CHAPTER 32 Uterine fibroids
Introduction
There continues to be an appalling paucity of research on this tumour and its treatment. This is of particular concern given that it occurs in more than two-thirds of women at some stage of their lives, and is the leading indication for the most common major gynaecological operation in women — hysterectomy. The annual cost of inpatient surgery for fibroids is estimated to be $US2.1 billion in the USA (Parker 2007), with additional indirect costs from outpatient treatment and lost time at work.
Incidence
The true incidence of uterine fibroids in the general female population is unknown. This is because most studies on fibroids have only included women with symptoms or women having treatments such as hysterectomy. Given that up to 50% of fibroids are asymptomatic (Buttram and Reiter 1981), the former approach can be expected to underestimate the true population incidence. However, studies of incidence based on histological examination of hysterectomy specimens may similarly overestimate the incidence of fibroids, as they concentrate the worst cases of menorrhagia where medical treatments have failed. Cramer and Patel (1990) found that 77% of hysterectomy specimens had fibroids on histology, although fibroids were only detected in 33% of patients clinically and 50% of patients by ultrasound examination.
The lifetime risk of a woman over 45 years of age developing fibroids is greater than 60%, with reported incidences in different studies varying from 30% to 70% for premenopausal women (Okolo 2008).
Aetiology
The precise aetiology of fibroids remains unknown. It is known that each fibroid is monoclonal, developing from a single cell. Electrophoresis patterns of glucose-6-phosphate dehydrogenase isoenzyme (A or B) demonstrated this in early studies (Townsend et al 1970). Later, polymerase chain reaction experiments confirmed monoclonality by the pattern of inactivation of the X-chromosome-linked phosphoglycerokinase gene (Hashimoto et al 1995). In the development of fibroid tumours, some form of initiation event must transform a normal myometrial cell into an abnormal ‘founding’ cell. Possible factors initiating this change include intrinsic myometrial abnormalities, elevated oestrogen receptors in the myometrium, hormonal changes and ischaemic injury (Parker 2007).
It has long been known that fibroid growth is dependent on both oestrogen and progesterone. Fibroids only occur after the menarche, and they normally show a reduction in size after the menopause. They display reversible shrinkage after treatment with gonadotrophin-releasing hormone (GnRH) agonists. Although circulating serum oestradiol and progesterone levels are not altered in women with fibroids, fibroids have increased local oestrogen levels relative to the surrounding normal myometrium, partly due to increased fibroid expression of the aromatase enzyme (Parker 2007). They also have increased oestrogen receptor levels and increased responsiveness to oestrogen compared with normal myometrial cells. As for progesterone, the receptor levels have been shown to be increased in fibroids relative to the myometrium (Brandon et al 1993), and to upregulate the levels of some proteins which influence cell proliferation, such as the antiapoptotic protein Bcl-2 (Matsuo et al 1999). During the secretory phase of the menstrual cycle, when serum progesterone levels are at their peak, the highest mitotic activity is seen in fibroids. Similarly, high mitotic activity is seen during pregnancy. Treatment with the antiprogestin, mifepristone, has been shown to cause a marked decrease in fibroid tumour volume.
A large number of growth factors are produced locally in uterine fibroids and the myometrium. Some of them are overexpressed in fibroids, partially due to an exaggerated response to oestrogen. Some of the growth factors that have been found to have a potential role in the promotion of fibroid growth include: transforming growth factor-β, epidermal growth factor, basic fibroblast factor, insulin-like growth factor, platelet-derived growth factor and vascular endothelial growth factor (VEGF). The differential effects of these growth factors on cell proliferation, angiogenesis, extracellular matrix production and apoptosis are due to a variety of mechanisms including altered receptor levels and signalling pathways. For a detailed review, see Fleischer et al (2008).
Epidemiology
Age is the most common risk factor for uterine fibroids (Marshall et al 1997), although many of the studies examining incidence are cross-sectional or retrospective, rather than prospective and longitudinal. Race has been identified as an epidemiological factor in fibroid development. Black women are three to five times more likely to have uterine fibroids than White, Asian or Hispanic women (Marshall et al 1997). In addition, the fibroids in Black women are larger, more numerous, more symptomatic and diagnosed at an earlier age.
There is evidence of hereditary factors influencing fibroid development, despite no specific gene being identified. The development of fibroids clusters in families, and ‘familial’ fibroids appear to be more likely to cause symptoms than ‘non-familial’ fibroids (Okolo 2008). Twin studies show an increased incidence of fibroids in monozygotic twin pairs compared with dizygotic twin pairs.
Uterine fibroids are more common in nulliparous women, presumably due to the increased number of cycles over their premenopausal lifespan compared with parous women. Increasing parity decreases the incidence and number of fibroids. It is a myth that fibroids increase in size during pregnancy. In pregnancy, fibroids initially increase in size in the first trimester, but then decrease in size for the remaining two trimesters, with an overall volume that is either reduced or unchanged throughout the pregnancy (Hammoud et al 2006). Obesity, diabetes mellitus, polycystic ovary syndrome and hypertension all appear to be associated with an increased risk of uterine fibroids. In these conditions, a combination of insulin resistance, increased androgen levels and increased peripheral conversion of circulating androgens to oestrogen may alter the ovarian steroid hormonal stimulus to the fibroids. Smoking decreases the risk of uterine fibroids, presumably through a reduction in oestrogen levels.
