Chapter 541 Urinary Lithiasis
Urinary lithiasis in children is less common in the USA than in other parts of the world. The wide geographic variation in the incidence of lithiasis in childhood is related to climatic, dietary, and socioeconomic factors. Approximately 7% of urinary calculi occur in children <16 yr of age. In the USA, many children with stone disease have a metabolic abnormality. The exceptions are patients with a neuropathic bladder (Chapter 536), who are prone to infection-initiated renal stones, and those who have urinary tract reconstruction with small or large intestine, which predisposes to bladder calculi. The incidence of metabolic stones is similar in boys and girls; they are most common in southeastern USA and are rare in African-Americans. In Southeast Asia, urinary calculi are endemic and are related to dietary factors. Contamination of formula with the organic base and unethical nitrogen-containing food additive melamine has been reported in China.
Stone Formation
Approximately 75% of all stones contain calcium as a major constituent, and 60% are composed of calcium oxalate. Most “spontaneous” stones are composed of calcium, oxalate, or phosphate crystals; others are due to uric acid, cystine, ammonium crystals, or phosphate crystals, or a combination of these substances (Table 541-1). The risk of stone formation increases in the presence of increasing concentrations of these crystals and is reduced with increasing concentrations of inhibitors. Renal calculi develop from crystals that form on the calyx and aggregate to form a calculus. Bladder calculi may be stones that formed in the kidney and traveled down the ureter, or they can form primarily in the bladder.
Table 541-1 CLASSIFICATION OF UROLITHIASIS
CALCIUM STONES (CALCIUM OXALATE AND CALCIUM PHOSPHATE)*
CYSTINE STONES
Cystinuria
STRUVITE STONES (MAGNESIUM AMMONIUM PHOSPHATE)
URIC ACID STONES
INDINAVIR STONES
MELAMINE
NEPHROCALCINOSIS
ACTH, adrenocorticotropic hormone.
Diagnosis
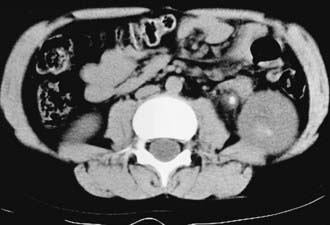
In a child with suspected renal colic, there are multiple imaging options. The most accurate study is an unenhanced spiral CT scan of the abdomen and pelvis (Fig. 541-1). This study takes only a few minutes to perform, has 96% sensitivity and specificity in delineating the number and location of calculi, and demonstrates whether the involved kidney is hydronephrotic. However, the radiation exposure is high. An alternative is to obtain a plain radiograph of the abdomen and pelvis plus a renal ultrasonogram. These studies can demonstrate hydronephrosis and possibly the calculus on the radiograph; however, the calculus is not visualized on sonography unless it is adjacent to the bladder. Consequently, the clinician needs to carefully balance the risks of CT imaging against the lower sensitivity of the plain abdominal film plus sonography.
In 2008 the Society for Pediatric Radiology initiated the Imaging Gently initiative to educate providers on the risks of radiologic imaging in children and to encourage the use of limited imaging in children, particularly those with suspected urolithiasis (http://www.pedrad.org/associations/5364/ig/). This approach was also advocated by the National Quality Forum. In a child with an already-diagnosed calculus, serial plain x-rays or renal ultrasonography can be used to follow the status of the calculus, such as whether it has grown or diminished in size or has moved. If a child has a renal pelvic calculus, a ureteropelvic junction obstruction should be suspected. In some cases, it can be difficult to determine whether hydronephrosis in such a child is secondary to an obstructing stone or the ureteropelvic junction obstruction, or both.
Metabolic Evaluation
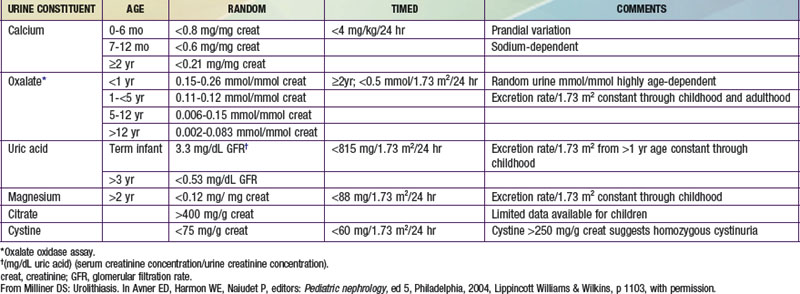
A metabolic evaluation for the most common predisposing factors should be undertaken in all children with urolithiasis, bearing in mind that structural, infectious, and metabolic factors often coexist. This evaluation should not be undertaken in a child who is in the process of passing a stone, because the altered diet and hydration status, as well as the effect of obstruction on the kidney, can alter the results of the study. The basic laboratory studies required are listed in Table 541-2, and the normal values for 24-hr urine collections are shown in Table 541-3. In children with hypercalciuria, further studies of calcium excretion with dietary calcium restriction and calcium loading are necessary.
