15 UPPER DIGESTIVE SEGMENT
Upper digestive segment: Mouth, esophagus, and stomach
We have divided the discussion of the digestive system into two components or chapters: Chapter 15 focuses on the upper digestive segment and includes the mouth, esophagus, and stomach. Chapter 16 describes the lower digestive segment (small and large intestines). This division is based on the distinctive functions of the upper digestive segment (swallowing and digestion) and lower digestive segment (absorption).
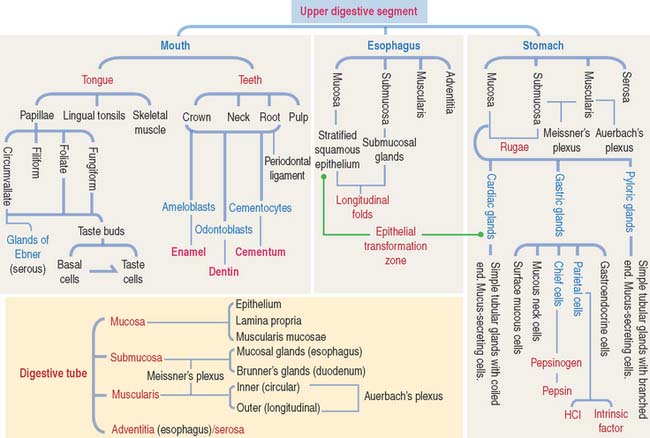
MOUTH
The mouth is the entrance to the digestive tube. Ingestion, partial digestion, and lubrication of the food, or bolus, are the main functions of the mouth and its associated salivary glands. We study the salivary glands in Chapter 17, Digestive Glands.
The soft palate and uvula are lined by a nonkeratinized stratified squamous epithelium extending into the oropharynx where it becomes continuous with the pseudostratified ciliated columnar epithelium of the upper respiratory tract. The submucosa is loose and contains abundant mucous and serous glands. Skeletal muscle fibers are present in the soft palate and uvula.
Tongue
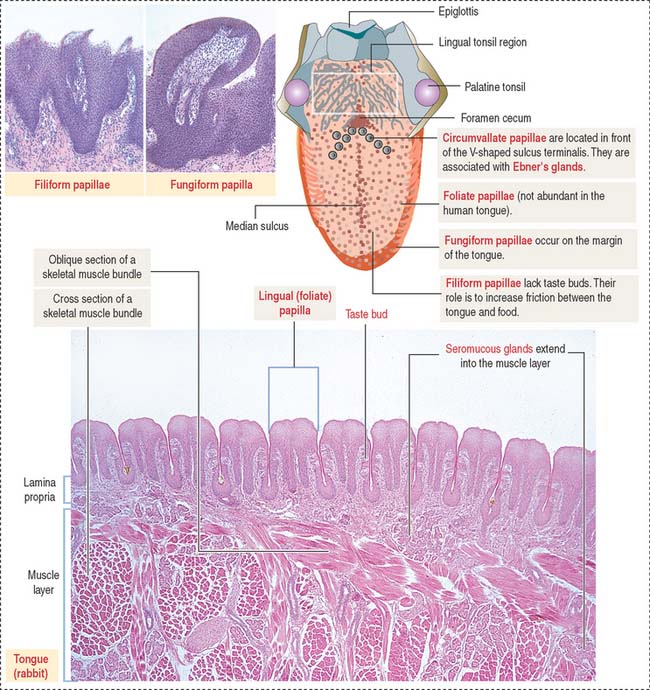
The dorsal surface of the tongue is covered by a nonkeratinized stratified squamous epithelium supported by a lamina propria associated with the muscle core of the tongue. Serous and mucous glands extend across the lamina propria and the muscle. Their ducts open into the crypts and furrows of the lingual tonsils and circumvallate papillae, respectively.
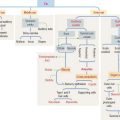
The dorsal surface of the tongue contains numerous mucosal projections called lingual papillae (Figure 15-1). Each lingual papilla is formed by a highly vascular connective tissue core and a covering layer of stratified squamous epithelium. According to their shape, lingual papillae can be divided into four types: (1) filiform papillae (narrow conical), the most abundant; (2) fungiform papillae (mushroom-shaped); (3) circumvallate papillae; and (4) foliate papillae (leaf-shaped), rudimentary in humans but well developed in rabbits and monkeys.
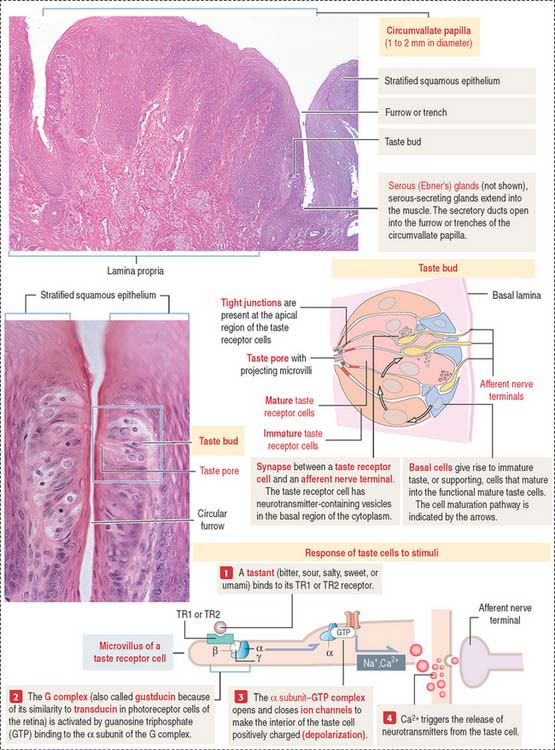
The sides of the circumvallate papilla and the facing wall of furrow contain several taste buds. Each taste bud, depending on the species, consists of 50 to 150 cells, with its narrow apical ends extending into a taste pore. A taste bud has three cell components (Figure 15-2): (1) taste receptor cells, (2) supporting cells (or immature taste cells), and (3) precursor cells (or basal cells).