The effects of exogenous sex steroid hormones on fibroid growth are particularly important as they comprise some of the most common treatments for menorrhagia, itself the most common symptom of uterine fibroids. Use of the combined oral contraceptive pill (COCP) has been associated with fibroid growth in some earlier studies (particularly older pill formulations with a higher oestrogen dosage), although the majority show no association or even a reduced risk of developing fibroids with COCP use. Therefore, fibroids are not a contraindication to the use of the COCP to treat menorrhagia. Both systemic and local administration of progestins to the uterus have been shown to be safe with fibroids. Depot medroxyprogesterone acetate injections reduce the risk of fibroids, and the levonorgestrel-containing intrauterine system (LNG-IUS; Mirena) reduces the overall volume of fibroid uteri. The LNG-IUS is also an effective treatment for menorrhagia associated with fibroids, although it is less effective than for dysfunctional uterine bleeding. In some patients, it may be difficult to insert the LNG-IUS due to cavity distortion by the fibroids. The effect of hormone therapy in postmenopausal women on fibroids has been reviewed by Ang et al (2001). They found that use of hormone therapy in women with fibroids caused continued fibroid growth, especially in the first 6 months of therapy, or at least a failure in normal postmenopausal regression. However, this did not correspond to an increase in symptoms.
Classification and Pathophysiology
Macroscopic appearance
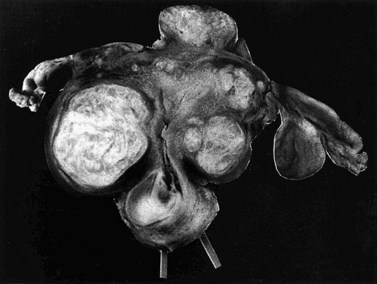
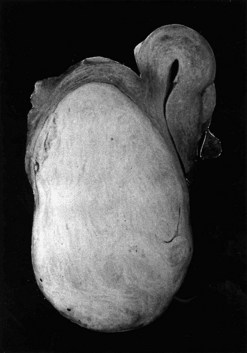
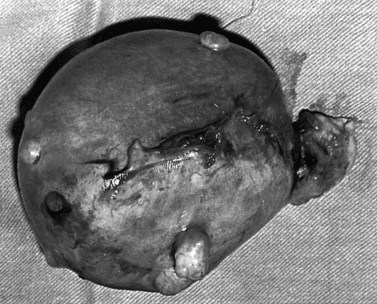
Fibroids have a characteristically firm fibrous texture, from which the clinical term ‘fibroid’ is presumably derived. They frequently become calcified; rarely, they become ossified so that they are rock hard. Fibroids are round or oval-shaped tumours with a characteristic white whorled appearance on cross-section. They may be single but are more commonly multiple, present in varying sizes and in different sites (Figure 32.1). Tiny ‘seedling’ fibroids are commonly seen in association with larger tumours, possibly accounting for the high recurrence rate after surgical removal at myomectomy. Surgical removal of over 120 individual fibroids from a single patient has been recorded in the literature!
Clinical classification
Subserosal fibroids
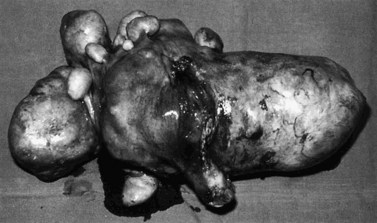
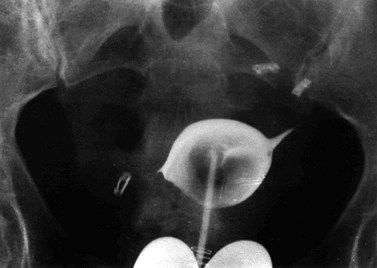
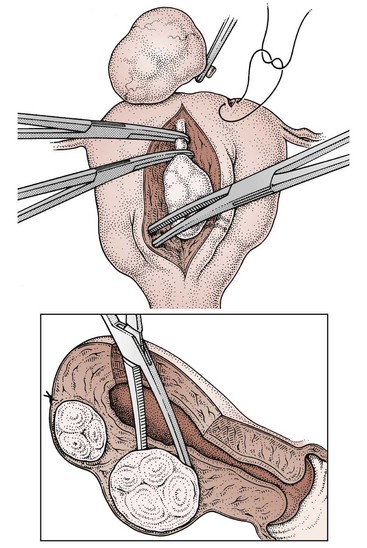
Subserosal fibroids project outwards from the uterine surface, covered with peritoneum, and may reach a very large size. Greater than 50% of the fibroid mass must project beyond the myometrium for the fibroid to be classified as subserosal. Many subserosal fibroids are pedunculated (Figure 32.2), making torsion a potential, although rare, complication. Sessile subserosal fibroids projecting from the fundal region may adhere to omentum or bowel, particularly if there has been coincidental inflammatory disease. Fibroids rarely become attached to the omentum; if they develop an alternative blood supply, they may become separated from the uterus, forming a so-called ‘parasitic’ fibroid. Subserosal fibroids arising from the lateral uterine wall may lie between the layers of the broad ligament where large tumours may displace the ureters, or bulge between the layers of the sigmoid mesocolon. Broad ligament fibroids arising from the lateral uterine wall differ from true broad ligament fibroids, which have no attachment to the uterus but have their origin in smooth muscle fibres within the broad ligament; for example, from the round ligament, ovarian ligament or perivascular connective tissue.
Submucosal fibroids
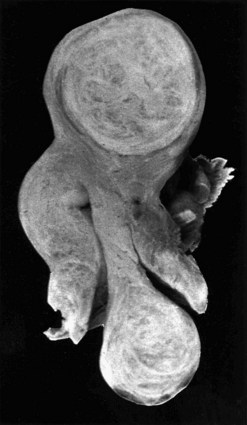
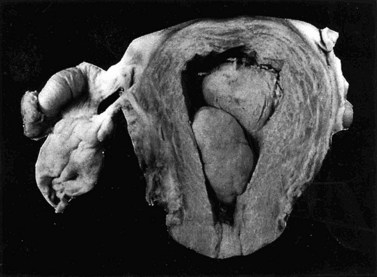
Submucosal fibroids are less common, comprising approximately 5% of all leiomyomata. By definition, more than 50% of the fibroid mass projects into the uterine cavity and is covered by endometrium. Submucosal fibroids may cause abnormal uterine bleeding and infertility. Uterine enlargement is not usually evident unless other fibroids are also present. Submucous fibroids are regarded as suitable for hysteroscopic resection. Pedunculated submucous fibroids on a long stalk may prolapse through the cervix (Figure 32.3), where they may cause intermenstrual bleeding or become ulcerated and infected.
