Use of Sedatives, Analgesics, and Neuromuscular Blockers
Critically ill patients often experience unpleasant sensations including pain, anxiety, dyspnea, and other forms of distress.1–4 This is particularly true for mechanically ventilated patients who make up a significant proportion of intensive care unit (ICU) patients. The ICU environment, and ICU care in general, is widely regarded as not ideally suited to patient comfort. For example, most patients may have surgical incisions and often have a variety of indwelling tubes and vascular catheters. Further, sleep disruption is common, sound and light levels are often excessive, and patient communication and mobility are impaired. Core principles of ICU care are to provide comfort to our patients and to relieve their suffering.5–8 Although nonpharmacologic interventions can improve patient comfort,9 clinicians have relied upon administration of analgesic and sedative medications to reduce the sensation of pain and anxiety, to control agitation, and to enhance tolerance of the ICU environment.10–12 However, clinical experience and research over the past decade have demonstrated the hazards associated with excessive or unnecessarily prolonged sedation and the importance of accurately achieving targeted analgesia and sedation.13–17 Newly revised clinical practice guidelines from the Society of Critical Care Medicine (SCCM)6 and other recent guidelines and reviews on sedation and analgesia18–23 highlight the importance of adopting a management approach that is patient-focused and strives to use the lowest effective dose of medications. The past decade has also brought a greater appreciation for the importance that development of delirium in the ICU has for long-term outcomes including downstream cognitive dysfunction.6,24–29 The topic of ICU delirium is reviewed in detail elsewhere in this textbook (Chapter 72). Finally, the use of neuromuscular blocking agents (NMBAs) to achieve therapeutic paralysis is undergoing reevaluation, particularly for optimizing management of acute respiratory distress syndrome (ARDS).30–33 The use of sedatives, analgesics, and NMBA in managing critically ill patients is reviewed in this chapter.
Surveys and clinical reports indicate that the majority of ICU patients experience pain, dyspnea, or other forms of distress and receive sedative and analgesic medications.10–12,34 Some of the key terms and definitions related to patient distress and neurobehavioral abnormalities in ICU patients are presented in Box 19.1.35 Much of the discomfort is related to various stressors. In one case series, being in pain, being unable to sleep, and having tubes in the nose or mouth were the most prominent stressors reported by recovering ICU patients.4 Clearly, providing relief from these and other issues is an important goal of analgesic and sedative therapy. Consequences of suboptimal analgesia and sedative therapy can include problems related to inadequately controlled pain and anxiety or excessive sedation. Agitation, defined as “excessive motor activity associated with internal tension,” is perhaps the most widely recognized manifestation of uncontrolled anxiety or pain and is a problem because it can result in dramatic events, such as self-removal of important tubes and catheters, or even violence directed toward care providers. Overt agitation is a common occurrence among critically ill patients with rates as high as 20% of patient-shifts and nearly three quarters of patients during ICU stay.35–38 Self-removal of critical indwelling tubes and vascular catheters is the most common manifestation of overt agitation, placing the patient in peril from loss of an artificial airway and positive-pressure ventilation, abrupt cessation of infusion of critical medications such as vasopressors, and the risk and discomfort associated with reinsertion of tubes and lines.39 In prospective studies, device removal rates range widely from 22 to 157 events per 1000 patient-days40,41 and have an associated added cost.42 Documentation of patient agitation has become commonplace with the broad use of scales that address both sedation and agitation.43 In addition to device removal and care provider assault, agitation is often accompanied by an intense stress response with catecholamine surge resulting in tachycardia, hypertension, and tachypnea.44,45 Withdrawal of continuous infusion sedative and analgesic medications to promote awakening is associated with a two- to fourfold increase in circulating catecholamines46 and can produce evidence of withdrawal that peaks within 6 hours.47 Adrenergic stress response has been linked to hypercoagulability, glucose intolerance, increased catabolism, immunosuppression, sleep disturbance, and delirium. Further this can precipitate adverse events such as myocardial ischemia, increased intracranial pressure (ICP), tachyarrhythmias, and complications of uncontrolled hypertension in susceptible individuals.48 Finally, it is also worth considering that overt agitation can be a marker for extreme distress that has a cause that may be life-threatening or that could be rapidly resolved upon recognition and management.21,35,36 Endotracheal tube malposition or obstruction, severe chest pain from myocardial ischemia, and tension pneumothorax are examples of conditions for which severe agitation in the nonverbal patient may be an important clue to an urgent dangerous situation. In fact, agitation was a common clinical antecedent prior to cardiopulmonary arrest in one study.49
Although the primary effect of analgesic and sedative medications is the relief of pain, anxiety, agitation, and related problems, such relief is usually accompanied by reduced level of consciousness. Excessive and unnecessarily prolonged impaired sensorium is associated with additional complications—as a result of excessively deep sedation (i.e., pressure sores or nerve compression from reduced movement), or as a result of sedation-related delayed recovery from critical illness and mechanical ventilation with higher rates of complications and interventions related to longer ICU length of stay (LOS). Further, long-term neuropsychological consequences of ICU sedation are increasingly recognized.25 Accordingly, it is critical to take a structured approach to managing analgesia and sedation, which includes carefully examining the specific patient-focused goals of therapy, using validated tools for evaluation and monitoring, and implementing management that explicitly targets using the lowest effective dose of the best analgesic and sedative medications for the specific patient. Some important components for analgesia and sedation management gleaned from prior reviews and current clinical practice guidelines are listed in Box 19.2.6,19–23
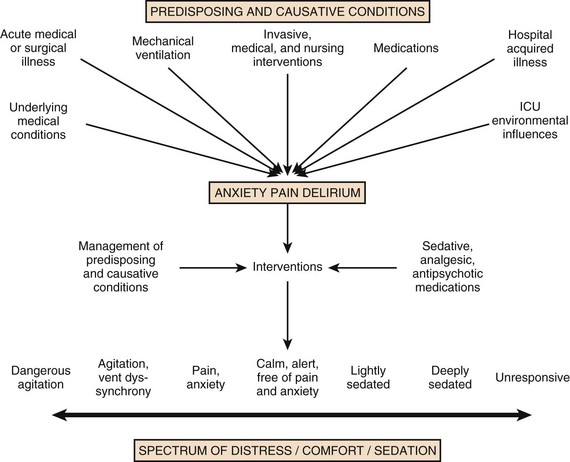
Analgesia and sedation are most effective when they are patient-focused because each patient and circumstances have unique features that may influence the response to therapy. Some of the predisposing and precipitating conditions that can contribute to the development of anxiety, pain, and delirium are noted in Figure 19.1.19,35 For example, a variety of underlying medical conditions—such as alcohol or substance abuse,50 psychiatric disorders, and chronic pain—can influence the likelihood of developing anxiety, pain, or delirium and can affect the response to therapy.37 Further, inadvertent discontinuation of home medications such as opioids, benzodiazepines, or antipsychotic medications because of lack of awareness of their prior use is not uncommon with unscheduled ICU admissions and is an important reason to perform detailed medication reconciliation with patients and their family members. Although the notion that “ICU psychosis” is actually caused by the ICU is misleading, it is clear that excessive noise, light, and repeated patient awakening to check vital signs or perform tests at night contribute to fragmented sleep and can provoke anxiety and delirium.51–53 Acute medical illness, surgical interventions, mechanical ventilation and the interface (i.e., tracheostomy tube, endotracheal tube, oronasal mask), and nursing interventions such as suctioning and turning can contribute to pain and discomfort.3,4,54 A variety of conditions and medications have been associated with the development of delirium and agitation, as displayed in Box 19.3.35
PAIN Management in the Intensive Care Unit
Pain is widely experienced in the ICU setting and can result from invasive procedures, surgery, and monitoring devices, as well as more direct stimuli from injury, inflammation, and immobility.55 Treating pain is a core component of the caregivers’ responsibility to relieve suffering and offers the physiologic benefits of relieving pain-induced altered glucose control, myocardial ischemia, immune system dysfunction, hypercoagulability, ventilator asynchrony, impaired ventilation, and disrupted sleep.55 Mechanisms for these downstream effects include neurohormonal derangements, catecholamine release, and stress response.55,56
Effective treatment of pain begins with the assumption that pain is widely present and that caregivers should systematically evaluate each patient for the presence of pain and its intensity and characteristics, on a repetitive basis.6,8,9,55 Caregivers tend to underrecognize pain and often fail to preemptively treat pain.3,57 Accordingly, clinicians should err on the side of presuming pain is present—particularly when considering that it is far more effective to prevent and manage pain at an early stage than after it has become severe. Ideally, pain should be described in regard to location, duration, type, exacerbating and relieving factors, and intensity. Pain is sometimes conceptualized by subtypes, including somatic, visceral, and neuropathic, because manifestations and management can be different.55 For example, somatic pain is typically dull and aching, often localized, and responds well to opioids and nonsteroidal anti-inflammatory agents (NSAIDs). In contrast, visceral pain is often cramping and colicky and may respond to anticholinergic therapy, although the burning and shooting neuropathic pain is often best treated with antidepressant and anticonvulsant agents.
Because pain is a subjective interpretation by an individual, the ability of that individual to convey the presence and magnitude of pain is of great value in guiding evaluation and management.58 Detailed verbal communication by ICU patients is often limited; however, use of simple tools such as the numerical rating scale or a series of cartoon faces ranging from smiling to crying can facilitate communication by pointing or nodding in the nonverbal but cognitively intact patient.59 A simple horizontal 0 to 10 numerical scale with enlarged font was judged to be most feasible and valid in one study of pain intensity rating scales.60 Other techniques to enhance the use of self-reporting of pain include adding descriptive words to the numerical scale, carefully explaining the tool and correct use with each encounter, providing glasses and hearing aids if necessary, allowing adequate time for instructions and patient response.58 Use of a scale that incorporates descriptive pictures, like the faces scale, can facilitate self-reporting of pain intensity regardless of potential language barriers.
