Urinary Tract Infections and Vesicoureteral Reflux
Urinary Tract Infections
Urinary tract infections (UTIs) are a common and significant source of morbidity in children. By 7 years of age, approximately 8% of girls and 2% of boys will have had at least one UTI.1,2 Children who have had at least one UTI are at risk for having a recurrence.3 The long-term sequelae include renal scarring, hypertension, chronic renal insufficiency, and pregnancy-related complications. Predisposing risk factors for UTIs include renal and bladder structural abnormalities as well as functional bladder and bowel dysfunction.4 Pediatric UTIs constitute a significant health burden and has been estimated to result in at least 13,000 hospital admissions with inpatient costs exceeding $180 million per year.5
Diagnosis
Localized clinical signs and symptoms are important clues in the diagnosis, but they are age dependent. Combinations of findings can be more useful than individual ones in identifying affected children.6 For example, neonates rarely present with symptoms specific to the urinary tract. Nonspecific symptoms of lethargy, irritability, temperature instability, anorexia, emesis, or jaundice predominate. Bacteremia is common with neonatal UTI, and a urine culture is an important aspect in the evaluation of neonatal sepsis.7 Confirmation of a UTI by microscopic examination and quantitative culture of a properly collected specimen is important. Older infants may present with nonspecific abdominal discomfort, emesis, diarrhea, poor weight gain including failure to thrive, and fever. Malodorous or cloudy urine may be reported by the parents.
Older children frequently present with dysuria and urinary frequency, urgency, and enuresis. Table 55-1 outlines the incidence of UTI symptoms as a function of age.8,9 As the symptoms can sometimes be obscure, it is important that care providers have a high index of suspicion in ill-appearing children. An unexplained high fever in an infant or toddler should prompt the clinician to obtain a urine sample.
TABLE 55-1
Presenting Symptoms in 200 Children with Urinary Tract Infection as a Function of Age
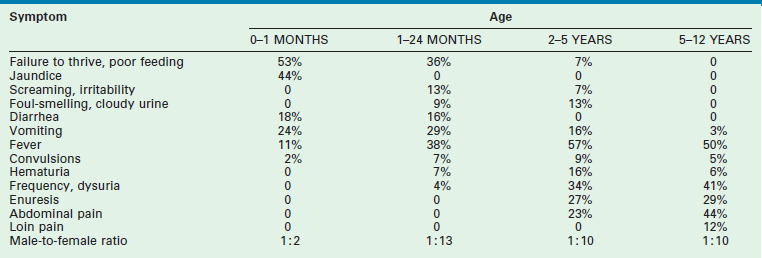
From Smellie JM, Hodson CJ, Edwards D, et al. Clinical and radiological features of urinary tract infection in childhood. BMJ 1964;2:1222; Bickerton MW, Duckett JW. Urinary tract infections in pediatric patients. AUA Update Service, Lesson 26, 1985;4:4.
Analysis of a properly collected urine sample is the cornerstone in the diagnosis of UTI.10 Errors in diagnosis most commonly result from failure to confirm a clinically suspected UTI by culture, or by reliance on a specimen that has been inadequately collected or mishandled. Specimens may be obtained by bag collection, clean catch, urethral catheterization, and suprapubic aspiration. Although invasive, urethral catheterization (or suprapubic aspiration) clearly offers the lowest risk of false-positive results.11 The results of a bag specimen or clean-catch specimen in a non-toilet-trained child are helpful only if negative.12 Bag specimens can be useful in an infant with a history of UTIs or structural abnormalities in whom a fever is present, but the suspicion for a UTI is otherwise low. Positive findings should be confirmed using a catheter or aspiration specimen unless the clinical presentation and laboratory findings are unequivocal. The accuracy of positive findings from a bag specimen in an infant has been estimated at 7.5%,13 whereas those of the midstream clean catch specimen varies with age: 42% under 18 months of age and 71% from 3 to 12 years of age.14 Specimens should be either analyzed and plated immediately, or placed on ice to minimize bacterial multiplication prior to testing.
The accepted gold standard for diagnosis remains the quantitative urine culture. The historically accepted criterion for diagnosis is greater than 105 colony-forming units per milliliter of a single bacterial species. The accuracy of such a positive finding on culture is estimated at 80% (single specimen) and 96% (confirmed by second culture).15 Table 55-2 outlines the probability of infection as a function of colony count and methods of collection that are used in children.16 One must avoid applying these criteria too strictly. The colony count varies as a function of hydration (dilution) and urinary frequency (bacterial multiplication time). One study of six untreated children with proven bacteriuria found colony counts to vary from 103 to 108 over a 24-hour period.17
TABLE 55-2
Criteria for Diagnosis of Urinary Tract Infections
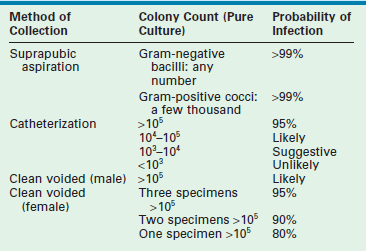
Modified from Hellerstein S. Recurrent urinary tract infection in children. Pediatr Infect Dis 1982;1:275.
Although clearly the most accurate laboratory test, urine culture results cannot provide an immediate diagnosis. As a result, initial treatment is generally guided by the urinalysis. Microscopic evaluation of a urine specimen should be done immediately on collection. This practice minimizes misleading ex vivo bacterial multiplication and deterioration of cellular elements. The identification of bacteria in an unspun urine specimen is very suggestive of significant bacteriuria.17 Pyuria (more than 10 leukocytes/mm3) is suggestive,18 but may also be seen in vaginitis, dehydration, calculi, trauma, chemical irritation, gastroenteritis, and viral immunization. Urinary Gram stain has been found to be reliable in detecting UTIs in young infants.19
A popular and indirect measurement of bacteriuria employs nitrite and leukocyte esterase analysis. Nitrate, normally present in urine, is converted to nitrite in the presence of bacteria. A positive nitrite reaction is indicative of bacteria with specificity and a positive predictive value approaching 100%.20 The nitrate-to-nitrite reaction requires a relatively long incubation period. Thus, urinary frequency and hydration may produce a false-negative result. Inadequate dietary nitrate and infection caused by nitrite-negative organisms may also cause false-negative reactions.21 The combination of nitrite and leukocyte esterase is more sensitive and specific than either alone.22 Overall, the combination of dipstick analysis and microscopic examination for bacteria have a sensitivity and negative predictive value approaching 100%.20
Classification
Laboratory studies designed to distinguish a lower tract from an upper tract UTI include antibody-coated bacteria assay, β2-microglobulin excretion, antibodies to Tamm–Horsfall protein, and urinary lactic dehydrogenase assay and procalcitonin.23,24 These tests are not sufficiently reliable for routine clinical use and thus are not typically employed. Direct culture by ureteral catheterization or percutaneous puncture is reliable, although cumbersome, and represents an option in complicated clinical situations. A quite useful study for localizing infection to the kidney is a radioisotope renal cortical scan (e.g., technetium-99m dimercaptosuccinic acid) during the initial presentation of the patient with a documented infection (Fig. 55-1).
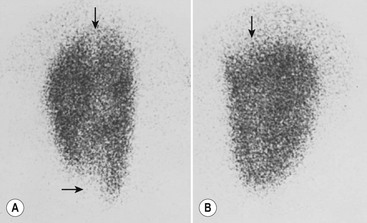
FIGURE 55-1 Technetium-99m dimercaptosuccinic acid (DMSA) scan. (A) The magnified view of the left kidney, seen by using a pinhole collimator, demonstrates defects in both poles that extend deep into the renal parenchyma (arrows), suggestive of acute pyelonephritis. (B) The right kidney has an upper pole defect (arrow) that may represent either acute or chronic pyelonephritis. (Courtesy of Michael J. Gelfand, MD.)
Epidemiology
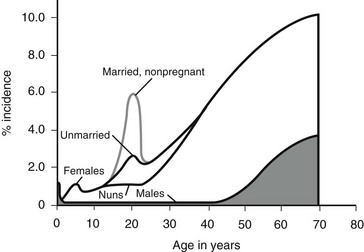
Figure 55-2 outlines the age- and gender-related incidence of UTIs. At all ages, with the exception of the neonatal period, the incidence of UTI is greater in females than in males. In both males and females, the incidence increases with advanced age. Although the male has one early peak in the newborn period, the female has two peaks, one at 3 to 6 years, and the other at the onset of sexual activity. The actual incidence of infection as a function of age and gender is difficult to determine from the literature. Table 55-3 summarizes the available data.16
TABLE 55-3
Incidence of Urinary Tract Infection as a Function of Age, Gender and Presence of Symptoms

a2.4–3.4% in premature infants.
Data compiled from multiple sources by Hellerstein S. Recurrent urinary tract infections in children. Pediatr Infect Dis 1982;1:271.
