CHAPTER 67 UPPER EXTREMITY FRACTURES: ORTHOPEDIC MANAGEMENT
Fractures and dislocations of the upper extremity can vary from benign, requiring minimal intervention, to life and limb threatening. The treatment plan is based on the injury pattern including location, associated neurologic or vascular injury, status of the soft tissues, mechanism, and other associated injuries. In this chapter, several key issues in the decision-making process are discussed, followed by description and treatment of specific injuries.
OPEN FRACTURES
The associated soft tissue injuries add an element of urgency to the treatment of fractures. A fracture is classified as “open” if the fracture or fracture hematoma communicates with the air via a wound in the soft tissues. This can be caused from the bone protruding through the skin, “inside out,” or if there is a penetrating mechanism causing an injury from the “outside in.” Regardless, the implication is that environmental contamination can increase the incidence of infection and fracture healing complications. If there is a wound in the same limb segment as the fracture, it should be considered open until proven otherwise. A classification system for open fractures appears in Table 1. Infection rates are reported at 0%–2% for Type I, 2%–7% for Type II, and 10%–25% for Type III overall. Rates for type III are subclassified as follows: IIIA, 7%; IIIB, 10%–50%; and IIIC, 25%–50%.
DISLOCATIONS
Neurovascular compromise is the reason for emergent reduction of the joint. Sciatic nerve injury has been reported to occur in 8%–19% of hip dislocations. Osteonecrosis is a known complication of hip dislocations as well, occurring in up to 17% of these injuries. This is due to the interruption of capsular blood supply from the increased tension caused by the dislocation. Other associated neurologic injuries can be seen in Table 2.
Table 2 Neurologic Injuries Associated with Upper Extremity Fractures
| Joint | Common Neurologic Injury | Deficit |
|---|---|---|
| Shoulder | Axillary nerve | Sensory deficit in deltoid region, weakness of deltoid and teres minor |
| Elbow | Posterior interosseous nerve | Weakness of wrist dorsiflexion |
| Knee | Peroneal nerve | Weakness of ankle and great toe dorsiflexion |
| Hip | Sciatic nerve | More frequently common peroneal portion giving dorsiflexion weakness |
GUNSHOT WOUNDS
Special attention is deserved here due to the need to make an important distinction for the treatment of these injuries. It is important to determine if the wound was inflicted by a “low-” or “high-velocity” weapon. The exact distinction is somewhat cloudy, but according to the Wound Ballistics Manual of the Office of the Surgeon General, muzzle velocity greater than 2500 ft/sec constitutes “high velocity.” This is important because the kinetic energy of the bullet varies directly with the square of its velocity and only linearly with its mass.
IMAGING STUDIES
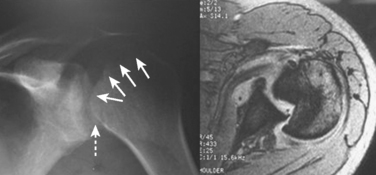
For fractures and dislocations, proper radiographic evaluation is essential before making any decisions regarding treatment of the injury. Ideally, two orthogonal views of the injured extremity should be obtained along with radiographs of the joints adjacent to the injured bone. Injuries to the shoulder girdle should have a minimum of three radiographic views in a standard trauma series, which includes anteroposterior (AP), scapular “Y,” and axillary views. Subtle injuries can be missed if adequate radiographs are not obtained. This is seen in Figure 1 of this missed posterior dislocation that was not properly diagnosed until a magnetic resonance image was obtained 6 months after the initial injury.
INJURIES TO SHOULDER GIRDLE AND HUMERUS
Scapula Fractures
A classification system was developed by Ada and Miller (Table 3), which divided fractures into those involving the acromion, spine and coracoid, type 1, glenoid neck, type 2, intra-articular glenoid fractures, type 3, and isolated scapular body fractures, type 4.
| Fracture Type | Anatomic Description/Location |
|---|---|
| 1A | Fracture through acromion |
| 1B | Fracture line through base of acromion or scapular spine |
| 1C | Fracture through coracoid process |
| 2A | Vertical glenoid neck fracture, lateral to base of acromion |
| 2B | Vertical glenoid neck fracture that extends up through scapular spine and supraspinatus fossa |
| 2C | Fracture line starts laterally at glenoid neck and propagates in transverse fashion through body exiting medially |
| 3 | Intra-articular glenoid fracture |
| 4 | Scapular body only |
Data from Ada JR, Miller ME: Scapular fractures: analysis of 113 cases. Clin Orthop 269:174–180, 1991.