Many ICU patients are cognitively impaired and self-reported pain intensity is not feasible. Accordingly, clinicians must rely upon observed behaviors that have been documented to correlate well with self-reported pain or to be associated with noxious stimuli.54,57,61–63 Investigators have combined behaviors into structured assessment tools. Interestingly, much of the early work in pain assessment for noncommunicative ICU patients was in infants and young children, resulting in the development of the COMFORT scale,64 the Face, Legs, Activity, Cry and Consolability (FLACC) observation tool,65 and others. Subsequently a variety of pain assessment tools for nonverbal critically ill adults have been developed, validated, and thoroughly reviewed.58,66,67 In the 2012 SCCM guidelines,6 published pain observation tools for adults in the ICU were identified as the Critical-Care Pain Observation Tool (CPOT),68 the Behavioral Pain Scale (BPS),69 the Behavioral Pain Scale—Nonintubated (BPS-NI),70 the Non-Verbal Pain Scale (NVPS),71 the Pain Behavioral Assessment Tool (PBAT),54,72 and the Pain Assessment, Intervention, and Notation (PAIN) tool.73 Extensive psychometric testing was performed including examination of scale development, testing of reliability, and testing of validity, feasibility, and scale relevance or impact of implementation in ICU patient outcomes yielding a maximum score of 25 and weighted score of 0 to 20. The CPOT had the highest weighted score of 14.7, followed by the BPS with 12.0,6 and these two scales are recommended in the 2012 SCCM guidelines as the most valid and reliable behavioral pain scales for monitoring pain in adult medical, postoperative, and trauma ICU patients who are unable to self-report and in whom motor function is intact and behaviors are observable.6 CPOT and BPS are displayed in Tables 19.1 and 19.2. Pain is often accompanied by changes in vital signs, with tachycardia, hypertension, and tachypnea being most common. However, changes in vital signs are inconsistently associated with pain, with many confounding factors that can mask anticipated changes—such as administration of beta blockers, as well as lack of specificity with many other causes of tachycardia, hypertension, and tachypnea.74 Accordingly, 2012 SCCM guidelines recommend against the use of vital signs (or observational pain scales that include vital signs) alone for pain assessment in adult ICU patients.6 However, it is acknowledged that vital signs may be an important cue to prompt further pain assessment.6 Other potentially useful approaches include evaluating pain risk profile, use of surrogate (such as family members) report,75 and performance of an analgesic trial.3,55,58
Table 19.1
Critical Care Pain Observation Tool (CPOT)
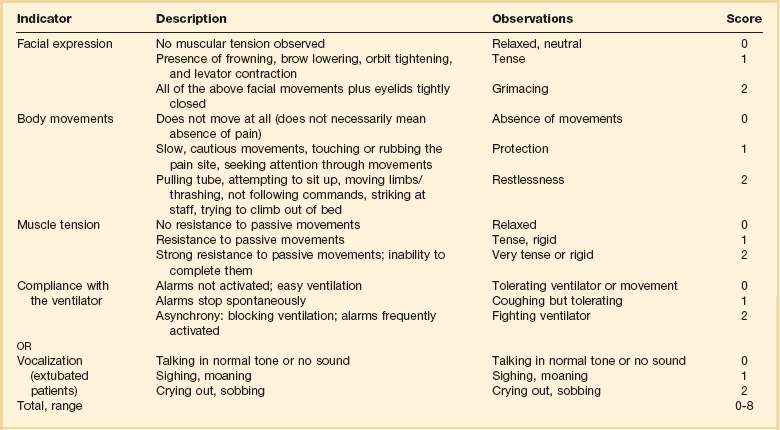
From Gelinas C, Fillion L, Puntillo KA, et al: Validation of the critical-care pain observation tool in adult patients. Am J Crit Care 2006;15(4):420-427; used with permission.
Table 19.2
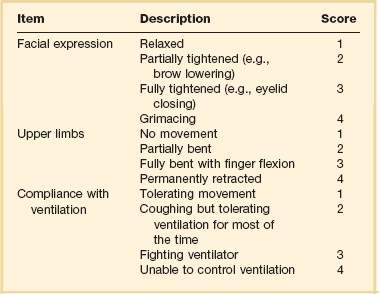
From Payen JF, Bru O, Bosson JL, et al: Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001;29(12):2258-2263; used with permission.
There are several important tenets for managing pain in the ICU. First, when initiating sedative treatment for mechanically ventilated ICU patients, analgesia should be the first step, followed by the addition of a sedative-anxiolytic agent. This “analgesia first” approach is suggested in the 2012 SCCM guidelines6 as well as many expert reviews.18–23,55 Second, the use of preemptive analgesia should be administered prior to the performance of interventions, such as chest tube removal, that are associated with having a high likelihood of producing pain.6 It is more effective to prevent pain or manage it at an earlier and milder stage than after pain has become severe.
Successful pain management in the ICU incorporates pharmacologic and nonpharmacologic treatment.9 Parenteral opioids are the primary agents used to treat pain in the ICU setting, but use of selected nonopioid and atypical agents can enhance pain relief and may reduce adverse effects. The major parenteral opioids for continuous infusion in ICU patients—fentanyl, morphine, hydromorphone, and remifentanil (see Table 19.4)9—are generally considered to be equivalent in regard to analgesic potential as well as class-based adverse effects such as respiratory depression or reduced level of consciousness. Important differences among agents in regard to lipid solubility, volume of distribution (Vd), half-life, clearance, metabolism, and active metabolites have important effects on onset, peak, and offset of action, potency, and the influence of renal or hepatic dysfunction.9 Some of the medication features that can influence opioid selection for a particular patient are noted in Table 19.3. For example, morphine sulfate is metabolized to several active metabolites, including morphine-6-glucuronide, which is as active as the parent compound and accumulates in renal failure, resulting in prolonged effects including analgesia but also somnolence and respiratory depression.76 The rapid equilibration of fentanyl from plasma to lipid-rich brain tissue is responsible for its rapid onset of action but its high lipophilicity also contributed to large Vd and delayed offset of action after prolonged infusion. Remifentanil has a small Vd, and rapid clearance with metabolism by esterases, yielding a short half-life and rapid offset of action that is independent of renal and hepatic function.77,78 Several opioids not listed deserve mention. Meperidine has the potential for neuroexcitatory adverse effects such as seizures79 and is not recommended for ICU use.6 Methadone can slow the development of tolerance to other opioid agents when coadministered but has unpredictable pharmacokinetics and is not administered by continuous infusion.6 Methadone is also a potent precipitating agent for prolonging the rate-corrected QT interval (QTc).80,81 Although opioids are most effective for pain management in the ICU patient, these agents have a well-documented propensity to produce adverse effects (AEs), which are reviewed, along with comments for each AE, in Table 19.4.9,55 Comprehensive pain management should include awareness of the likely adverse effects and how best to avoid them and to recognize and manage them.
Table 19.3
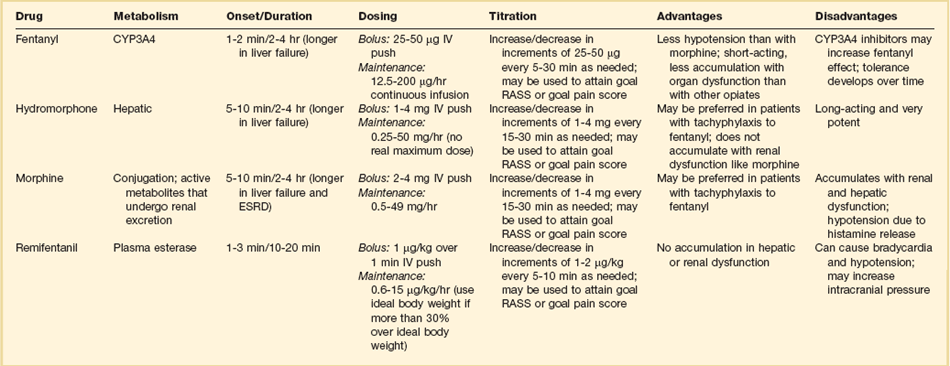
Table 19.4
| Potential Adverse Effect | Comment |
| Allergy | Rare; most common with morphine |
| Arrhythmias | QTc prolongation with methadone |
| CNS depression | Accumulation of active morphine metabolite in renal failure |
| Constipation | Start bowel regimen early if sustained opioid administration is likely Enteral naloxone is sometimes effective, but reverses analgesia |
| Cough | Most common with fentanyl and similar agents |
| Dry mouth | Use oral lubricants |
| Histamine release | Uncommon with fentanyl and similar agents Slow infusion rate Treat with combination of H1 and H2 agonists |
| Hyperalgesia | Paradoxic increase in pain sensitivity More common with short-acting opioids |
| Myoclonus | Consider reduced opioid dose or switch agent; hydrate |
| Nausea/vomiting | Administer antiemetic until tolerance develops |
| Neurotoxicity | Avoid meperidine—associated with highest incidence Can occur with morphine or hydromorphone, particularly at higher doses or in renal failure |
| Opioid dependency | Withdrawal symptoms can develop—usually coincide with clearance Prevent by tapering; reinstitute agent if withdrawal occurs |
| Pruritus | Consider naloxone, serotonic antagonists such as ondansetron |
| Rigidity | Most common with fentanyl and similar agents but can occur with any agent at high dose |
| Serotonin syndrome | Reported with meperidine and methadone Avoid concomitant serotonin reuptake inhibitor |
Adapted from Erstad BL, Puntillo K, Gilbert HC, et al: Pain management principles in the critically ill. Chest. 2009;135(4):1075-1086; used with permission.
It is often advantageous to use the lowest effective dose of an opioid analgesic, particularly when intolerable side effects are present. Additionally, there are clinical settings in which a nontraditional approach may be more effective, safer, and better tolerated. Employment of alternative or complementary approaches such as adding nonopioid or atypical analgesic agents, use of epidural or other neuraxial alternatives to parenteral opioid, and use of complementary nonpharmacologic interventions should be considered in selected cases.9
Nonopioid and Atypical Agents
The use of nonopioid medications is best approached from the perspective of differentiating neuropathic pain for which anticonvulsants, antidepressants, and other agents play a primary role versus non-neuropathic pain for which opioids are the first line of therapy and other agents are used to reduce opioid dosage and reduce side effects. There is compelling evidence that the addition of oral gabapentin or carbamazepine to opioids provides superior pain relief for neuropathic pain in ICU patients when compared to opioids alone,82,83 and this use is strongly recommended in this setting in the 2012 SCCM guidelines.6 Pharmacotherapy for neuropathic pain has been extensively studied in randomized controlled trials (RCTs) in non-ICU patients, and medications including tricyclic antidepressants, selective serotonin receptor inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), anticonvulsants, tramadol, dextromethorphan, ketamine, topical capsaicin, lidocaine patch or gel, cannabinoids, and other agents have shown efficacy, albeit often in trials with small sample size and with associated side effects.84
Nonopioid adjuvant therapy in non-neuropathic pain can help reduce the quantity of opioids administered and potentially decrease the incidence and severity of opioid-related side effects. Such agents have been extensively studied in various non–critically ill populations—primarily postoperative patients, with efficacy demonstrated in meta-analyses of RCTs for corticosteroids, NSAIDs, acetaminophen, ketamine, and others.85–88 There is relatively limited research in ICU patients and most of the RCTs performed with ICU patients have been in the postoperative setting. Best studied in the ICU population are IV (intravenous) acetaminophen, cyclooxygenase inhibitors, and ketamine.89–91 As a result of the limited evidence base in ICU patients, the 2012 SCCM guidelines generally support this approach as a weak recommendation for the addition of nonopioids for non-neuropathic pain.6 The potential benefits as well as risks of nonopioids for ICU use are further discussed in reviews published in 2009.9,55
Regional Anesthesia and Analgesia
Although systemic administration of opioids for pain management in the ICU is typically by the IV route, regional approaches can offer advantages in selected cases.9 Options for regional anesthesia and analgesia (RAA) include continuous epidural analgesia (CEA) and anesthesia (central neuraxial techniques) and continuous peripheral nerve or plexus block. With medication delivery in close proximity to the spinal cord or nerves, these techniques can promote parenteral opioid sparing, which is associated with less respiratory depression and gastrointestinal hypomotility and conveys other benefits. However, these techniques introduce new issues related to needle and catheter placement and maintenance, as well as physiologic alterations related to local anesthetic block and epidural drug delivery. Critically ill patients are often at higher risk for complications due to coagulopathy, thrombocytopenia, sepsis, and hemodynamic instability. Peripheral nerve blocks are particularly useful for anatomically localized pain such as limb injury or operation. However, of all the various RAA techniques, the greatest experience in ICU patients is with CEA.
