tumour resection. Br J Ophthalmol. 2001;85:1213.
106 Puusaari I, Damato B, Kivela T. Transscleral local resection versus iodine brachytherapy for uveal melanomas that are large because of tumour height. Graefe’s Arch Clin Exp Ophthalmol. 2007;245:522.
107 Fuchs E. Das Sarcom des Uvealtractus. Wien: Braumuller; 1882.
108 Zimmerman LE, McLean IW. An evaluation of enucleation in the management of uveal melanomas. Am J Ophthalmol. 1979;87:741.
109 Seigel D, et al. Survival rates after enucleation of eyes with malignant melanoma. Am J Ophthalmol. 1979;87:761.
110 Char DH, Phillips TL. Pre-enucleation irradiation of uveal melanoma. Br J Ophthalmol. 1985;69:177.
111 Augsburger JJ, et al. The effect of pre-enucleation radiotherapy on mitotic activity of choroidal and ciliary body melanomas. Ophthalmology. 1987;94:1627.
112 Luyten GP, et al. No demonstrated effect of pre-enucleation irradiation on survival of patients with uveal melanoma. Am J Ophthalmol. 1995;119:786.
113 Char DH, Phillips TL. The potential for adjuvant radiotherapy in choroidal melanoma. Arch Ophthalmol. 1982;100:247.
114 The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: Initial mortality findings. COMS report no. 10. Am J Ophthalmol. 1998;125:779.
115 Kersten RC, Tse DT, Anderson RL, Blodi FC. The role of orbital exenteration in choroidal melanoma with extrascleral extension. Ophthalmology. 1985;92:436.
116 Constable IJ, Koehler AM, Schmidt RA. Proton irradiation of simulated ocular tumors. Invest Ophthalmol. 1975;14:547.
117 Gragoudas ES. The Bragg peak of proton beams for treatment of uveal melanoma. Int Ophthalmol Clin. 1980;20:123.
118 Goitein M, Miller T. Planning proton therapy of the eye. Med Phys. 1983;10:275.
119 Daftari I, Phillips TL. Design, development, and performance of an adapter for simulation of ocular melanoma patients in supine position for proton beam therapy. Rev Sci Instrum. 2003;74:3093.
120 Castro JR, Quivey JM. Clinical experience and expectations with helium and heavy ion irradiation. Int J Radiat Oncol Biol Phys. 1977;3:127.
121 Daftari IK, et al. New UCSF proton ocular beam facility at the Crocker Nuclear Laboratory Cyclotron (UC Davis). Nucl Instr Meth Phys Res. 1996;A380:597.
122 Gragoudas ES, et al. Intraocular recurrence of uveal melanoma after proton beam irradiation. Ophthalmology. 1992;99:760.
123 Munzenrider JE, et al. Conservative treatment of uveal melanoma: local recurrence after proton beam therapy. Int J Radiat Oncol Biol Phys. 1989;17:493.
124 Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years, The Weisenfeld lecture. Invest Ophthal Visual Sci. 2006;47(11):4666.
125 Gragoudas E, et al. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002;120(12):1665.
126 Gragoudas ES, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol. 2000;118:773.
127 Dendale R, et al. Proton beam radiotherapy for uveal melanoma: results of Curie Institut-Orsay proton therapy center (ICPO). Int J Radiat Oncol Biol Phys. 2006;65(3):780.
128 Kodjikian L, et al. Survival after proton-beam irradiation of uveal melanomas. Am J Ophthalmol. 2004;137:1002.
129 Fuss M, et al. Proton radiation therapy for medium and large choroidal melanoma: Preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys. 2001;49(4):1053.
130 Damato B, et al. Proton beam radiotherapy of choroidal melanoma: The Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys. 2005;62:1405.
131 Egan KM, et al. The risk of enucleation after proton beam irradiation of uveal melanoma. Ophthalmology. 1989;96(9):1377.
132 Egger E, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 2003;55(4):867.
133 Marucci L, et al. Conservation treatment of the eye: conformal proton reirradiation for recurrent uveal melanoma. Int J Radiat Oncol Biol Phys. 2006;64(4):1018.
134 Seddon JM, et al. Visual outcome after proton beam irradiation of uveal melanoma. Ophthalmology. 1986;93:674.
135 Char DH, et al. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology. 1993;100:1547.
136 Char D, Castro JR, Kroll SM, et al. Five-year follow-up of helium ion therapy for uveal melanoma. Arch Ophthalmol. 1990;108:209.
