CHAPTER 23 Use of Bone Graft Substitutes and Bioactive Materials in Treatment of Distal Radius Fractures
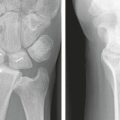
The goals of treating distal radius fractures include anatomical reduction, stable fixation to allow early movement, reliable and rapid osteosynthesis, and restoration of pain-free function. Bone healing can be delayed by the presence of large metaphyseal defects or voids that are often seen in distal radius fractures. These defects, as well as segmental defects or osteoporotic bone, are amenable to bone grafting. Autologous bone graft has been frequently used in the treatment of distal radius fractures to aid in healing in those cases. Over the past several years there has been significant interest in the development of biomaterials that can augment fracture healing to preclude the need for autologous graft.
Economically, the high market value of these products has created a stimulus for their development.1 As such, physicians may now choose from several products that are now commercially available. Unfortunately, there is currently little consensus on the indications for use of bone graft substitutes and a paucity of comparative studies between products. Rigorous comparison of the commercially available bone graft substitutes has proved difficult because of their diversity and the lack of standardized assays. Furthermore, comparable clinical studies have not been performed.2 The U.S. government has contributed to this confusion as regulatory control of the different types of products, even similar products, has fallen under different agencies within the Food and Drug Administration.3 This review will cover the indications and currently available materials for use as bone replacements in the treatment of distal radius fractures.
Bone Graft Properties
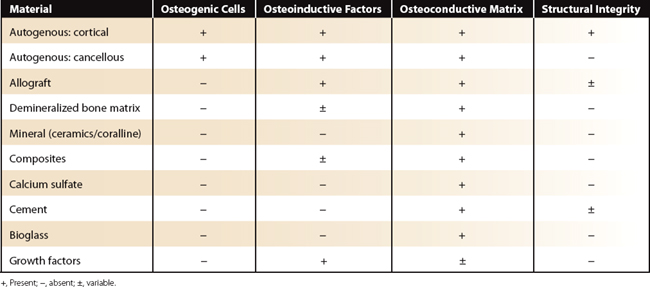
An ideal bone graft possesses four important properties: (1) osteogenic cells, which are naturally occurring cells with the potential to differentiate into bone forming cells; (2) osteoinductive factors, which are proteins, including growth factors, that stimulate and signal new bone growth; (3) osteoconductive matrix providing a scaffold for new bone growth; and (4) structural integrity.4 Both cancellous and cortical autogenous bone graft possess the first three properties. Although cortical grafts are able to provide structural integrity, they are less osteogenic and osteoinductive when compared with cancellous grafts. Iliac crest autologus graft has long been the gold standard of bone grafts.
Alternatives to autogenous graft, or bone graft substitutes, are judged by their ability to provide aspects of these four components. Substitutes now available include allograft bone, demineralized bone matrix (DBM), synthetic ceramic mineral substitutes, and recombinant bone morphogenetic proteins BMP-7 and BMP-2.1 Unfortunately, comparison of one substitute with another or with autogenous graft is difficult. Each substitute is made of unique materials and participates in healing in different ways. Additionally, there are no standardized assays specific for osteoinduction and osteoconduction in humans, making it impossible to accurately quantify their role in bone healing.
Indications
The distal radius has been the center of attention for the development of many of the bone graft substitutes because it is a common area to fracture with a relative lack of associated confounding variables.5 The indications for the use of bone grafts or graft substitutes in the treatment of distal radius fractures, however, have not been clearly defined. Additionally, although autogenous graft has been proved to be beneficial,6–9 there is no clear consensus with regard to bone graft substitutes.
The goal of treatment is to restore alignment of the radius and provide stability with minimal compromise of hand function.10 This can be accomplished with either a cast, external fixator, or a variety of internal fixation techniques, depending on the fracture pattern, degree of displacement, stability of the fracture, patient age, and physical demands. Fracture healing is typically not a problem, because the fracture involves metaphyseal bone with ample vascularity.11 However, comminuted fractures or fractures in osteoporotic bone often result in cortical comminution and metaphyseal defects, which if left unsupported can lead to collapse of the distal fragments and loss of alignment. Additionally, osteopenic or osteoporotic bone can limit fixation and result in loss of reduction after internal fixation. In either case, healing will typically occur but may result in a shortened or malaligned radius, which can produce pain, stiffness, and loss of strength.12,13 It is in these osteoporotic or comminuted fractures that bone graft substitutes provide structural support or act as a scaffold for new bone formation and are particularly desirable.