Similar to other pain management issues, there is more published research to support the use of RAA in noncritical care settings, and most ICU studies focus on patient who are postoperative or have sustained injury. The recent evidence-based review undertaken to develop the 2012 SCCM guidelines provides the most relevant and current basis for use of RAA in ICU patients.6 Specific patient populations were examined, and only thoracic epidural anesthesia/analgesia for postoperative analgesia in patients undergoing abdominal aortic surgery received a strong recommendation as a superior alternative to IV opioids.92 Interestingly, high-level evidence was also available to compare lumbar epidural analgesia to IV opioids in the same population and no benefit was demonstrated.93 Patients with traumatic rib fractures are the other population for which benefit from RAA was demonstrated for ICU patients. Specifically, use of thoracic epidural analgesia was associated with superior pain control and reduced incidence of pneumonia—but more hypotension—compared to parenteral opioids,94,95 and its use is supported as a weak recommendation in this setting.6 The evidence was insufficient to recommend thoracic epidural analgesia for intrathoracic or nonvascular abdominal surgical procedures.6 Similarly, the lack of high-quality research prevented a recommendation regarding the use of RAA for medical ICU patients.6
Complementary Techniques
Nonpharmacologic interventions such as music, relaxation, and provision of information can provide complementary pain relief and can be considered.9,55,96–98 However, although they are low cost, easy to provide, and safe, they have a relatively weak body of evidence to support their routine use, and they are not “high impact,” providing only limited opioid-sparing or pain intensity reduction capability.9,55,96
Sedation Management
Sedative drugs have formed the cornerstone for patient comfort and tolerance of the ICU environment for decades by calming anxiety, providing amnesia, and controlling agitation. There have been changing paradigms from preferential use of moderate- to long-acting benzodiazepines5 and acceptance of prolonged deep sedation a decade ago, to patient-focused sedation with careful monitoring, analgesia first, and more recently to an emphasis on the use of nonbenzodiazepine sedative agents and using the lightest level of sedation possible.6 Management of sedation should be preceded by examining individual patient characteristics and effective pain control. It incorporates establishing the goals of sedative therapy, monitoring of the depth of sedation, selecting and administering the best agent(s) for the particular patient, and using protocolized management of sedation that includes structured elements to assure use of the lowest effective dose and lightest level of sedation. Common goals of therapy include relief of anxiety, control of frank agitation, promotion of patient-ventilator synchrony, and elimination of awareness when an NMBA is used. Monitoring of the depth of sedation is now routinely performed in most ICUs and is applied to titrate medications to a targeted level of consciousness and to avoid excessive or prolonged sedation.
Monitoring the depth of sedation is most commonly performed by observing patient behaviors, often after varying levels of stimulation, using a structured sedation scale.43,67,99 The domain being tested is that of arousal or level of consciousness and typically ranges from alert to comatose. Ramsay and coworkers developed the first widely utilized sedation scale for ICU patients—a 6-level scale based upon response to the assessor’s voice and to physical stimulation.100 Subsequent scale development has introduced assessment of agitation, ranging from calm to combative. Useful features of sedation-agitation scales include multidisciplinary development; ease of administration, recall, and interpretation; well-defined discrete criteria for each level; sufficient sedation levels for effective drug titration; assessment of agitation; demonstration of good inter-rater reliability for relevant patient populations; and evidence of validity.43 Recently the SCCM guideline task force performed a careful analysis of the sedation scales that have undergone validation analysis in ICU patients, including the Ramsay Sedation Scale (RSS),100 the New Sheffield Sedation Scale,101 the Observer’s Assessment of Alertness/Sedation Scale (OAA/S),102 the Sedation Intensive Care Score (SEDIC),103 the Motor Activity Assessment Scale (MAAS),104 the Adaption to the Intensive Care Environment (ATICE) tool,105 the Minnesota Sedation Assessment Tool (MSAT),106 the Vancouver Interaction and Calmness Scale (VICS),107 the Sedation Agitation Scale (SAS)108 and the Richmond Agitation Sedation Scale (RASS).109 Of these, the RASS had the highest total weighted psychometric score (19 of 20 possible points), followed by the SAS (16.5 points), and these two instruments are recommended in the 2012 SCCM guidelines as the most valid and reliable sedation assessment tools for measuring quality and depth of sedation in adult ICU patients.6 The RASS, developed by a multidisciplinary group led by Sessler, is displayed in Table 19.5.109 The RASS incorporates assessment of arousal in response to 2 levels of stimulation (voice, physical), presence or absence of cognition, sustainability of response, and the presence and intensity of agitated behavior into a 10-level scale subcategorized with positive (agitation) and negative (sedation) values.109 The SAS, developed by Riker and colleagues, is displayed in Table 19.6.108 Both RASS and SAS have been extensively tested for inter-rater reliability and validity in a cross section of ICU populations, correlated with subjective and objective measures of sedation, and evaluated for ease of use in clinical settings.6,67,108–112 The introduction of a sedation scale into clinical practice has been demonstrated to result in higher quality sedation with fewer hours of oversedation, as well as reduced utilization of sedative and analgesic drug doses, shorter duration of mechanical ventilation, and reduced use of vasopressors.113,114 Structured assessment and management of agitation and pain were similarly associated with less agitation, quicker extubation, and fewer nosocomial infections.115
Table 19.5
Richmond Agitation Sedation Scale (RASS)

From Sessler CN, Gosnell MS, Grap MJ, et al: The Richmond Agitation Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-1344; used with permission.
Table 19.6
Sedation Agitation Scale (SAS)
| Score | Term | Description |
| 7 | Dangerous agitation | Pulling at endotracheal tube, trying to remove catheters, climbing over bedrail, striking at staff, thrashing side to side |
| 6 | Very agitated | Does not calm despite frequent verbal reminding of limits; requires physical restraints; biting endotracheal tube |
| 5 | Agitated | Anxious or mildly agitated; attempts to sit up; calms down to verbal instructions |
| 4 | Calm and cooperative | Calm, awakens easily, follows commands |
| 3 | Sedated | Difficult to arouse, awakens to verbal stimuli or gentle shaking but drifts off again, follows simple commands |
| 2 | Very sedated | Arouses to physical stimuli but does not communicate or follow commands; may move spontaneously |
| 1 | Unarousable | Minimal or no response to noxious stimuli; does not communicate or follow commands |
From Riker RR, Picard JT, Fraser GL: Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med 1999;27(7):1325-1329; used with permission.
Objective measures of cerebral function have been developed to quantify the level of consciousness as reflected by the cortical electrical activity based upon processed electroencephalographic (EEG) signals.67,116 Raw EEG signals are difficult to interpret, but proprietary mathematical algorithms have been developed and incorporated into commercially available devices to simplify interpretation of the power and frequency of the signal by converting this into a numerical display ranging from 0 (flat EEG) to 100 (fully alert). The Bispectral Index (BIS), Narcotrend Index (NI), Patient State Index (PSI), and State Entropy (SE) are examples of processed EEG monitors that have been used to assess level of consciousness in ICU patients.6 The addition of assessing the “evoked potential” to an auditory signal (i.e., auditory evoked potentials [AEPs])—displayed as the Auditory Evoked Response Index [AAI]) incorporates measurement of latency and amplitude of the evoked electrical potential.117 Collectively, these devices offer the advantage of continuous display of data, which can be used to rapidly detect unanticipated changes in level of consciousness, such as accidental cessation of propofol infusion.67 However, validation studies of these objective tools against sedation scales and other parameters has yielded inconsistent results.6,67,118 A particularly challenging problem has been filtering out electromyographic (EMG) signals generated by activity of muscles of the head that lie between the brain and the EEG electrodes.119 Additionally, demonstration of added value to justify widespread use of this expensive monitoring technology when compared to routine sedation scale monitoring has been lacking in RCTs of ICU patients. Accordingly, the use of objective measures of brain function to assess the depth of sedation in noncomatose adult ICU patients who are not receiving NMBAs is not recommended in the 2012 SCCM guidelines.6 There are unique clinical circumstances for which objective monitoring may be superior to routine clinical monitoring, including patients who are receiving NMBAs and the guidance of titration of anticonvulsant medications to achieve burst suppression.6
Sedative Drug Therapy
Sedative medications are widely used in the ICU setting and although various agents can be administered by other routes, most are given IV—either by continuous infusion or intermittent bolus. Traditionally, benzodiazepines have formed the cornerstone of ICU sedation,5 but recent clinical trial results and meta-analyses suggest that nonbenzodiazepines such as propofol and dexmedetomidine may be associated with better results such as less delirium and shorter ventilator time.6 Benzodiazepines activate γ-aminobutyric acid (GABA) A receptors, producing anxiolytic, amnestic, sedating, and hypnotic effects.6,120 Benzodiazepines cause respiratory depression and systemic hypotension—problems that are particularly prominent in the elderly. Although diazepam, midazolam, and lorazepam have been employed for ICU sedation over the years, diazepam is now used infrequently. Key pharmacokinetic and clinical features of midazolam and lorazepam are displayed in Table 19.7. Delayed offset of action is relatively common with these drugs for a variety of reasons including tissue saturation after prolonged administration, impaired metabolism due to hepatic dysfunction, and in the case of midazolam, accumulation of active renally cleared metabolites.6,120,121 Continuous infusion of lorazepam can lead to accumulation of the diluent vehicle, propylene glycol, causing toxicity that is characterized by metabolic acidosis and acute kidney injury, and accompanied by development of an increased osmolal gap.122,123
Table 19.7
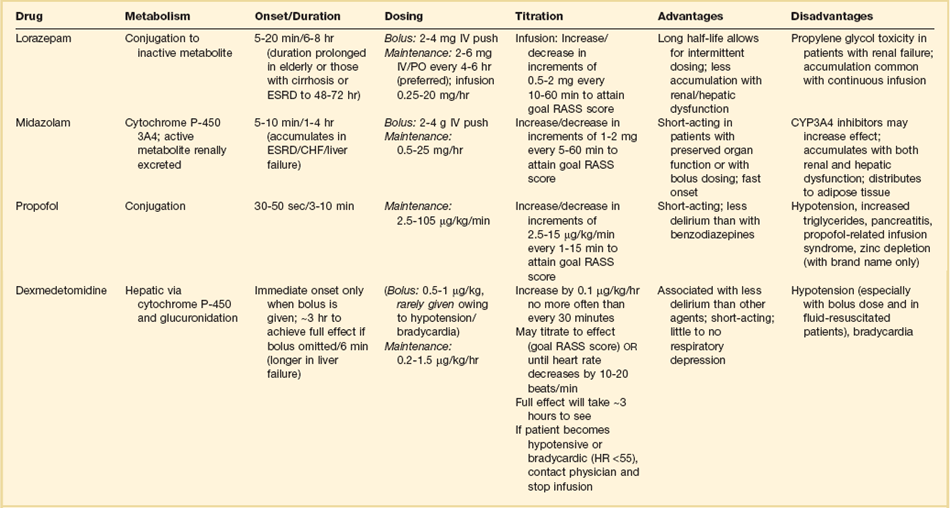
Propofol is perhaps the most widely utilized agent administered for continuous IV sedation in the United States.124 Propofol binds to GABA receptors as well as glycine, nicotinic, and muscarinic receptors producing effects similar to those from benzodiazepines.6,125 Like benzodiazepines, propofol has no analgesic effects. Propofol is highly lipid soluble, quickly crossing the blood-brain barrier and rapidly redistributing into peripheral tissues. These features result in rapid onset and offset of action (see Table 19.7). However, prolonged administration can result in tissue saturation that can ultimately produce delayed offset of action. Similar to benzodiazepines, propofol commonly causes respiratory depression as well as systemic hypotension. Because propofol is dissolved in a lipid emulsion, higher rates of propofol infusion can produce hypertriglyceridemia and acute pancreatitis. Each milliliter of propofol solution contains 1.1 kcal of nutrition, and thus an infusion rate of about 40 mL/hour results in more than 1000 kcal of nutrition in 24 hours. Propofol infusion syndrome (PRIS) is an uncommon complication that is typically associated with prolonged infusion of high-dose propofol (i.e., >70 µg/kg/minute), although cases are reported at lower infusion rates or briefer administration. PRIS is characterized by metabolic acidosis, hypotension, cardiac arrhythmias, hypertriglyceridemia, and less commonly with acute kidney injury, rhabdomyolysis, liver dysfunction, and hyperkalemia.6,126,127