Taste is initiated when soluble chemicals, called tastants, diffuse through the taste pore and interact with the G-protein α, β, and γ subunits (called gustducin) linked to the taste receptors (designated TR1 and TR2), present in the apical microvilli of the taste receptor cells. As we discussed in Chapter 3, Cell Signaling, guanosine triphosphate (GTP) binding to the α subunit of the G-protein complex activates target molecules (ion channels in the taste receptor cells). Ionic changes within taste cells cause either depolarization (see Figure 15-2) or hyperpolarization of the receptor cells. An increase in intracellular Ca2+ triggers the release of neurotransmitters at the afferent synapse with the afferent nerve terminal. Some taste receptor cells respond to only one of the basic taste substances. Others are sensitive to more than one taste substance.
TOOTH
Each of the several types of teeth has a distinctive shape and function: incisors are specialized for cutting; canines, for puncturing and holding; and molars, for crushing.
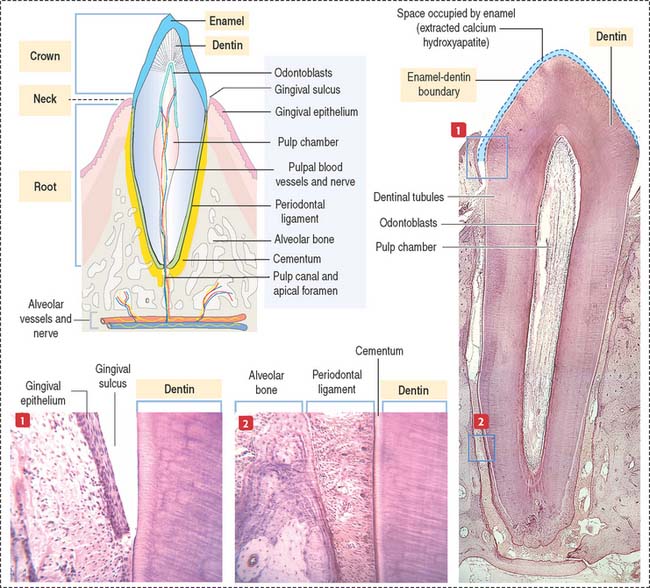
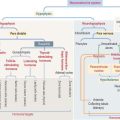
Each tooth consists of a crown and either single or multiple roots (Figure 15-3). The crown is covered by highly calcified layers of enamel and dentin. The outer surface of the root is covered by another calcified tissue called cementum. The dentin forms the bulk of the tooth and contains a central chamber filled with soft tissue, the pulp. The pulp chamber opens at the apical foramen into the bony alveolar process by the root canal. Blood vessels, nerves, and lymphatics enter and leave the pulp chamber through the apical foramen. Myelinated nerve fibers run along with the blood vessels.
TOOTH DEVELOPMENT
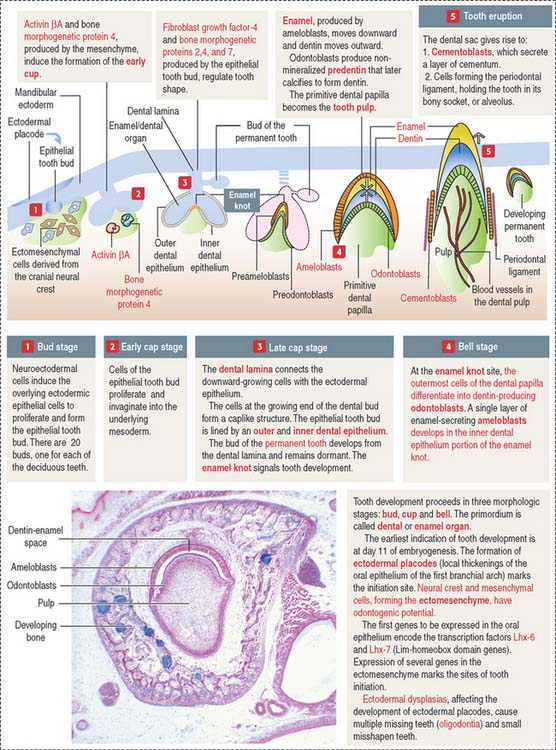
The ectoderm, cranial neural crest, and mesenchyme contribute to the development of the tooth (Figure 15-4). Ameloblasts derive from the ectoderm. Odontoblasts derive from the cranial neural crest. Cementocytes derive from the mesenchyme.
Secreted signaling molecules—activin βA, fibroblast growth factor, and bone morphogenetic proteins—mediate the interaction between the dental epithelium and the mesenchyme during tooth morphogenesis. Figure 15-4 illustrates the relevant steps of tooth development.
ODONTOBLASTS
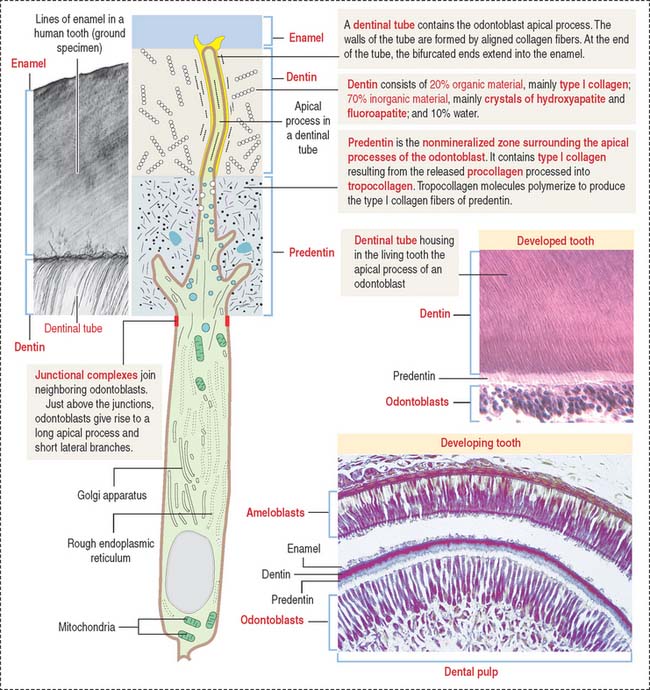
The odontoblast is a columnar epithelial-like cell located at the inner side of the dentin, in the pulp cavity (Figure 15-5). The apical cell domain is embedded in predentin, a nonmineralized layer of dentin-like material. The apical domain projects a main apical cell process that becomes enclosed within a canalicular system just above the junctional complexes linking adjacent odontoblasts.