Management of these fractures is often nonsurgical as poor healing is an infrequent complication due to the rich blood supply from the investing rotator cuff musculature. Nonoperative management consists of admission for a period of 24 hours to assess pulmonary and cardiac status. A brief period of sling immobilization is initiated for comfort, followed by passive range of motion. Most fractures are united by 6 weeks such that active mobilization and strengthening can ensue safely. Maximal functional recovery can take 6–12 months.
Proximal Humerus Fractures
Proximal humerus fractures are common injuries, especially with our aging population. The majority of these injuries are minimally displaced or nondisplaced and can be treated conservatively. Factors to take into consideration in the treatment plan are age of the patient, hand dominance, bone quality, fracture type, and fracture displacement. Associated injuries in the multitrauma patient are also important in the decision-making process.
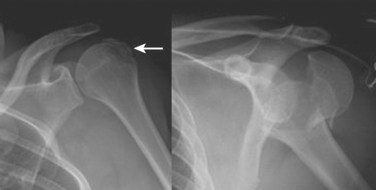
These fractures are classified according to the description by Neer. He described six variations of displaced proximal humerus fractures, and defined displacement as greater than 1 cm or 45 degrees of angulation. The anatomic “parts” consist of the anatomic neck, surgical neck, and greater and lesser tuberosities. Despite poor interobserver reliability, this is the most frequently used classification system. Figure 2 depicts various fracture patterns that are both considered “two-part fractures.” The portion of the humerus that is displaced has clinical relevance due to the varying blood supply of the proximal humerus. Displacement of the anatomic neck for example, has a high chance of disrupting the blood supply and adversely affecting outcome, regardless of the treatment chosen.
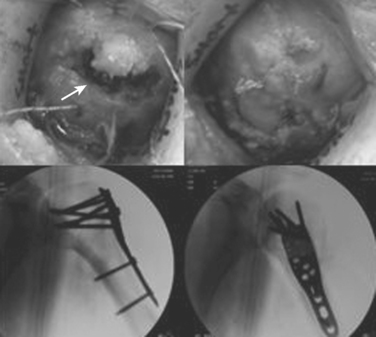
Treatment varies from a period of immobilization in a sling followed by supervised physical therapy with close radiographic evaluation in nondisplaced or impacted fractures. Open reduction and internal fixation (ORIF) with aggressive therapy to reduce postoperative stiffness is preferable in fractures with greater displacement. The greater tuberosity fracture in Figure 2 was treated with suture fixation to the shaft, whereas the anatomic neck fracture underwent ORIF with a plate and screws as seen in Figure 3. In both cases, rapid aggressive physical therapy was possible postoperatively.
Humeral Shaft Fractures
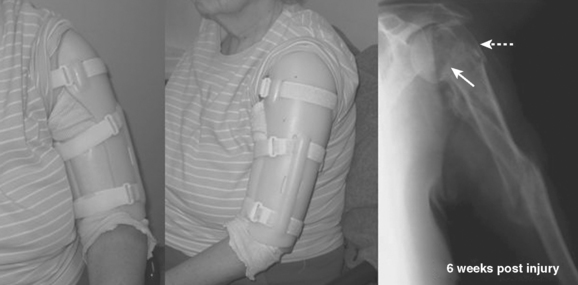
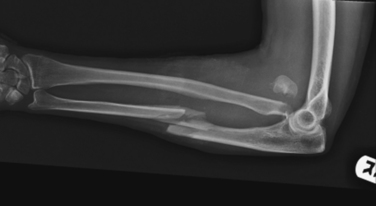
Historically, treatment of these fractures has been successful with nonoperative management; initial splinting is followed by placement in a functional brace in the subacute phase. Gravity serves as an important factor in reduction and maintenance of alignment with this method of treatment. The complication rates of nerve injury and delayed union or nonunion have been lower than those reported for operative treatment. In one study of 922 patients treated with functional bracing, only 3% of fractures failed to heal and 98% of patients regained near-full motion of the shoulder and elbow. Even in severely displaced or comminuted fractures such as the one seen in Figure 4, functional bracing may be the treatment of choice to minimize the risks associated with a major surgical procedure.