Pathogenesis of Specific Renal Calculi
Calcium Oxalate and Calcium Phosphate Calculi
Most urinary calculi in children in the USA are composed of calcium oxalate and/or calcium phosphate. The most common metabolic abnormality in these patients is normocalcemic hypercalciuria. Between 30% and 60% of children with calcium stones have hypercalciuria without hypercalcemia. Other metabolic aberrations that predispose to stone disease include hyperoxaluria, hyperuricosuria, hypocitruria, heterozygous cystinuria, hypomagnesuria, hyperparathyroidism, and renal tubular acidosis (Chapter 523).
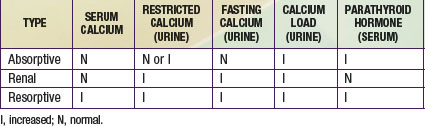
Hypercalciuria may be absorptive, renal, or resorptive. The primary disturbance in absorptive hypercalciuria is intestinal hyperabsorption of calcium. In some children, an increase in 1,25-dihydroxyvitamin D is associated with the increased calcium absorption, and in others the process is independent of vitamin D. Renal hypercalciuria refers to impaired renal tubular reabsorption of calcium (Chapter 513.8). Renal leak of calcium causes mild hypocalcemia, which triggers an increased production of parathyroid hormone, with increased intestinal absorption of calcium and increased mobilization of calcium stores. Resorptive hypercalciuria is uncommon and is found in patients with primary hyperparathyroidism. Excess parathyroid hormone secretion stimulates intestinal absorption of calcium and mobilization of calcium stores. A brief summary of the metabolic evaluation of children with hypercalciuria is shown in Table 541-4.
Enteric hyperoxaluria refers to disorders such as inflammatory bowel disease (Chapter 328), pancreatic insufficiency (Chapter 342), and biliary disease, in which there is gastrointestinal malabsorption of fatty acids, which bind intraluminal calcium and form salts that are excreted in the feces. Normally, calcium forms a complex with oxalate to reduce oxalate absorption, but if calcium is unavailable, there is increased absorption of unbound oxalate.
Renal tubular acidosis (RTA) is a syndrome involving a disturbance of acid-base balance within the kidney that can be classified into three types, one of which predisposes to renal calculi that typically are calcium phosphate (Chapter 523). In type 1, the distal nephron does not secrete hydrogen ion into the distal tubule. The urine pH is never <5.8, and hyperchloremic hypokalemic acidosis results. Patients acquire nephrolithiasis, nephrocalcinosis, muscle weakness, and osteomalacia. Type 1 RTA can be an autosomal dominant disorder, but more often it is acquired and associated with systemic diseases such as Sjögren syndrome, Wilson disease, primary biliary cirrhosis, and lymphocytic thyroiditis, or it results from amphotericin B, lithium, or toluene (an organic solvent associated with glue sniffing).
From 5-8% of patients with cystic fibrosis (Chapter 395) have urolithiasis. Typically the stones are calcium, and they often become manifest in adolescence or young adulthood. Microscopic nephrocalcinosis also occurs in younger children with the disease. These patients do not have hypercalciuria, and the propensity for urolithiasis has been speculated to result from an inability to excrete a sodium chloride load or from intestinal malabsorption.
Struvite Calculi
Urinary tract infections (Chapter 532) caused by urea-splitting organisms (most often Proteus spp, and occasionally Klebsiella spp, Escherichia coli, Pseudomonas spp, and others) result in urinary alkalinization and excessive production of ammonia, which can lead to the precipitation of magnesium ammonium phosphate (struvite) and calcium phosphate. In the kidney, the calculi often have a staghorn configuration, filling the calyces. The calculi act as foreign bodies, causing obstruction, perpetuating infection, and causing gradual kidney damage. Patients with struvite stones also can have metabolic abnormalities that predispose to stone formation. These stones often are seen in children with neuropathic bladder dysfunction, particularly those who have undergone an ileal conduit procedure (Chapter 536). Struvite stones can form in the reconstructed bladder of children who have undergone urinary tract reconstruction with augmentation cystoplasty or continent diversion, or both.
Uric Acid Calculi
Hyperuricosuria can result from various inborn errors of purine metabolism that lead to overproduction of uric acid, the end product of purine metabolism in humans. Children with the Lesch-Nyhan syndrome (Chapter 83) and patients with glucose-6-phosphatase deficiency (Chapter 81) form urate calculi as well. In children with short-bowel syndrome, and particularly those with ileostomies, chronic dehydration and acidosis sometimes are complicated by uric acid lithiasis.
Treatment
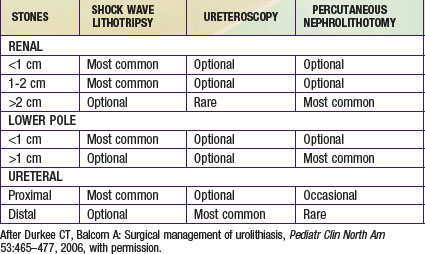
If the calculus does not pass or seems unlikely to pass or if there is associated urinary tract infection, removal is necessary (Table 541-5). Lithotripsy of bladder, ureteral, and small renal pelvic calculi using the holmium laser through a flexible or rigid ureteroscope is quite effective. Extracorporeal shock wave lithotripsy has been successfully applied to children with renal and ureteral stones, with a success rate of >75%. Another alternative is percutaneous nephroscopy, in which access to the renal pelvis is obtained percutaneously, and the calculi are broken down by ultrasonic lithotripsy. In cases in which these modalities are suboptimal, an alternative is laparoscopic removal; this procedure can be performed using the da Vinci robot.