Coronal dentin dysplasia (also known as dentin dysplasia, type II) is a rare inherited autosomal defect characterized by abnormal development of dentin, extremely short roots (rootless teeth), and obliterated pulp chambers.
Cementum
The cementum meets the enamel at the cementoenamel junction and separates the crown from the root at the neck region of the tooth. The outermost layer of the cementum is uncalcified and is produced by cementoblasts in contact with the periodontal ligament, a collagen- and fibroblast-rich and vascularized suspensory ligament holding the tooth in the sockets of the alveolar bone (see Figure 15-3). The strength of the periodontal ligament fibers gives teeth mobility and strong bone attachment, both useful in orthodontic treatment.
AMELOBLASTS
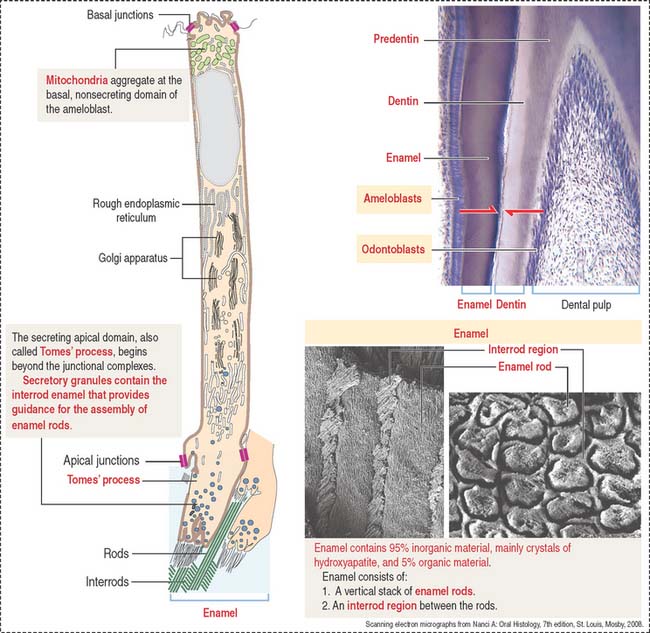
Ameloblasts are enamel-producing cells present only during tooth development. The ameloblast (Figure 15-6) is a polarized columnar cell with mitochondria and a nucleus present in the basal region of the cell. The supranuclear region contains numerous cisternae of rough endoplasmic reticulum and Golgi apparatus.
GENERAL ORGANIZATION OF THE DIGESTIVE, OR ALIMENTARY, TUBE
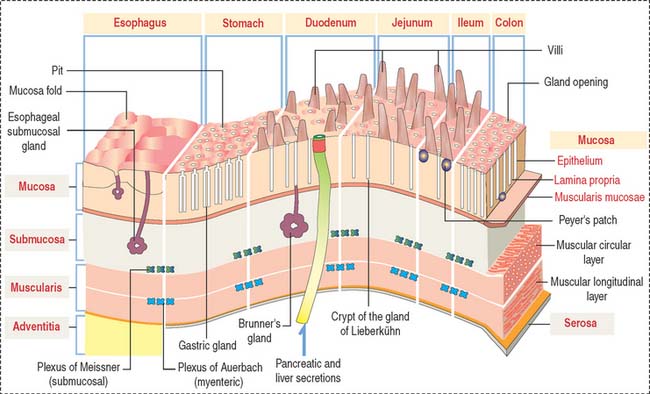
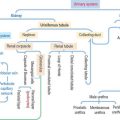
After the oral cavity, the digestive tube is differentiated into four major organs: esophagus, stomach, small intestine, and large intestine. Each of these organs is made up of four concentric layers (Figure 15-7): (1) the mucosa, (2) the sub-mucosa, (3) the muscularis, and (4) the adventitia, or serosa.
Lymphatic nodules and scattered immunocompetent cells (lymphocytes, plasma cells, and macrophages) are present in the lamina propria. The lamina propria of the small and large intestines is a relevant site of immune responses (see Chapter 16, Lower Digestive Segment).
The muscularis contains two layers of smooth muscle: the smooth muscle fibers of the inner layer are arranged around the tube lumen (circular layer); fibers of the outer layer are disposed along the tube (longitudinal layer). Contraction of the smooth fibers of the circular layer reduces the lumen; contraction of the fibers of the longitudinal layer shortens the tube. Skeletal muscle fibers are present in the upper esophagus and the anal sphincter.
Microvasculature of the digestive tube
We start our discussion with the microvasculature of the stomach. The microcirculation of the small intestine and differences from the gastric microcirculation are discussed in Chapter 16, Lower Digestive Segment (see Figure 16-3).