Pathophysiology
Host Factors
The establishment of clinical infection and its consequent injury to the urinary tract results from a complex interplay between host resistance and bacterial virulence. As a general rule, UTI-causing organisms originate from the feces of their host. Conceptually, four levels of defense are identifiable: periurethral, bladder, ureterovesical junction, and renal papillae.9 These concepts are illustrated in Figure 55-3.
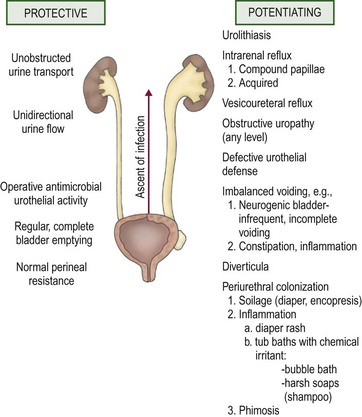
FIGURE 55-3 Host factors that protect the urinary tract from infection and abnormalities that potentiate the establishment of invasive bacterial infection.
Bacteria generally possess an ability to adhere to vaginal mucosal cells in order to readily establish infection.25 The resultant periurethral colonization then allows replication and migration, which ultimately lead to transurethral invasion to the bladder. Healthy girls have low bacterial colonizations of the periurethral region. Girls prone to UTIs experience greater colonization, especially prior to a new episode of UTI. Moreover, the cultured organism from the introital region belongs to the same strain as that from the urine during the UTI that ensues. Periurethral bacterial colonization is correspondingly low in UTI patients after resolution of recurrent UTIs.26 A similar mechanism may apply to bacterial adherence in the prepuce of males.27 This may explain why 92% of male infants less than 6 months old with a UTI are uncircumcised.28
A number of bladder defense mechanisms help maintain sterile urine. The most critical is the act of regular and complete voiding. The healthy bladder is capable of eliminating 99% of instilled bacteria and leaves a small residual urine that minimizes the inoculum at the onset of the following cycle.29 High intravesical pressure may also potentiate infection in children. In the absence of an elevated residual urine, uninhibited bladder contractions are associated with an increased risk of recurrent UTI, which may be lessened by anticholinergic therapy.30 Dysfunctional elimination syndrome with abnormal voiding habits and constipation can affect the development of UTI as well.31 The acidic pH of urine, as well as its osmolality, further discourages bacterial growth.32 The uroepithelial cells of healthy individuals suppress bacterial growth and are capable of killing bacteria. The uroepithelial cells secrete a mucopolysaccharide substance that, upon coating the surface of the uroepithelium, provides an additional barrier to uroepithelial adherence.29 Glycosaminoglycans are continuously shed and thus function to entrap and eliminate bacteria.
Abnormalities at the ureterovesical junction (UVJ) and altered ureteral peristalsis may allow vesicoureteral reflux (VUR), which potentiates but is not always necessary for upper tract invasion. Distortion of the pyramids allows renal parenchymal invasion, which results in irreversible renal injury. The anatomy of the renal papillae usually prevents intrarenal reflux (Fig. 55-4).9 Structural abnormalities that potentiate infection include phimosis, obstructive uropathy at any level (e.g., ureteropelvic and UVJ obstructions, posterior urethral valves [PUV]), VUR, bladder diverticula, urinary calculi or foreign bodies, and the renal papillary anatomy.
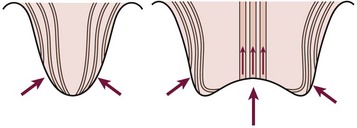
FIGURE 55-4 The normal oblique insertion of the collecting ducts onto the surface of simple papillae prevent intrarenal reflux (left). Collecting duct insertion onto the surface of compound papillae may allow intrarenal reflux (right). (From Ransley PG. Intrarenal reflux: Anatomic, dynamic and radiological studies. Urol Res 1977;5:61.)
Bacterial Factors
Several bacterial factors may potentiate a UTI and are outlined in Box 55-1.9,33 O antigens are lipopolysaccharides that are part of the cell wall. They are thought to be responsible for many of the systemic symptoms associated with infection. Of the more than 150 strains of Escherichia coli identified by O antigens, nine are responsible for the majority of UTIs.
K antigens are also polysaccharides, and their presence on Gram negative bacterial capsules is considered to be an important virulence factor. They are thought to protect against phagocytosis, to inhibit the induction of a specific immune response, and to facilitate bacterial adhesion. Bacterial strains causing UTI exhibit considerably more K antigen than those isolated from the feces. Urease, a virulence factor especially prominent with Proteus species, allows the breakdown of urea to ammonium. This process alkalinizes the urine and facilitates stone formation. Such bacteria are generally incorporated into the stone structure, making eradication extremely difficult. Mannose-resistant pili are important adherence factors. They promote adherence to uroepithelial cells as well as renal epithelial cells. This factor appears to counter the normal cleansing action of urine flow and allows tissue invasion and bacterial proliferation. That these factors truly are associated with virulence is shown in Figure 55-5.
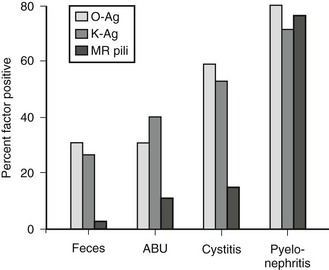
FIGURE 55-5 Presence of bacterial virulence factors as a function of the clinical setting. More invasive infections are associated with a high incidence of virulence factors, implicating these factors in pathogenesis. MR, mannose resistant; Ag, antigen; ABU, asymptomatic bacteriuria. (From Mannhardt W, Schofer O, Schulte-Wisserman H. Pathogenic factors in recurrent urinary tract infection and renal scar formation in children. Eur J Pediatr 1986;145:330.)
Increasingly invasive urinary infections are associated with bacteria with a high number of virulence factors. Figure 55-6 demonstrates the pathophysiologic changes of renal injury that can occur in the absence of significant host factors. Colonization of the feces with a virulent organism allows periurethral colonization and ultimately bladder entry. Uroepithelial adherence promotes bacterial proliferation and tissue invasion. This series of events is facilitated by the presence of one or more host factors (see Fig. 55-3).
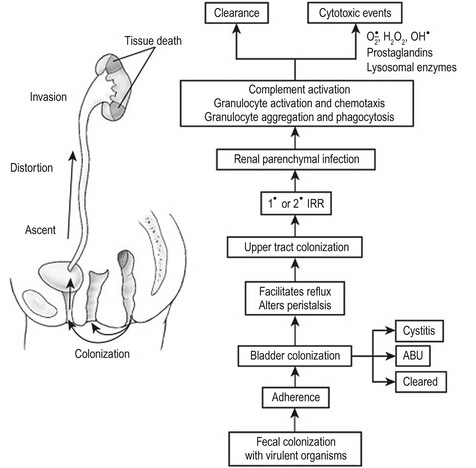
FIGURE 55-6 The pathogenesis of destructive infection is shown. The process is facilitated by, but does not require, defects in the host protective factors outlined in Fig. 55-3. IRR, intrarenal reflux; ABU, asymptomatic bacteriuria.
Investigation
Rationale for Radiographic Imaging
Although many patients with UTI do not develop serious illness, the pediatric caregiver must be cognizant of several important risks. Urinary abnormalities are found in approximately 50% of children up to the age of 12 years who present with UTI.34 VUR is found in up to 35% and obstructive lesions in 8%. Nonobstructive, nonrefluxing lesions are found in 7%.
While renal scars develop in about 13% of girls and 5% of boys with unspecified infection,35 they develop in up to 43% of kidneys involved in acute pyelonephritis.36 A recent meta-analysis assessing the prevalence of renal scarring in children after an initial episode of UTI found that 57% had acute renal cortical changes consistent with pyelonephritis in the acute phase and 15% had evidence of renal scarring on the follow-up Tc-99m dimercaptosuccinic acid (DMSA) scan.37 Pyelonephritic scarring is responsible for 11% of childhood hypertension cases38 and a majority of cases of severe hypertension.39 Although hypertension is most common with bilateral scarring, it is also seen with unilateral scarring. 40 Pyelonephritic scarring is also an important cause of end-stage renal failure in childhood and may require specific pretransplantation treatment, especially if associated with reflux.41 Additionally, approximately 50% of patients will suffer from recurrent UTI.33
Current Controversies
The standard of care for many years has been to perform imaging studies in children to assess for structural abnormalities that might lead to recurrent UTIs and renal scarring. It was felt that not only infants 42 but older children, particularly males, should be investigated after the initial infection.43 Several evidence-based guidelines have been published over the years.44 Controversy persists, however, with many researchers proposing even less aggressive approaches to the child with a UTI. Recent published guidelines from the American Academy of Pediatrics recommends deferring a voiding cystourethrography (VCUG) until after the second documented UTI in children ages 2 to 24 months if the ultrasound (US) is normal and the child has a complete clinical response to treatment.45 The urologic community has significant concerns about the paradigm shift made in these guidelines and the methodology employed in the meta-analysis upon which these recommendations were based.46
Imaging Studies
The initial radiographic evaluation ideally includes a fluoroscopic VCUG to precisely grade vesicoureteral reflux and assess for bladder and urethral abnormalities. Follow-up studies in females can be adequately performed with a radionuclear cystogram (RNC) that allows a somewhat lower radiation exposure to the ovaries. The exception is the girl with neurogenic bladder, ectopic ureter, bladder diverticulum, or ureterocele documented by ultrasound or previous cystograms. Radionuclide scanning using DMSA is readily available and can be used to diagnose acute pyelonephritis,47 detect renal scarring, and assess differential renal function.48
Treatment
Acute Phase
The treatment of an acute UTI is dependent on the clinical presentation. Ill-appearing children with pyelonephritis should be treated immediately and aggressively with broad-spectrum antimicrobial therapy and intravenous hydration. Initial empiric therapy should be initiated with broad-spectrum parenteral antibiotics until cultures return. Prompt, effective treatment is the most important factor in preventing permanent renal injury.49 Further therapy is dictated by culture and sensitivity findings. Indications for inpatient parenteral antibiotic therapy include very young age (<3 months), unusual or multi-resistant pathogens, persistent vomiting, concern for compliance with treatment, and/or significant urinary tract anomalies.
