Use of ultrasound in the evaluation and treatment of intraabdominal hypertension and abdominal compartment syndrome
Overview
Intraabdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are well-documented causes of morbidity and mortality in the critically ill. IAH and ACS may affect almost every organ system.1 The World Society of the Abdominal Compartment Syndrome (www.wsacs.org) has set forth a number of definitions based on clinical evidence and expert opinion. Intraabdominal pressure (IAP) is normally 5 to 7 mm Hg in adults. IAH is defined as sustained or repeated pathologic evaluation of IAP greater than or equal to 12 mm Hg. This is further subdivided into four grades based on pressure value. ACS is sustained IAP greater than 20 mm Hg, associated with new organ dysfunction/failure.1 Usual causes of IAH and ACS include intraabdominal hemorrhage, pneumoperitoneum from perforated viscus, and third spacing of fluid during massive resuscitation (Figure 46-1).
Pathophysiologic effects
Cardiovascular
Cardiac dysfunction occurs in patients with IAH and ACS because of increased intrathoracic pressure (ITP) resulting from upward diaphragm displacement. This increased ITP causes a significant decrease in venous return, thereby reducing cardiac output. There is a clear association between IAP and ITP. Fifty percent of the IAP is transmitted and affects the ITP.2 Cheatham et al3 point out that catheter-based hemodynamic measures, such as pulmonary artery occlusion pressure and central venous pressure, therefore have significant limitations as indexes of volume status in the face of IAH. They proposed the idea of using volumetric measurements, such as end-diastolic volume index, in directing adequate resuscitation in these patients. Anticipating the relationship between IAP and ITP and possibly using volume indexes for estimation of preload could help to prevent inadequate resuscitation and inappropriate use of vasoactive agents in patients with IAH. Several ultrasound (US) methods are used to guide therapeutic strategies in the face of IAH. Resuscitation efforts geared toward a right ventricular, end-diastolic volume index, goal-directed model have been shown to reduce the incidence of multiple organ failure and death.4
Respiratory
IAH results in increased ITP by pulmonary parenchymal compression and reduced chest wall compliance. Parenchymal compression leads to pulmonary hypertension. To overcome the alveolar compression and atelectasis, positive end-expiratory pressure (PEEP) is increased or added to maintain oxygenation and ventilation. Aggressive PEEP can result in not only opening up atelectatic areas of the lung but also overdistending normal lung and inhibiting adequate ventilation.1,5
Renal
An early sign of end-organ hypoperfusion resulting from IAH and/or ACS is decreased urine output. Renal dysfunction usually occurs with oliguria at 15 mm Hg and anuria at 30 mm Hg in euvolemic patients with no underlying kidney disease.6 This may occur at lower IAP in those who are already hypovolemic or in those with baseline pathology. Dysfunction may be due to compression of the renal parenchyma and vein, along with decreased renal perfusion pressures, leading thus to microcirculatory alterations and decreased urine output.7 Urine output is dependent on the renal filtration gradient (FG) or the glomerular filtration pressure (GFP) minus the proximal tubular pressure (PTP), which can be estimated as mean arterial pressure (MAP) minus two times the IAP [FG = GFP − PTP = MAP − (2 × IAP)].1,6
Ultrasound and other diagnostic adjuncts
Intravesicular pressure
Measurements are performed at the end of expiration in a supine position with the transducer zeroed at the iliac crest in the midaxillary line by using 25 mL of saline instilled into the bladder. The measurement is taken in mm Hg between 30 to 60 seconds after the saline is instilled and catheter clamped. All abdominal muscle contractions should be absent during measurements.8,9
Intragastric pressure
Intragastric pressure (IGP) monitoring has been used, in place of bladder pressure monitoring, in several experimental models to obtain abdominal pressure readings.10,11 There is a close relationship between IAP and IGP, with potential for inaccuracy in patients with ileus and in those who are being enterally fed.11
Abdominal ultrasonography
Recently, abdominal US has been used to identify patients with IAH and ACS. Cavaliere’s group12 examined the ultrasonographic detection of changes in the dimensions of abdominal veins—in the setting of simulated increased IAP in normal volunteers—as a marker of ACS. They used a pelvic binder to create external compression, inducing mild IAH. Dimensions of multiple abdominal veins were estimated by B-mode, while pulsed wave Doppler measured peak blood flow velocities at the end of expiration. The inferior vena cava (IVC) below the renal veins, the right suprahepatic vein, the portal vein (PV), the right external iliac vein, and the segmental branches of the right renal artery were measured. Significant changes in IVC and PV diameters were noted. Diameters were noted to be decreased about 10%, whereas the cross-sectional area of the IVC was decreased about 25%. No significant changes were evident in peak Doppler velocities of the studied vessels, but significant variability between individuals existed. Studies using computed tomography scans revealed distorted IVC shape and decreased diameter as well as flattening of renal vasculature in ACS.13 Further research should include ultrasonographic analysis of IVC and PV in patients with IAH and ACS.