Vascular supply
Perfusion studies with injections of 133Xe radiocontrast and gadolinium-enhancement contrast material during magnetic resonance imaging (MRI) show reduced blood flow through fibroids compared with myometrium, but increased blood flow in a compressed rim around the fibroid. Immunohistochemical studies of the microvascular density of fibroids demonstrate reduced vessel area staining in fibroids compared with the myometrium (Casey et al 2000), and with the difference increasing after the menopause (Weston et al 2005); this is probably due to relatively faster collapse of the myometrial cellular and extracellular matrix volume relative to that of the fibroid. Electron microscopy of corrosion vessel casts of fibroids performed by Walocha et al (2003) shed even greater insight into the vasculature of fibroids. A single larger artery usually provides the major blood supply to the fibroid, sending small nutrient arteries to penetrate the capsule. The vasculature of fibroids is reduced compared with the surrounding myometrium, but is most markedly reduced in smaller fibroids, with the only area of greater vascularity than the myometrium being the compressed rim of perifibroid tissue around the periphery of larger fibroids. Walocha et al (2003) proposed an initial regression of the vasculature within smaller fibroids, followed by an intense proangiogenic response, whereby the vascular rim around the fibroid acts as the source for neovascularization of the growing fibroid.
The relative avascularity of the fibroid core appears to be at odds with the finding of increased VEGF in fibroids compared with the myometrium. However, the VEGF within the interior of the fibroid may be trapped in a biologically inactive fashion within the expanded extracellular matrix of the fibroid. A gene expression microarray experiment performed by Weston et al (2003) specifically targeting angiogenesis-related genes found a reduced level of two angiogenesis promoters (CTGF and CYR61) and increased expression of collagen IVa 2; a precursor for the angiogenesis inhibitor, canstatin. This triad of gene expression differences may, in part, explain the relatively avascular fibroid tissue.
Microscopic appearance
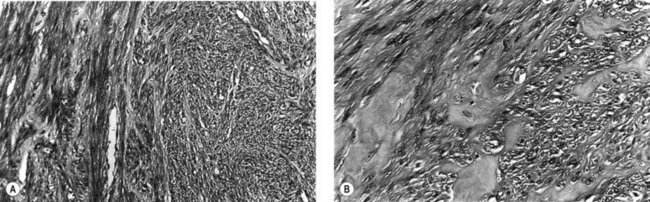
Microscopically, fibroids are composed of smooth muscle cell bundles, also arranged in whorl-like patterns (Figure 32.4A), admixed with a variable amount of connective tissue, although the latter rarely predominates. The relatively poor blood supply to individual fibroids may result in degenerative changes, particularly within large tumours. Hyaline degeneration (Figure 32.4B) is the most common, resulting in a smoother and more homogeneous consistency, which may become cystic if liquefaction occurs. Much more rarely, fatty change may develop. This is distinct from the true lipoma of the uterus, which is extremely uncommon. Calcification (Figure 32.5) is a later consequence of degeneration secondary to circulatory impairment. It characteristically occurs after the menopause, although it may occur earlier in subserosal fibroids with narrow pedicles.
Crow et al (1995) examined the microscopic appearance of uterine fibroids after treatment with GnRH agonists. They found considerable variation between fibroids after a course of treatment and subsequent myomectomy or hysterectomy. Decreases in the extracellular matrix, in particular, cause extreme crowding of tumour nuclei, resulting in a densely cellular appearance of such fibroids on microscopic examination. For the inexperienced pathologist, this will be worrying in terms of possible malignancy. There should not, however, be mitotic activity, cytological atypia or coagulative necrosis.
Clinical Presentation
Abnormal bleeding/menorrhagia
The proportion of uterine fibroids causing abnormal uterine bleeding — menorrhagia being the most common abnormal pattern observed — is uncertain but is usually quoted as 30–50%. The exact mechanism by which fibroids cause menorrhagia is unknown. The many theories include: increase in endometrial surface area (Figure 32.6), increased vascularity of the uterus, vascular congestion due to fibroid compression of the myometrial venous drainage, abnormal uterine contractility, dysregulation of growth factors causing disordered angiogenesis of the uterine vasculature, and ulceration of the endometrial layer overlying submucous fibroids.
As fibroids are so common, and so often asymptomatic, menorrhagia in a woman with uterine fibroids should not automatically be ascribed to the fibroids themselves. Other possible aetiologies for menorrhagia should always be considered and excluded, including coagulopathies such as von Willebrand’s disease (Munro and Lukes 2005). In a study of self-reported bleeding patterns by 878 women (aged 35–49 years) screened for the presence and size of myomas, Wegienka et al (2003) reported findings on the association between fibroids and bleeding pattern. Of women with fibroids, 46% reported ‘gushing’ episodes during their periods, compared with 28% without fibroids. Gushing episodes and length of periods were related to the size of the myomas, but not to their number or location. The worst bleeding was found with fibroids over 5 cm in diameter, with women using three more pads or tampons on heavy bleeding days than women with smaller fibroids.
