CHAPTER 64 UPPER EXTREMITY VASCULAR TRAUMA
Trauma to the upper extremities can have devastating results. The experience of wartime has taught many lessons about etiology, diagnosis, and treatment of upper extremity injury. Understanding the lessons learned from history provides the basis for treatment today.
Hemorrhage from injured blood vessels has been a well-known consequence of injury for several millennia. The diagnosis was made even in ancient times, but the treatment options were limited. Styptics were prepared from vegetable or mineral material and applied to bleeding vessels. Archigenes first advocated amputation of gangrenous extremities above the line of demarcation with linen ligatures placed on the vessels in the first century ACE.1 Celsus records the first account of vessel ligation to establish hemostasis in 25 ACE1. These ancient recommendations were lost in later centuries, and mass cautery became the standard practice for extremity hemorrhage control until the late 1400s. Ambroise Pare reestablished the technique of amputation above the line of demarcation with linen ligatures of the injured vessels in 1552.1 The tourniquet was introduced in 1674 by Morel. Direct vessel ligation was the treatment of choice by the 19th century, but the results were often disappointing. There was a 100% mortality associated with ligation of the aorta in 10 patients, a 77% mortality with ligation of the common iliac artery in 68 patients, and in 31 patients a 40% mortality when the femoral artery was ligated.2
The first known successful repair of an injured artery was performed in 1759 by Hallowell.3 In 1889, Jassinowsky performed arterial reconstruction in animals and proved that interrupted silk sutures could successfully repair the carotid artery.4 Murphy in Chicago was the first to successfully perform an end-to-end anastomosis on a femoral artery in 1897. He had firm beliefs that successful repair in vascular trauma required complete asepsis, atraumatic technique, temporary clamping of the vessel, accurate approximation, and meticulous hemostasis and cleansing of the wound.2,3 Carrel and Guthrie described the basic techniques still used today for end-to-end anastomosis and lateral suture during the early 1900s. The use of veins as conduits for repair or bypass of arterial vessels was reported by Goyanes (1906) and Lexer (1907).3,4 The unacceptably high thrombosis rate of these repair techniques precluded their widespread use.
DeBakey and Simeone5 published the management results of 2471 combat arterial injuries in World War II in 1946. Ligation continued to be the primary treatment during this conflict. However, 81 cases of suture repair were performed with an overall lower amputation rate, 35.8% versus 49%, when compared with ligation. This was perhaps the first indication that suture repair could be successful and improve outcome. Other factors stressed in the report included meticulous technique, wound debridement, delayed primary closure, and antibiotic use.
The Korean War began with a similar plan toward arterial injury. Patients were arriving at care facilities earlier than in any prior war because of improved evacuation techniques. An aggressive approach was then adopted, and surgical research teams were developed in both the Army and Navy to demonstrate the feasibility of acute arterial repair in wartime. Hughes (1958), Jahnke and Seeley (1953), Shannon and Howard (1955), and Spencer and Grewe (1955) then presented successful data. The amputation rate dropped to 13% in Hughes’s series after most patients had suture repair of their injuries.6
These wartime advances in vascular injury treatment crossed over into the civilian sector during the 1950s. Ferguson et al.7 reported their success with suture repair of civilian arterial injuries from 1950 to 1959. Their amputation rate of 13.6% compared well with the military experience. Early diagnosis and treatment, debridement of devitalized vessel, intimal approximation, and achievement of distal pulses were considered vital to the success of arterial repair.
The Vietnam War provided further opportunity for advancement in the treatment of acute arterial injury. A vascular registry was established at Walter Reed hospital to track soldiers with arterial injuries. The patients were again evacuated quickly, and over 98% of 1000 patients reported by Rich et al.8 underwent arterial repair. The amputation rate once again was 13.5% and consistent with the Korean War experience, despite more severe injuries encountered.