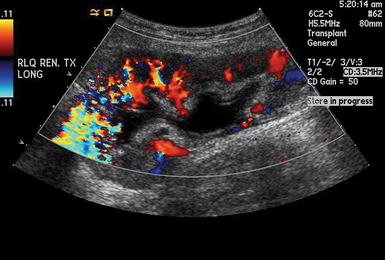
Renal duplex ultrasound
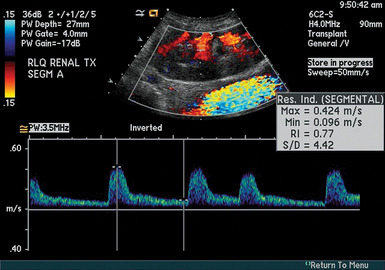
Duplex US of the renal arteries and their branches has been used in patients with various kidney diseases to estimate their renal resistive index (RI).14 RI is defined as [(peak systolic velocity − end-diastolic velocity)/(peak systolic velocity)] and correlates with renal function. Pulsed wave Doppler measurements are obtained with the sample volume adjusted to 1.5 to 2 mm and the angle to 0 degrees when evaluating interlobar arteries, whereas the angle should be 60 degrees or less when scanning renal arteries. Signals are obtained from the interlobar arteries along the border of the medullary pyramids (Figure 46-2).15 Several measurements at multiple points in the organ are obtained, whereas average values are used to calculate the RI (Figure 46-3). The RI method is technically demanding and has many limitations (e.g., optimization of Doppler signals and pertinent waveforms, proper identification of arcuate and intramedullary arteries, etc.).14 The normal mean RI value is approximately 0.60 (in patients without underlying kidney disease), whereas values greater than 0.8 usually indicate parenchymal disease.16 Of note, in the above-mentioned Cavaliere et al13 study, subjects with mild IAH had increased renal RI values. Although the role of RI role in the evaluation of vascular-interstitial abnormalities is debatable, the method may aid in identifying ACS at the renal microvascular level.
Focused transthoracic echocardiography
Significant cardiovascular alterations occur with IAH and ACS. Transthoracic echocardiography (TTE) is used in patients with IAH and ACS to assess cardiac function, intraventricular filling, and intravascular volume status. TTE aids clinicians in garnering information and then basing resuscitation efforts on volumetric measurements.4 Invasive methods of assessing cardiac function and fluid status include the use of pulmonary artery catheters, although no survival benefits have been documented.17,18 TTE is a noninvasive and safe alternative to invasive measures of volume status.
Ultrasound in the treatment of abdominal compartment syndrome
Decompressive laparotomy (DL) has long been the definitive treatment of ACS. A meta-analysis of 18 studies published between 1972 and 2004 analyzed the effects DL on patients with ACS.19 These 18 studies yielded a total of 250 patients who had been treated for ACS with DL. Of the 250 patients noted, 161 were found to have before and after IAP recorded in relation to their DL. As expected, there was a statistically significant drop in IAP from a mean of 34.6 mm Hg to a mean of 15.5 mm Hg. The mortality in these studies, after DL, ranged from 22% to 100%, with a mean of 49.2%, although some improvements were noted in cardiac output, urine output, and respiratory function, as evidenced by improved partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2).