Pelvic pain and pressure symptoms
Fibroids do not usually give rise to pain. In a cohort study of 635 non-care-seeking women, symptoms of dyspareunia, dysmenorrhoea and non-cyclic pelvic pain were examined for their association with the presence and size of uterine fibroids (Lippman et al 2003). The 96 women with fibroids had only a slight increase in non-cyclic pain or dyspareunia, but no increase in dysmenorrhoea, and no relationship was found between the size or total volume of fibroids and pain symptoms. Where fibroids are associated with pain or abdominal discomfort, it is usually described as ‘pressure’ or a ‘dragging’ sensation. It is surprising that even in the presence of multiple fibroids, menstruation may be painless.
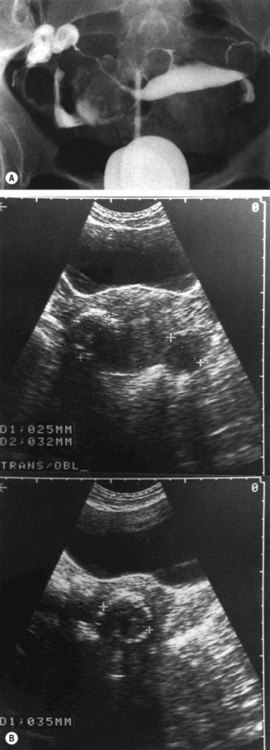
The location and size of fibroids determines symptoms from pressure on adjacent organs. A posterior wall fibroid can cause lower back pain, or constipation and tenesmus if the rectosigmoid is compressed. Anterior wall fibroids compressing the bladder may cause urinary frequency, nocturia and urgency in some women. It has been shown that reduction in size of such fibroids after either GnRH agonists or uterine artery embolization (UAE) causes a decrease in associated urinary symptoms (Parker 2007). Cervical fibroids causing compression of the bladder outlet may present with acute retention of urine (Figure 32.7), although this is rare.
Reproductive outcomes
Infertility/miscarriage
Fibroids are associated with infertility, although it has been estimated that fibroids are only responsible for 2–3% of cases (Buttram and Reiter 1981). Increasing maternal age increases the likelihood of both fibroids and infertility, making it a possible confounding factor. It is not known why fibroids increase infertility. Hypothetical mechanisms include interference with embryo implantation or sperm/embryo transport due to distortion of the cavity or tubal ostium, and an altered uterine environment due to increased local oestrogen levels or a local inflammatory effect. Embryo implantation may be reduced due to endometrial oedema, atrophy or ulceration, which in turn could be due to either the pressure effects of the uterine fibroid or its effects on the local blood supply. There have been series indicating postoperative pregnancy rates of 50–60% in women after myomectomy, but these studies have usually been uncontrolled or poorly controlled.
The effect of fibroids on assisted reproductive technology (ART) has been the subject of many studies, often with conflicting results. In an excellent systematic literature review, Klatsky et al (2008) summarized the evidence for the effects of fibroids on the results of in-vitro fertilization (IVF). It is generally accepted that subserosal fibroids, with more than 50% of their mass outside the myometrial border, are unlikely to have an adverse effect on IVF outcomes or to increase the risk of miscarriage. This was confirmed by Eldar-Geva et al (1998), with subserosal fibroids not influencing treatment outcome after ART. However, in a series of 88 patients, treatment outcome after ART was impaired with either submucosal fibroids or intramural fibroids, even without uterine cavity distortion. Established wisdom is that submucosal fibroids should undergo hysteroscopic resection prior to ART. Submucosal fibroids, if not removed, are associated with an overall 70% reduction in pregnancy rate and 72% reduction in implantation rate compared with controls (Klatsky et al 2008), as well as an approximate doubling of the risk of miscarriage. Intramural fibroids are where controversy begins. Surgery to remove them is more risky and involved than that for submucosal fibroids, while the evidence is for a smaller and less consistent effect. Some authors (Khaund and Lumsden 2008) believe that intramural fibroids have an adverse effect on fertility where they cause distortion of the uterine cavity, and should be removed if they do so. Others (Klatsky et al 2008), citing disparity of study results, possible biases including publication bias and the small effect, feel that more prospective studies of intramural fibroids are required before a strong recommendation on desired treatment can be given. Their cumulative results from 18 controlled studies showed a slight decrease in implantation rate (18% vs 22%) and clinical pregnancy rate (37% vs 41%), and an increase in miscarriage rate (8% vs 15%) associated with intramural fibroids.
Pregnancy complications
The best-available evidence points to an increased risk of caesarean section delivery with fibroids, mainly due to malpresentation of the fetus. Incoordinate uterine action and labour dystocia were increased in at least one study on pregnant women with fibroids, but fibroids are not a contraindication to a trial of labour. The results for placenta praevia and placental abruption are conflicting, with some studies showing an increased risk and other studies showing no increased risk. Preterm labour and delivery are more common with uterine fibroids (odds ratio 1.5–1.9). At least four studies showed an increased risk of postpartum haemorrhage with uterine fibroids (Klatsky et al 2008). Despite the considerable evidence of adverse pregnancy outcomes with uterine fibroids, it should be noted that the data are often conflicting in different studies. Also, confounding factors such as advanced maternal age, and ascertainment bias from increased use of ultrasound in complicated pregnancies, need to be considered.
In approximately 5% of pregnant women with fibroids, the fibroids undergo red degeneration. This is a form of coagulative necrosis resulting in a haemorrhagic, meaty, cut surface and areas of cystic degeneration or mucinous change. One hypothesis is that changes in orientation of the myoma to its vascular supply during uterine growth cause obstruction of the vascular supply and ischaemic necrosis of the fibroid tissue (Parker 2007). Red degeneration causes severe abdominal pain and mild fever, and often requires a short hospital stay and treatment with non-steroidal anti-inflammatory drugs, such as ibuprofen. A similar clinical presentation can occur after GnRH agonist treatment or uterine artery embolisation UAE.
Malignant transformation in fibroids: where is the evidence?