Dexmedetomidine is a selective α2-receptor agonist with sedative properties similar to benzodiazepines and propofol, but has a number of unique features. In contrast to other sedatives, dexmedetomidine has analgesic properties and can be opioid-sparing; is sympatholytic, potentially producing prominent bradycardia as well as hypotension at higher doses; is associated with a more alert state relative to anxiolytic properties; and has minimal effect on respiratory drive.128,129 It does not bind GABA receptors. Selected pharmacokinetic and clinical characteristics are displayed in Table 19.7. The most common adverse effects are hypotension and bradycardia. Hypotension can be particularly prominent when a loading dose is used—although hypertension can also occur. Clinical trial results suggest that when compared directly to midazolam, dexmedetomidine may be associated with lower prevalence of delirium.130 The 2012 SCCM guidelines suggest that dexmedetomidine may be preferred over benzodiazepines in mechanically ventilated patients who have delirium and require continuous IV infusion of sedative medications.6
Management of Sedation
Sedative drug therapy is an important component of sedation and analgesia management as discussed earlier and outlined in Box 19.2. Careful selection and titration of sedative drugs are critical to achieve optimal patient comfort while avoiding the adverse impact of excessive or unnecessarily prolonged sedation. The use of structured approaches to sedation and analgesia via algorithms and protocols has achieved a prominent place in ICU practice. There are several common themes. One theme is to tailor the sedation to the specific patient, including medication selection based upon sedation goals (such as rapid arousal), avoidance of side effects (such as hypotension), and consideration of elimination issues (such as avoiding midazolam in a patient with impaired renal function). Although all of the sedative drugs listed in Table 19.7 have a role in ICU sedation management, emerging evidence appears to favor using propofol or dexmedetomidine in preference to benzodiazepines in patients for whom continuous infusion sedation is needed—as suggested in the 2012 SCCM guidelines.6 A recent meta-analysis limited to moderate or high-quality RCTs demonstrated slightly longer ICU LOS with benzodiazepines, in comparison to propofol or dexmedetomidine.6 In a 2008 meta-analysis of 16 RCTs that compared propofol to alternative agents for moderate to long duration sedation (i.e., >2 days), propofol was associated with shorter duration of mechanical ventilation and shorter ICU LOS.131 In a 2010 meta-analysis of 24 RCTs comparing dexmedetomidine to alternative agents, ICU LOS was shorter with dexmedetomidine and duration of mechanical ventilation was not significantly different.132 Two recent studies deserve additional comment. A multicenter RCT comparing dexmedetomidine to midazolam by Riker and colleagues demonstrated that dexmedetomidine was associated with shorter time to extubation, lower prevalence of delirium, and fewer infections but no difference in ICU LOS, mortality rate, or sedation quality. Dexmedetomidine was less potent than midazolam and was associated with more bradycardia but less hypertension.130 Jakob and coworkers recently reported two phase-3 multicenter RCTs that compared dexmedetomidine to midazolam and to propofol.133 Patients randomized to dexmedetomidine had shorter duration of mechanical ventilation than with midazolam, but not with propofol. Patient interaction (communication, arousability, cooperation) was better with dexmedetomidine than with either other agent, but hypotension and bradycardia were more common.
A second major theme is to focus first on analgesia, as discussed earlier. The 2012 SCCM guidelines suggest that analgesia-first sedation be used in mechanically ventilated adult ICU patients.6 Asking first about pain, before the patient has been sedated and cognitively impaired, is advantageous. Additionally, several RCTs have demonstrated analgesia-based sedation to be associated with shorter duration of mechanical ventilation in comparison to hypnotic-based sedation.134–136 Finally, Strom and associates randomized mechanically ventilated adult ICU patients to bolus morphine (analgesia-based, limited sedation) versus morphine plus propofol for the first 48 hours, then midazolam (sedation + analgesia), and found shorter duration of mechanical ventilation and ICU LOS but threefold more agitation when sedative drugs were minimized.16
A third major theme is to strive for a light level of consciousness and to avoid accumulation of medications and their active metabolites, thus reducing the likelihood of delayed awakening. This approach is strongly recommended in the 2012 SCCM guidelines,6 based in part on studies like those of Strom and associates.16 The underlying rationale for this approach arises from the observation by Kollef and colleagues that use of continuous IV sedation and analgesia is associated with delayed recovery from respiratory failure and longer LOS.13 Although a variety of sedation management strategies have been demonstrated to shorten the duration of mechanical ventilation, the most robust data support the use of a sedation protocol or the implementation of daily interruption of sedation.6,20 In an early RCT, Brook and coworkers found the use of a nurse-implemented protocol that emphasized intermittent therapy to be associated with shorter duration of mechanical ventilation, reduced ICU and hospital LOS, and fewer tracheostomies compared to usual management.14
The following year, Kress and colleagues showed in an RCT that daily interruption of IV sedation (DIS) until the patient was alert or agitated and then restarting the medications at 50% of the original dose was accompanied by more rapid recovery from respiratory failure, as well as performance of fewer tests for unexplained altered mental status.15 Interestingly, not all of the benefit could be attributable to reduced accumulation of medications or active metabolites because total propofol dosage was similar between groups although significantly less midazolam was used with daily interruption.15 This prompted speculation that the DIS might also permit earlier identification of sufficient respiratory reserve for ventilator weaning—“wake up and breathe.”137 Girard and colleagues subsequently demonstrated that the strategy of combining DIS with performance of a spontaneous breathing trial (SBT) led to faster recovery from respiratory failure as well as reduced all-cause 1-year mortality rate compared to SBT alone.138 Abrupt withdrawal of sedative (and sometimes analgesic) medications is accompanied by severalfold increases in circulating catecholamines as well as tachycardia and hypertension.46 Experts have expressed concern that this unmasked stress response might be associated with cardiac ischemia.139 In a prospective study, DIS was not accompanied by electrocardiographic or cardiac enzyme evidence of myocardial ischemia in at-risk patients.46 Questions have also been raised as to potential impact of this abrupt withdrawal of medications designed to provide comfort on downstream neuropsychological disturbances such as posttraumatic stress disorder (PTSD).139 An additional study by Kress and colleagues demonstrated no increase in subsequent PTSD and, actually, trends for better neuropsychological health months after daily interruption,140 perhaps as a result of the added opportunities to establish concrete memories during awakening.141 There are populations for whom complete cessation of IV sedatives and analgesics is probably unwise. Girard and colleagues incorporated a safety screen into their protocol and avoid performing DIS in patients (a) receiving sedative infusion for active seizures or alcohol withdrawal, (b) receiving escalating doses due to ongoing agitation, (c) receiving NMBA, (d) having evidence of myocardial ischemia within the past 24 hours, or (e) having elevated ICP.138 It may be that alcoholic patients are at particular risk for poor tolerance of daily interruption because patients randomized to this intervention had worse outcomes compared to a protocol approach in an RCT conducted in an ICU with a high prevalence of patients with alcohol use disorders.142
A variety of other structured approaches have been examined in prospective trials, although many have utilized a less robust two-phase approach. Protocols that utilize an evidence-based nurse-implemented algorithm that seeks to minimize medication doses has been linked to shorter duration of mechanical ventilation,143–148 shorter ICU and hospital LOS,144,147,148 lower direct drug costs,144,146 less opioid medication,148 less medication-induced coma,148 and lower 30-day mortality rate.148 These interventions appear to be less effective in Australian ICUs, perhaps as a result of higher nurse : patient ratios.149,150 Other algorithms apply active pharmacist input,151 or focus on pain and agitation.115 There are few multicenter RCTs that compare several approaches—more are needed. In a preliminary study,152 Mehta and associates found DIS to be equivalent to a sedation protocol, setting the stage for a larger Canadian RCT. Carson and colleagues found continuous infusion propofol with DIS to be superior to intermittent lorazepam.153
In summary, a variety of structured approaches to sedation management are effective for speeding recovery from respiratory failure and critical illness. Practical considerations for establishing a sedation protocol include multidisciplinary development, integrating published algorithms into the local environment, and sustaining a durable approach despite the challenges of insufficient time and resources.20 Finally, emphasis on protocolized light sedation brings additional opportunities such as linkage to SBTs138 and to early mobilization,154 but new challenges such as managing patient agitation16 and consequences such as self-extubation.138 The 2012 SCCM guidelines recommend using a multidisciplinary ICU team approach with provider education, preprinted protocols and order sets, and ICU rounds checklists to facilitate pain, agitation, and delirium management guidelines and protocols.6
Neuromuscular Blocking Agents
In contrast to analgesic and sedative medications, neuromuscular blocking agents (NMBAs) are administered relatively infrequently in the management of mechanically ventilated ICU patients. In an international study of mechanically ventilated patients, 13% of patients received NMBAs.155 However, up to 55% of patients with ARDS in modern clinical trials receive an NMBA at some point in their management,156 with even higher rates with interventions such as high-frequency oscillatory ventilation (HFOV).157 The most common indication for an NMBA is to facilitate mechanical ventilation in particularly challenging scenarios such as severe status asthmaticus or ARDS. NMBAs can enhance oxygenation, reduce the risk of barotrauma, and improve synchronization of the patient and ventilator.30,158 Additional data suggest that pulmonary and systemic inflammation may be reduced, although the mechanism(s) are not established.159 Indications for use of NMBAs include facilitation of mechanical ventilation, control of ICP, management of status epilepticus, reduction of oxygen consumption from muscle rigidity or shivering, the presence of an open surgical abdomen, prevention of lactic acidosis in conditions such as tetanus or neuroleptic malignant syndrome, and empirically in patients with severe ARDS (PaO2:FIO2 ratio < 120 mm Hg). Lacking robust RCTs, it is noteworthy that previously published guidelines assigned a grade of C (weak) for the recommendation to use an NMBA for these indications.30 Most use of NMBAs is driven by improving control of a particular problem such as patient-ventilator asynchrony or elevated ICP that persists despite use of less invasive measures.30,31,160 However, results of a 2010 multicenter RCT support the empiric administration of cisatracurium to all patients with fulminant ARDS (PaO2:FIO2 < 120 mm Hg) who do not have contraindications.32 The findings and limitations of this provocative study are discussed further.