The use of autogenous bone graft has been shown to be advantageous to support metaphyseal defects after distal radius fractures.6–9 In addition to providing structural support, autogenous graft can accelerate and augment bone healing due to the presence of growth factors and viable osteoblasts. However, use of autogenous bone graft has been associated with donor site morbidity, including infection, blood loss, and pain, as well as increased surgical time, hospital stay, and cost.14 These potential disadvantages have helped lead to the development of numerous bone graft substitutes.
Graft Substitutes
There are several graft substitutes available for orthopaedic use in the United States. These include allograft, demineralized allograft bone matrix, mineral derived graft, composite graft, calcium sulfate, injectable cement, bioactive glass, and growth factors. These substitutes vary with regard to their osteoconductive properties, osteoinductive properties, structural strength, and rate of disappearance (Table 23-1).
Allograft
Allograft is available in various forms, sizes, and shapes, depending on the individual need. Corticocancellous graft can be used to reconstruct larger defects or in cases where significant structural support is needed. Cancellous graft is better for smaller defects, especially in metaphyseal areas, and is particularly useful for the distal radius.15 It can be used to augment autograft, or it can be mixed with autograft in order to introduce osteoprogenitor cells.
The disadvantages of allograft include its variable quality and potential for disease transmission. There have been several documented cases of bacterial infections transmitted by allografts and even a documented case of human immunodeficiency virus transmission.1 When compared with autograft, other disadvantages include lack of osteogenic cells, fewer osteoinductive factors, and less structural integrity. Still, in a recent prospective and randomized study, no significant differences regarding fracture union or outcomes were noted when comparing allograft cancellous chips and autologous iliac crest bone grafting.16
Demineralized Bone Matrix
DBM is allograft bone that has been demineralized and processed by chemical and/or radiation treatment, leaving collagen as well as bone morphogenetic proteins and growth factors. It has been shown to be osteoinductive in an animal model and is therefore thought to maintain the presence of growth factors despite processing.17 However, the potential for osteoinduction is variable, even among individual batches of the same product.
DBM is available from several manufacturers, differing in its form and carrier. Forms available include those that can be injected or molded. Various carrier agents have also been used, including saline, glycerol, gelatin, polymers, hyaluronic acid, and collagen. Despite significant debate regarding the best form and carrier, no consensus exists. There has been concern regarding glycerol toxicity in an animal model, although this has not been seen in humans.18
Minerals
Calcium phosphate mineral grafts are osteoconductive grafts that are composed of hydroxyapatite, tricalcium phosphate, or a combination of the two. Most of these grafts, other than coralline hydroxyapatite, are ceramics that are made by heating mineral salts to high temperatures in a process known as sintering. This process increases strength but reduces the resorption and remodeling of the material. There has been significant, but unsubstantiated, concern about the slow rate of radiographic disappearance when used clinically. The rate of resorption depends on the chemical composition as well as material factors such as the surface area and pore size. Tricalcium phosphates of greater pore size allow better resorption by allowing osteoclasts into the pores, but this renders them mechanically weaker. The ideal pore size is between 150 and 500 μm.19 Newer materials have been developed with increased porosity to increase the rate of resorption. This rate of resorption may or may not correlate with remodeling, depending on the material and the environment.1
Coralline hydroxyapatite is produced by a thermochemical reaction between Pacific coral and ammonium phosphate. This process converts the majority of the coral’s calcium carbonate into hydroxyapatite, which is more slowly desorbed. Technically, this is not a ceramic because it does not undergo sintering. The pores are interconnected similar to cancellous bone, allowing bone ingrowth. Newer products have been produced with calcium carbonate on the surface to improve bone ingrowth and potentially graft resorption. True osteoclastic resorption does not occur and the graft disappears slowly. Similar to the ceramics, there is minimal remodeling. It has been shown to be useful to augment fixation of distal radius fractures.20
Calcium phosphate mineral grafts function purely as an osteoconductive implant on which bone will grow. Osteoinduction may be introduced when grafting is combined with autogenous platelets from a special autotransfusion process. They may be used to fill defects, although structural fixation is also needed. As a group, these grafts are brittle with little tensile strength, limiting their use to nonloaded defects or defects in which the bending, shear, and torsional stresses are neutralized with internal or external fixation.21 Other disadvantages include variable quality and slow resorption/remodeling.