For the ‘worried well’, the diagnosis of a uterine fibroid naturally raises the issue ‘Can it become cancer, doctor?’. Some women continue to have a hysterectomy or a myomectomy ‘just in case’ the uterine fibroid grows into a uterine sarcoma. Gard et al (1999) reviewed uterine leiomyosarcomas in Australia, conducting a retrospective survey of major teaching hospitals in Sydney and Melbourne. They identified 49 patients treated over a 28-year period, equating to approximately one leiomyosarcoma of the uterus per million Australian women per year. There is considerable doubt regarding whether or not a myomectomy would be protective in any case, as most leiomyosarcomas are believed to arise de novo, unrelated to existing benign fibroids. Malignant transformation of fibroids is believed to be exceedingly rare; genetic differences between uterine fibroids and leiomyosarcomas point to distinct origins. Given that leiomyosarcomas can also arise outside the uterus, even a hysterectomy may not be completely protective of this rare tumour. Removing asymptomatic fibroids from women on this basis cannot be justified. Until proven otherwise, sarcomatous ‘change’ in a fibroid is a myth. Even rapid myoma growth almost never indicates a uterine sarcoma. No cases of sarcoma were found in a study of 198 fibroid uteri with an increase in size greater than 6 weeks over a 1-year period (Parker et al 1994).
Investigations for Uterine Fibroids
Ultrasound
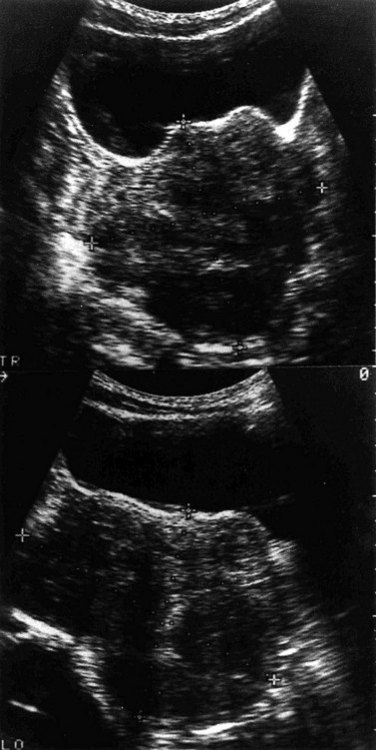
Ultrasound should always be used to confirm or clarify the nature of a pelvic mass. Both the transabdominal and transvaginal route should be taken in examining possible fibroids. Larger fibroid uteri may only be fully visible via the abdominal route, but smaller lesions and submucosal fibroids may be better visualized via the vagina. Ultrasound is rapid, inexpensive and uses no ionizing radiation, making it the undisputed first-line imaging investigation for fibroids. Even in relatively unskilled hands, it will diagnose pregnancy and differentiate between cystic and solid lesions (Figure 32.8). With appropriate training and experience, the site and nature of a pelvic mass may be predicted with accuracy in over 80% of cases. Fibroids appear as an enlarged uterus with patchy echogenicity. Serial ultrasound examination is of value in monitoring fibroid size during medical or conservative treatment. The volume of individual fibroids or of the whole uterus can be calculated by measuring the diameter in three planes at right angles and using the formula 4/3πr3, where r is the radius.
Treatment Methods
Medical management
Medical treatment of menorrhagia associated with fibroids
Since fibroids are often associated with menorrhagia, the effect of medical treatments for menorrhagia, with respect to both symptoms and fibroid growth, is important. As mentioned above, oral contraceptive pills with a high dose of oestrogen have been reported to cause fibroid growth. Later studies with current lower oestrogen-containing pills have shown either no change or a reduction in size. Similarly, the de-novo incidence of fibroids in users of the oral contraceptive pill appears to be decreased or no different compared with controls. Thus, the use of the oral contraceptive pill to treat menorrhagia in the setting of a fibroid uterus is not contraindicated (Benagiano and Primiero 2006).
The LNG-IUS is highly effective for the treatment of menorrhagia. It is also effective for the treatment of menorrhagia associated with fibroids, although less effective than for dysfunctional uterine bleeding. There is an increased risk of expulsion or failed insertion with fibroids, particularly where they cause cavity distortion. Regarding the effect of the LNG-IUS on fibroid growth, the data are conflicting (Benagiano and Primiero 2006). Despite initial reports of regression in the size of fibroids, subsequent studies have shown no decrease in size.
Gonadotrophin-releasing hormone analogues
For women nearing the menopause who are particularly keen to avoid surgery, the use of GnRH analogues as a stand-alone therapy, with ‘add-back’ therapy to reduce menopausal symptoms, has been proposed as a viable alternative to surgical treatment. Low-dose oestrogen/progesterone combinations, oral progesterone alone and tibolone have been used to ameliorate the vasomotor symptoms associated with GnRH agonist gonadal suppression, but without compromising fibroid shrinkage (Sankaran and Manyonda 2008).
Antiprogesterone treatment
The use of antiprogesterone RU486, mifepristone, has been reported to reduce fibroid size and fibroid-related symptoms, making it a potentially promising new medical treatment. However, there have been varying reports of increased incidence of endometrial hyperplasia following prolonged use of mifepristone, due to its antiprogestin effects on the endometrium (Tropeano et al 2008). Recurrence rates after cessation of therapy are likely to be high, and risk to any potential pregnancies makes its use for women with infertility particularly troublesome. Further studies are required with larger numbers of patients over longer periods to reliably determine the long-term efficacy and safety of treatment.
Asoprisnil, a selective progesterone receptor modulator (SPRM) with mixed agonist/antagonist effects, has also been used to treat fibroids. It inhibits the proliferation of endometrial cells, unlike mifepristone, theoretically reducing the risk of endometrial hyperplasia with treatment. Only one randomized, double-blind, placebo-controlled trial of asoprisnil for the treatment of fibroids has been reported (Chwalisz et al 2007). It found that asoprisnil treatment for 3 months reduced fibroid-related symptoms and size in a dose-dependent manner, without hypo-oestrogenic symptoms or endometrial hyperplasia. Clearly, longer-term and larger trials are needed, but the initial results are promising. Other SPRMs are likely to be trialled for the treatment of fibroids over the next few years.