INCIDENCE
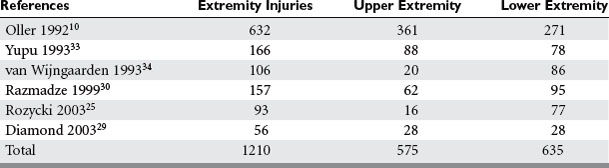
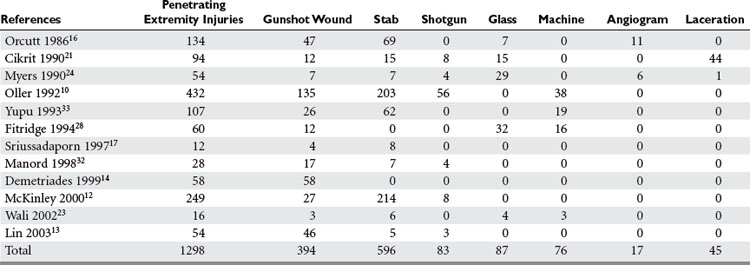
The overall incidence of vascular injury is quite low among all extremity injuries in both civilian and military settings. Beebe and DeBakey9 reported a 0.25% incidence of arterial injury among all wounds in American troops in World War I. DeBakey and Simeone5 reported a 0.96% incidence of arterial injury in World War II. The data on vascular trauma in the civilian population reported by Oller et al.10 shows a 3.7% incidence of vascular trauma among 1148 injuries in over 26,000 patients in a state registry. The overall incidence of upper extremity vascular trauma is also low. Pillai et al. reported 21 (3.3%) arterial injuries in 643 cases of upper-extremity trauma.11 However, the extremities are the most common site of vascular injury. Rich et al.8 found that 93% of all vascular injuries occurred in the extremities of American casualties in the Vietnam War. The relative incidence of vascular injury in upper versus lower extremities varies according to the series reviewed. Civilian series demonstrate upper extremity vascular injury incidence between 17% and 53%, as compared with lower extremity involvement, but when series are combined the incidence is almost equal (Table 1).
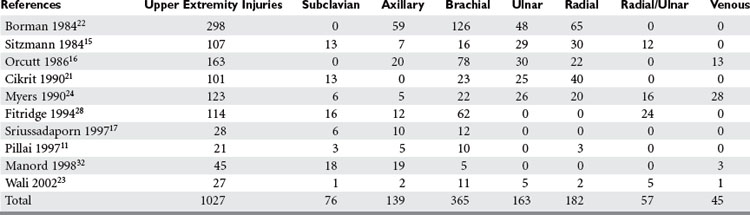
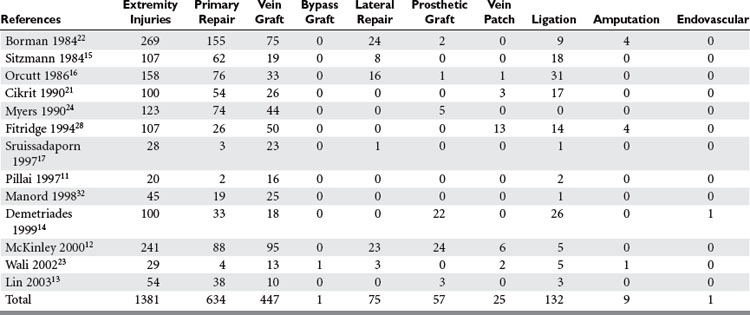
Most upper extremity vascular injuries involve the arterial system, including the subclavian, axillary, brachial, ulnar, and radial arteries (Table 2). Associated bone, nerve, and soft tissue injuries also occur and substantially impact ultimate outcome. As in much of trauma, vascular injury to the upper extremity is mainly a disease of young males. Subclavian arterial injury accounts for 3%–9% of all vascular extremity injuries. The brachial artery is the most commonly injured vessel in the upper extremity. Brachial artery injury ranges from 12%–48% of all extremity vascular traumas and radial and ulnar artery injury among all upper extremity vascular traumas is 16%–17% (see Table 2).
MECHANISM OF INJURY
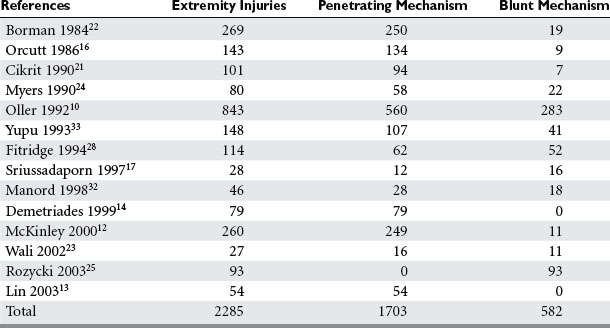
Penetrating trauma is the most common mechanism in upper extremity vascular injury. A penetrating mechanism of injury in military series is greater than 90% routinely, and consists of explosive shrapnel and high-velocity gunshot wounds. Civilian series demonstrate a penetrating mechanism for extremity vascular trauma in approximately 70%–85% of cases (Table 3). Blunt trauma comprises approximately 15%–30% of extremity vascular trauma seen in the civilian population (Table 4).