Once clinically stabilized and afebrile for 24–48 hours, patients with pyelonephritis and sensitive organisms may complete the course of parenteral culture-specific antibiotics on an outpatient basis, via a peripherally inserted central catheter (PICC) and a home-based nursing service. Some studies have shown that initial parenteral therapy followed by oral antibiotics can be as effective as a prolonged course of intravenous therapy in preventing renal scarring.50,51 Patients with obstruction or renal abscess who do not become afebrile or have persistent severe symptoms require repeat upper tract imaging. An abscess or obstructed kidney may require percutaneous, or very rarely, open drainage.
Initial empiric therapy in less toxic appearing children without vomiting can be performed with oral broad-spectrum antibiotics (e.g., cephalosporins) after obtaining a reliable urine culture. Short treatment courses appear to be insufficient for treatment of childhood UTI.52 Therefore, we prefer a 7–10-day course dictated by culture and sensitivity results.53 Retention by the toilet-trained patient due to fear of voiding or dysuria may be managed with phenazopyridine (Pyridium) and hydration, and allowing the child to void while sitting in a tub of warm clear bath water.
Prophylactic Antibiotics
Patients who have recurrent UTIs or those who are managed nonoperatively for VUR are often treated with long-term suppressive antibiotics. It is unclear whether the benefit outweighs the risks, particularly in low-grade VUR without nephropathy.45,54 The urothelial injury from an infection takes several months to fully recover. As a result, irritative voiding symptoms, such as dysuria, incontinence, and frequency, may persist despite the finding of sterile urine. A propensity for re-infection also exists.
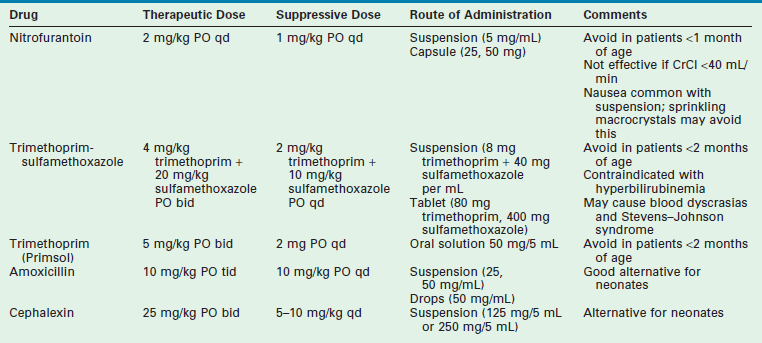
Patients without obvious urinary tract structural abnormalities should be evaluated for functional bowel and bladder dysfunction. Regular and complete elimination is mandatory to diminish the risk of recurrence. Along with aggressive treatment of constipation and voiding dysfunction, three months of antibiotic suppression therapy may help to break the cycle. Table 55-4 outlines the characteristics of the drugs that are most commonly used for suppression.
Vesicoureteral Reflux
VUR refers to the retrograde passage of urine from the bladder into the ureter. Although VUR was first discovered in the late 1800s, its clinical importance has only been recognized in the last five decades. Hutch’s studies, reported in the 1950s, demonstrated the pathophysiologic changes with VUR in children.55 These reports and Hodson’s observations in 1959 regarding the association between VUR, UTI, and pyelonephritic scarring set the stage for the modern era of reflux management.56
Although most commonly diagnosed during the evaluation for UTI, VUR may also be discovered during evaluations for hypertension, proteinuria, voiding dysfunction, or chronic renal insufficiency. In addition, VUR has been identified in asymptomatic patients with prenatally-detected hydronephrosis as well as through sibling screening.57
Pathophysiology
Figure 55-7 depicts the various anatomic components of the competent UVJ as well as the abnormalities most often implicated in the genesis of VUR. The normal UVJ is characterized by an oblique entry of the ureter into the bladder and a length of submucosal ureter providing a high ratio of tunnel length to ureteral diameter. This anatomic configuration provides a predominantly passive valve mechanism.58,59 As the bladder fills and the intravesical pressure rises, the resulting bladder wall tension is applied to the roof of the ureteral tunnel. This results in a compression of the ureter which prevents retrograde passage of urine. Intermittent increases in bladder pressure, such as the act of voiding, upright posture, activity, and coughing, are met with an equal and immediate increase in resistance to retrograde urine flow. This effect is supplemented by the active effects of ureterotrigonal muscle contraction and ureteral peristalsis. 59,60
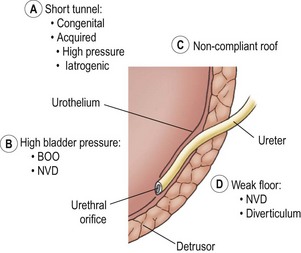
FIGURE 55-7 Components of the competent ureterovesical junction. Those abnormalities most often implicated in the etiology of vesicoureteral reflux are outlined. BOO, bladder outlet obstruction; NVD, neurovesical dysfunction.
Ureters with marginal tunnels can be made to reflux during infection due to UVJ distortion, loss of compliance of the valve roof, and intravesical hypertension. Excessively high intravesical pressure in neurovesical dysfunction (NVD) or bladder outlet obstruction (BOO) may also potentiate reflux as may a structurally weak detrusor floor (e.g., diverticulum or ureterocele). As the submucosal ureter tends to lengthen with age, the ratio of tunnel length to ureteral diameter increases, and the propensity for reflux may disappear.58,59
Of critical importance is the concept of intrarenal reflux (IRR), which has been demonstrated clinically61 as well as experimentally.62 The usually oblique entry of the papillary ducts onto the surface of simple papillae inhibits IRR. In contrast, the papillary duct entrance onto compound papillae facilitates IRR (see Fig. 55-4). The critical pressure for IRR is considered to be about 35 mmHg in compound papillae.62,63 Experimentally, this same pressure may cause scar formation in the absence of infection.62,64 When occurring intravesically, this pressure has been associated with an increased risk of renal deterioration. Higher pressure is thought to be necessary to induce IRR in simple papillae. The combination of infection and IRR is particularly devastating. Focal scarring appears to result from the difference in susceptibility of the renal papillae to IRR. The polar distribution of compound papillae corresponds closely to the predominant occurrence of renal scarring in the upper and lower poles of the kidney.
Classification
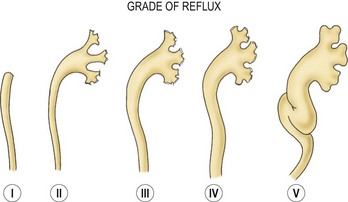
The most clinically pertinent classification systems, however, have attempted to quantitate the degree of reflux. At the present time, VUR is graded from I to V according to the international classification system diagrammed in Figure 55-8.65 This classification system is based not only on the proximal extent of retrograde urine flow and ureteral and pelvic dilatation, but also on the resultant anatomy of the calyceal fornices. This system is currently universally accepted and used extensively in the literature.
Epidemiology
The incidence of VUR in otherwise normal children is thought to be quite low.66 A much higher incidence of VUR, between 30–40%, is reported in patients undergoing evaluation for UTI.67–70 It is important to note that the incidence rises as age decreases.71 Thus, the infant who is most vulnerable to the combination of UTI and VUR is precisely the pediatric patient in whom this combination is most likely to occur.
Although females account for the majority of reflux patients, a few important characteristics of males with VUR require comment. Although males account for approximately 14% of patients with VUR,72 an increased incidence of VUR (30%) is found in those males presenting with UTI.69 Boys with VUR tend to present at a relatively young age (25% under 3 months) and younger children tend to have the more severe degrees of reflux.72
Multiple studies have documented a significant risk of VUR in family members of patients with reflux. The reported risk of sibling reflux ranges from 27–34%,73–75 while as much as 66% of offspring of women with reflux also have VUR.76 As a result of these studies, it has been suggested that siblings, especially those under 2 years of age, should have a screening investigation.56,73,76 Another option is to perform a renal and bladder ultrasound in younger siblings and defer the VCUG if the ultrasound is normal, unless they have a documented UTI.