In children with urolithiasis, the underlying metabolic disorder should be addressed (Table 541-6). Because lithiasis results from a too-high concentration of specific substances in the urine, maintaining a continuous high urine output by maintaining a high fluid intake often is an effective method of preventing further stones. The high fluid intake should be continued at night, and usually it is necessary for the child to get up at least once at night to urinate and drink more water.
Table 541-6 SUGGESTED THERAPY FOR UROLITHIASIS CAUSED BY METABOLIC ABNORMALITIES
| METABOLIC ABNORMALITY | INITIAL TREATMENT | SECOND-LINE TREATMENT |
|---|---|---|
| Hypercalciuria | Reduction of dietary Na+ | Potassium citrate |
| Dietary calcium at RDA | Neutral phosphate | |
| Thiazides | ||
| Hyperoxaluria | Adjustment of dietary oxalate | Neutral phosphate* |
| Potassium citrate | Magnesium | |
| Pyridoxine* | ||
| Hypocitric aciduria | Potassium citrate | |
| Bicarbonate | ||
| Hyperuricosuria | Alkalinization | Allopurinol |
| Cystinuria | Alkalinization | Tiopronin (Thiola) |
| Reduction of dietary Na+ | D-Penicillamine | |
| Captopril |
RDA, recommended dietary allowance.
* Initial therapy in primary hyperoxaluria.
From Milliner DS: Urolithiasis. In Avner ED, Harmon WE, Niaudet P, editors: Pediatric nephrology, ed 5, Philadelphia, 2004, Lippincott Williams & Wilkins, p 1104, with permission.
Al-Marhoon MS, Sarhan OM, Awad BA, et al. Comparison of endourological and open cystolithotomy in the management of bladder stones in children. J Urol. 2009;181:2684-2687.
Lam HS, Ng PC, Chu WCW, et al. Renal screening in children after exposure to low dose melamine in Hong Kong: cross sectional study. BMJ. 2008;337:12991.
Landau EH, Shenfeld OZ, Pode D, et al. Extracorporeal shock wave lithotripsy in prepubertal children: 22-year experience at a single institution with a single lithotriptor. J Urol. 2009;182:1835-1839.
Lottmann H, Gagnadoux MF, Daudan M. Urolithiasis in children. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. ed 2. Philadelphia: Saunders; 2010:631-661.
McNally MA, Pyzik PL, Rubenstein JE, et al. Empiric use of potassium citrate reduces kidney stone incidence with the ketogenic diet. Pediatrics. 2009;124:e300-e304.
The Medical Letter. Drugs for kidney stones. Med Lett. 2010;52(1352):93-94.
Nelson CP, Diamond DA, Cendron M, et al. Extracorporeal shock wave lithotripsy in pediatric patients using a late generation portable lithotripter: experience at Children’s Hospital Boston. J Urol. 2008;180:1865-1868.
Passerotti C, Chow JS, Silva A, et al. Ultrasound versus computerized tomography for evaluating urolithiasis. J Urol. 2009;182:1829-1834.
Persaud AC, Stevenson MD, McMahon DR, et al. Pediatric urolithiasis: clinical predictors in the emergency department. Pediatrics. 2009;124:888-893.
Preminger GM, Tiselius HG, Assimos DG, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178:2418-2434.
Riccabona M, Avni FE, Blickman JG, et al. Imaging recommendations in paediatric uroradiology. Minutes of the ESPR Uroradiology Task Force session on childhood obstructive uropathy, high-grade fetal hydronephrosis, childhood haematuria, and urolithiasis in childhood. ESPR Annual Congress, Edinburgh, UK, June 2008. Pediatr Radiol. 2009;39:891-898.
Sas DJ, Hulsey TC, Shatat IF, et al. Increasing incidence of kidney stones in children evaluated in the emergency department. J Pediatr. 2010;157:132-137.
Shouman AM, Ziada AM, Ghoneim IA, et al. Extracorporeal shock wave lithotripsy monotherapy for renal stones >25 mm in children. Urology. 2009;74:109-111.
Srivastava T, Winston MJ, Auron A, et al. Urine calcium/citrate ratio in children with hypercalciuric stones. Pediatr Res. 2009;66:85-90.
Vezzoli G, Soldati L, Gambara G. Hypercalciuria revisited: one or many conditions? Pediatr Nephrol. 2008;23:503-506.
Worcester EM, Coe FL. Calcium kidney stones. N Engl J Med. 2010;363(10):954-962.
Zhu SL, Li JH, Chen L, et al. Conservative management of pediatric nephrolithiasis caused by melamine-contaminated milk powder. Pediatrics. 2009;123:e1099-e1102.