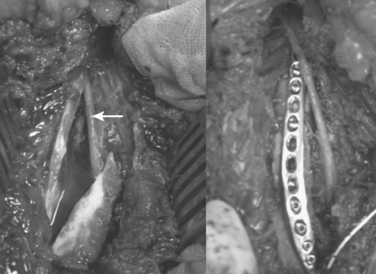
Treatment of fractures with associated nerve injury remains controversial. The reported incidence of radial nerve palsy varies from 1.8% to 24% with shaft fractures. The nerve is most commonly contused with a neuropraxia that recovers in greater than 70% of reported cases. Transverse fractures of the middle third are more likely to have a neuropraxia than spiral fractures of the distal third, which have a higher incidence of laceration or entrapment of the radial nerve as can be seen in Figure 5.
ELBOW
Distal Humerus Fractures
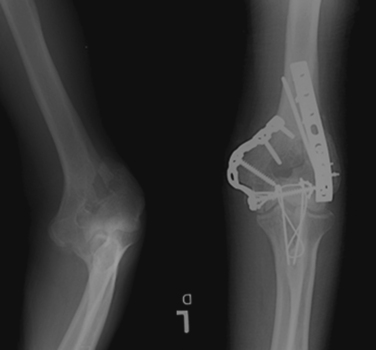
When there is a displaced intra-articular component, operative treatment is the only way one can restore articular congruity. Nondisplaced or minimally displaced fractures can be treated nonoperatively if they are stable enough to allow for early range-ofmotion (ROM) exercises. If the joint is not restored to its anatomic position, it will become painful as the high joint reactive forces lead to premature arthrosis.
In adults the goals of reconstruction are aimed at re-creating the articular surface, and then re-establishing the medial and lateral columns of the humerus, Figure 6. An olecranon osteotomy is usually necessary in order to provide adequate exposure of the articular surface, after which the ulnar nerve is identified and retracted. Transposition of the nerve at the time of surgery is somewhat controversial at present.
In experienced hands, these fractures can be treated operatively with good to excellent results in most patients.
Elbow Dislocation
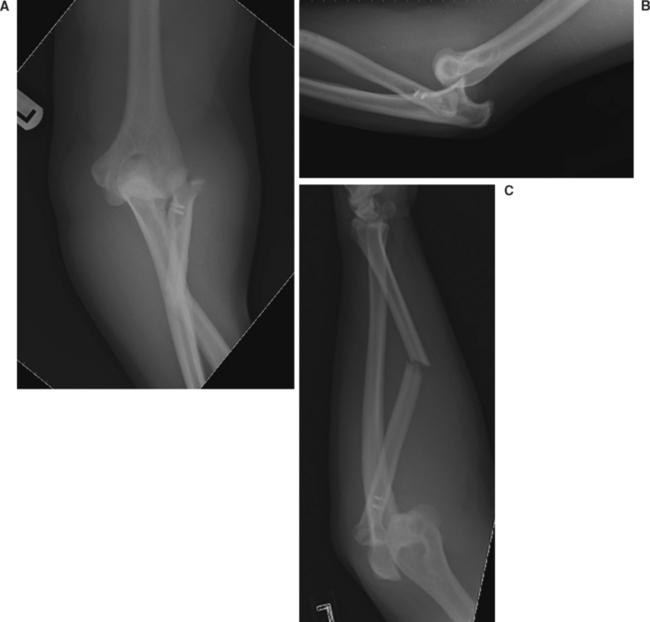
Radiographs of the elbow are used to confirm the clinical diagnosis of dislocation. An elbow dislocation that does not involve a fracture is known as a simple dislocation, and is classified according to the direction of the dislocation. Dislocation with associated fracture(s) of the radial head/neck and/or coronoid process (i.e., complex dislocation) must be determined on imaging studies because they have implications for definitive treatment (Figure 7).