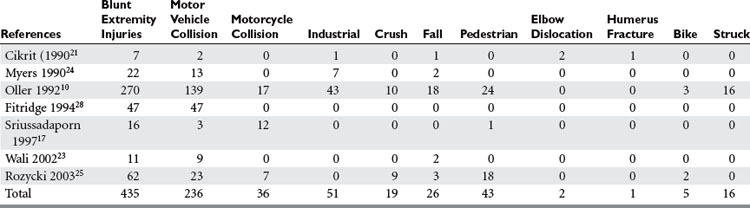
Of all penetrating extremity injuries, the most recent civilian data show stabbings to be the most common with gunshot wounds second. Other sources of penetrating trauma include glass, industrial/farming incidence, and shotgun blasts (Table 5). Military series include high-velocity mechanisms of penetrating trauma from gunshots, bombs, and mine explosions. The high-velocity mechanism of military weapons may account for more extensive associated injuries, similar to blunt trauma.
McKinley et al.12 collected data on 260 patients over a 19-year period with proximal axillary and subclavian artery injuries; only 11 patients had a blunt mechanism for their injury. In this study, stab wounds accounted for 82% of the penetrating trauma,12 while Lin et al.13 reviewed penetrating trauma to the subclavian artery and found gunshot wounds (85%) to be the most prevalent wounding agent. Demetriades et al.14 reviewed their data for subclavian and axillary penetrating injuries and again gunshots were the most common mechanism.
DIAGNOSIS
Ischemic changes were associated with absent pulses in over 70% of patients with upper extremity vascular trauma.15–17 Hematomas and bruits were less common than other hard signs. Neurologic deficits are common, but these should not always be attributed to nerve damage unless they persist after vascular repair, or a damaged nerve is directly visualized, as vascular insufficiency itself can give rise to these findings.
Noninvasive tests have been used to evaluate injured extremities for potential vascular injury, and include ankle:brachial pressure readings and duplex ultrasound. Limitations to this technology include the inability to provide the skilled individuals 24/7, and the lack of demonstrated reliability in complex injuries with large hematomas and bulky dressings. Johansen and colleagues18 have found the ankle:brachial index (ABI) to have a high sensitivity and diagnostic accuracy for vascular injury. Duplex scanning combines B-mode ultrasound and Doppler technology to allow both visual and auditory evaluation of the blood vessel. Bynoe et al.19 found duplex scanning to be both sensitive and specific when used to evaluate 198 injured extremities for vascular injury, with 20 documented cases of vascular trauma. This technique requires a highly skilled individual to perform and interpret, and limited availability of personnel and access to the extremity involved can limit its usefulness.
Conventional contrast arteriography remains the gold standard for the evaluation of injured extremities for vascular trauma, and should only be performed in hemodynamically stable patients as it requires transport of the patient to the radiology suite. With newer techniques for treatment of some vascular injuries, arteriography may also be therapeutic, allowing such interventional techniques as embolization and endovascular stenting to be performed. For many years, simple proximity of any asymptomatic injury to a major extremity artery mandated arteriography. Frykberg et al.20 demonstrated a 10.5% incidence of injury in penetrating trauma when vessel proximity to an injury was the only indication for arteriography, although all injuries were nonocclusive with a largely benign natural history.20 They concluded that in the absence of hard signs, proximity alone should not be an indication for arterial imaging or investigation. Certainly high-velocity penetrating mechanisms or complex blunt mechanisms may justify more liberal use of imaging.11 Civilian studies currently demonstrate that 18%–57% of patients with extremity vascular trauma have arteriography performed preoperatively.11,17,21–25 Complications from arteriography occur 2%–4% of the time, although major complications typically occur in far less than 1% of cases. Arteriography can also be performed more quickly, safely and cheaply in the emergency center or operating room by direct arterial injection of contrast by the surgeon.