Hysterectomy
The route selected for hysterectomy will depend on the size of the uterus, the situation of the fibroids and the history of any previous surgical procedures. Abdominal hysterectomy with ovarian conservation (Figure 32.9) will be the procedure of choice where fibroids are very large. For gynaecologists who favour vaginal hysterectomy, consideration should be given to preoperative shrinkage with a GnRH agonist, as described below. Similarly, a reduction in volume and vascularity may facilitate removal of a very large uterus by the abdominal route. Abdominal hysterectomy is rendered difficult in the presence of large fibroids arising from the cervix or situated in the broad ligament. It is also more complicated if there are adhesions from previous myomectomies, or from associated endometriosis or pelvic inflammatory disease. Difficult access to the pelvis during hysterectomy for large fibroids will be rendered easier by prior enucleation of the fibroids. The ureters may be vulnerable during an operation to remove a broad ligament fibroid, and their pathway must always be identified. Regardless of the direction of displacement, they are always extracapsular. In the case of a large cervical fibroid, an alternative approach is hemisection of the uterus, followed by enucleation of the fibroid in order to gain access to the uterine arteries and cervix. These techniques are described in detail elsewhere (Monaghan 1986).
Abdominal myomectomy
Complications
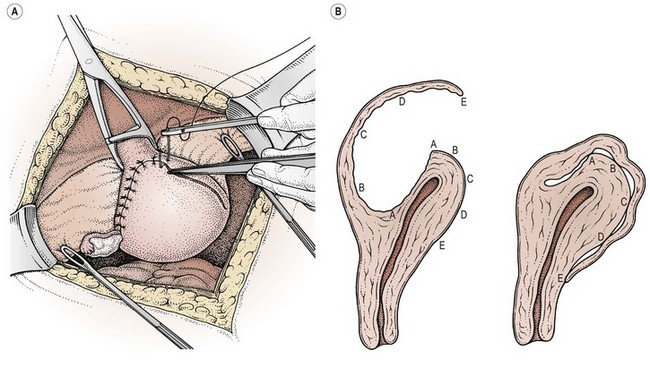
The two major problems associated with abdominal myomectomy are intraoperative blood loss (which can be severe and may necessitate a hysterectomy to control bleeding in 1–2% of patients) and postoperative adhesion formation. The risk of adhesions is increased with posterior and multiple uterine incisions. They are usually a consequence of difficulties with haemostasis and oozing from incision lines. Adhesions after incisions on the posterior wall are potentially more serious because of involvement of the uterine tubes and ovaries. To minimize adhesion formation, as many fibroids as possible should be removed through a single incision (Figure 32.10), preferably at the fundus or anterior uterine wall. Some authorities recommend removal of posterior wall fibroids via the uterine cavity through an incision on the anterior abdominal wall, although this causes a deliberate breach of the uterine cavity and entails a risk of intrauterine adhesions. Bonney (Monaghan 1986) described a ‘hood’ method of closure of the cavity left after enucleation of a large single posterior tumour (Figure 32.11), suturing the redundant flap of serosal-covered myometrium over the fundus and low down on to the anterior abdominal wall to avoid adhesion formation. Keeping tissues moist, using wet packs to displace the bowel and minimal tissue handling is thought to reduce adhesions. The use of barrier agents, such as Interceed, Seprafilm and Gore-Tex, to reduce adhesions has been the subject of a recent Cochrane review (Ahmad et al 2008). While the use of Interceed does reduce postoperative adhesion formation after surgery, there is insufficient evidence to show that the reduced adhesion rates correspond with increased fertility and pregnancy rates.
Both mechanical and medical methods have been described to reduce intraoperative blood loss. Preoperative treatment with GnRH analogues for at least 3 months prior to either hysterectomy or myomectomy has been shown to increase both preoperative and postoperative haemoglobin and haematocrit (Lethaby et al 2000). This also reduced the size of the fibroids by approximately 50%, and reduced pelvic symptoms, operating time, duration of hospital stay, blood loss and rate of requiring a vertical incision. Disadvantages of the use of GnRH analogues include the cost, menopausal symptoms during treatment and bone demineralization with prolonged use. While an increased risk of fibroid recurrence has been reported, due to shrinkage of smaller fibroids making them undetectable at surgery, this was not confirmed in a systematic review. While the use of GnRH analogues has been criticized for making the pseudocapsule plane of cleavage between the fibroid and the surrounding myometrium less well defined, this has not been the authors’ experience. Intraoperative interventions to reduce blood loss during myomectomy have undergone a Cochrane review by Kongnyuy and Wiysonge (2007). One such method is the use of Bonney’s myomectomy clamp (Figure 32.11A), which is placed across the lower uterus to occlude the uterine arteries. It can be used in conjunction with ring forceps to occlude the ovarian blood supply. An alternative is the use of a rubber tourniquet or catheter, placed around the uterus through an incision in the broad ligament at the level of the lower segment. During a long operation, intermittent release of the clamps or tourniquet is recommended every 10–20 min to prevent ischaemic damage to the tissues. Many intraoperative techniques have been advocated to reduce blood loss. Those that have been found to be effective (with weighted mean difference in blood loss with their use given in parentheses) include intramyometrial vasopressin (−299 ml), intramyometrial bupivacaine plus epinephrine (−69 ml), pericervical tourniquet (−1870 ml) and vaginal misoprostol (−149 ml).