A particularly important subset of patients with reflux includes those who have secondary reflux. Most have NVD or BOO as the primary disease. Many patients, however, have reflux not because of increased bladder pressure alone, but rather because UVJ deficiency is associated with other congenital anomalies, such as imperforate anus,77 ureterocele,78 and bladder exstrophy. Although many patients with PUV have reflux due to or exacerbated by high intravesical pressure, as demonstrated by VUR resolution after valve ablation or vesicostomy, the incidence of VUR in PUV patients is only approximately 50%. Many have congenitally abnormal ureteral insertions.79
Although a significant incidence of NVD exists in patients with imperforate anus, this is not a prerequisite for VUR.80 The diagnosis of VUR in imperforate anus thus assumes a critical importance to the pediatric surgeon. Not only may the association of NVD potentiate increased severity of reflux and the development of infection, the presence of a rectourethral or rectovesical fistula provides the opportunity for severe urinary contamination. Consequently, we believe that the patient with a rectovesical or rectourethral fistula should be managed with a completely diverting colostomy rather than a loop.
In addition to these structural associations, important functional associations are found as well, including florid NVD, as seen in myelodysplasia,81 and a variety of more subtle voiding disturbances.82–84 A particularly important subset of VUR patients are those who have uninhibited detrusor contractions (UDCs). Three important components of maturation are found with successful toilet training: (1) growth in bladder volume; (2) development of volitional control over the striated muscle sphincter; and (3) control over bladder smooth muscle. Delay in this maturation leads to UDCs. Many children with reflux and recurrent UTI have UDCs. Such involuntary or uninhibited bladder contractions are not caused by neurologic disease. Intense voluntary constriction of the striated sphincter occurs in an attempt to ensure continence and results in excessively high intravesical pressures. Pressures often exceeding 150 cmH2O have been observed resulting in intravesical distortions, such as diverticula, saccules, trabeculations, and abnormal ureteral orifices.85 Reflux occurred in almost half of the children studied with UDC and UTI.
Thus, all patients with VUR should be screened for frequency, urgency, and incontinence, which suggest UDC’s and voiding dysfunction. Vincent’s curtsy, a squatting maneuver spontaneously employed to prevent incontinence, is particularly suggestive.84 That these UDCs may cause reflux is suggested by an enhanced resolution of reflux with anticholinergic drug therapy. Equally important is the potential for UDCs to cause a false-negative cystogram.
Diagnostic Evaluation
VUR is diagnosed with a cyclic voiding cystourethrogram (VCUG), with either radiopaque contrast medium or radioisotope.86,87 Body temperature contrast material, which is not excessively concentrated, is instilled into the bladder through a small catheter by gravity with modest pressure in a non-anesthetized child.
Natural History
The natural history of VUR is extremely variable, and ranges from spontaneous resolution without nephropathy to clinically silent scar formation to recurrent pyelonephritis with hypertension and end-stage renal disease (ESRD). Numerous factors may contribute to the potential for resolution, including the patient’s age, the grade of reflux, the appearance of the ureteral orifice, the length of the ureteral submucosal tunnel, and the intravesical dynamics. The American Urological Association Pediatric Vesicoureteral Reflux Guidelines Panel in 1997 analyzed 26 reports comprising 1,987 patients with conservative follow-up, to estimate the probability of reflux resolution (Fig. 55-9).88 In general, a lower reflux grade correlated with a better chance of spontaneous resolution.
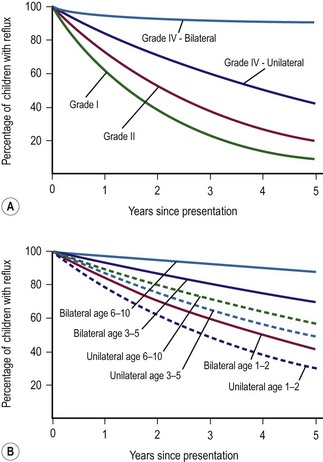
FIGURE 55-9 (A) Percentage chance of reflux persistence, grades I, II, and IV, for 1 to 5 years after presentation. (B) Percentage chance of reflux persistence by age at presentation, grade III, for 1 to 5 years after presentation. (From Elder JS, Peters CA, Arant BS, et al. Report on the Management of Primary Vesicoureteral Reflux in Children. Baltimore: American Urological Association; 1997.)
Younger children are thought to have better prognoses for resolution of VUR, particularly infant males in the first year of life. This may be due to a heightened degree of trigonal growth, but the diminishing prominence of UDCs with age is also likely. Spontaneous resolution is relatively independent of grade in secondary reflux, implicating management of primary bladder dysfunction as the primary prognostic variable (Fig. 55-10).89
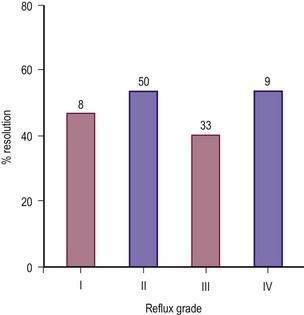
FIGURE 55-10 The rate of spontaneous resolution of secondary vesicoureteral reflux as a function of reflux grade. (From Cohen RA, Roston MG, Belman AB, et al. Renal scarring and vesicoureteral reflux in children with myelodysplasia. J Urol 1990;144:541.)
Renal injury due to VUR may take the form of focal scarring, generalized scarring with atrophy, and failure of renal growth.90 As a result, kidneys drained by refluxing ureters should be observed not only for scarring but also for renal growth with serial upper tract imaging by ultrasound.91 Reflux-induced renal injury is usually a result of the association of VUR with UTI.92 It has been generally considered that such injury is most likely in children under the age of 2 years.71 It is now clear, however, that the risk of renal injury from VUR extends well beyond this age.70,92–94 Reflux can also cause renal injury in the absence of UTI because of the pressure effects from NVD and BOO. The ability of high intravesical pressure, when associated with VUR, to cause renal injury has been confirmed experimentally.95
Significant renal injury in the absence of BOO, NVD, and UTI can be found in infants.96,97 The ureteral bud theory postulates that VUR associated with displacement of the ureteral orifice is associated with anomalies of renal differentiation.98 Such ureters probably do not arise from the appropriate segment of the Wolffian duct and consequently make ectopic contact with the nephrogenic cord, resulting in abnormal renal development. Although this mechanism may be present in some patients, it is now clear that congenital VUR-associated renal injury in the absence of BOO, NVD, and UTI may occur in the presence of a normally positioned ureteral orifice.99 This finding implies that pressure from in utero VUR may injure the developing kidney.
In a longitudinal study of 923 children, high-pressure bladder dynamics, severity of reflux, and frequency of UTIs were the chief contributing factors in the development of new scars or the worsening of old scars.94 Children with low-grade VUR were relatively unlikely to develop progressive renal injury when compared with those children with grades IV and V reflux. A similar relationship is seen in infants and children with secondary reflux. When monitoring these patients for progression of renal injury as an indicator of success of a therapeutic regimen, one must be cognizant of the fact that sonographic evidence of new renal injury may take several months to become apparent.
There is little consensus regarding the long-term sequelae of minor renal scars detected by high-resolution renal cortical scans. However, in a small number of patients, a spectrum of symptomatic nephropathy exists, most notably renal parenchymal hypertension and ESRD. The significance and predominance of reflux nephropathy (RN) as a cause of renal parenchymal hypertension has been reviewed.100 Approximately 30–65% of childhood hypertension is associated with RN. RN is an important cause of end-stage renal failure in children and adults.101–103 The 2008 report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) lists reflux nephropathy as being the primary diagnosis in 5% of 9854 children who received transplants over the previous 20 years.2
Some patients so affected may not have had an evident prior infection or will have the first recognized infection at or near the time of diagnosis of ESRD.101 As histologic evidence of chronic pyelonephritis is found, preceding infection is likely, underscoring the silent progressive nature of RN and the need for meticulous long-term follow-up of children with VUR. Glomerular lesions play an important role in the progression of RN. There is a clear association between RN, ‘heavy’ proteinuria, and glomerular lesions that resemble focal segmental glomerulosclerosis.104 Although the mechanisms of this disease remain uncertain, immunologic injury, macromolecular trapping with mesangial injury, vascular alterations with hypertension, and glomerular hyperfiltration have been implicated. The latter theory of glomerular hyperfiltration is presently favored.
Treatment
Nonoperative management of VUR (Box 55-2) is successful in most patients. Such management may be considered in four stages: (1) diagnostic evaluation; (2) avoidance of infection; (3) voiding dysfunction treatment; and (4) active surveillance. Diagnostic evaluation has been previously reviewed. However, it is important to stress that the exclusion and treatment of voiding dysfunction and BOO is imperative. Patients with problematic uninhibited detrusor contraction can be managed with low-dose anticholinergic medication, such as oxybutynin hydrochloride. Side effects are manageable and include constipation and facial flushing/dry mouth. Voiding dysfunction with retentive characteristics may require timed/double voiding, alpha blockers, biofeedback, and in severe cases, intermittent catheterization (IC). Secondary reflux from neurovesical dysfunction often requires aggressive bladder management with IC and anti-cholinergics.