Anatomic Location of Injury and Injury Grading
The brachial artery begins at the inferior border of the teres major and extends to just below the antecubital fossa. The first branch, the profunda, extends posteriorly through the medial and long heads of the triceps muscle along with the radial nerve to supply the posterior compartment. The superior ulnar collateral artery courses medially along with the ulnar nerve to supply a portion of the posterior compartment. A final branch, the inferior ulnar collateral, travels toward the medial epicondyle of the elbow. The median nerve follows the path of the brachial artery as it travels to the elbow. The brachial artery follows the inner border of the biceps muscle as it extends down the arm. Two brachial veins, venae comitantes, accompany the brachial artery. Extensive collateral circulation at the elbow is a result of the anastomosis of the branches of the brachial artery with inferior vessels.
The innermost layer of the artery and vein is the intima, which consists of endothelial cells. Smooth muscle and elastic fibers make up the middle layer or media and the outer layer is the adventitia, which consists of connective tissue. Forces placed on the vessels will result in a variety of injury patterns. The particular injury will influence the way the patient presents with the injury and how a diagnosis may need to be made. The force of the wounding agent will also affect the extent of the injury and the options available for treatment. High-velocity gunshot wounds can cause significant soft tissue damage and disrupt more than the primary vessels but the collaterals as well. In fact, the blast effect from high velocity weapons can damage vessels even without directly hitting them.3
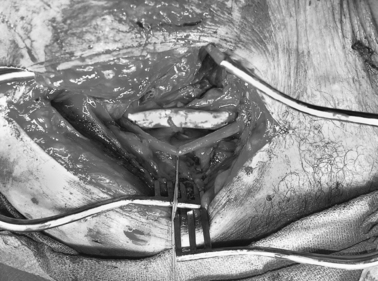
Lacerations and transections are the most common types of extremity vascular injury (Figure 1). A full-thickness tear in the wall with the vessel still intact is a laceration and can often be repaired by simple lateral suture. Hemorrhage is typically more pronounced with a laceration because the smooth muscle contracts and keeps the vessel open. These injuries may present a diagnostic dilemma because the pulse may be intact distal to the injury. A transection is a full-thickness cut through the vessel with disruption of the vessel. The ends of the vessel tend to constrict and bleeding will stop. Loss of the distal pulse with ischemia to the extremity is usually evident. The extent of the surrounding tissue destruction and or vessel loss will influence treatment strategies.
Finally, if the surrounding soft tissues contain the bleeding from an injured vessel, a pseudoaneurysm or arteriovenous fistula may arise. Pulses may be intact, and so the diagnosis is sometimes hard to make. When found at the time of surgery these injuries are treated with resection and repair. The more chronic they become, the more complications may occur from direct pressure and erosion into surrounding structures, or delayed rupture and hemorrhage. Surgical repair of these chronic lesions is also more difficult than injuries found acutely. McKinley12 reported transection and false aneurysm to be the two most common types of injury found in the subclavian and axillary arteries.
SURGICAL MANAGEMENT
Nonoperative management of asymptomatic and nonocclusive extremity vascular injuries is feasible and safe. Dennis et al.26 prospectively followed 43 patients with intimal flaps, arterial narrowing or small false aneurysms, and arteriovenous fistulas that fulfilled their criteria for nonoperative management, and were successful in avoiding surgery in 89%. To date, all published reports of this management have documented similar success rates, and not a single instance of limb loss or limb morbidity has yet been reported.
Endovascular techniques have proven efficacy and safety in the definitive treatment of upper extremity vascular injuries. The subclavian and axillary arteries are the vessels most amenable to this approach. The morbidity associated with an open repair of these vessels can be avoided with endovascular stenting. A guidewire must be able to traverse the lesion in order for a stent to be placed and repair the injury. Xenos et al.27 presented their experience with five endovascular stents placed for subclavian and axillary artery injuries. The complication rate and long-term patency were comparable to open surgical repair. The small numbers and absence of long-term follow-up mandate further study before this approach can be widely applied.