137 Linstadt D, Castro JR, Char DH, et al. Long-term results of helium ion irradiation of uveal melanoma. Int J Radiat Oncol Biol Phys. 1990;19:613.
138 Castro JR, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 1997;39:989.
139 Tsuji H, et al. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a phase I/II dose-escalation study. Int J Rad Oncol Biol Phys. 2007;67:857.
140 Char DH, et al. Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle (proton or helium ion) therapy. Ophthalmology. 2002;109:1850.
141 Perret C, et al: Die Behandlung intraokularer melanome mit protonen and Paul Scherrer Institute (PSI). Juli 1988. ID PSI/2000.
142 Sheen MA. Development of the eye proton therapy planning program. 20th PTCOG Meeting. 1994;29:16.
143 Weber DC, et al. Proton beam radiotherapy versus fractionated stereotactic radiotherapy for uveal melanomas: a comparative study. Int J Radiat Oncol Biol Phys. 2005;63(2):373.
144 Dieckmann K, et al. LINAC based stereotactic radiotherapy of uveal melanoma: 4 years clinical experience. Radiother Oncol. 2003;67(2):199.
145 Tokuuye K, et al. Fractionated stereotactic radiotherapy for choroidal melanoma. Radiother Oncol. 1997;43:87.
146 Mueller A, et al. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology. 2000;107:1381.
147 Simonova G, et al. Leksell gamma knife treatment of uveal melanoma. J Neurosurg. 2002;97:635.
148 Marchini G, et al. Gamma knife stereotactic radiosurgery for uveal melanoma: Clinical results after 2 years. Stereotact Funct Neurosurg. 1996;66:208.
149 Zehetmayer M, et al. Local tumor control and morbidity after one to three fractions of stereotactic external beam irradiation for uveal melanoma. Radiother Oncol. 2000;55:135.
150 Fakiris AJ, et al. Gamma-knife-based stereotactic radiosurgery for uveal melanoma. Stereotact Funct Neurosurg. 2007;85(2-3):106.
151 Liscak R, Vladyka V. Radiosurgery in ocular disorders: Clinical applications. Prog Neurol Surg. 2007;20:324.
152 Henderson MA, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy in the treatment of uveal melanoma. Technol Cancer Res Treat. 2006;5(4):411.
153 Pendl G, et al. Gamma knife radiosurgery for uveal melanomas: an 8-year experience. J Neurosurg. 2000;93(Suppl.3):184.
154 Zytkovicz A, et al. Peripheral dose in ocular treatments with Cyberknife and Gamma Knife radiosurgery compared to proton radiotherapy. Phys Med Biol. 2007;52(19):5957.
155 Nag S, et al. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys. 2003;56:544.
156 Almony A, et al. Tilting of radioactive plaques after initial accurate placement for treatment of uveal melanoma. Arch Ophthalmol. 2008;126(1):65.
157 Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579.
158 Packer S, et al. Long-term results of iodine 125 irradiation of uveal melanoma. Ophthalmology. 1992;99:767.
159 Fontanesi J, et al. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys. 1993;26:619.
160 Harbour JW, et al. Metastatic risk for distinct patterns of postirradiation local recurrence of posterior uveal melanoma. Ophthalmology. 1997;104:1785.
161 Gunduz K, et al. Plaque radiotherapy of uveal melanoma with predominant ciliary body involvement. Arch Ophthalmol. 1999;117:170.
162 De Potter P, et al. Impact of enucleation versus plaque radiotherapy in the management of juxtapapillary choroidal melanoma on patient survival. Br J Ophthalmol. 1994;78:109.
163 Gunduz K, et al. Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am J Ophthalmol. 1999;127:579.
164 Karlsson UL, et al. Recurrence of posterior uveal melanoma after 60-Co episcleral plaque therapy. Ophthalmology. 1989;96:382.
165 Karvat A, et al. The treatment of choroidal melanoma with 198-Au plaque brachytherapy. Radiother Oncol. 2000;59:153.
166 Petrovich A, et al. Episcleral plaque radiotherapy in the treatment of uveal melanomas. Int J Radiat Oncol Biol Phys. 1992;24:247.