Good hydration, perineal hygiene, and bowel management are crucial and apply to all patients. With the exception of the asymptomatic toilet-trained child with low-grade reflux and normal kidneys, most children will benefit from low-dose continuous suppressive antibiotics (Table 55-4). It is important to remember that the prophylactic dose is approximately one-quarter the dose to treat an acute infection. Although generally well tolerated and having been a common practice for decades, the long-term implications of chronic antimicrobial suppression remain incompletely investigated and are actively debated.4,45,105 Currently a prospective, multicenter, double-blind, placebo-controlled clinical trial of 600 patients funded by the National Institutes of Health is underway called the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR study).106 This may help further define the role of antibiotic prophylaxis in the management of VUR. Until more evidence-based guidelines are published, the clinician must use caution in treating children with risk factors for recurrent UTI and renal scarring, including VUR.
Once a nonoperative regimen is selected, the patient is committed to long-term, strict surveillance. Renal imaging is performed every six to 12 months, depending on the age at diagnosis and the stability of the disease. Attention is directed at both renal growth as well as the detection of focal scarring. Voiding cystourethrography is generally performed no more often than once a year. The child’s growth, renal function, and blood pressure are monitored. The role of urodynamics has been previously outlined. Cystoscopy is rarely necessary except at the time of antireflux surgery when it is performed to exclude urothelial inflammation and to confirm the position and number of ureteral orifices.
The American Urological Association (AUA) Pediatric Vesicoureteral Reflux Guidelines Panel published their recommendations for management of VUR in children in 1997 and updated their guidelines in 2010. For VUR in an infant diagnosed after a febrile UTI or found to be high grade (III–V), then continuous antibiotic prophylaxis is still recommended. It is also recommended for an older child (>1 year of age) with recurrent febrile UTI, bowel/bladder dysfunction, and/or renal cortical anomalies. For asymptomatic older children with normal kidneys, suppression is an option. Breakthrough UTI management guidelines are summarized in Table 55-5.4,88 There is little solid evidence or consensus about the management of VUR in older school-age patients or about the length of time that the clinician should observe nonoperatively before recommending surgery. The actual decision must be carefully individualized after a thorough discussion of all the treatment options with the parents.
TABLE 55-5
Breakthrough UTI Management According to the 2010 American Urological Association Guidelines
| Clinical Scenario | Recommendation (R)/Option (O) |
| Symptomatic BT-UTI | R: Change of therapy guided by scenario |
| Patient on CAP with febrile BT-UTI | R: Consider open or endoscopic surgical intervention |
| Patient on CAP with single febrile BT-UTI without evidence of existing or new renal cortical abnormalities | O: Change to alternative antibiotics is an option before surgical intervention |
| Patient not on CAP with febrile UTI | R: Initiation of CAP |
| Patient not on CAP with afebrile UTI | R: Initiation of CAP |
| All patients with BT-UTI | O: Open or endoscopic surgical intervention |
BT, breakthrough; CAP, continuous antibiotic prophylaxis.
Adapted from Peters CA, Skoog SJ, Arant BS Jr, et al. Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J Urol 2010;184:1134.
The established principles of successful ureteral reimplantation include: (1) adequate ureteral exposure and mobilization; 2) meticulous preservation of the blood supply; and (3) creation of a valvular mechanism whose submucosal tunnel length to ureteral diameter ratio exceeds 4 : 1. These goals can be attained by a variety of procedures, most commonly via an open Pfannensteil approach but also laparoscopically and robotic-assisted (Fig. 55-11).
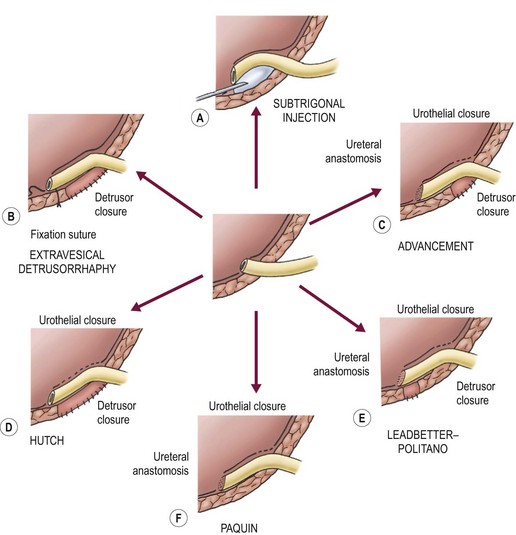
FIGURE 55-11 Conceptual comparison of techniques to correct reflux. A common theme is the achievement of a long length of intravesical ureter based on a strong detrusor floor and covered with compressible urothelium.
Important differences exist between these operative procedures. Variables include (1) presence or absence of a ureteral anastomosis; (2) need for detrusor closure; (3) transgression of urothelium; and (4) whether the neohiatus is fashioned by an appropriately sized detrusor incision or by the closure of the detrusor around the ureter. Performance of a ureteral anastomosis increases the risk of postoperative obstruction, whereas the need for detrusor closure increases the risk of diverticula. Table 55-6 outlines the specific advantages and disadvantages of some representative procedures. Three of the most commonly employed operations for the treatment of primary VUR are diagramed in Figures 55-12 to 55-14.
TABLE 55-6
Specific Advantages and Disadvantages of Commonly Performed Antireflux Procedures
| Procedure | Advantages | Disadvantages |
| Subtrigonal injection | Endoscopic procedure | Material injected: Teflon—migration, granuloma formation Collagen—uncertain durability |
| Extravesical detrusorrhaphy | Bladder never opened No hematuria No ureteral anastomosis Minimal bladder spasms Endoscopically accessible ureteral orifices |
|
| Advancement | Avoids complications of neohiatus formation in Leadbetter–Politano reimplantation | |
| Cohen (transtrigonal) | Transtrigonal: difficult to access ureter endoscopically | |
| Glenn–Anderson | Glenn–Anderson: limited length of tunnel achievable | |
| Hutch | No ureteral anastomosis Good alternative with large associated congenital diverticulum |
|
| Leadbetter–Politano | Excellent ureteral tunnel dimensions with endoscopically accessible ureteral orifices | Risk of ureteral obstruction Risk of sigmoid colon injury with left reimplantation |
| Paquin | Versatility, extremely useful during complex reconstructive procedures |
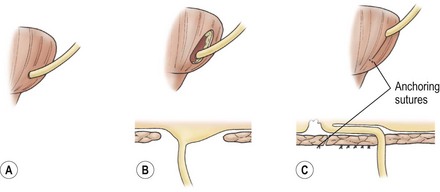
FIGURE 55-12 The extravesical detrusorrhaphy antireflux technique conceptually viewed from behind the bladder. (A) The detrusor is incised. (B) The dissection is continued until the plane between urothelium and muscle has been developed. (C) The ureter is advanced and fixed into position with anchoring sutures. The detrusor is closed.
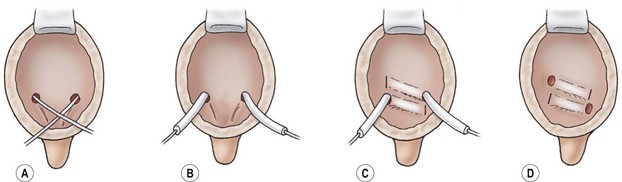
FIGURE 55-13 The Cohen cross-trigonal ureteral reimplantation. (A) The ureter is intubated and the mucosa is incised circumferentially around the ureteral orifice. (B) The ureters are dissected from the muscular attachments and mobilized until free within the retroperitoneum. (C) Cross-trigonal tunnels are created by scissor dissection. (D) The ureteral anastomoses are completed.
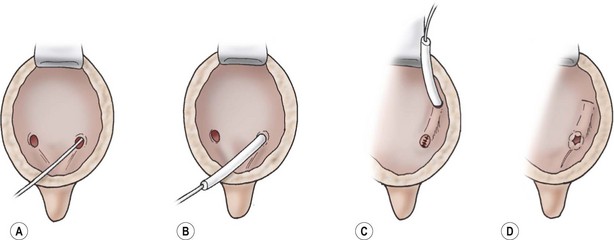
FIGURE 55-14 The Leadbetter–Politano ureteral reimplantation. (A) The ureter is intubated. (B) The ureter is mobilized. The hiatus is dilated, and the retroperitoneal ureter is mobilized. Under direct vision the peritoneum is reflected from the outer surface of the bladder. (C) The neohiatus is created, and the ureter is internalized into the bladder. The tunnel is created by scissor dissection, and the original hiatus is closed. (D) The ureteral anastomosis is completed.
In general, excellent results are nearly uniformly attainable with an open approach and early reports of robotic-assisted laparoscopic outcomes are encouraging.107 A meta-analsysis in 1997 of 86 reports, including 6472 patients (8563 ureters), found overall success for an open ureteral reimplantation to be 96%.88 Success was achieved in 99% with grade I, 99.1% with grade II, 98.3% with grade III, 98.5% with grade IV and 80.7% in grade V.