The issue of mode of delivery in subsequent pregnancies following myomectomy where the uterine cavity has been breached is controversial. While common advice given to patients is that all myomectomies involving breach of the uterine cavity mandate caesarean sections in subsequent pregnancies, the evidence for this advice is limited (Parker 2007). During pregnancy and labour, rupture of the uterus after fibroid surgery is an extremely rare event. However, rupture after laparoscopic myomectomy may be higher than after abdominal myomectomy.
Hysteroscopic resection
Submucosal fibroids (such as those illustrated in Figure 32.12) are best removed hysteroscopically. Resection of the fibroid via the hysteroscope may be combined with endometrial ablation for the relief of menorrhagia if the woman has completed childbearing. Hysteroscopic surgery has been used for the removal of lesions measuring up to 7 cm in diameter, although many authorities would set a maximum of 3–4 cm (Agdi and Tulandi 2008). With larger fibroids, GnRH analogues can be used to shrink the tumour prior to surgery. Preoperatively, some surgeons use intravaginal misoprostol to soften and dilate the cervix, and allow easier insertion of the instruments. The uterine cavity is distended with a non-conductive glycine solution, and continuous irrigation with an infusion pump is employed to maintain visibility. A loop electrode is placed distal to the myoma, and sliced towards the cervix under vision. At all times, the loop should remain under vision to reduce the risk of perforation of the uterus and damage to bowel or other internal organs. Any uterine perforation necessitates abandoning the procedure. Lengthy procedures carry the risk of ‘water intoxication’ and electrolyte disturbances, and a strict check should be made on the amount of glycine absorbed. The major advantage of hysteroscopic resection is the avoidance of major surgery, as well as easier access to submucosal fibroids compared with the abdominal route.
Some authors (Donnez et al 1990) advocate the use of the neodynium:YAG laser for larger fibroids, after prior shrinkage with a GnRH analogue. Laser ablation can also be used for smaller fibroids.
Laparoscopic myomectomy
Despite laparoscopic myomectomy being performed for almost two decades, there is little evidence and considerable debate about its place in the treatment of uterine fibroids. It is still not clear how the complications of the procedure compare with open myomectomy, and reported results from studies and case series are highly operator dependent. The purported advantages of the technique include shorter hospital stay, less postoperative pain, faster recovery and better visualization of adjacent organs (Agdi and Tulandi 2008). However, there have been reports of a higher risk of recurrence of fibroids, due to the inability to palpate the uterus and remove smaller coexisting fibroids laparoscopically. There is also a possible increased risk of uterine rupture in pregnancy and labour. Larger fibroid uteri may be impossible to remove laparoscopically, although preoperative shrinkage with GnRH analogues may help in these cases. The procedure is challenging, with skills only available at a few centres, and is time consuming compared with an open myomectomy, subjecting the patient to a longer period of anaesthesia.
Uterine artery embolization
Transarterial embolization for the successful treatment of obstetric and gynaecological haemorrhage has been practised for 30 years. It has also been used subsequently to treat uterine arteriovenous malformations. The initial report of the use of UAE to treat symptomatic fibroids was by Ravina et al in 1995. Its use has increased in popularity since then, and has been the subject of thorough recent reviews (Hickey and Hammond 2008, Tropeano et al 2008).
Technique
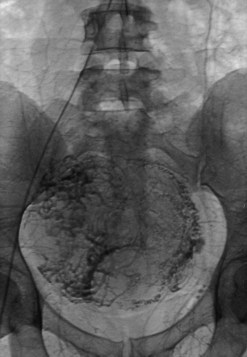
UAE is a percutaneous, image-guided procedure performed by an interventional radiologist. With regional anaesthesia or sedation, an angiographic catheter is placed under X-ray guidance into the uterine arteries via the common femoral artery. Embolic agents (usually polyvinyl alcohol particles or tris-acryl gelatin microspheres) are then injected into both uterine arteries to occlude them. Occlusion is confirmed angiographically and the catheters are removed (Figure 32.13). While the normal myometrium rapidly re-establishes its blood supply due to an extensive collateral circulation, the fibroids are supplied by end-arteries and undergo necrosis. The procedure takes approximately 1 h, and exposes the woman’s ovaries to 20 rads, the equivalent of most routine diagnostic imaging procedures. The date of the last menses should be assessed, and a urine pregnancy test should be performed prior to the procedure to exclude pregnancy. Pretreatment with GnRH agonists may hamper UAE treatment for fibroids and is contraindicated, unlike for surgery.
MRI-guided focused ultrasound
MRI-guided focused ultrasound uses high-intensity ultrasound waves directed to a precise area of tissue via MRI (performed concurrently), causing a temperature rise sufficient to induce coagulative necrosis in the tissue (Tropeano et al 2008). The aim is controlled localized thermal ablation of the fibroid tissue, while leaving the surrounding normal tissues undamaged. The ultrasound waves are delivered to the fibroids via the anterior abdominal wall while the patient is sedated and lying prone on the MRI table. The procedure takes approximately 3 h, but patients go home 1 h post procedure and return to work in 48 h.
Future Directions
Development of non-surgical treatments for uterine fibroids, as well as improvement in understanding of pathogenesis, is hampered by a lack of suitable in-vitro or in-vivo models. Most studies in the literature consist of observational comparisons between fibroid and adjacent normal myometrial tissue. The best available animal model is the Eker rat model, in which 65% of the female TscEK/+ carriers develop fibroids (Walker and Stewart 2005), but their applicability to fibroids in humans is still unclear and they are expensive to maintain. In-vitro models using smooth muscle cells isolated from the fibroids and adjacent myometrium have also been used. However, recent gene expression microarray work has shown that the differences in gene expression patterns of the two cell types diminish over time in culture (Zaitseva et al 2006), including the oestrogen and progesterone receptors that are critical to fibroid growth in vivo. This makes work from in-vitro cultures more difficult to translate into clinically relevant data.