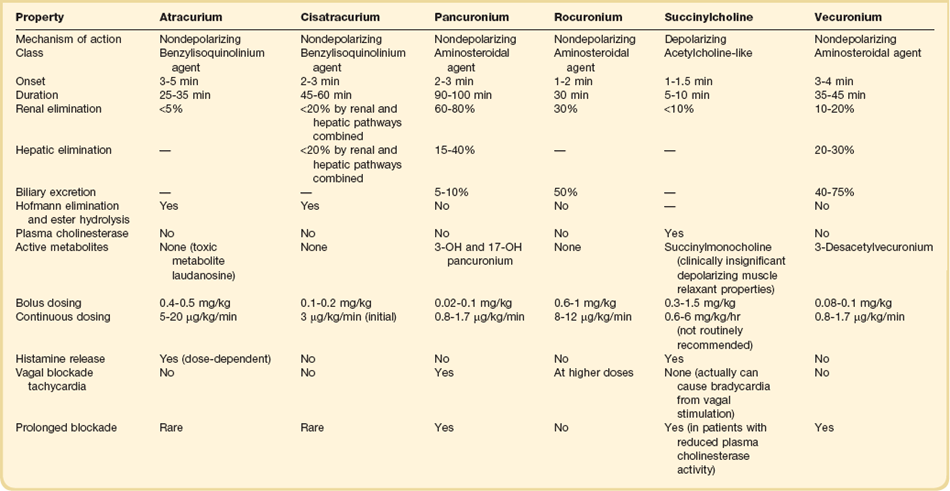
Comparison of Agents (Table 19.8)
The neuromuscular junction consists of a prejunctional motor nerve ending separated from a postjunctional membrane—or motor endplate—of skeletal muscle by a synaptic cleft. Acetylcholine (ACh) is released from vesicles in the prejunctional motor ending and bind nicotinic cholinergic receptors of the motor endplate, producing a change in membrane potential as a result of influx of sodium and efflux of potassium. Calcium is released from the sarcoplasmic reticulum, producing muscle action potential. Depolarizing NMBAs such as succinylcholine bind the receptors and cause the ion channel to remain open, producing depolarization and potentially causing sufficient potassium efflux to produce hyperkalemia. Additional depolarizations cannot occur until the agent diffuses from the receptors. Succinylcholine is the prototypical paralytic for rapid sequence intubation, due to its rapid onset and short duration of action.161 However, it can precipitate malignant hypertension and can cause hyperkalemia, which can lead to fatal arrhythmias.162 The cause of this adverse event is thought to be up-regulation of ACh receptors along the skeletal muscle membrane. Stimulation of these additional receptors by succinylcholine results in potassium efflux, leading to hyperkalemia. Hence, caution should be used when succinylcholine is considered for patients at risk for up-regulation of ACh receptors such as upper or lower motor denervation, chemical denervation (by muscle relaxants, drugs, or toxins), immobilization, infection, direct muscle trauma, muscle tumor, muscle inflammation, and burn injury. Furthermore, succinylcholine should be avoided in patients with renal dysfunction, as hyperkalemia is potentiated in this population. Additionally, succinylcholine has been associated with increases in intracranial and intraocular pressure and should be used with caution in patients with acute ocular or traumatic brain injury.163
The nondepolarizing agents are bulky molecules with ACh-like moieties that bind the ACh receptors without ion flow or depolarization. Broadly, the nondepolarizing NMBAs are divided into two structural classes, aminosteroidal and benzylisoquinolinium agents. The aminosteroidal NMBAs incorporate an ACh-like structure onto a steroid backbone.164 Much like other steroidal drugs, elimination of the aminosteroidal agents is organ dependent. Pancuronium, the first aminosteroidal NMBA, antagonizes not only nicotinic ACh receptors, but also muscarinic receptors.165 This results in vagal blockade and tachycardia, making it less ideal for patients with significant cardiac disease.30 Compared to other aminosteroidal NMBAs, pancuronium has a long half-life, which allows for sustained neuromuscular blockade to be achieved with intermittent bolus dosing.30 Because of the long half-life, organ-dependent metabolism, and production of active metabolites, pancuronium has a high propensity to accumulate in critically ill patients. Newer aminosteroidal NMBAs, such as vecuronium and rocuronium, offer advantages over pancuronium as they do not antagonize muscarinic receptors. Vecuronium is an intermediate-acting aminosteroidal NMBA with both renal and hepatic elimination. It offers utility both for procedural and sustained paralysis, but like pancuronium, can accumulate in patients with organ dysfunction. Rocuronium has the fastest onset and shortest duration of action of the aminosteroidal NMBAs, making it ideal for rapid sequence intubation in circumstances when succinylcholine is contraindicated. Like all aminosteroidal NMBAs, it exhibits organ dependent clearance; however, when used intermittently for procedural paralysis, this is rarely of clinical significance.
In contrast to the aminosteroidal NMBAs, benzylisoquinolinium agents are nonsteroidal agents that are degraded by plasma esterase and Hofmann degradation.164,165 Atracurium was the first benzylisoquinolinium NMBA and was advantageous in that it was an intermediate-acting paralytic with organ-independent clearance. However, atracurium causes significant histamine release and thus must be used with caution in patients with hypotension or shock. Additionally, patients with organ dysfunction are at increased risk of seizures when atracurium is used for sustained paralysis due to accumulation of a toxic metabolite, laudanosine.31 Cisatracurium is the cis-cis isomer of atracurium and represents approximately 15% of the atracurium conformation.164 Like atracurium, cisatracurium undergoes clearance via plasma esterase and Hofmann degradation but is devoid of histamine release and toxic metabolites.
Monitoring
Monitoring of the patient during chemical paralysis is performed to ensure safety and effectiveness of the intervention. Goals of monitoring include achieving the targeted depth of sedation to accomplish therapeutic goals while reducing the likelihood of undesired prolonged paralysis or acute quadriplegic myopathy (AQM) and also avoiding other potential complications. Different targets for NMBAs may vary from merely reduced spontaneous movement or better synchronization of spontaneous breathing with mechanical ventilation to apnea for fully passive ventilation or complete cessation of all skeletal muscle activity. Accordingly, observation of limb and respiratory muscle activity as well as review of graphic display of ventilator data should be performed to confirm the therapeutic target is reached. Testing of deep tendon reflexes may be helpful as they disappear as 100% blockade is reached. Monitoring the depth and duration of paralysis is important in order to optimize neuromuscular blockade for each individual patient and to avoid complications of therapy.30,31,160,166 Such monitoring typically includes clinical evaluation as well as an objective measure of the extent of blockade using peripheral nerve stimulation (PNS) testing by evaluating the response to “train-of-four” (TOF) stimulation. In TOF testing, four equal brief electrical charges are delivered every 0.5 second from a nerve stimulator device that is attached to leads placed on the skin overlying a superficial nerve that innervates an easily observed muscle. The most common placement is over the ulnar nerve with observation of contraction of the adductor pollicis muscle or over the facial nerve with observation of contraction of the orbicularis oculi muscle. It is estimated that if less than 75% of receptors are blocked, the four muscular contractions are equal in intensity and that once more than 90% of receptors are blocked no contractions occur. A common safety goal is for titration of the NMBA so that one to three muscle contractions are present, thus potentially avoiding excessive dosing. It is worth considering, however, that clinical effectiveness (such as patient-ventilator synchrony) is sometimes achieved while TOF reveals all four contractions. In contrast, in order to achieve apnea or complete cessation of muscular activity, TOF of zero may be necessary. In this circumstance, periodically decreasing the NMBA infusion rate until TOF of one or more may be necessary to avoid excessive dosing.
Despite the intuitive value of monitoring the depth of NMBA dosing, clinical trials that compare PNS monitoring to clinical monitoring alone have not consistently demonstrated added value. In one RCT, Rudis and coworkers demonstrated more rapid recovery to four of four TOF contractions and to spontaneous breathing as well as fewer episodes of delayed recovery when vecuronium infusion was titrated to achieve one of four TOF contractions in comparison to patients with clinical monitoring alone.167 Patients with impaired renal function had the slowest recovery. In contrast, PNS monitoring did not lead to better outcomes in prospective trials of patients who received atracurium168 or cisatracurium.169 Further, intentional dosing of cisatracurium to achieve TOF of zero only marginally prolonged the recovery time.170 Finally, no PNS monitoring was performed in the 2010 placebo-controlled RCT in which 178 patients received a 48-hour cisatracurium infusion and there was no difference in the rate of ICU paresis compared to the placebo group.32 Unfortunately, maintenance of TOF more than zero does not guarantee AQM will not occur. Most reported cases developed despite documented appropriate TOF results. Accordingly, although the 2002 SCCM guidelines recommended that all patients receiving NMBA should be assessed both clinically and by TOF monitoring (grade B recommendation) with a goal of adjusting the degree of NMBA to achieve 1 to 2 twitches (grade C recommendation), the validity of this recommendation is most compelling when vecuronium infusion is used but less so for atracurium or cisatracurium given subsequent published reports.30 Monitoring of patients who are receiving NMBAs should also address prevention and early detection of venous thromboembolism (VTE), pressure ulcers, corneal ulcers, patient awareness, and inadequately controlled pain and anxiety.