At our institution, we prefer the extravesical detrusorrhaphy approach (see Fig. 55-12).108–110 Because the lumen of the bladder is not entered, there is no postoperative hematuria, with minimal bladder spasms and a short hospitalization. The absence of a uretero-vesical anastomosis decreases the risk of postoperative obstruction. No ureteral stents, suprapubic catheters, or perivesical drains are needed. The urethral catheter is removed on the first day after unilateral surgery and the second day following bilateral reimplantation. Once the patient voids, he/she is discharged on oral antibiotics for 1 week, then is placed back on suppression for 3 months.
The four major principles of a successful extravesical detrusorrhaphy are: (1) complete mobilization of the ureter from the peritoneal reflection to the UVJ, leaving a wide sheath of its peri-adventitial blood supply; (2) distal fixation of the ureter with long-acting absorbable sutures; (3) wide mobilization of the detrusor muscle flaps to enable firm approximation of the detrusor over the ureter; and (4) development of a sufficient tunnel length. The use of the extravesical detrusorrhaphy has been successfully expanded to include a tapered excisional megaureter repair,109 reimplantation of the ureters associated with paraureteral Hutch diverticula,111 as well as correction of VUR associated with duplicated collecting systems.112
Complications of ureteral reimplantation are rare.88,113 The most common complication is de novo contralateral reflux,114 while the most common technical complications are ureteral obstruction, persistent reflux, and diverticula formation. Persistent reflux may be caused by an insufficient tunnel length to ureteral diameter ratio. However, the greatest risk for postoperative reflux is related to the high-pressure voiding dynamics due to uninhibited bladder contraction, detrusor sphincter dyssnergia, and/or urinary retention. Ureteral obstruction may be due to ureteral kinking (at the neohiatus or obliterated umbilical artery), an excessively high placed neohiatus, construction of a tight neohiatus, twisting, anastomotic stricture, devascularization, and tight tunnel.
With attention directed toward the avoidance of technical complications and the selection of a procedure associated with the lowest complication rate, ureteral reimplantation remains a safe and highly successful operation. The extravesical approach for bilateral ureteral reimplantation has been questioned because of a reportedly high incidence of postoperative urinary retention.115 In our experience with a large group of patients, we have found acceptable rates of temporary incomplete bladder emptying (4%) which is transient and has minimal morbidity.116 Risk factors appear to be infants under age one and girls with large, thin-walled bladders and preexisting retentive voiding dysfunction.
In 1984, a minimally invasive endoscopic procedure for the correction of VUR was reported (Fig. 55-15). This subureteral transurethral injection (STING) utilized polytetrafluoroethylene (Teflon) and has been used successfully outside the USA for many years.117 Many different injectable materials have subsequently been investigated and reported.118–120 This ambulatory procedure performed under a brief general anesthetic has low morbidity and children may return to full activity as soon as the next day. The initial success rates were promising; however, they did not quite match those of ureteral reimplantation.121
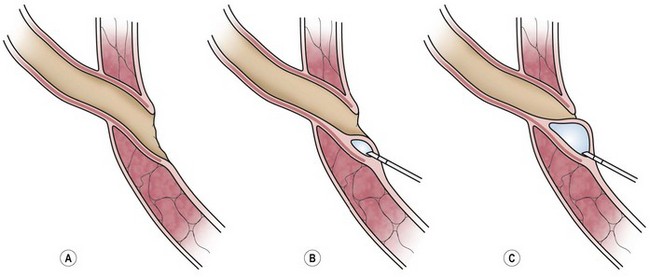
FIGURE 55-15 Technique of subureteric transurethral injection of dextranomer/hyaluronic acid copolymer (STING procedure). (Figure used with permission from the Salix Corporation®, Raleigh, NC, USA.)
In 2001, the Food and Drug Administration approved dextranomer/hyaluronic acid copolymer (Deflux) as the first injectable substance indicated in the USA for grades I-IV vesicoureteral reflux.122 This substance is biodegradable, has no immunogenic properties, and does not seem to have potential for malignant transformation. Results vary, but most studies show success rates of around 70–80% for abolishing reflux.122–125 Higher success rates with larger injected volumes and multiple injection sites have been reported.126 Long-term reflux resolution rates appear to be stable, but recurrence has been found a year after the procedure.127
Other complications such as ureteral obstruction appear to be very low. Dysuria, gross hematuria, and urinary frequency occasionally occur, but are self-limiting. Flank pain and fever are rare. In our institution, a VCUG and renal ultrasound are obtained 3 months after the procedure, but the cystogram is not subsequently repeated if the patient remains asymptomatic off antibiotic prophylaxis. Patients having a febrile UTI after an apparently successful procedure should be re-investigated with a VCUG to assess for recurrent reflux.128
References
1. Hellstrom, A, Hanson, E, Hansson, S, et al. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Child. 1991; 66:232–234.
2. Montini, G, Tullus, K, Hewitt, I. Febrile urinary tract infections in children. N Engl J Med. 2011; 365:239–250.
3. Panaretto, K, Craig, J, Knight, J, et al. Risk factors for recurrent urinary tract infection in preschool children. J Paediatr Child Health. 1999; 35:454–459.
4. Peters, CA, Skoog, SJ, Arant, BS, Jr., et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol. 2010; 184:1134–1144.
5. Freedman, AL. Urologic diseases in North America Project: Trends in resource utilization for urinary tract infections in children. J Urol. 2005; 173:949–954.
6. Shaikh, N, Morone, NE, Lopez, J, et al. Does this child have a urinary tract infection? JAMA. 2007; 298:2895–2904.
7. Hoberman, A, Chao, HP, Keller, DM, et al. Prevalence of urinary tract infection in febrile infants. J Pediatr. 1993; 123:17–23.
8. Smellie, J, Hodson, C, Edwards, D, et al. Clinical and radiological features of urinary infection in childhood. Br Med J. 1964; 14(5419):1222–1226.
9. Bickerton, MW, Duckett, JW. Urinary tract infections in pediatric patients. AUA. 4, 1985. [update series].
10. Liao, JC, Churchill, BM. Pediatric urine testing. Pediatr Clin North Am. 2001; 48:1425–1440.
11. Pollack, CV, Jr., Pollack, ES, Andrew, ME. Suprapubic bladder aspiration versus urethral catheterization in ill infants: Success, efficiency and complication rates. Ann Emerg Med. 1994; 23:225–230.
12. Li, PS, Ma, LC, Wong, SN. Is bag urine culture useful in monitoring urinary tract infection in infants? J Paediatr Child Health. 2002; 38:377–381.
13. Edelmann, CM, Ogw, JE, Fine, BP, et al. The prevalence of bacteriuria in full-term and premature newborn infants. J Pediat. 1973; 82:125–132.
14. Aronson, AS, Gustafson, B, Svenningsen, NW. Combined suprapubic aspiration and clean voided urine examination in infants and children. Acta Paediatr Scand. 1973; 62:396–400.
15. Iravani, A. Treatment of urinary tract infections in young women. AUA. 12, 1993. [update series].
16. Hellerstein, S. Recurrent urinary tract infections in children. Pediatr Infect Dis. 1982; 1:271–281.
17. Pryles, CV, Lustik, B. Laboratory diagnosis of urinary tract infection. Pediatr Clin North Am. 1971; 18:233.
18. Hoberman, A, Wald, ER, Reynolds, EA, et al. Pyuria and bacteriuria in urine specimens obtained by catheter from young children with fever. J Pediatr. 1994; 124:513–519.
19. Gorelick, MH, Shaw, KN. Screening tests for urinary tract infection in children: A meta-analysis. Pediatrics. 1999; 104:e54.
20. Lohr, JA, Portilla, MG, Geuder, TG, et al. Making a presumptive diagnosis of urinary tract infection by using a urinalysis performed in on-site laboratory. J Pediatr. 1993; 122:22–25.
21. Durbin, WA, Peters, G. Management of urinary tract infections in infants and children. Pediatr Infect Dis. 1984; 3:564–574.
22. Liptak, GS, Campbell, J, Stewart, R, et al. Screening for urinary tract infection in children with neurogenic bladders. Am J Physical Med & Rehab. 1993; 72:122–126.
23. Sheldon, CA, Gonzalez, R. Differentiation of upper and lower urinary tract infections: How and when? Med Clin North Am. 1984; 68:321–333.
24. Pecile, P, Romanello, C. Procalcitonin and pyelonephritis in children. Curr Opin Infect Dis. 2007; 20:83–87.
25. Fowler, J, Stamey, T. Studies of introital colonization in women with recurrent urinary infections. VII. The role of bacterial adherence. J Urol. 1977; 117:472–476.
26. Stamey, TA, Mihara, G. Studies of introital colonization in women with recurrent urinary tract infection. VI. Analysis of segmented leukocytes in vaginal vestibule in relation to enterobacterial colonization. J Urol. 1976; 116:72–73.