KEY POINTS
Agdi M, Tulandi T. Endoscopic management of uterine fibroids. Best Practice and Research Clinical Obstetrics and Gynaecology. 2008;22:707-716.
Ahmad G, Duffy JM, Farquhar C et al 2008 Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database of Systematic Reviews 2: CD000475.
Ang WC, Farrell E, Vollenhoven B. Effect of hormone replacement therapies and selective estrogen receptor modulators in postmenopausal women with uterine leiomyomas: a literature review. Climacteric. 2001;4:284-292.
Benagiano G, Primiero FM. Uterine leiomyomata: medical treatment. In: Brosens I, editor. Uterine Leiomyomata: Pathogenesis and Management. London: Taylor and Francis, 2006.
Brandon DD, Bethea CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. American Journal of Obstetrics and Gynecology. 1993;169:78-85.
Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology and management. Fertility and Sterility. 1981;36:433-445.
Casey R, Rogers PAW, Vollenhoven BJ. An immunohistochemical analysis of fibroid vasculature. Human Reproduction. 2000;15:1469-1475.
Chwalisz K, Larsen L, Mattia-Goldberg C, et al. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertility and Sterility. 2007;87:1399-1412.
Cramer SF, Patel A. The frequency of uterine leiomyomas. Americal Journal of Clinical Pathology. 1990;94:435-438.
Crow J, Gardner RL, McSweeney G, et al. Morphological changes in uterine leiomyomas treated by GnRH agonist goserilin. International Journal of Gynaecological Pathology. 1995;14:235-248.
Donnez J, Gillerot S, Bougonjon D, et al. Neodynium:YAG laser hysteroscopy in large submucous fibroids. Fertility and Sterility. 1990;54:999-1003.
Eldar-Geva T, Meagher S, Healy DL, et al. Effect of intramural, subserosal and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertility and Sterility. 1998;70:687-691.
Fleischer R, Weston GC, Vollenhoven BJ, et al. Pathophysiology of fibroid disease: angiogenesis and regulation of smooth muscle proliferation. Best Practice and Research Clinical Obstetrics and Gynaecology. 2008;22:603-614.
Gard GB, Mulvany NJ, Quinn MA. Management of uterine leiomyosarcoma in Australia. Australia and New Zealand Journal of Obstetrics and Gynaecology. 1999;39:93-98.
Hammoud AO, Asaad R, Berman J, et al. Volume change of uterine myomas during pregnancy: do myomas really grow? Journal of Minimally Invasive Gynecology. 2006;13:386-390.
Hashimoto K, Azuma C, Kamiura S, et al. Clonal determination of uterine leiomyomas by analysing differential inactivation of the X-chromosome-linked phosphoglycerokinase gene. Gynecologic and Obstetric Investigation. 1995;40:204-208.
Hickey M, Hammond I. What is the place of uterine artery embolisation in the management of symptomatic uterine fibroids? Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;48:360-368.
Khaund A, Lumsden MA. Impact of fibroids on reproductive function. Best Practice and Research Clinical Obstetrics and Gynaecology. 2008;22:749-760.
Klatsky PC, Tran ND, Caughey AB, et al. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. American Journal of Obstetrics and Gynecology. 2008;198:357-366.
Kongnyuy EJ, Wiysonge CS 2007 Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database of Systematic Reviews 1: CD005355.
Lethaby A, Vollenhoven B, Sowter M 2000 Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2: CD000547.
Lippman SA, Warner M, Samuels S, et al. Uterine fibroids and gynaecologic pain symptoms in a population-based study. Fertility and Sterility. 2003;80:1488-1494.
Marshall LM, Spiegelman D, Barbieri R, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstetrics and Gynecology. 1997;90:967-973.
Matsuo H, Kurachi O, Shimomura Y, et al. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57(Suppl 2):49-58.
Monaghan JM. Bonney’s Gynaecological Surgery, 9th edn. London: Baillière Tindall; 1986.
Munro MG, Lukes AS. Abnormal uterine bleeding and underlying haemostatic disorders: report of a consensus process. Fertility and Sterility. 2005;84:1335-1337.
Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Practice and Research Clinical Obstetrics and Gynaecology. 2008;22:571-588.
Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstetrics and Gynecology. 1994;83:414-418.
Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertility and Sterility. 2007;87:725-736.
Ravina JH, Herbreteau D, Cirau-Vigneron N, et al. Arterial embolisation to treat uterine myomata. The Lancet. 1995;346:671-672.
Reiter RC, Wagner PL, Gambone JC. Routine hysterectomy for large asymptomatic uterine leiomyomata: a reappraisal. Obstetrics and Gynecology. 1992;79(4):481-484.
Sankaran S, Manyonda IT. Medical management of fibroids. Best Practice and Research Clinical Obstetrics and Gynaecology. 2008;22:655-676.
Townsend DE, Sparkes RS, Baluda MC, et al. Unicellular histogenesis of uterine leiomyomas as determined by electrophoresis by glucose-6-phosphate dehydrogenase. American Journal of Obstetrics and Gynecology. 1970;107:1168-1173.
Tropeano G, Amoroso S, Scambia G. Non-surgical management of uterine fibroids. Human Reproduction Update. 2008;14:259-274.
Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589-1592.
Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Human Reproduction. 2003;18:1088-1093.
Wegienka G, Baird DD, Hertz-Picciotto I, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstetrics and Gynecology. 2003;101:431-437.
Weston G, Trajstman AC, Gargett CE, et al. Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Molecular Human Reproduction. 2003;9:541-549.
Weston GC, Cattrall F, Lederman F, et al. Differences between the pre-menopausal and post-menopausal uterine fibroid vasculature. Maturitas. 2005;51:343-348.
Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro cuture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Molecular Human Reproduction. 2006;12:187-207.