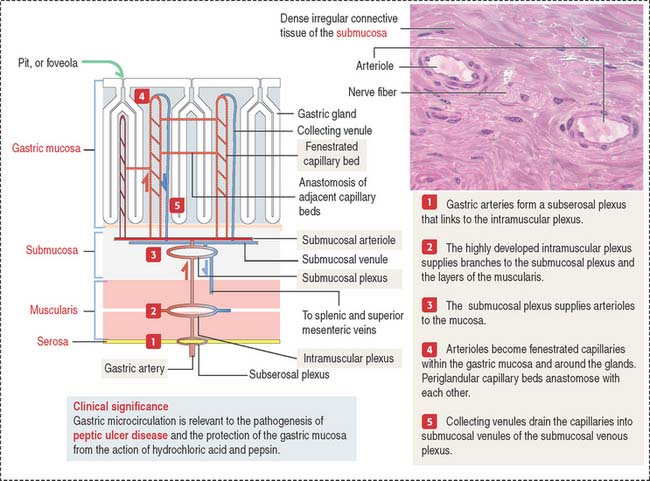
Blood and lymphatic vessels and nerves reach the walls of the digestive tube through the supporting mesentery or the surrounding tissues. After entering the walls of the stomach, arteries organize three arterial networks: the subserosal, intramuscular, and submucosal plexuses (Figure 15-8). Some branches from the plexuses run longitudinally in the muscularis and submucosa; other branches extend perpendicularly into the mucosa and muscularis.
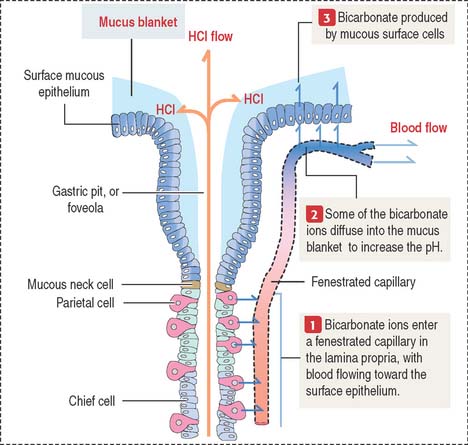
In the mucosa, arterioles derived from the submucosal plexus supply a bed of fenestrated capillaries around the gastric glands and anastomose laterally with each other. The fenestrated nature of the capillaries facilitates bicarbonate delivery to protect the surface epithelial cells against hydrochloric acid damage (see Figure 15-17).
Collecting venules descend from the mucosa into the submucosa as veins, leave the digestive tube through the mesentery, and drain into the splenic and superior mesenteric veins. Mesenteric veins drain into the portal vein, leading to the liver (see Chapter 17, Digestive Glands).
Clinical significance: Gastric microcirculation and gastric ulcers
As we discuss later in this chapter, gastric microcirculation plays a significant role in the protection of the integrity of the gastric mucosa. A breakdown in this protective mechanism, including mucus and bicarbonate secretion, allows the destructive action of hydrochloric acid and pepsin and bacterial infection, leading to peptic ulcer disease (PUD). PUD includes a group of disorders characterized by a partial or total loss of the mucosal surface of the stomach or duodenum or both.
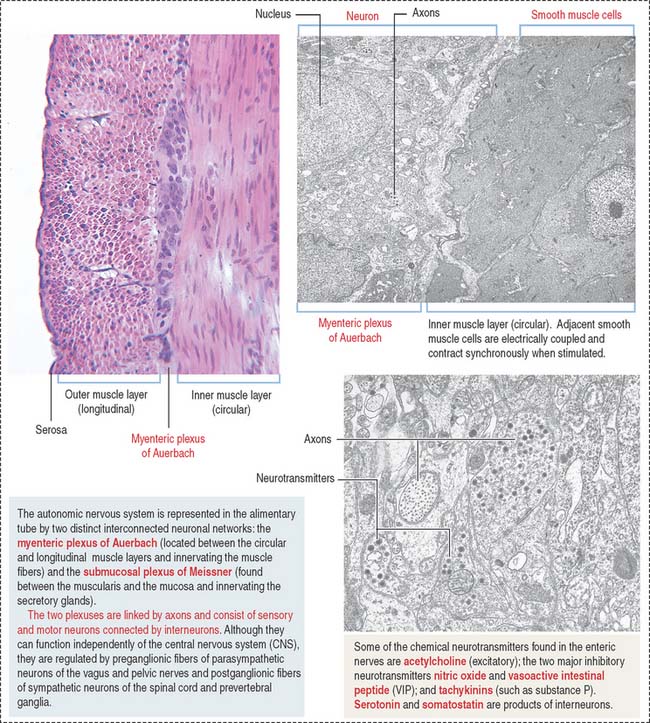
Nerve supply of the digestive tube
The intrinsic or enteric innervation is represented by two distinct interconnected neuronal circuits formed by sensory and motor neurons linked by inter-neurons: (1) the submucosal plexus of Meissner, present in the submucosa; and (2) the myenteric plexus of Auerbach (Figure 15-9), located between the inner circular and outer longitudinal layers of the muscularis.
ESOPHAGUS
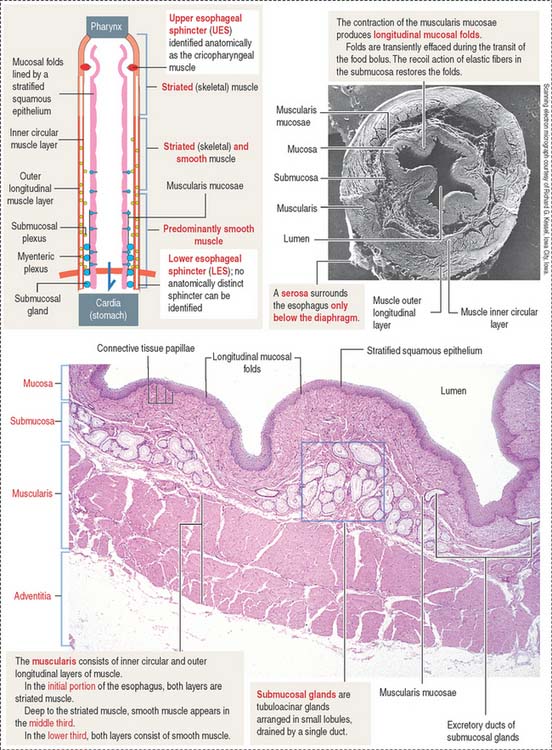
The esophageal mucosa consists of a stratified squamous epithelium overlying a lamina propria with numerous connective tissue papillae (Figure 15-10). The muscularis mucosae is not present in the upper portion of the esophagus, but it becomes organized near the stomach. Both the mucosa and the submucosa in the undistended esophagus form longitudinal folds that give the lumen an irregular outline. As the bolus of food moves down the esophagus, the folds disappear transiently and then are restored by the recoil of the elastic fibers of the submucosa.