167 Quivey JM, et al. 125I plaque therapy for uveal melanoma. Analysis of the impact of time and dose factors on local control. Cancer. 1996;77:2356.
168 Lean EK, et al. Episcleral radioactive plaque therapy: initial clinical experience with 56 patients. Am J Clin Oncol. 1990;13:185.
169 Quivey JM, Augsburger J, Snelling L, et al. I-125 plaque therapy for uveal melanoma: Analysis of the impact of time and dose factors on local control. Cancer. 1996;77:2356.
170 Moore R. Choroidal sarcoma treated by intra-ocular insertion of radon seeds. Br J Ophthalmol. 1930;14:145.
171 Stallard H. Radiotherapy for malignant melanoma of the choroid. Br J Ophthalmol. 1966;50:147.
172 Sealy R, et al. The treatment of ophthalmic tumors with low energy sources. Br J Radiol. 1976;49:551.
173 Luxton G, Astrahan MA, Melvin A, et al. Dosimetric calculations and measurements of gold plaque ophthalmic irradiators using iridium-192 and iodine-125 seeds. Int J Radiat Oncol Biol Phys. 1988;15:167.
174 Straatsma BR, Fine SL, Earle JD, et al. The Collaborative Ocular Melanoma Study Research Group. Enucleation versus plaque irradiation for choroidal melanoma. Ophthalmology. 1988;95:1000.
175 Lommatzsch PK, Lommatzsch R. Treatment of juxtapapillary melanomas. Br J Ophthalmol. 1991;75:715.
176 Tjho-Heslinga RE, et al. Results of ruthenium irradiation of uveal melanoma. Radiother Oncol. 1993;29:33.
177 Ling CC, et al. Computer assisted treatment planning for 125-I ophthalmic plaque radiotherapy. Int J Radiat Oncol Biol Phys. 1989;17:405.
178 Weaver KA. The dosimetry of I-125 seed plaques. Med Phys. 1986;13:78.
179 Williamson J. Monte Carlo evaluation of specific dose constants in water for I-125 seeds. Med Phys. 1988;15:686.
180 Fiedler JA, et al. A magnetic resonance imaging-based treatment planning method for episcleral brachytherapy. Endocurietherapy/Hyperthermia Oncol. 1993;9:201.
181 Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:886.
182 Nath R, et al. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys. 1995;22:209.
183 Collaborative Ocular Melanoma Study Group. the COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report no. 28. Arch Ophthalmol. 2006;124:1684.
184 Collaborative Ocular Melanoma Study—Quality of Life Study Group. quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5 year results from the COMS: COMS QOLS report no. 3. Arch Ophthalmol. 2006;124:226.
185 Straatsma BR, et al. Mortality after deferral of treatment or no treatment for choroidal melanoma. Am J Ophthalmol. 2003;136(1):47.
186 Daftari IK, Char DH, Verhey LJ, et al. Anterior segment sparing to reduce charged particle radiotherapy complications in uveal melanoma. Int J Radiat Oncol Biol Phys. 1997;39:997.
187 Collaborative Ocular Melanoma Study Group. the COMS randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol. 2004;138(6):936.
188 Avery RB, et al. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol. 2008;126(2):207.
189 Coupland SE, et al. Metastatic choroidal melanoma to the contralateral orbit 40 years after enucleation. Arch Ophthalmol. 1996;114:751.
190 Kirk HQ. Delayed metastasis from choroidal melanoma. Surv Ophthalmol. 1966;11:651.
191 Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651.
192 Diener-West M, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: COMS report no. 26. Arch Ophthalmol. 2005;123(12):1639.
193 Collaborative Ocular Melanoma Study Group. incidence of cataract and outcomes after cataract surgery in the first 5 year after iodine 125 brachytherapy in the COMS: COMS report no. 27. Ophthalmology. 2007;114(7):1363.
194 Char DH, et al. Long term visual outcome of radiated uveal melanomas in eyes eligible for randomisation to enucleation versus brachytherapy. Br J Ophthalmol. 1996;80:117.
195 Melia BM, et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years. COMS report no. 16. Ophthalmology. 2001;108:348.
196 Collaborative Ocular Melanoma Study Group. Ten-year follow-up of fellow eyes of patients enrolled in collaborative ocular melanoma study randomized trials. COMS Report No. 22. Ophthalmology. 2004;111(5):966.