Complications
The primary safety concerns with NMBAs are for prolonged paralysis due to persistent drug effect or from AQM. Both circumstances result in significant weakness and are considerations in the broad differential diagnosis of weakness in the ICU patient. Causes of weakness range widely from severe electrolyte disturbances like hypermagnesemia or hypophosphatemia to critical illness polyneuropathy, steroid myopathy, or Guillain-Barré syndrome. A variety of factors can increase the risk for prolonged recovery from NMBAs, including organ dysfunction and resulting impaired clearance of the parent compound, accumulation of active metabolites, drug-drug interactions, and other medical conditions. Drugs that potentiate or antagonize the actions of nondepolarizing NMBAs are listed in Box 19.4. There is also considerable variability in duration of effect among NMBAs. The aminosteroids are eliminated by a combination of renal clearance and hepatic metabolism to a number of compounds, some of which are active and are eliminated in the urine. Accordingly, dysfunction of liver or kidneys is associated with prolonged paralysis.31,171 Resolution of paralysis is not predictable with vecuronium, particularly with prolonged infusion.171,172 This contrasts sharply with the benzylisoquinoliniums with recovery times of about 1 hour, even despite prolonged infusion169 and with higher doses.170 Perhaps most instructive is a prospective head-to-head comparison of vecuronium and cisatracurium in ICU patients in which average recovery time was fivefold longer with vecuronium.173 Close monitoring of depth of sedation using TOF PNS appears to reduce the incidence of prolonged paralysis with vecuronium167 but is of less certain value with atracurium or cisatracurium.168,169
AQM is a potentially devastating form of NMBA-related weakness in which the muscle weakness can be severe and quite protracted.30 AQM is characterized by weakness of the limb and trunk muscles with sparing of the extraocular muscles as well as preserved sensory function and cognition. Understanding of the mechanisms underlying AQM have evolved from suspicion that myonecrosis alone was responsible to that of altered muscle membrane properties with loss of motor protein myosin and myosin-associated thick filament proteins. Work by Larsson and associates has demonstrated that a combination of myofibrillar protein degradation plus down-regulation of protein synthesis at the transcriptional level is prominent.174 Key predisposing factors for ICU-acquired weakness appear to be functional denervation—such as from NMBAs and corticosteroids. The argument has been made that prolonged deep sedation may be another source of immobilization.33 Additional risk factors for ICU-acquired polyneuropathy and myopathy include sepsis and other forms of systemic inflammation, hyperglycemia, malnutrition, and prolonged immobility. Although both aminosteroid and benzylisoquinolinium agents have been linked to AQM in case reports, it is noteworthy that in the relatively limited prospective clinical trial literature of NMBA in ICU patients, AQM is described in about 5% of patients who received vecuronium but none of the more than 200 patients who were administered atracurium or cisatracurium infusion.169 Further, in the largest multicenter RCT of cisatracurium versus placebo, there was no difference in the incidence of ICU-acquired weakness.32 Finally, some experts consider the causality of NMBA—particularly short-duration NMBA—for producing ICU-acquired weakness to be far from certain.33
Evolving Role in Acute Respiratory Distress Syndrome
NMBAs have been utilized for years to optimize gas exchange in patients with severe ARDS. Numerous RCTs demonstrate and other observational studies indicate that as many as one half of ARDS patients receive at least intermittent NMBA, with the most common indication being refractory hypoxemia. Sustained increases in PaO2:FIO2 ratio have been demonstrated in small RCTs.158,159 Interestingly, preliminary studies also have demonstrated lower levels of proinflammatory cytokines in bronchoalveolar lavage fluid and circulating blood in patients randomized to cisatracurium, in contrast to placebo.159 In 2010, Papazian and coworkers published the results of a French multicenter RCT in which 340 patients with severe ARDS (PaO2:FIO2 < 150 mm Hg) were randomized to receive a 48-hour infusion of cisatracurium or placebo.32 Patients randomized to cisatracurium had better outcomes including lower rate of death at 28 days and trends for lower rates of death in the ICU and the hospital, more ventilator-free days and organ failure–free days, and fewer barotraumas. Post hoc analysis showed that the mortality rate benefit was limited entirely to the patients with most profound hypoxemia (PaO2:FIO2 < 120 mm Hg), although the relationship of other key outcomes to baseline oxygenation was not reported. Impressively, there was no difference in rates of ICU paresis, and limb weakness was identical for cisatracurium and placebo groups. A number of methodologic issues are worth noting, including no use of PNS for monitoring, use of very deep sedation (Ramsay Sedation Score = 6), use of ketamine for sedation in 20% of patients, open label use of cisatracurium in 56% of placebo patients, the use of very modest PEEP despite severe hypoxemia, and the high barotrauma for placebo patients. Clinicians are eager for additional RCTs to confirm the findings.
References
1. Puntillo, KA, Arai, S, Cohen, NH, et al. Symptoms experienced by intensive care unit patients at high risk of dying. Crit Care Med. 2010; 38:2155–2160.
2. Desbiens, NA, Wu, AW, Broste, SK, et al. Pain and satisfaction with pain control in seriously ill hospitalized adults: Findings from the SUPPORT research investigations. Crit Care Med. 1996; 24(12):1953–1961.
3. Puntillo, KA. Pain experiences of intensive care unit patients. Heart Lung. 1990; 19(5 Pt 1):526–533.
4. Novaes, MA, Knobel, E, Bork, AM, et al. Stressors in ICU: Perception of the patient, relatives and health care team. Intensive Care Med. 1999; 25(12):1421–1426.
5. Jacobi, J, Fraser, GL, Coursin, DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30(1):119–141.
6. Barr, J, Fraser, GL, Puntillo, K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41(1):263–306.
7. Sessler, CN. Comfort and distress in the ICU: Scope of the problem. Sem Respir Crit Care Med. 2001; 22(2):111–113.
8. Sessler, CN. Progress toward eliminating inadequately managed pain in the ICU through interdisciplinary care. Chest. 2009; 135(4):894–896.
9. Erstad, BL, Puntillo, K, Gilbert, HC, et al. Pain management principles in the critically ill. Chest. 2009; 135(4):1075–1086.
10. Arroliga, A, Frutos-Vivar, F, Hall, J, et al. Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest. 2005; 128(2):496–506.
11. Payen, JF, Chanques, G, Mantz, J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: A prospective multicenter patient-based study. Anesthesiology. 2007; 106(4):687–695.
12. Mehta, S, McCullagh, I, Burry, L. Current sedation practices: Lessons learned from international surveys. Anesthesiol Clin. 2011; 29(4):607–624.
13. Kollef, MH, Levy, NT, Ahrens, TS, et al. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest. 1998; 114(2):541–548.
14. Brook, AD, Ahrens, TS, Schaiff, R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999; 27(12):2609–2615.
15. Kress, JP, Pohlman, AS, O’Connor, MF, Hall, JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342(20):1471–1477.
16. Strom, T, Martinussen, T, Toft, P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010; 375(9713):475–480.
17. Fraser, GL, Riker, RR. Comfort without coma: Changing sedation practices. Crit Care Med. 2007; 35(2):635–637.
18. Martin, J, Heymann, A, Basell, K, et al. Evidence and consensus-based German guidelines for the management of analgesia, sedation and delirium in intensive care—short version. Ger Med Sci. 2010; 8:Doc02.
19. Sessler, CN, Varney, K. Patient-focused sedation and analgesia in the ICU. Chest. 2008; 133(2):552–565.
20. Sessler, CN, Pedram, S. Protocolized and target-based sedation and analgesia in the ICU. Crit Care Clin. 2009; 25(3):489–513.
21. Riker, RR, Fraser, GL. Altering intensive care sedation paradigms to improve patient outcomes. Crit Care Clin. 2009; 25(3):527–538.
22. Honiden, S, Siegel, MD. Analytic reviews: Managing the agitated patient in the ICU: Sedation, analgesia, and neuromuscular blockade. J Intensive Care Med. 2010; 25(4):187–204.
23. Patel, SB, Kress, JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012; 185(5):486–497.
24. Ely, EW, Shintani, A, Truman, B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291(14):1753–1762.
25. Girard, TD, Jackson, JC, Pandharipande, PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010; 38(7):1513–1520.
26. Morandi, A, Brummel, NE, Ely, EW. Sedation, delirium and mechanical ventilation: The “ABCDE” approach. Curr Opin Crit Care. 2011; 17(1):43–49.
27. Gunther, ML, Morandi, A, Ely, EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin. 2008; 24(1):45–65.
28. Bledowski, J, Trutia, A. A review of pharmacologic management and prevention strategies for delirium in the intensive care unit. Psychosomatics. 2012; 53(3):203–211.
29. Jones, SF, Pisani, MA. ICU delirium: An update. Curr Opin Crit Care. 2012; 18(2):146–151.
30. Murray, MJ, Cowen, J, DeBlock, H, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002; 30(1):142–156.
31. Warr, J, Thiboutot, Z, Rose, L, et al. Current therapeutic uses, pharmacology, and clinical considerations of neuromuscular blocking agents for critically ill adults. Ann Pharmacother. 2011; 45(9):1116–1126.
32. Papazian, L, Forel, JM, Gacouin, A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363(12):1107–1116.
33. Puthucheary, Z, Rawal, J, Ratnayake, G, et al. Neuromuscular blockade and skeletal muscle weakness in critically ill patients: Time to rethink the evidence? Am J Respir Crit Care Med. 2012; 185(9):911–917.
34. Wunsch, H, Kahn, JM, Kramer, AA, Rubenfeld, GD. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009; 37(12):3031–3039.
35. Sessler, CN, Grap, MJ, Brophy, GM. Multidisciplinary management of sedation and analgesia in critical care. Semin Respir Crit Care Med. 2001; 22(2):211–226.
36. Jaber, S, Chanques, G, Altairac, C, et al. A prospective study of agitation in a medical-surgical ICU: Incidence, risk factors, and outcomes. Chest. 2005; 128(4):2749–2757.
37. Woods, JC, Mion, LC, Connor, JT, et al. Severe agitation among ventilated medical intensive care unit patients: Frequency, characteristics and outcomes. Intensive Care Med. 2004; 30(6):1066–1072.
38. Fraser, GL, Prato, BS, Riker, RR, et al. Frequency, severity, and treatment of agitation in young versus elderly patients in the ICU. Pharmacotherapy. 2000; 20(1):75–82.
39. Sessler, CN, Glass, C, Grap, MJ. Unplanned extubation: Incidence, predisposing factors, and management. J Crit Illness. 1994; 9:609–619.