Composites
An additional composite is made up of bovine collagen, hydroxyapatite, and tricalcium phosphate. This material is osteoconductive but can be used along with aspirated autogenous bone marrow to make it osteoinductive and potentially osteogenic. Because it provides no structural support, it appears to be useful in fractures that have been stabilized but contain bone defects. It has been shown to be a viable alternative to autogenous bone graft in the treatment of fractures, including those of the distal radius.22,23
Calcium Sulfate
Calcium sulfate, also known as plaster of Paris, has been used to fill bone defects for many years. It acts as an osteoconductive filler for bone defects, but, unlike ceramics, the rate of resorption is rapid, with some variation depending on the formulation, configuration, and amount of material used. It is available in various forms including pellets, blocks, and in an injectable paste. Resorption occurs by both dissolution and replacement by bone.24 It is indicated to fill metaphyseal defects but requires supplemental fixation.
Cement
Injectable cements are made up of calcium phosphate and have the advantage of complete filling of defects. They are either injected or molded into a defect in a liquid or putty form and solidify within several minutes without generating significant heat. The solid form provides structural support with compressive strength that is greater than cancellous bone, although it poorly resists torsion or shear.25
Bone cements have been shown to remodel with cutting cones and osteoclastic activity, because they strongly resemble the mineral phase of bone due to the presence of dahllite.25 Radiographic disappearance of the material by 30% to 60% has been seen at 1 year, with cortical remodeling preceding medullary remodeling.26
Several studies have evaluated the use of calcium phosphate cement in the treatment of distal radius fractures. An initial study demonstrated improved clinical outcome compared with casting alone.27 A larger, multicenter, prospective study compared bone cement (with or without Kirschner wires [K-wires]) to controls treated with casting or external fixation (with or without K-wires).26 This demonstrated earlier functional return in the cement group without loss of measured radiographic parameters. However, long-term outcomes were similar.
Bioactive Glass
Bioactive glasses are a combination of silica, calcium, and phosphate materials that form a bond between the graft and host tissue. The silica is rapidly broken down with the release of calcium and phosphate. This process may prove useful for the delivery of newly derived growth factors. These substitutes are osteoconductive but do not provide structural support.
Growth Factors
Inflammation is the earliest stage of fracture healing and begins as a result of the hematoma from fracture bleeding. Cells within this initial fibrin clot secrete growth factors, which regulate the early events of fracture healing. These factors stimulate and regulate bone formation. One of the main limitations of the majority of the currently available graft substitutes is the lack of this osteoinductive or osteogenic potential. This is currently the most intense area of research with regard to bone graft substitutes.1
Autogenous bone marrow has been used to fill this deficit. It can be used to augment many of the synthetic or allogenic substitutes to add osteoinductive factors as well as potentially osteogenic cells.28 Typically harvested from the ilium with limited morbidity, it must be used immediately in order to maintain the viability of the existing cells. Marrow has been shown to contain both osteoprogenitor cells and growth factors.29
More recently, recombinant growth factors such as the bone morphogenetic proteins and synthetic peptides have been introduced, primarily for the augmentation of spinal fusion. The recombinant forms of BMP-7 (osteogenic protein-1 [OP1]; Stryker, Kalamazoo, MI) and BMP-2 (Infuse; Medtronic Sofamor Danek, Memphis, TN) have been approved for selected trauma indications.30,31 These recombinant bone morphogenetic proteins are produced in hyperphysiological concentrations and therefore may have the potential to impart an immunological reaction. In addition, these proteins require an additional substitute to act as a carrier and to provide an osteoconductive matrix. Antibodies to these factors, and also the carriers, have been seen in humans, but it is unclear if this represents a clinical problem.30 Newer techniques will certainly continue to develop and, along with genetic engineering, will most likely represent the future of bone graft substitution.