Surgical repair is the standard treatment for upper extremity vascular trauma after several decades of published experience. General principles of vascular surgery apply to the exposure, preparation and repair of the injured vessel. The injured extremity can provide obstacles to exposure and identification of vasculature. Proximal and distal control of the injured vessel must be established. A detailed inspection of the wound and determination of vessel loss and surrounding soft tissue injury is done. The subclavian artery and proximal axillary artery can be difficult to surgically expose. Demetriades et al.14 reported the use of an infraclavicular incision with extension into the deltopectoral groove to approach over 50% of these injuries. Another 48% were successfully exposed by adding a sternotomy or thoracotomy to the infraclavicular incision. Lin et al.13 exposed most injuries using a supraclavicular incision on the left and a median sternotomy with infraclavicular extension, when necessary, on the right. McKinley et al.12 exposed most vascular injuries with a supraclavicular or limited sternotomy incision with sternoclavicular dislocation.
Several methods can then be employed to repair the blood vessel (Table 6). Lateral suture repair can be performed if a simple laceration without tissue loss is found and the vessel is still intact, although this is rarely feasible for the relatively small brachial, radial, and ulnar arteries, which also tend to spasm after injury and manipulation. Mobilization of the vessel with debridement of the ends and a primary end-to-end anastomosis can be performed on transected vessels. Recent studies show this is performed in up to 69% of cases.13,15,17,21–23,28 Uncomplicated penetrating injuries are more amenable to primary vascular repair than are blunt or complex injuries.17,23,28 An interposition graft may be needed if extensive vessel loss occurs or mobilization for primary repair may cause undue tension or require ligation of collateral vessels. An autogenous vein is the recommended conduit for interposition grafting, and is used in up to 82% of cases requiring grafts. Prosthetic grafts are preferable when adequate vein is not available, or in unstable patients with multiple associated injuries when time is critical (Figure 2). In these circumstances, the risk of infection is outweighed by these considerations. In reviewing their treatment of subclavian and axillary injuries, Demetriades et al.14 and McKinley et al.12 found that over 20 prosthetic grafts were necessary for repair. Primary end-to-end anastomosis was performed in approximately one-third of these cases, as was interposition vein graft. Prosthetic graft was used as frequently as vein graft by Demetriades et al.12–14 Extra-anatomic bypass may be necessary when the native vascular bed is contaminated or involved in extensive tissue destruction, in which setting it is recommended that the graft be tunneled through uninvolved clean tissue planes. Externally stented prosthetic grafts should be used for this approach. Finally, ligation of the injured vessel may be a last-resort option in unstable patients with other life-threatening problems as a damage control measure. Temporary intraluminal arterial shunts are another option to allow limb salvage in these dire circumstances, as a means of maintaining extremity perfusion until the patient has been stabilized to the point that they may return to the operating room for definitive vascular repair. Ligation was used more frequently as management of radial and ulnar arterial injuries. Either artery may be ligated as long as the other is intact, and an intact palmar arch can be confirmed by performing an Allen test, or by observing brisk back-bleeding from the distal arterial segment.13,15,20,28,29
Primary amputation at the time of initial presentation may sometimes be an option to consider in patients with such severely injured upper extremities that prolonged and costly attempts at limb salvage are judged unlikely to succeed. The initial clinical examination is of major importance in this most difficult and challenging decision process, which should always involve the entire multidisciplinary team of trauma, vascular, plastic, and orthopedic surgeons. Most commonly a primary amputation is performed when the extremity is severely mangled without chance for recovery of limb function, and the potential for life-threatening complications related to attempted salvage are prohibitive. Primary amputation of the upper extremity is reported in up to 14% of cases of upper extremity trauma.17,22,25,28,30 Secondary amputation is usually related to infectious complications from attempted salvage, and/or continued vascular, bony or soft tissue injury, or long-term disability from irreversible nerve injury and a nonfunctional extremity. Amputation of the upper extremity is less common than in lower extremity trauma, because of the more extensive collateral circulation, because problems with protective sensation, motor function and length discrepancy are easier to accommodate, and because limb prosthetics are not as advanced and useful.