An acute elbow dislocation should be initially managed by prompt closed reduction under adequate analgesia and muscle relaxation. This can usually be achieved in the emergency room with intramuscular or intravenous medication(s). Several reduction techniques have been described, but they all involve correcting the medial-lateral displacement, followed by longitudinal traction and flexion of the forearm. A “clunk” is often felt with reduction of the joint.
Coronoid Fractures
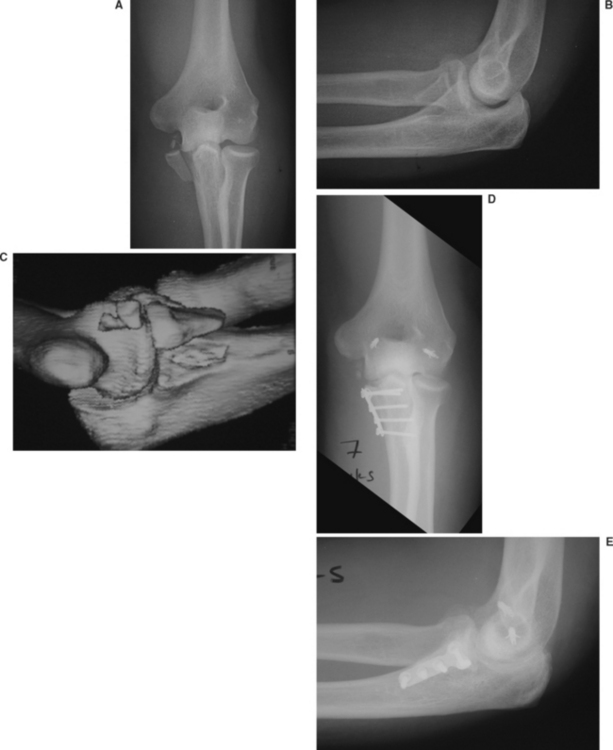
The coronoid process of the ulna serves as an anterior buttress of the greater sigmoid notch and is the attachment site for the anterior bundle of the medial collateral ligament and anterior capsule. Therefore it has an important role in providing stability to the elbow. Coronoid fractures are common, and are associated with 10% of elbow dislocations. Classically, three types have been identified to occur in the coronal plane and are best seen on a lateral elbow x-ray: type I, tip avulsion; type II, less than 50%; and type II, greater than 50%. More recently, an oblique or vertical fracture line about the anteromedial coronoid is also recognized to cause instability of the ulnohumeral joint (Figure 8). This fragment is best seen on computed tomography scan. In general, types I and II coronoid fractures can be managed without fixation of the fragment itself. Because displaced type III or anteromedial fractures are associated with elbow instability, they should be treated with internal fixation and as part of the overall surgical management of elbow dislocation. Failure to stabilize these fractures will result in recurrent elbow subluxation or dislocation.
FOREARM
Monteggia Fracture
Monteggia fracture is a fracture of the proximal ulna with dislocation of the radial head. It occurs in 1%–2% of all forearm fractures. Bado described four types of injury patterns: type I, apex anterior ulnar fracture and anterior radial head dislocation; type II, apex posterior ulnar fracture and posterior radial head dislocation; type III, proximal ulnar metaphyseal fracture and lateral radial head dislocation; and type IV, proximal ulnar and radial shaft fractures and anterior radial head dislocation (Figure 9). The key to successful outcome of Monteggia fracture is anatomic reduction and fixation of the ulna fracture. Upon reduction of the ulna, there is typically concomitant stable relocation of the radial head. Irreducible radial head dislocations can be associated with posterior interosseous nerve entrapment. In type IV lesions, open reduction and internal fixation of both the ulna and radius are required.