With treatment protocols (e.g., Surviving Sepsis Campaign20) encouraging aggressive resuscitation, IAH is increasingly observed by intensivists. To avoid the morbidity of a laparotomy, there has been a renewed interest into minimally invasive treatments for significant IAH and ACS, particularly in those in whom a laparotomy is not otherwise indicated. Nonoperative management of IAH and ACS includes five alternate therapies, all aimed at reduction of IAP: (1) evacuation of intraluminal contents, (2) evacuation of intraabdominal space-occupying lesions, (3) improvement of abdominal wall compliance, (4) optimization of fluid administration, and (5) optimization of systemic and regional tissue perfusion.21,22 Each of these therapies consists of escalating interventions, from noninvasive to more invasive, with the final step consisting of DL.
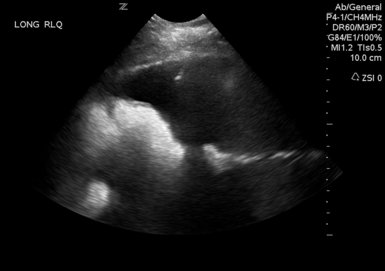
To facilitate the evacuation of intraabdominal space-occupying lesions, Cheatham advocated performance of a diagnostic abdominal ultrasound to identify the space-occupying lesion or fluid collection.21 Hemoperitoneum, ascites, intraabdominal abscess, retroperitoneal hemorrhage, and free air can all be space-occupying lesions that can raise the IAP.20 Many studies examined the utility of percutaneous drainage of these fluid collections and the resultant decreased IAP.15,23–26 These studies have shown a significant decrease in IAP and resolution of IAH/ACS (without DL) in many of the patients studied. Also, many intensivists (nonsurgeons) are more willing to attempt ultrasound- or CT-guided percutaneous drainage as an initial treatment of ACS.
A recent study of percutaneous drainage for the treatment of ACS revealed success rates of 81% (25/31) in avoiding DL.24 In this study, successful management of IAH/ACS with percutaneous drainage was associated with drainage greater than or equal to 1000 mL of fluid or decrease in IAP greater than or equal to 9 mm Hg in the first 4 hours after drain placement. The authors recommended that in patients with significant IAH or patients with ACS, ultrasound should be performed to confirm the presence of free intraabdominal fluid or blood. If a sufficient fluid pocket is identified that would allow safe placement of a drainage catheter, this should be performed (Figure 46-4). A caveat is when using US to decompress someone who is actively bleeding and who would be more safely treated in the operating room or in an interventional radiology/angiography suite.
Pearls and highlights
• Normal IAP is between 5 and 7 mm Hg. IAH is defined as a sustained elevation of IAP greater than or equal to 12 mm Hg. ACS is defined as IAP greater than 20 mm Hg with signs of new organ dysfunction/failure.
• Although ACS is a clinical diagnosis, multiple diagnostic adjuncts exist.
• Abdominal US detects space-occupying lesions and/or fluid collections, leading thus to more conservative therapeutic strategies by avoiding a possible DL.
• TTE evaluates fluid status and guides resuscitation based on cardiac volumetric indexes. Inferior vena cava deformability with respiration may represent a sign of poor volume status or increased IAP.
• The definitive treatment for ACS is DL.
• Minimally invasive techniques (e.g., US-guided percutaneous drainage) are suggested to be effective at lowering IAP and avoiding the morbidities associated with an open abdomen. We would recommend this approach in patients with pancreatitis and liver failure, where accumulation of ascites leads to increased IAP and organ failure.
References
1. Cheatham, ML, Malbrain, ML, Kirkpatrick, A, et al. Definitions and pathophysiological implications of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011; 77(Suppl 1):S6–S11.