MORBIDITY AND COMPLICATIONS MANAGEMENT
The loss of function of the upper extremity as a result of trauma is multifactorial. This multifactorial nature can influence recognition, evaluation, diagnosis, and treatment of upper extremity injury. Miller and Welch31 specifically looked at limb loss as associated with ischemic times in canines with vascular disruption and reported a linear correlation between the time interval to revascularization and risk of limb loss. This correlation has since been confirmed in numerous experimental and clinical studies and emphasizes the essential importance of prompt diagnosis and treatment of vascular injuries. Associated subclavian vein and brachial plexus injury is high.12,13 Associated nerve injury to the brachial plexus was identified in approximately 30% of patients in the previous series. Fitridge et al.28 evaluated a patient population with a much higher blunt mechanism of injury to the subclavian and axillary vessels. Seventy-nine percent of their patients had associated major musculoskeletal trauma and only 32% of patients with subclavian/axillary vascular injuries had normal limb function after the 12-month follow-up. The extent of the nerve injury has a significant impact on the functional recovery of the extremity. Sitzmann et al.15 had a lower incidence of associated nerve injury with brachial artery injury, whereas Cikrit et al.21 reported a 65% incidence of associated nerve injury, which was the highest for all upper extremity vascular injuries in their series. Fitridge et al.28 reported a 64% incidence of brachial plexus injury associated with subclavian/axillary arterial injuries, and only a 14% incidence of nerve injury associated with brachial artery injury. Associated nerve injury is found in as many as 58% of patients with radial and ulnar artery injury,15 and the median nerve is most commonly injured. Late disability from upper extremity artery injury is related to the extent of nerve injury. Manord et al.32 have shown that statistically significant recovery is possible, but continued disability is related to the extent of the initial nerve injury, which can be difficult to determine in the acute setting. Fracture of the distal humerus and proximal radius or ulna can be associated with brachial artery injury. Overall skeletal injury in association with an upper extremity vascular injury ranges from 11%–41%.21,22,24,33
MORTALITY
Experience from combat settings has shown that decreases in mortality correlate with decreased time from wounding to definitive care.8 As time for evacuation went from 10 hours in World War II to 65 minutes in Vietnam, mortality among soldiers reaching medical care alive dropped from 8% to 1.8%. Patients with injuries to the subclavian and axillary vessels presented in shock or with no signs of life in 23%–53% of cases.12–15 Demetriades et al.14 found isolated vein injuries in 20 patients, in whom the mortality was 50%, while isolated arterial injury resulted in a mortality rate of only 20%. In current civilian series, shock was seen in 15%–38% of patients with upper extremity vascular injury and is more commonly seen in patients with subclavian and axillary injuries.14,15 Not surprisingly, patients presenting with shock had higher mortality.13,14 Mortality associated with radial and ulnar vascular injury is low.
CONCLUSIONS AND ALGORITHM
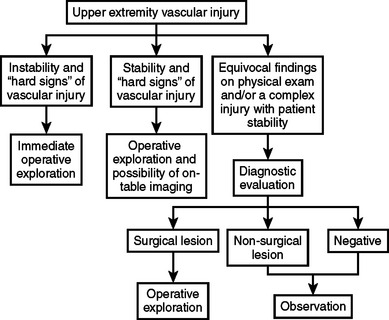
Vascular injury to the upper extremity poses a variety of diagnostic and therapeutic challenges. Penetrating trauma is the most common mechanism. Diagnosis and most treatment decisions can often be made with clinical examination alone. Many diagnostic modalities are available for further evaluation of the extremity with equivocal findings. Nonoperative management is appropriate in certain situations but surgery remains the primary mode of treatment for upper extremity vascular trauma. Continued innovation in endovascular techniques will change the management strategies for certain extremity vascular injuries in the future. The functional recovery of a patient’s upper extremity is related primarily to the associated neurologic injury and not the vascular injury. The algorithm in Figure 3 can be applied to upper extremity vascular trauma.
1 Schwartz AM. The historical development of methods of hemostasis. Surgery. 1974;76:849-866.
2 Murphy JB. Resection of the arteries and veins injured in continuity—end-to-end suture—experimental clinical research. Med Rec. 1897;51:73-88.
3 Rich NM. Vascular trauma. Surg Clin North Am. 1973;53:1367-1392.
4 Dale WA. The beginnings of vascular surgery. Surgery. 1974;76:849-866.
5 DeBakey ME, Simeone FA. Battle injuries of the arteries in World War II: an analysis of 2,471 cases. Ann Surg. 1946;123:534-579.
6 Hughes CW. Arterial repair during the Korean War. Ann Surg. 1958;147:555-561.
7 Ferguson IA, Byrd WM, McAfee DK. Experiences in the management of arterial injuries. Ann Surg. 1961;153:980-986.
8 Rich NM, Baugh JH, Hughes CW. Acute arterial injuries in Vietnam: 1,000 cases. J Trauma. 1970;10:359-369.
9 Beebe GW, DeBakey ME. Battle Casualties: Incidence, Mortality, and Logistic Considerations. Springfield, IL: Charles C. Thomas, 1952.