The submucosa contains a network of collagen and elastic fibers and many small blood vessels. At the lower end of the esophagus, submucosal venous plexuses drain into both the systemic venous system and the portal venous system. An increase in pressure in the portal venous system, caused by chronic liver disease, results in dilation of the submucosal venous sinuses and the formation of esophageal varices. Rupture of the varices or ulceration of the overlying mucosa can produce hemorrhage into the esophagus and stomach, often causing vomiting (hematemesis).
The submucosal tubuloacinar glands, found in the submucosa just beneath the muscularis mucosae, are organized into small lobules drained by a single duct (see Figure 15-10). The acini are lined by two secretory cell types: a mucous and a serous cell type, the latter with secretory granules containing lysozyme.
The composition of the inner circumferential (or circular) and outer longitudinal layers of the muscularis shows segment-dependent variations. In the upper third of the esophagus, both layers consist of striated muscle. In the middle third, smooth muscle fibers can be seen deep to the striated muscle. In the lower third, both layers of the muscularis contain smooth muscle cells.
STOMACH
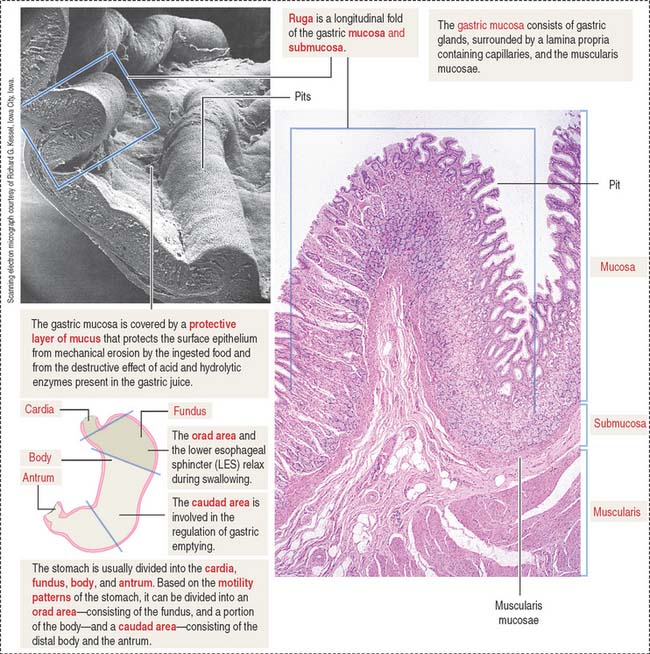
Four regions are recognized in the stomach: (1) the cardia, a 2- to 3-cm-wide zone surrounding the esophageal opening; (2) the fundus, projecting to the left of the opening of the esophagus; (3) the body, an extensive central region; and (4) the pyloric antrum (Greek pyloros, gatekeeper), ending at the gastroduodenal orifice. Based on the motility characteristics of the stomach, the orad area, consisting of the fundus and the upper part of the body, relaxes during swallowing. The caudad area, consisting of the lower portion of the body and the antrum, participates in the regulation of gastric emptying.
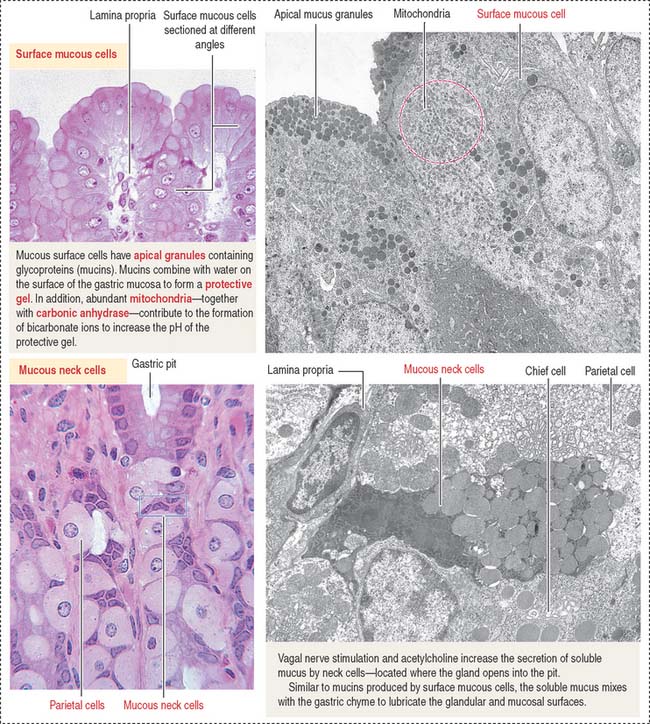
The empty stomach shows gastric mucosal folds, or rugae, covered by gastric pits or foveolae (Figure 15-11). A gastric mucosal barrier, produced by surface mucous cells, protects the mucosal surface. The surface mucous cells contain apical periodic acid–Schiff (PAS)–positive granules and are linked to each other by apical tight junctions.