27. Roberts, JA. Pathogenesis of nonobstructive urinary tract infections in children. J Urol. 1990; 144:475–480.
28. Rushton, HG, Majd, M. Pyelonephritis in male infants: How important is the foreskin? J Urol. 1992; 148:733–738.
29. Parsons, CL, Greenspan, C, Mullholland, SG. The primary antibacterial defense mechanism of the bladder. Invest Urol. 1975; 13:72–78.
30. Koff, SA, Murtagh, DS. The uninhibited bladder in children: Effect of treatment on recurrence of urinary infection and on vesicoureteral reflux resolution. J Urol. 1983; 130:1138–1141.
31. Koff, SA, Wagner, TT, Jayanthi, VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998; 160:1019–1022.
32. Asscher, AW, Sussman, M, Waters, WE, et al. Urine as a medium for bacterial growth. Lancet. 1966; 2:1037–1041.
33. Mannhardt, W, Schofer, O, Schulte-Wiserman, H. Pathogenic factors in recurrent urinary tract infection and renal scar formation in children. Eur J Pediatr. 1986; 145:330–336.
34. Smellie, J, Normand, I. Urinary tract infection: Clinical aspects. In: Johnston JH, Williams DI, eds. Paediatric Urology. London: Butterworths; 1982:95.
35. Hanson, L. Prognostic indicators in childhood urinary infection. Kidney Int. 1982; 21:659–667.
36. Rushton, HG, Majd, M, Jantausch, B, et al. Renal scarring following reflux and nonreflux pyelonephritis in children: Evaluation with 99 mtechnetium-dimercaptosuccinic acid scintigraphy. J Urol. 1992; 147:1327–1332.
37. Shaikh, N, Ewing, AL, Bhatnagar, S, et al. Risk of renal scarring in children with a first urinary tract infection: A systematic review. Pediatrics. 2010; 126:1084–1091.
38. Wallace, D, Rothwell, D, Williams, D. The long term follow up of surgically treated vesicoureteral reflux. Br J Urol. 1978; 50:479–484.
39. Still, J, Cottom, D. Severe hypertension in childhood. Arch Dis Child. 1967; 42:34–39.
40. Scott, J. Hypertension, reflux and renal scarring. In: Johnston JH, ed. Management of Vesicoureteral Reflux. Baltimore: Wiliams and Wilkins; 1984:60.
41. Sheldon, CA, Geary, DF, Shely, EA, et al. Surgical consideration in childhood end-stage renal disease. Pediatr Clin North Am. 1987; 34:1187–1207.
42. Cascio, S, Chertin, B, Yoneda, A, et al. Acute renal damage in infants after first urinary tract infection. Pediatr Nephrol. 2002; 17:503–505.
43. Chon, CH, Lai, FC, Shortliffe, LM. Pediatric urinary tract infections. Pediatr Clin North Am. 2001; 48:1441–1459.
44. UTI Guideline Team C. Evidence-based care guideline for medical management of first urinary tract infection in children 12 years of age or less. (Cincinnati Children’s Hospital Medical Center) Guidelines 2006;7:1–23.
45. Roberts, KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610.
46. Wan, J, Skoog, SJ, Hulbert, WC, et al. Section on Urology response to new guidelines for the diagnosis and management of UTI. Pediatrics. 2012; 129:e1051–e1053.
47. Craig, JC, Wheeler, DM, Irwig, L, et al. How accurate is dimercaptosuccinic acid scintigraphy for the diagnosis of acute pyelonephritis? A meta-analysis of experimental studies. J Nucl Med. 2000; 41:986–993.
48. Kogan, BA, Kay, R, Wasnick, RJ, et al. 99mTc-DMSA scanning to diagnose pyelonephritic scarring in children. Urology. 1983; 21:641–644.
49. Hiraoka, M, Hashimoto, G, Tsuchida, S, et al. Early treatment of urinary infection prevents renal damage on cortical scintigraphy. Pediatr Nephrol. 2003; 18:115–118.
50. Hoberman, A, Wald, ER, Hickey, RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999; 104:79–86.
51. Montini, G, Toffolo, A, Zucchetta, P, et al. Antibiotic treatment for pyelonephritis in children: Multicentre randomised controlled non-inferiority trial. BMJ. 2007; 335:386.
52. Johnson, CE, Maslow, JN, Fattlar, DC, et al. The role of bacterial adhesions in the outcome of childhood urinary tract infections. Amer J Dis Children. 1993; 147:1090–1093.
53. Keren, R, Chan, E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 109, 2002. [E70–E70].
54. Mattoo, TK. Evidence for and against urinary prophylaxis in vesicoureteral reflux. Pediatr Nephrol. 2010; 25:2379–2382.
55. Hutch, JA, Bunge, RG, Flocks, RH. Vesicoureteral reflux in children. J Urol. 1955; 74:607–620.
56. Hodson, CJ, Edwards, D. Chronic pyelonephritis and vesico-ureteric reflux. Clin Radiol. 1960; 11:219–231.
57. Noe, HN. The current status of screening for vesicoureteral reflux. Pediatr Nephrol. 1995; 9:638–641.
58. King, LR, Kazmi, SO, Belman, AB. Natural history of vesicoureteral reflux: Outcome of a trial of nonoperative therapy. Urol Clin North Am. 1974; 1:441–455.
59. Stephens, FD, Lenaghan, D. Anatomical basis and dynamics of vesicoureteral reflux. J Urol. 1962; 87:669–680.
60. Eckman, H, Jacobsson, B, Kock, NG, et al. High diuresis: A factor in preventing vesicoureteral reflux. J Urol. 1966; 95:511–515.
61. Rolleston, GL, Maling, TM, Hodson, CJ. Intrarenal reflux and the scarred kidney. Arch Dis Child. 1974; 49:531–539.
62. Hodson, CJ, Maling, TM, McManamon, PH, et al, The pathogenesis of reflux nephropathy. Br J Radiol (suppl) 1975;(Suppl. 13):1–26.
63. Thomsen, H, Talner, LB, Higgins, CB. Intrarenal backflow during retrograde pyelography with graded intrapelvic pressure. A radiologic study. Invest Radiol. 1982; 17:593–603.
64. Hodson, CJ, Twohill, SA. The time factor in the development of sterile reflux scarring following high pressure vesicoureteral reflux. Contr Nephrol. 1984; 39:358–369.
65. Medical versus surgical treatment of primary vesicoureteral reflux: Report of the International Reflux Study Committee. Pediatrics. 1981; 67:392–400.
66. Arant, BS, Jr. Vesicoureteric reflux and renal injury. Am J Kid Dis. 1991; 17:49–511.
67. Mattoo, TK. Vesicoureteral reflux and reflux nephropathy. Adv Chronic Kidney Dis. 2011; 18:348–354.
68. Bourchier, D, Abbott, GD, Maling, TM. Radiological abnormalities in infants with urinary tract infections. Arch Dis Child. 1984; 59:620–624.
69. Sargent, MA, Stringer, DA. Voiding cystourethrography in children with urinary tract infection: The frequency of vesicoureteric reflux is independent of the specialty of the physician requesting the study. AJR. 1995; 164:1237–1241. [164].
70. Benador, D, Benador, N, Slosman, D, et al. Are younger children at highest risk of renal sequelae after pyelonephritis? Lancet. 1997; 349:17–19.
71. Ditchfield, M, deCampo, JF, Nolan, TM, et al. Risk factors in the developement of early renal cortical defects in children with urinary tract infection. AJR. 1994; 162:1393–1397.
72. Deckter, RM, Roth, DR, Gonzales, ET. Vesicoureteral reflux in boys. J Urol. 1988; 40:1089–1091.
73. Noe, HN. The long-term results of prospective sibling reflux screening. J Urol. 1992; 148:1739–1742.
74. Wan, J, Greenfield, SP, Ng, M, et al. Sibling reflux: A dual center retrospective study. J Urol. 1996; 156:677–679.
75. Chertin, B, Puri, P. Familial vesicoureteral reflux. J Urol. 2003; 169:1804–1808.
76. Noe, HN, Wyatt, RJ, Peeden, JN, Jr., et al. The transmission of vesicoureteral reflux from parent to child. J Urol. 1992; 148:1869–1871.
77. McLorie, GA, Sheldon, CA, Fleisher, M, et al. The genitourinary system in patients with imperforate anus. J Pediatr Surg. 1987; 22:1100–1104.
78. DeFoor, W, Minevich, E, Tackett, L, et al. Ectopic ureterocele: clinical application of classification based on renal unit jeopardy. J Urol. 2003; 169:1092–1094.
79. Henneberry, MD, Stephens, FD. Renal hypoplasia and dysplasia in infants with posterior urethral valves. J Urol. 1980; 123:912–915.
80. Sheldon, CA, Cormier, M, Crone, K, et al. Occult neurovesical dysfunction in children with imperforate anus. J Pediatr Surg. 1991; 26:49–54.
81. Agarwal, SK, Khoury, AE, Abramson, RP, et al. Outcome analysis of vesicoureteral reflux in children with myelodysplasia. J Urol. 1997; 157:980–982.