40. Carrion, MI, Ayuso, D, Marcos, M, et al. Accidental removal of endotracheal and nasogastric tubes and intravascular catheters. Crit Care Med. 2000; 28(1):63–66.
41. Mion, LC, Minnick, AF, Leipziq, R, et al. Patient-initiated device removal in intensive care units: A national prevalence study. Crit Care Med. 2007; 35(12):2714–2720.
42. Fraser, GL, Riker, RR, Prato, BS, Wilkins, ML. The frequency and cost of patient-initiated device removal in the ICU. Pharmacotherapy. 2001; 21(1):1–6.
43. Sessler, CN. Sedation scales in the ICU. Chest. 2004; 126:1727–1730.
44. Kong, KL, Willatts, SM, Prys-Roberts, C, et al. Plasma catecholamine concentration during sedation in ventilated patients requiring intensive therapy. Intensive Care Med. 1990; 16:171–174.
45. Lindenmayer, JP. The pathophysiology of agitation. J Clin Psychiatry. 2000; 61(Suppl 14):5–10.
46. Kress, JP, Vinayak, AG, Levitt, J, et al. Daily sedative interruption in mechanically ventilated patients at risk for coronary artery disease. Crit Care Med. 2007; 35(2):365–371.
47. Korak-Leiter, M, Likar, R, Oher, M, et al. Withdrawal following sufentanil/propofol and sufentanil/midazolam. Sedation in surgical ICU patients: Correlation with central nervous parameters and endogenous opioids. Intensive Care Med. 2005; 31(3):380–387.
48. Epstein, J, Breslow, MJ. The stress response of critical illness. Crit Care Clin. 1999; 15(1):17–33.
49. Schein, RM, Hazday, N, Pena, M, et al. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990; 98(6):1388–1392.
50. de Wit, M, Jones, DG, Sessler, CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010; 138(4):994–1003.
51. Li, SY, Wang, TJ, Vivienne Wu, SF, et al. Efficacy of controlling night-time noise and activities to improve patients’ sleep quality in a surgical intensive care unit. J Clin Nurs. 2011; 20(3-4):396–407.
52. Xie, H, Kang, J, Mills, GH. Clinical review: The impact of noise on patients’ sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care. 2009; 13(2):208.
53. van de Leur, JPO, van der Schans, CP, Loef, BG, et al. Discomfort and factual recollection in intensive care unit patients. Crit Care. 2004; 8(6):R467–R473.
54. Puntillo, KA, Morris, AB, Thompson, CL, et al. Pain behaviors observed during six common procedures: Results from Thunder Project II. Crit Care Med. 2004; 32(2):421–427.
55. Mularski, RA, Sessler, CN, Schmidt, GA. Pain management in the intensive care unit. In: Rathmell J, Ballantye J, Fishman S, eds. Bonica’s Management of Pain. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:1587–1602.
56. Willis, WD, Westlund, KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997; 14(1):2–31.
57. Puntillo, KA, Wild, LR, Morris, AB, et al. Practices and predictors of analgesic interventions for adults undergoing painful procedures. Am J Crit Care. 2002; 11(5):415–429.
58. Puntillo, K, Pasero, C, Li, D, et al. Evaluation of pain in ICU patients. Chest. 2009; 135(4):1069–1074.
59. Jensen, MP, Karoly, P, Braver, S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986; 27(1):117–126.
60. Chanques, G, Viel, E, Constantin, JM, et al. The measurement of pain in intensive care unit: Comparison of 5 self-report intensity scales. Pain. 2010; 151(3):711–721.
61. Payen, JF, Bosson, JL, Chanques, G, et al. and DOLOREA Investigators: Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: A post hoc analysis of the DOLOREA study. Anesthesiology. 2009; 111(6):1308–1316.
62. Puntillo, KA, Miaskowski, C, Kehrle, K, et al. Relationship between behavioral and physiological indicators of pain, critical care patients’ self-reports of pain, and opioid administration. Crit Care Med. 1997; 25(7):1159–1166.
63. Herr, K, Coyne, PJ, Key, T, et al. Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Manage Nurs. 2006; 7(2):44–52.
64. Ambuel, B, Hamlett, KW, Marx, CM, Blumer, JL. Assessing distress in pediatric intensive care environments: The COMFORT scale. J Pediatr Psychol. 1992; 17(1):95–109.
65. Merkel, SI, Voepel-Lewis, T, Shayevitz, JR, Malviya, S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997; 23(3):293–297.
66. Li, D, Puntillo, K, Miaskowski, C. A review of objective pain measures for use with critical care adult patients unable to self-report. J Pain. 2008; 9(1):2–10.
67. Sessler, CN, Grap, MJ, Ramsay, MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008; 12(Suppl 3):S2.
68. Gelinas, C, Fillion, L, Puntillo, KA, et al. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006; 15(4):420–427.
69. Payen, JF, Bru, O, Bosson, JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001; 29(12):2258–2263.
70. Chanques, G, Payen, JF, Mercier, G, et al. Assessing pain in non-intubated critically ill patients unable to self report: An adaptation of the behavioral pain scale. Intensive Care Med. 2009; 35(12):2060–2067.
71. Odhner, M, Wegman, D, Freeland, N, et al. Assessing pain control in nonverbal critically ill adults. Dimens Crit Care Nurs. 2003; 22(6):260–267.
72. Li, D, Miaskowki, C, Burkhardt, D, Puntillo, K. Evaluations of physiologic reactivity and reflexive behaviors during noxious procedures in sedated critically ill patients. J Crit Care. 2009; 24(3):472.
73. Puntillo, KA, Stannard, D, Miaskowski, C, et al. Use of a pain assessment and intervention notation (P. A. I. N. ) tool in critical care nursing practice: Nurses’ evaluations. Heart Lung. 2002; 31(4):303–314.
74. Gelinas, C, Johnston, C. Pain assessment in the critically ill ventilated adult: Validation of the critical-care pain observation tool and physiologic indicators. Clin J Pain. 2007; 23(6):497–505.
75. Desbiens, NA, Mueller-Rizner, N. How well do surrogates assess the pain of seriously ill patients? Crit Care Med. 2000; 28(5):1347–1352.
76. Bion, JF, Logan, BK, Newman, PM, et al. Sedation in intensive care: Morphine and renal function. Intensive Care Med. 1986; 12(5):359–365.
77. Wilhelm, W, Kreuer, S. The place for short-acting opioids: Special emphasis on remifentanil. Crit Care. 2008; 12(Suppl 3):S5.
78. Battershill, AJ, Keating, GM. Remifentanil: A review of its analgesic and sedative use in the intensive care unit. Drugs. 2006; 66(3):365–385.
79. Hagmeyer, KO, Mauro, LS, Mauro, VF. Meperidine-related seizures associated with patient-controlled analgesia pumps. Ann Pharmacother. 1993; 27(1):29–32.
80. Riker, RR, Fraser, GL. Adverse events associated with sedatives, analgesics, and other drugs that provide patient comfort in the intensive care unit. Pharmacotherapy. 2005; 25(5 Pt 2):8S–18S.
81. Ehret, GB, Voide, C, Gex-Fabry, M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone—High frequency in hospitalized patients and risk factors. Arch Intern Med. 2006; 166(12):1280–1287.
82. Pandey, CK, Bose, N, Garg, G, et al. Gabapentin for the treatment of pain in Guillain-Barré syndrome: A double-blinded, placebo-controlled crossover study. Anesth Analg. 2002; 96(6):1719–1723.
83. Pandey, CK, Raza, M, Tripathi, M, et al. The comparative evaluation of gabapentin and carbamazepine for pain management in Guillain-Barré syndrome patients in the intensive care unit. Anesth Analg. 2005; 101(1):220–225.
84. Eisenberg, E, Peterson, D. Neuropathic pain pharmacotherapy. In: Rathmell J, Ballantye J, Fishman S, eds. Bonica’s Management of Pain. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:1194–1207.
85. De Oliveira, GS, Jr., Agarwal, D, Benzon, HT. Perioperative single dose ketorolac to prevent postoperative pain: A meta-analysis of randomized trials. Anesth Analg. 2012; 114(2):424–433.
86. Maund, E, McDaid, C, Rice, S, et al. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: A systematic review. Br J Anaesth. 2011; 106(3):292–297.
87. De Oliveira, GS, Jr., Almeida, MD, Benzon, HT, McCarthy, RJ. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology. 2011; 115(3):575–588.
88. Laskowski, K, Stirling, A, McKay, WP, Lim, HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesthesia. 2011; 58(10):911–923.
89. Memis, D, Inal, MT, Kavalci, G, et al. Intravenous acetaminophen reduced the use of opioids compared with oral administration after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2005; 19:306–309.
90. Maddali, MM, Kurian, E, Fahr, J. Extubation time, hemodynamic stability, and postoperative pain control in patients undergoing coronary artery bypass surgery: An evaluation of fentanyl, remifentanil, and nonsteroidal anti-inflammatory drugs with propofol for perioperative and postoperative management. J Clin Anesth. 2006; 18:605–610.
91. Guillou, N, Tanguy, M, Seguin, P, et al. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003; 97:843–847.
92. Nishimori, M, Ballantyne, JC, Low, JH. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. (3):2006.
93. Block, BM, Liu, SS, Rowlingson, AJ, et al. Efficacy of postoperative epidural analgesia: A meta-analysis. JAMA. 2003; 290:2455–2463.
94. Bulger, EM, Edwards, T, Klotz, P, et al. Epidrual analgesia improves outcome after multiple rib fractures. Surgery. 2004; 136(2):426–430.
95. Carrier, FM, Turgeon, AF, Nicole, PC, et al. Effect of epidural analgesia in patients with traumatic rib fractures: A systemic review and meta-analysis of randomized controlled trials. Can J Anaesth. 2009; 56:230–242.
96. Cepeda, MS, Carr, DB, Lau, J, Alvarez, H. Music for pain relief. Cochrane Database Syst Rev. (2):2006.
97. Kwekkeboom, KL, Gretarsdottir, E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006; 38(3):269–277.
98. Suls, J, Wan, CK. Effects of sensory and procedural information on coping with stressful medical procedures and pain: A meta-analysis. J Consult Clin Psychol. 1989; 57(3):372–379.
99. Riker, RR, Fraser, GL. Monitoring sedation, agitation, analgesia, neuromuscular blockade, and delirium in adult ICU patients. Sem Respir Crit Care Med. 2001; 22:189–198.
100. Ramsay, MA, Savege, TM, Simpson, BR, Goodwin, R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974; 2:656–659.
101. Olleveant, N, Humphris, G, Roe, B. A reliability study of the modified new Sheffield Sedation Scale. Nurs Crit Care. 1998; 3(2):83–88.
102. Chernik, DA, Gillings, D, Laine, H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J Clin Psychopharmacol. 1990; 10(4):244–251.