Cardia region
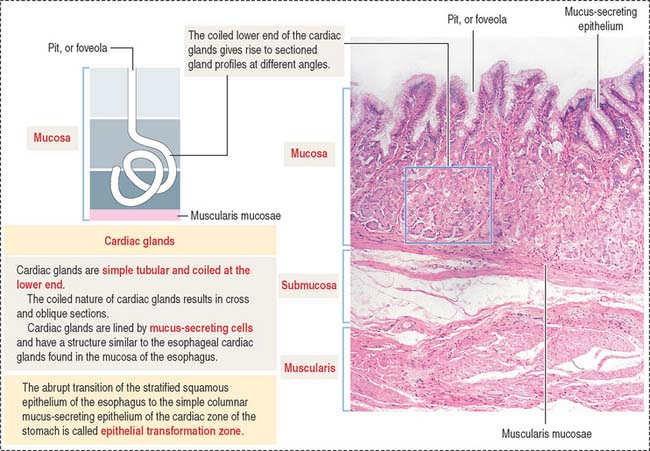
Glands of the cardia region are tubular, with a coiled end and an opening continuous with the gastric pits (Figure 15-12). A mucus-secreting epithelium lines the cardiac glands.
Functions of the gastric gland
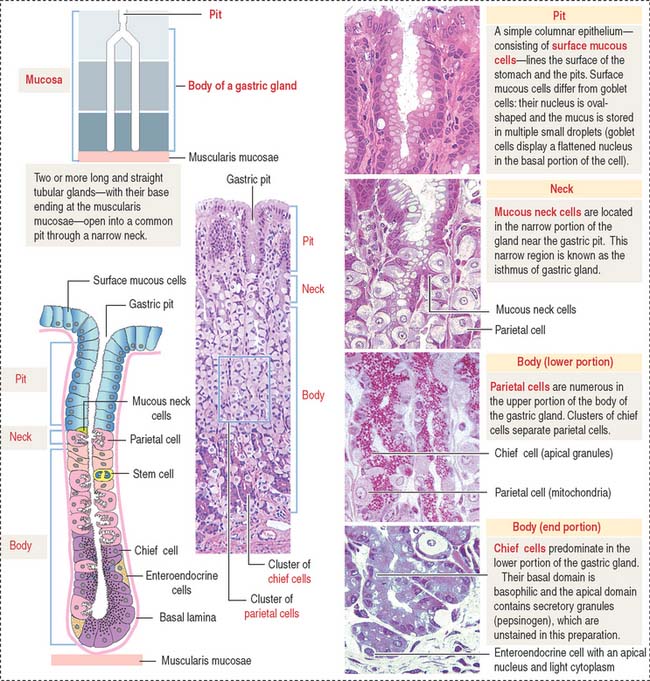
A gastric gland consists of three regions (Figure 15-13): (1) the pit, or foveola, lined by surface mucous cells; (2) the neck, containing mucous neck cells, mitotically active stem cells, and parietal cells; and (3) the body, representing the major length of the gland. The upper and lower portions of the body contain different proportions of cells lining the gastric gland.
The surface mucous cells line the surface of the gastric mucosa and the gastric pits (Figure 15-14; see also Figure 15-13).
The gastric glands proper house five major cell types: (1) mucous neck cells (see Figure 15-13), (2) chief cells (also called peptic cells), (3) parietal cells (also called oxyntic cells), (4) stem cells, and (5) gastroenteroendocrine cells (called enterochromaffin cells because of their staining affinity for chromic acid salts).
The upper portion of the main body of the gastric gland contains abundant parietal cells. Chief cells and gastroenteroendocrine cells predominate in the lower portion (see Figure 15-13).
The gastric mucosa of the fundus-body has two classes of mucus-producing cells (see Figure 15-14): (1) the surface mucous cells lining the pit and (2) the mucous neck cells located at the opening of the gastric gland into the pit. Both cells produce mucins, glycoproteins with high molecular mass. A mucus layer, containing 95% water and 5% mucins, forms an insoluble gel that attaches to the surface of the gastric mucosa, forming a 100-μm-thick protective gastric mucosal barrier. This protective mucus blanket traps bicarbonate ions and neutralizes the microenvironment adjacent to the apical region of the surface mucous cells to an alkaline pH.
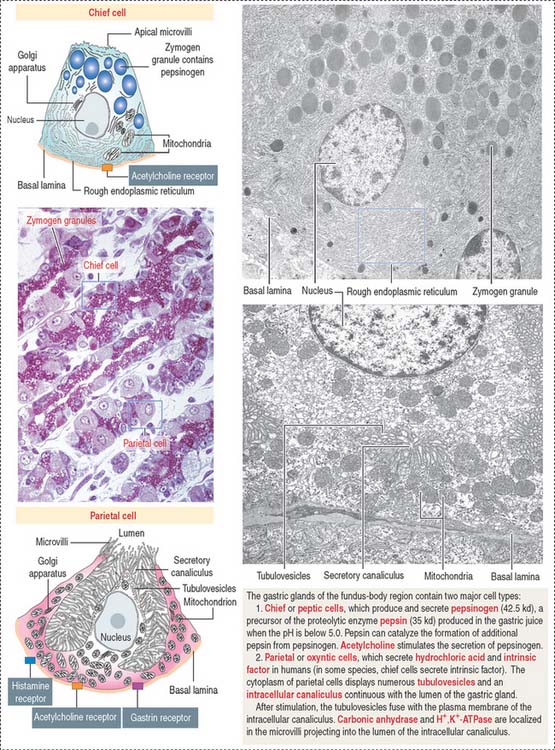
Chief cells (Figure 15-15) predominate in the lower third of the gastric gland. Chief cells are not present in cardiac glands and are seldom found in the pyloric antrum. Chief cells have a structural similarity to the zymogenic cells of the exocrine pancreas: the basal region of the cytoplasm contains an extensive rough endoplasmic reticulum. Pepsinogen-containing secretory granules (zymogen granules) are observed in the apical region of the cell. Pepsinogen, a proenzyme stored in the zymogen granules, is released into the lumen of the gland and converted in the acid environment of the stomach to pepsin, a proteolytic enzyme capable of digesting most proteins. Exocytosis of pepsinogen is rapid and stimulated by feeding (after fasting).