1. Ladd AL, Pliam NB. Bone graft substitutes in the radius and upper limb. J Am Soc Surg Hand.. 2003;3(4):227-245.
2. Ladd AL, Pliam NB. The role of bone graft and alternatives in unstable distal radius fracture treatment. Orthop Clin North Am.. 2001;32(2):337-351.
3. Bauer TW, Smith ST. Bioactive materials in orthopaedic surgery: overview and regulatory considerations. Clin Orthop Relat Res.. 2002;395:11-22.
4. Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg.. 1995;3:1-8.
5. Owen RA, Meltron LJ, Johnson KA, et al. Incidence of Colles’ fracture in a North American community. Am J Public Health.. 1982;72:605-607.
6. Swigart CR, Wolfe SW. Limited incision open techniques for distal radius fracture management. Orthop Clin North Am.. 2001;32:317-327.
7. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg [Am].. 1988;13:372-377.
8. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intra-articular fractures of the distal radius. J Hand Surg [Am].. 1990;15:1-11.
9. Seitz WH, Froimson AI, Leb R, Shapiro JD. Augmented external fixation of unstable distal radius fractures. J Hand Surg [Am].. 1991;16:1010-1016.
10. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. J Bone Joint Surg Am.. 2003;85(3):552-564.
11. Segalman KA, Clark GL. Un-united fractures of the distal radius: a report of 12 cases. J Hand Surg [Am].. 1998;23:914-919.
12. Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg Am.. 1986;68:647-659.
13. Jupiter JB. Current concepts review: fractures of the distal end of the radius. J Bone Joint Surg Am.. 1991;73:461-469.
14. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma.. 1989;3:192-195.
15. Herrera M, Chapman CB, Roh M, et al. Treatment of unstable distal radius fractures with cancellous allograft and external fixation. J Hand Surg [Am].. 1999;24:1269-1278.
16. Rajan GP, Fornaro J, Trentz O, Zellweger R. Cancellous allograft versus autologous bone grafting for repair of comminuted distal radius fractures: a prospective, randomized trial. J Trauma.. 2006;60:1322-1329.
17. Urist MR, Silverman BF, Burning K, et al. The bone induction principle. Clin Orthop Relat Res.. 1967;53:243-283.
18. Bostrom MP, Yang X, Kennan M, et al. An unexpected outcome during testing of commercially available demineralized bone graft materials: how safe are the nonallograft components? Spine.. 2001;26:1425-1428.
19. Geissler WB. Bone graft substitutes in the upper extremity. Hand Clin.. 2006;22(3):329-339.
20. Wolfe SW, Pike L, Slade JFIII, Katz LD. Augmentation of distal radius fracture fixation with coralline hydroxyapatite bone graft substitute. J Hand Surg.. 1999;24:816-827.
21. Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res.. 2002;395:44-52.
22. Chapman MW, Bucholz R, Cornell C. Treatment of acute fractures with a collagen-calcium phosphate graft material: a randomized clinical trial. J Bone Joint Surg.. 1997;79:495-502.
23. Scaglione PH, Buchman MT. Collagraft bone substitute in upper extremity fractures: a preliminary study. Surg Forum.. 1997;48:563-565.
24. Kelly CM, Wilkins RM, Gitelis S, et al. The use of surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res.. 2001;382:342.
25. Constantz BR, Ison IC, Fulmer MT, et al. Skeletal repair by in situ formation of the mineral phase of bone. Science.. 1995;24:1796-1799.
26. Cassidy C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with conventional fixation in distal radius fractures: a randomized study. J Bone Joint Surg Am.. 2003;85:2127-2137.
27. Jupiter JB, Winters S, Sigman S, et al. Repair of five distal radius fractures with an investigational cancellous bone cement: a preliminary report. J Orthop Trauma.. 1997;11:110-116.
28. Burwell RG. The function of bone marrow in the incorporation of a bone graft. Clin Orthop Relat Res.. 1985;200:125-141.
29. Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res.. 2001;19:117-125.
30. Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am.. 2001;83(Suppl 1):S151-S158.
31. Grovender M, Scimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am.. 2002;84:2123-2134.