82. Koff, SA. Relationship between dysfunctional voiding and reflux. J Urol. 1992; 148:1703–1705.
83. Chandra, M, Maddix, H, McVicar, M. Transient urodynamic dysfunction of infancy: Relationship to urinary tract infections and vesicoureteral reflux. J Urol. 1996; 155:673–677.
84. van Gool, JD, Hjalmas, K, Tamminen-Mobius, T, et al. Historical clues to the complex of dysfunctional voiding, urinary tract infection and vesicoureteral reflux. The International Study in Children. J Urol. 1992; 148:1699–1702.
85. Koff, SA, Lapides, J, Piazza, DH. Association of urinary tract infections and reflux with uninhibited bladder contractions and voluntary sphincteric obstruction. J Urol. 1979; 122:373–376.
86. Paltiel, HJ, Rupich, RC, Kiruluta, HG. Enhanced detection of vesicoureteral reflux in infants and children with use of cyclic voiding cystourethrography. Radiology. 1992; 184:753–755.
87. Lebowitz, RL. The detection and characterization of vesicoureteral reflux in child. J Urol. 1992; 148:1640–1642.
88. Elder, JS, Peters, CA, Arant, BS, Jr., et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. 1997; 157:1846–1851.
89. Cohen, RA, Rushton, HG, Belman, AB, et al. Renal scarring and vesicoureteral reflux in children with myelodysplasia. J Urol. 1990; 144:541–544.
90. Smellie, JM, Normand, ICS. Reflux nephropathy in childhood. In: Hodson J, Kincaid-Smith P, eds. Reflux Nephropathy. New York: Masson; 1979:14.
91. Claesson, I, Jacobsson, B, Olsson, T, et al. Assessment of renal parenchymal thickness in normal children. Acta Radiol Diagn. 1981; 22:305–314.
92. Smellie, JM, Ransley, PG, Normand, ICS, et al. Development of new renal scars: A collaborative study. Br Med J. 1985; 290:1957–1960.
93. McLorie, GA, McKenna, PH, Jumper, BM, et al. High grade vesicoureteral reflux: Analysis of observational therapy. J Urol. 1990; 144:537–540.
94. Shimada, K, Matsui, T, Ogino, T, et al. Renal growth and progression of reflux nephropathy in children with vesicoureteral reflux. J Urol. 1988; 140:1097–1100.
95. Ransley, PG, Risdon, RA. Reflux and renal scarring. Br J Radiol Suppl. 1978; 14:1.
96. Yeung, CK, Godley, ML, Dhillon, HK, et al. The characteristics of primary vesico-ureteric reflux in male and female infants with pre-natal hydronephrosis. Br J Urol. 1997; 80:319–327.
97. Marra, G, Barbieri, G, Dell’Agnola, CA, et al. Congenital renal damage associated with primary vesicoureteral reflux detected prenatally in male infants. J Pediatrics. 1994; 124:726–730.
98. Makie, GG, Stephens, FD. Duplex kidneys: A correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975; 114:274–280.
99. Najmaldin, A, Burge, DM, Atwell, JD. Reflux nephropathy secondary to intrauterine reflux. J Pediatr Surg. 1990; 25:387–390.
100. Cortez, J, Sheldon, CA. Focal and diffuse renal parenchymal lesions associated with hypertension: the urologic surgeon’s approach to evaluation and managemen. In: Loggie J, ed. Pediatric and Adolescent Hypertension. Cambridge: Blackwell Scientific; 1991:217.
101. Salvatierra, O, Kountz, SL, Belzer, FO. Primary vesicoureteral reflux and end-stage renal disease. JAMA. 1973; 226:1454–1456.
102. McEnery, PT, Alexander, SR, Sullivan, K, et al. Renal transplantation in children and adolescents: The 1992 annual report of the North American pediatric Renal Transplant Cooperative Study. Ped Nephr. 1993; 7:711–720.
103. Avner, ED, Chavers, B, Sullivan, K, et al. Renal transplantation and chronic dialysis in children and adolescents: The 1993 annual report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 1995; 9:61–73.
104. Hinchliffe, SA, Kreczy, A, Ciftci, AO, et al. Focal and segmental glomerulosclerosis in children with reflux nephropathy. Ped Pathology. 1994; 14:327–338.
105. Williams, GJ, Wei, L, Lee, A, et al. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. (3):2006.
106. Mathews, R, Carpenter, M, Chesney, R, et al. Controversies in the management of vesicoureteral reflux: The rationale for the RIVUR study. J Pediatr Urol. 2009; 5:336–341.
107. Casale, P, Kojima, Y. Robotic-assisted laparoscopic surgery in pediatric urology: an update. Scand J Surg. 2009; 98:110–119.
108. Zaontz, MR, Maizels, M, Sugar, EC, et al. Detrusorrhaphy: Extravesical ureteral advancement to correct vesicoureteral reflux in children. J Urol. 1987; 138:947–949.
109. Wacksman, J, Gilbert, A, Sheldon, CA. Results of the renewed extravesical reimplant for surgical correction of vesicoureteral reflux. J Urol. 1992; 148:359–361.
110. Minevich, E, Sheldon, CA. Extravesical detrusorrhaphy (ureteroneocystostomy). AUA Updates. 2001. [XX].
111. Jayanthi, VR, McLorie, GA, Khoury, AE, et al. Extravesical detrusorrhaphy for refluxing ureters associated with paraureteral diverticula. Urology. 1995; 45:664–666.
112. Minevich, E, Tackett, L, Wacksman, J, et al. Extravesical common sheath detrusorrhaphy (ureteroneocystotomy) and reflux in duplicated collecting systems. J Urol. 2002; 167:288–290.
113. Gibbons, MD, Gonzales, ET. Complications of antireflux surgery. Urol Clin North Am. 1983; 10:489–501.
114. Minevich, E, Wacksman, J, Lewis, AG, et al. Incidence of contralateral vesicoureteral reflux following unilateral extravesical detrusorrhaphy (ureteroneocystostomy). J Urol. 1998; 159:2126–2128.
115. Fung, LC, McLorie, GA, Jain, U, et al. Voiding efficiency after ureteral reimplantation: A comparison of extravesical and intravesical techniques. J Urol. 1995; 153:1972–1975.
116. Minevich, E, Aronoff, D, Wacksman, J, et al. Voiding dysfunction after bilateral extravesical detrusorrhaphy. J Urol. 1998; 160:1004–1006.
117. O’Donnell, B, Puri, P. Treatment of vesicoureteric reflux by endoscopic injection of Teflon. Br Med J (Clin Res Ed). 1984; 289:7–9.
118. Leonard, MP, Canning, DA, Peters, CA, et al. Endoscopic injection of glutaraldehyde cross-linked bovine dermal collagen for correction of vesicoureteral reflux. J Urol. 1991; 145:115–119.
119. Smith, DP, Kaplan, WE, Oyasu, R. Evaluation of polydimethylsiloxane as an alternative in the endoscopic treatment of vesicoureteral reflux. J Urol. 1994; 152:1221–1224.
120. Diamond, DA, Caldamone, AA. Endoscopic correction of vesicoureteral reflux in children using autologous chondrocytes: Preliminary results. J Urol. 1999; 162:1185–1188.
121. Chertin, B, Kocherov, S. Long-term results of endoscopic treatment of vesicoureteric reflux with different tissue-augmenting substances. J Pediatr Urol. 2010; 6:251–256.
122. Elder, JS, Diaz, M, Caldamone, AA, et al. Endoscopic therapy for vesicoureteral reflux: A meta-analysis. I. Reflux resolution and urinary tract infection. J Urol. 2006; 175:716–722.
123. Higham-Kessler, J, Reinert, SE, Snodgrass, WT, et al. A review of failures of endoscopic treatment of vesicoureteral reflux with dextranomer microspheres. J Urol. 2007; 177:710–714.
124. Yucel, S, Gupta, A, Snodgrass, W. Multivariate analysis of factors predicting success with dextranomer/hyaluronic acid injection for vesicoureteral reflux. J Urol. 2007; 177:1505–1509.
125. Kirsch, AJ, Perez-Brayfield, M, Smith, EA, et al. The modified sting procedure to correct vesicoureteral reflux: Improved results with submucosal implantation within the intramural ureter. J Urol. 2004; 171:2413–2416.
126. Kalisvaart, JF, Scherz, HC, Cuda, S, et al. Intermediate to long-term follow-up indicates low risk of recurrence after Double HIT endoscopic treatment for primary vesico-ureteral reflux. J Pediatr Urol. 2012; 8:359–365.
127. Lee, EK, Murphy, JP, Gatti, JM, et al. Long term follow-up of Dextranomer/Hyaluronic acid for reflux: Late failure warrants continued follow-up. J. Urol. 2009; 181:1869–1875.
128. Traxel, E, DeFoor, W, Reddy, P, et al. Risk factors for urinary tract infection after dextranomer/hyaluronic acid endoscopic injection. J Urol. 2009; 182:1708–1712.