Parietal cells predominate near the neck and in the upper segment of the gastric gland and are linked to chief cells by junctional complexes. Parietal cells produce the hydrochloric acid of the gastric juice and intrinsic factor, a glycoprotein that binds to vitamin B12. Vitamin B12 binds in the stomach to the transporting binding protein intrinsic factor. In the small intestine, the vitamin B12-intrinsic factor complex binds to intrinsic factor receptor on the surface of enterocytes in the ileum and is transported to the liver through the portal circulation.
Autoimmune gastritis is caused by autoantibodies to H+,K+-dependent ATPase, a parietal cell antigen, and intrinsic factor. Destruction of parietal cells causes a reduction in hydrochloric acid in the gastric juice (achlorhydria) and a lack of synthesis of intrinsic factor. The resulting vitamin B12 deficiency disrupts the formation of red blood cells in the bone marrow, leading to a condition known as pernicious anemia, identified by examination of peripheral blood as megaloblastic anemia characterized by macrocytic red blood cells and hypersegmented large neutrophils (see Chapter 6, Blood and Hematopoiesis).
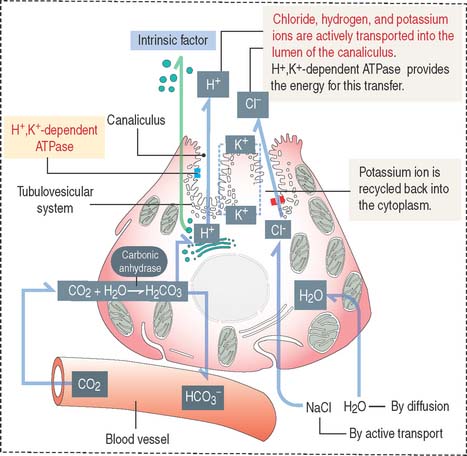
Parietal cells have three distinctive features (see Figure 15-15): (1) abundant mitochondria, which occupy about 40% of the cell volume and provide the adenosine triphosphate (ATP) required to pump H+ ions into the lumen of the intracellular canaliculus; (2) an intracellular canaliculus, formed by an invagination of the apical cell surface and continuous with the lumen of the gastric gland, which is lined by numerous microvilli; and (3) an H+,K+-dependent ATPase-rich tubulovesicular system, which is distributed along the secretory canaliculus during the resting state of the parietal cell.
Secretion of hydrochloric acid by parietal cells
Parietal cells produce an acidic secretion (pH 0.9 to 2.0) rich in hydrochloric acid, with a concentration of H+ ions one million times greater than that of blood (Figure 15-16). The release of H+ ions and Cl− by the parietal cell involves the membrane fusion of the tubulovesicular system with the intracellular canaliculus.
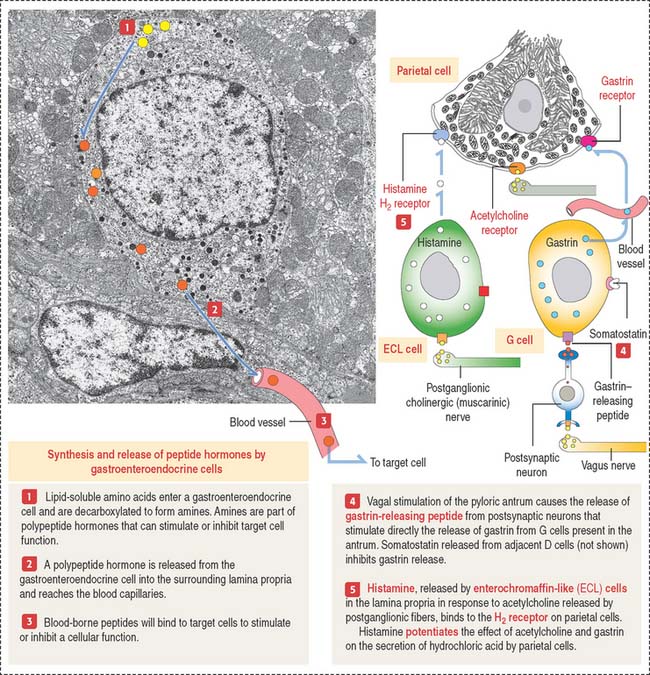
The parasympathetic mediator acetylcholine and the peptide gastrin, produced by enteroendocrine cells of the pyloric antrum, stimulate parietal cells to secrete HCl (see Figure 15-19). Acetylcholine also stimulates the release of gastrin. Histamine potentiates the effects of acetylcholine and gastrin on parietal cell secretion after binding to the histamine H2 receptor. Histamine is produced by enterochromaffin-like (ECL) cells within the lamina propria surrounding the gastric glands. Cimetidine is an H2 receptor antagonist that inhibits histamine-dependent acid secretion.