WRIST
Distal Radius Fracture
In light of the limitations of external fixation, open reduction and internal fixation have become a more widely used technique. Exposing the fracture through a dorsal or volar approach allows direct visualization and manipulation of the fracture fragments. A number of plate and screw constructs are available for both dorsal and volar fixation. Dorsally applied hardware has associated complications, such as tendon irritation or rupture and the possible need for removal of the hardware after fracture healing. Thus, the volar approach has recently become more popular. Newer fixed-angle plating systems allow early therapy to regain wrist and finger motion. However, the amount of soft tissue dissection and the extramedullary position of the implant still pose some disadvantages in treating wrist fractures.
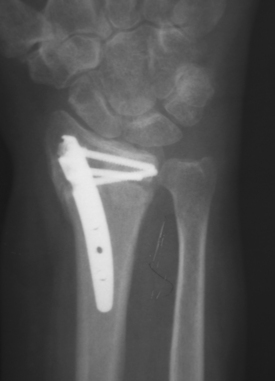
Intramedullary devices such as the Micronail (Wright Medical Technology, Inc., Memphis, TN) attempt to counteract some of the disadvantages noted with open reduction and plating. By residing within the medullary canal, these devices minimize or eliminate soft tissue irritation and pin track infection, yet maintaining fracture reduction and alignment (Figure 10).
Perilunate Dislocations
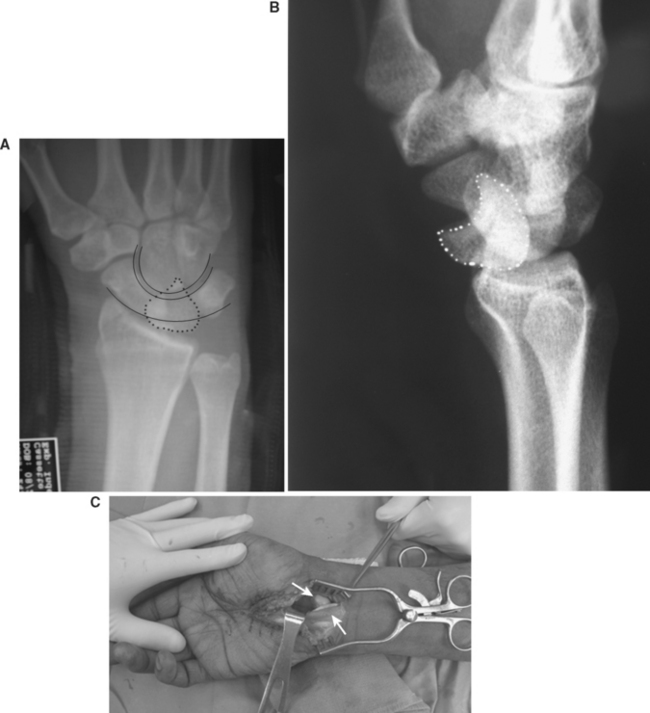
The typical presentation of an acute perilunate dislocation includes pain and swelling about the wrist. Deformity may be more subtle than expected. The carpus is usually displaced dorsally. In a lunate dislocation, the lunate can come to lie within the carpal tunnel; therefore, thorough neurovascular assessment of the upper extremity is important. A well-taken wrist series is the key to the diagnosis. Posterior-anterior view will show disruption of the normal carpal arcs (Figure 11A). Lateral radiograph will reveal loss of colinearity between the capitate, lunate, and the radius (Figure 11B). Traction radiographs may be indicated to further assess the injury pattern.
Once the diagnosis of a perilunate dislocation is made, treatment consists of immediate closed manipulation to achieve reduction and immobilization. Reduction is usually undertaken with the patient under intravenous sedation. The arm is first suspended in longitudinal traction. With the wrist extended and maintaining traction, a thumb is used to push the lunate back into its fossa as the wrist is then flexed to reduce the capitate over and into the concavity of the lunate. Failure to achieve a reduction via closed means often indicates interposed volar capsule and necessitates an urgent open procedure. A patient with an irreducible perilunate dislocation at minimum must undergo an urgent temporizing extended carpal tunnel release to decrease the pressure on the median nerve (Figure 11C). Failure to do so could result in permanent median nerve dysfunction.