10 Oller DW, Rutledge R, Clancy T, et al. Vascular injuries in a rural state: a review of 978 patients from a state trauma registry. J Trauma. 1992;32:740-746.
11 Pillai L, Luchette FA. Upper-extremity arterial injury. Am Surg. 1997;63:224-228.
12 McKinley AG, Carrim ATO, Robbs JV. Management of proximal axillary and subclavian artery injuries. Br J Surg. 2000;87:79-85.
13 Lin PH, Koffron AJ, Guske PJ, et al. Penetrating injuries of the subclavian artery. Am J Surg. 2003;185:580-584.
14 Demetriades D, Chahwan S, Gomez H, et al. Penetrating injuries to the subclavian and axillary vessels. J Am Coll Surg. 1999;188:290-295.
15 Sitzmann JV, Ernst CB. Management of arm arterial injuries. Surgery. 1984;96:895-901.
16 Orcutt MB, Levine BA, Gaskill HV, et al. Civilian vascular trauma to the upper extremity. J Trauma. 1986;26:63-67.
17 Sriussadaporn S. Vascular injuries of the upper arm. J Med Assoc Thai. 1997;80:160-168.
18 Johansen K, Lynch K, Paun M, et al. Non-invasive vascular tests reliably exclude occult arterial trauma in injured extremities. J Trauma. 1991;31:515-519.
19 Bynoe RP, Miles WAS, Bell RM, et al. Noninvasive diagnosis of vascular trauma by duplex ultrasonography. J Vasc Surg. 1991;14:346-352.
20 Frykberg ER, Crump JM, Vines FS, et al. A reassessment of the role of arteriography in penetrating proximity extremity trauma: a prospective study. J Trauma. 1989;29:1041-1050.
21 Cikrit DF, Dalsing MC, Bryant BJ, et al. An experience with upper-extremity vascular trauma. Am J Surg. 1990;160:229-233.
22 Borman KR, Snyder WH, Weigelt JA. Civilian arterial trauma of the upper extremity, an 11 year experience in 267 patients. Am J Surg. 1984;148:796-799.
23 Wali MA. Upper limb vascular trauma in the Asir region in Saudi Arabia. Ann Thorac Cardiovasc Surg. 2002;8:298-301.
24 Myers SI, Harward TRS, Maher DP, et al. Complex upper extremity vascular trauma in an urban population. J Vasc Surg. 1990;12:305-309.
25 Rozycki GS, Tremblay LN, Feliciano DV, et al. Blunt vascular trauma in the extremity: diagnosis, management and outcome. J Trauma. 2003;55:814-824.
26 Dennis JW, Frykberg ER, Crump JM, et al. New perspectives on the management of penetrating trauma in proximity to major limb arteries. J Vasc Surg. 1990;11:84-92.
27 Xenos ES, Freeman M, Steven S, et al. Covered stents for injuries of subclavian and axillary arteries. J Vasc Surg. 2003;38:451-454.
28 Fitridge RA, Raptis S, Miller JH, et al. Upper extremity arterial injuries: experience at the Royal Adelaide Hospital, 1969 to 1991. J Vasc Surg. 1994;20:941-946.
29 Diamond S, Gaspard D, Katz S. Vascular injuries to the extremities in a suburban trauma center. Am Surg. 2003;10:848-851.
30 Razmadze A. Vascular injuries of the limbs: a fifteen-year Georgian experience. Eur J Vasc Endovasc Surg. 1999;18:235-239.
31 Miller HH, Welch CS. Quantitative studies on the time factor in arterial injuries. Ann Surg. 1949;130:428-438.
32 Manord JD, Garrard L, Kline DG, et al. Management of severe proximal vascular and neural injury of the upper extremity. J Vasc Surg. 1998;27:43-49.
33 Yupu L, Yaotian H, Rensheng LL, et al. Management of major arterial injuries of limbs: a study of 166 cases. Cardiovasc Surg. 1993;1:486-488.
34 M van Wijngaarden, L Omert & A Rodriguez, et al Management of blunt vascular trauma to the extremities. Surg Gynecol Obstet. 177: 41–48