103. Binnekade, JM, Vroom, MB, de Vos, R, et al. The reliability and validity of a new and simple method to measure sedation levels in intensive care patients: A pilot study. Heart Lung. 2006; 35:137–143.
104. Devlin, JW, Boleski, G, Mlynarek, M, et al. Motor Activity Assessment Scale: A valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999; 27(7):1271–1275.
105. De Jonghe, B, Cook, D, Griffith, L, et al. Adaptation to the Intensive Care Environment (ATICE): Development and validation of a new sedation assessment instrument. Crit Care Med. 2003; 31(9):2344–2354.
106. Weinert, C, McFarland, L. The state of intubated ICU patients: Development of a two-dimensional sedation rating scale for critically ill adults. Chest. 2004; 126(6):1883–1890.
107. De Lemos, J, Tweeddale, M, Chittock, D. Measuring quality of sedation in adult mechanically ventilated critically ill patients: The Vancouver interaction and calmness scale. J Clin Epidemiol. 2000; 53:908–919.
108. Riker, RR, Picard, JT, Fraser, GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999; 27(7):1325–1329.
109. Sessler, CN, Gosnell, MS, Grap, MJ, et al. The Richmond Agitation Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–1344.
110. Ely, EW, Truman, B, Shintani, A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003; 289(22):2983–2991.
111. Pun, BT, Gordon, SM, Peterson, JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: A report from two medical centers. Crit Care Med. 2005; 33(6):1199–1205.
112. Brandl, KM, Langley, KA, Riker, RR, et al. Confirming the reliability of the sedation-agitation scale administered by ICU nurses without experience in its use. Pharmacotherapy. 2001; 21(4):431–436.
113. Costa, J, Cabre, L, Molina, R, Carrasco, G. Cost of ICU sedation: Comparison of empirical and controlled sedation methods. Clin Intensive Care. 1994; 5(Suppl):17–21.
114. Botha, JA, Mudholkar, P. The effect of a sedation scale on ventilation hours, sedative, analgesic and inotropic use in an intensive care unit. Crit Care Resuscitation. 2004; 6:253–257.
115. Chanques, G, Jaber, S, Barbotte, E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006; 34(6):1691–1699.
116. Friedman, D, Claassen, J, Hirsch, LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009; 109:506–523.
117. Lu, CH, Man, KM, Ou-Yang, HY, et al. Composite auditory evoked potential index versus bispectral index to estimate the level of sedation in paralyzed critically ill patients: A prospective observational study. Anesth Analg. 2008; 107:1290–1294.
118. LeBlanc, JM, Dasta, JF, Kane-Gill, SL. Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother. 2006; 40:490–500.
119. Vivien, B, DiMaria, S, Ouattara, A, et al. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003; 99:9–17.
120. Young, CC, Prielipp, RC. Benzodiazepines in the intensive care unit. Crit Care Clin. 2001; 17:843.
121. Bauer, TM, Ritz, R, Haberthur, C, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet. 1995; 346(8968):145–147.
122. Yaucher, NE, Fish, JT, Smith, HW, Wells, JA. Propylene glycol-associated renal toxicity from lorazepam infusion. Pharmacotherapy. 2003; 23(9):1094–1099.
123. Barnes, BJ, Gerst, C, Smith, JR, et al. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy. 2006; 26(1):23–33.
124. Wunsch, H, Kahn, JM, Kramer, AA, Rubenfeld, GD. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009; 37(12):3031–3039.
125. Marik, PE. Propofol: Therapeutic indications and side-effects. Curr Pharm Design. 2004; 10(29):3639–3649.
126. Fong, JJ, Sylvia, L, Ruthazer, R, et al. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008; 36(8):2281–2287.
127. Diedrich, DA, Brown, DR. Analytic reviews: Propofol infusion syndrome in the ICU. J Intensive Care Med. 2011; 26(2):59–72.
128. Bhana, N, Goa, KL, McClellan, KJ. Dexmedetomidine. Drugs. 2000; 59(2):263–268.
129. Gerlach, AT, Murphy, CV, Dasta, JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009; 43(12):2064–2074.
130. Riker, RR, Shehabi, Y, Bokesch, PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009; 301(5):489–499.
131. Ho, KM, JY, Ng. The use of propofol for medium and long-term sedation in critically ill adult patients: A meta-analysis. Intensive Care Med. 2008; 34(11):1969–1979.
132. Tan, JA, Ho, KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis. Intensive Care Med. 2010; 36(6):926–939.
133. Jakob, SM, Ruokonen, E, Grounds, RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012; 307(11):1151–1160.
134. Breen, D, Karabinis, A, Malbrain, M, et al. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: A randomised trial. Crit Care. 2005; 9(3):R200–210.
135. Park, G, Lane, M, Rogers, S, Bassett, P. A comparison of hypnotic and analgesic based sedation in a general intensive care unit. Br J Anaesth. 2007; 98(1):76.
136. Rozendaal, FW, Spronk, PE, Snellen, FF, et al. Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: A centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med. 2009; 35(2):291–298.
137. Sessler, CN. Wake up and breathe. Crit Care Med. 2004; 32:1413–1414.
138. Girard, TD, Kress, JP, Fuchs, BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008; 371(9607):126–134.
139. Heffner, JE. A wake-up call in the intensive care unit. N Engl J Med. 2000; 342(20):1520–1522.
140. Kress, JP, Gehlbach, B, Lacy, M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003; 168(12):1457–1461.
141. Jones, C, Griffiths, RD, Humphris, G, Skirrow, PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001; 29(3):573–580.
142. de Wit, M, Gennings, C, Jenvey, WI, Epstein, SK. Randomized trial comparing daily interruption of sedation and nursing-implemented sedation algorithm in medical intensive care unit patients. Crit Care. 2008; 12(3):R70.
143. De Jonghe, B, Bastuji-Garin, S, Fangio, P, et al. Sedation algorithm in critically ill patients without acute brain injury. Crit Care Med. 2005; 33(1):120–127.
144. Mascia, MF, Koch, M, Medicis, JJ. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000; 28(7):2300–2306.
145. Brattebo, G, Hofoss, D, Flaatten, H, et al. Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. Qual Safety Health Care. 2004; 13(3):203–205.
146. MacLaren, R, Plamondon, JM, Ramsay, KB, et al. A prospective evaluation of empiric versus protocol-based sedation and analgesia. Pharmacotherapy. 2000; 20(6):662–672.
147. Quenot, JP, Ladoire, S, Devoucoux, F, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007; 35(9):2031–2036.
148. Skrobik, Y, Ahern, S, Leblanc, M, et al. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010; 111(2):451–463.
149. Bucknall, TK, Manias, E, Presneill, JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med. 2008; 36(5):1444–1450.
150. Elliott, R, McKinley, S, Aitken, LM, Hendrikz, J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive Care Med. 2006; 32(10):1506–1514.
151. Marshall, J, Finn, CA, Theodore, AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008; 36(2):427–433.
152. Mehta, S, Burry, L, Martinez-Motta, JC, et al. A randomized trial of daily awakening in critically ill patients managed with a sedation protocol: A pilot trial. Crit Care Med. 2008; 36(7):2092–2099.
153. Carson, SS, Kress, JP, Rodgers, JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006; 34(5):1326–1332.
154. Schweickert, WD, Pohlman, MC, Pohlman, AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomized controlled trial. Lancet. 2009; 373(9678):1874–1882.
155. Arroliga, A, Frutos-Vivar, F, Hall, J, et al. Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest. 2005; 128(2):496–506.
156. Mercat, A, Richard, JC, Vielle, B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2008; 299(6):646–655.
157. Sessler, CN. Sedation, analgesia, and neuromuscular blockade for high-frequency oscillatory ventilation. Crit Care Med. 2005; 33(3 Suppl):S209–S216.
158. Gainnier, M, Roch, A, Forel, JM, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004; 32(1):113–119.
159. Forel, JM, Roch, A, Marin, V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006; 34(11):2749–2757.
160. Gehr, LC, Sessler, CN. Neuromuscular blockade in the intensive care unit. Semin Respir Crit Care Med. 2001; 22(2):175–188.
161. Walz, JM, Zayaruzny, M, Heard, SO. Airway management in critical illness. Chest. 2007; 131(2):608–620.
162. Martyn, JA, Richtsfeld, M. Succinylcholine-induced hyperkalemia in acquired pathologic states: Etiologic factors and molecular mechanisms. Anesthesiology. 2006; 104(1):158–169.
163. McManus, MC. Neuromuscular blockers in surgery and intensive care, Part 2. Am J Health Syst Pharm. 2001; 58(24):2381–2395.
164. Tuba, Z, Maho, S, Vizi, ES. Synthesis and structure-activity relationships of neuromuscular blocking agents. Curr Med Chem. 2002; 9(16):1507–1536.
165. Brunton LL, Goodman LS, Blumenthal D, Buxton I, eds. Goodman and Gilman’s Manual of Pharmacology and Therapeutics. New York: McGraw-Hill, 2007.
166. Sessler, CN. Train-of-four to monitor neuromuscular blockade? Chest. 2004; 126(4):1018–1022.
167. Rudis, MI, Sikora, CA, Angus, E, et al. A prospective, randomized, controlled evaluation of peripheral nerve stimulation versus standard clinical dosing of neuromuscular blocking agents in critically ill patients. Crit Care Med. 1997; 25(4):575–583.
168. Strange, C, Vaughan, L, Franklin, C, Johnson, J. Comparison of train-of-four and best clinical assessment during continuous paralysis. Am J Respir Crit Care Med. 1997; 156(5):1556–1561.
169. Baumann, MH, McAlpin, BW, Brown, K, et al. A prospective randomized comparison of train-of-four monitoring and clinical assessment during continuous ICU cisatracurium paralysis. Chest. 2004; 126(4):1267–1273.
170. Lagneau, F, D’Honneur, G, Plaud, B, et al. A comparison of two depths of prolonged neuromuscular blockade induced by cisatracurium in mechanically ventilated critically ill patients. Intensive Care Med. 2002; 28(12):1735–1741.
171. Segredo, V, Caldwell, JE, Matthay, MA, et al. Persistent paralysis in critically ill patients after long-term administration of vecuronium. N Engl J Med. 1992; 327(8):524–528.
172. Segredo, V, Caldwell, JE, Wright, PM, et al. Do the pharmacokinetics of vecuronium change during prolonged administration in critically ill patients? Br J Anaesth. 1998; 80(6):715–719.
173. Prielipp, RC, Coursin, DB, Scuderi, PE, et al. Comparison of the infusion requirements and recovery profiles of vecuronium and cisatracurium 51W89 in intensive care unit patients. Anesth Analg. 1995; 81(1):3–12.
174. Larsson, L, Li, X, Edstrom, L, et al. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: Mechanisms at the cellular and molecular levels. Crit Care Med. 2000; 28:34–45.