CONCLUSION
Trauma to the upper extremity can result in a variety of injury patterns, ranging from minor sprains to complex open fracture-dislocations. Often the injury can be treated or at least temporized by immobilization or splinting which will help with pain control and also minimize further damage to the extremity. Proper patient assessment and imaging studies are essential to initiating treatment. Although life-threatening injuries take precedence over the limb, a long-arm posterior splint can usually be applied expeditiously to the injured arm without interfering with the resuscitation effort.
Ada JR, Miller ME. Scapular fractures: analysis of 113 cases. Clin Orthop. 1991;269:174-180.
Althausen PL, Lee MA, Finkemeier CG. Scapulothoracic dissociation: diagnosis and treatment. Clin Orthop Relat Res. 2003;416:237-244.
Bado JL. The Monteggia lesion. Clinical Orthopaedics & Related Research. 1967;50:71-86.
Blazar PE. Dislocations/instability. In: Beredjiklian PK, Bozentka DJ, editors. Review of Hand Surgery. Philadelphia: Saunders; 2004:139-150.
Boyer MI, Galatz LM, Borrelli JJr, et al. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
Egol KA, Connor PM, Karunakar MA, Sims SH, Bosse MJ, Kellam JF. The floating shoulder: clinical and functional results. J Bone Joint Surg [Am]. 2001;83:1188-1194.
Gustilo RB, Merkow RL, Tempelton D. Current concepts review: the management of open fracture. J Bone Joint Surg. 1990;72-A:299-304.
Hawkins RJ, Angelo RL. Displaced proximal humeral fractures: selecting treatment, avoiding pitfalls. Orthop Clin North Am. 1987;18:421-431.
Hughes SP. Antibiotics penetration into bone in relation to the immediate management of open fractures: a review. Acta Orthopaed Belg. 1992;58(1):217-221.
Jeon IH, Oh CW, Park BC, Ihn JC, Kim PT. Minimal invasive percutaneous Herbert screw fixation in acute unstable scaphoid fracture. Hand Surg. 2003;8(2):213-218.
Johansen K, Sangeorzan B, Copass MK. Traumatic scapulothoracic dissociation: case report. J Trauma. 1991;31:147-149.
Knapp TP, Patzakis MJ, Lee J, Seipel PR, Abdollahi K, Reisch RB. Comparison of intravenous and oral antibiotic therapy in the treatment of fractures caused by low-velocity gunshots. A prospective, randomized study of infection rates. J Bone Joint Surg [Am]. 1996;78:1167-1171.
Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg [Am]. 1980;5(3):226-241.
Neer CSII. Displaced proximal humeral fractures: I. Classification and evaluation. J Bone Joint Surg [Am]. 1970;52:1077-1089.
Driscoll SW. Elbow dislocations. In: Morrey BF, editor. The Elbow and Its Disorders. Philadelphia: WB Saunders; 2000:409-420.
Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
Pugh DM, Wild LM, Schemitsch EH, King GJ, McKee MD. Standard surgical protocol to treat elbow dislocations with radial head and coronoid fractures. J Bone Joint Surg [Am]. 2004;86-A(6):1122-1130.
Regan WD, Morrey BF. Coronoid process and Monteggia fractures. In: Morrey BF, editor. The Elbow and Its Disorders. Philadelphia: WB Saunders; 2000:396-408.
Ring D, Quintero J, Jupiter JB. Open reduction and internal fixation of fractures of the radial head. Journal of Bone & Joint Surgery—American Volume. 2002;84-A(10):1811-1815.
Rozental TD, Beredjiklian PK, Bozentka DJ. Longitudinal radioulnar dissociation. J Am Acad Orthop Surg. 2003;11(1):68-73.
Sarmiento A, Zagorski JB, Zych GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg [Am]. 2000;82:478-486.
Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
Tan V, Capo J, Warburton M. Distal radius fixation with an intramedullary nail. Tech Hand Upper Extrem Surg. 2005;9(4):195-201.