2. Wauters, J, Wilmer, A, Valenza, F, Abdomino-thoracic transmission during ACS: facts and figures. Acta Clin Belg Supp. 2007; 1:200–205.
3. Cheatham, ML, Malbrain, ML, Cardiovascular implications of abdominal compartment syndrome. Acta Clin BeIg Suppl. 2007;62:98–112.
4. Cheatham, M, Safcsak, K, Block, E, et al. Preload assessment in patients with an open abdomen. J Trauma. 1999; 46:16–22.
5. Malhotra, A, Hillman, D, Obesity and the lung: 3. Obesity, respiration and intensive care. Thora. 2008; 63:925–931.
6. Shenasky, JH. The renal hemodynamic and functional effects of external counterpressure. Surg Gynecol Obstet. 1972; 134:253–258.
7. Papavramidis, TS, Marinis, AD, Pliakos, I, et al, Abdominal compartment syndrome – Intra-abdominal hypertension: defining, diagnosing, and managing. J Emerg Trauma.Shoc. 2011; 4:279–291.
8. Cheatham, ML, Malbrain, ML, Kirkpatrick, A, et al, Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–1732.
9. Cheatham, ML, Malbrain, ML, Kirkpatrick, A, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007; 33:951–962.
10. Kron, IL, Harman, PK, Nolan, SP. The measurement of intraabdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984; 199:28–30.
11. Decramer, M, De Troyer, A, Kelly, S, et al. Regional differences in abdominal pressure swings in dogs. J Appl Physiol. 1984; 57:1682–1687.
12. Cavaliere, F, Cina, A, Biasucci, D, et al. Sonographic assessment of abdominal vein dimensional and hemodynamic changes induced in human volunteers by a model of abdominal hypertension. Crit Care Med. 2011; 39:344–348.
13. Patel, A, Lall, CG, Jennings, SG, et al. Abdominal compartment syndrome. AJR Am J Roentgenol. 2007; 189:1037–1043.
14. Tublin, ME, Bude, RO, Platt, JF, The resistive index in renal doppler sonography: where do we stand. AJR Am J Roentgeno. 2003; 180:885–892.
15. Umgelter, A, Reindl, W, Franzen, M, et al, Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med. 2009;35:152–156.
16. Keogan, M, Kliewer, M, Hertzberg, B, et al, Renal resistive indexes: variability in Doppler US measurement in a healthy population. Radiolog. 1996; 199:165–169.
17. Richard, C, Warszawski, J, Anguel, N, et al, Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAM. 2003; 290:2713–2720.
18. Harvey, S, Harrison, DA, Singer, M, et al, Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomized controlled trial. Lance. 2005; 366:472–477.
19. De Waele, JJ, Hoste, EA, Malbrain, ML, Decompressive laparotomy for abdominal compartment syndrome—a critical analysis. Crit Care. 2006;10:R51.
20. Dellinger, RP, Levy, MM, Carlet, JM, et al, Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Car. 2008; 36:1394–1396.
21. Cheatham, ML. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009; 33:1116–1122.
22. Cheatham, ML. Abdominal compartment syndrome. Curr Opin Crit Care. 2009; 15:154–162.
23. Corcos, AC, Sherman, HF. Percutaneous treatment of secondary abdominal compartment syndrome. J Trauma. 2001; 51:1062–1064.
24. Parra, MW, Al-Khayat, H, Smith, HG, et al, Paracentesis for resuscitation-induced abdominal compartment syndrome: an alternative to decompressive laparotomy in the burn patient. J Traum. 2006; 60:1119–1121.
25. Reed, SF, Britt, RC, Collins, J, et al. Aggressive surveillance and early catheter-directed therapy in the management of intra-abdominal hypertension. J Trauma. 2006; 61:1359–1363.
26. Kimball, EJ, Rollins, MD, Mone, MC, et al. Survey of intensive care physicians on the recognition and management of intra-abdominal hypertension and abdominal compartment syndrome. Crit Care Med. 2006; 34:2340–2348.