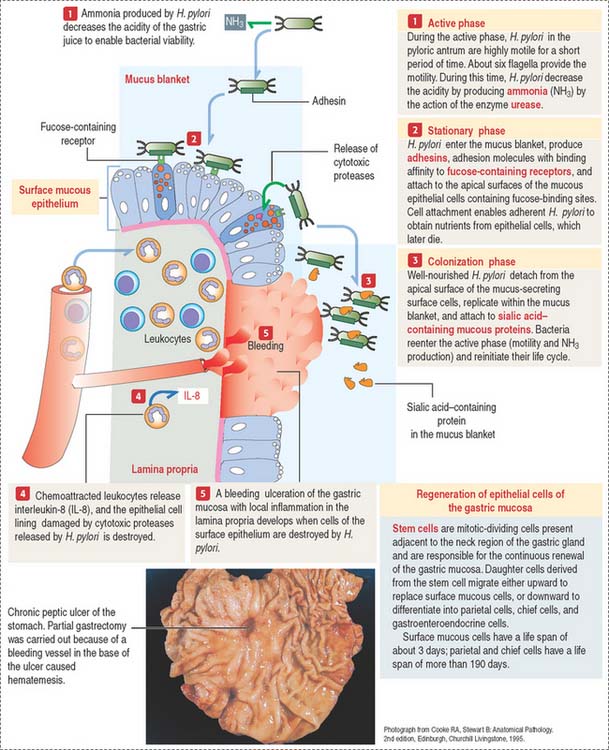
Clinical significance: Helicobacter pylori infection
It is convenient to regard the gastric juice as a combination of two separate secretions: (1) an alkaline mucosal gel protective component, produced by surface mucous cells and mucous neck cells; and (2) HCl and pepsin, two parietal–chief cell-derived potentially aggressive components. The protective component is constitutive; it is always present. The aggressive component is facultative because hydrochloric acid and pepsin levels increase above basal levels after food intake.
The viscous, highly glycosylated gastric mucus blanket—produced by surface mucous cells and mucous neck cells—maintains a neutral pH at the epithelial cell surfaces of the stomach. In addition, the mitochondrial-rich surface mucous cells (see Figure 15-14) produce HCO3− ions diffusing into the surface mucus gel. Recall the clinical significance during chronic vomiting of Na+, K+, and Cl− present in the protective mucosal barrier and gastric juice (see section on functions of the gastric gland).
HCO3− ions, produced by parietal cells, enter the fenestrated capillaries of the lamina propria. Some of the HCO3− ions diffuse into the mucus blanket and neutralize the low pH created by the HCl content of the gastric lumen at the vicinity of the surface mucous cells (Figure 15-17).
However, the mucus blanket lining the gastric epithelium, in particular in the pyloric antrum, is the site where the flagellated bacterium Helicobacter pylori resides in spite of the hostile environment.
Three phases define the pathogenesis of H. pylori (Figure 15-18):
Gastroenteroendocrine cells
Gastroenteroendocrine cells are members of the APUD system, so called because of the amine precursor uptake and decarboxylation property of amino acids (Figure 15-19). Because not all the cells accumulate amine precursors, the designation APUD has been replaced by DNES (for diffuse neuroendocrine system).
Neuroendocrine mediators are released from nerve terminals. Acetylcholine is released at the terminals of postganglionic cholinergic nerves. Gastrin-releasing peptide is released by postsynaptic neurons activated by stimulation of the vagus nerve (see Figure 15-19).
We consider six major gastrointestinal peptide hormones: secretin, gastrin, cholecystokinin (CCK), glucose-dependent insulinotropic peptide, motilin, and ghrelin.
Gastrin is produced by G cells located in the pyloric antrum. Three forms of gastrin have been described: little gastrin, or G17 (which contains 17 amino acids), big gastrin, or G34 (which contains 34 amino acids), and minigastrin, or G14 (which consists of 14 amino acids). G cells produce primarily G17. The duodenal mucosa in humans contains G cells producing mainly G34. The neuroendocrine mediator gastrin-releasing peptide regulates the release of gastrin. Somatostatin, produced by adjacent D cells, inhibits the release of gastrin (see Figure 15-19).
Gastrin stimulates the growth of ECL cells of the stomach. Continued hypersecretion of gastrin results in hyperplasia of ECL cells. ECL cells produce histamine by decarboxylation of histidine. Histamine binds to the histamine H2 receptor on parietal cells to potentiate the effect of gastrin and acetylcholine on HCl secretion (see Figure 15-19). Histamine H2 receptor blocking drugs (such as cimetidine [Tagamet] and ranitidine [Zantac]) are effective inhibitors of acid secretion.
Plasma levels of ghrelin are high in patients with Prader-Willi syndrome (caused by abnormal gene imprinting; see section on epigenetics in Chapter 20, Spermatogenesis). Severe hypotonia and feeding difficulties in early infancy, followed by obesity and uncontrollable appetite, hypogonadism, and infertility are characteristics of Prader-Willi syndrome.
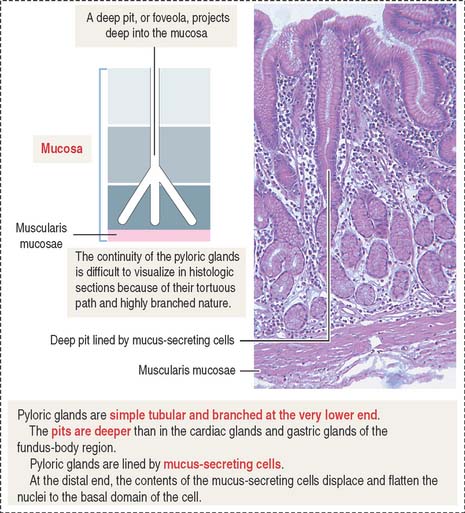
Pyloric glands
Pyloric glands differ from the cardiac and gastric glands in the following layers: (1) the gastric pits, or foveolae, are deeper and extend halfway through the depth of the mucosa; and (2) pyloric glands have a larger lumen and are highly branched (Figure 15-20).
Mucosa, submucosa, and muscularis of the stomach
The submucosa consists of dense irregular connective tissue in which collagenous and elastic fibers are abundant. A large number of arterioles, venous plexuses, and lymphatics are present in the submucosa. Also present are the cell bodies and nerve fibers of the submucosal plexus of Meissner.
Based on motility functions, the stomach can be divided into two major regions: the orad (Latin os [plural ora], mouth; ad, to; toward the mouth) portion, consisting of the fundus and part of the body, and the caudad (Latin cauda, tail; ad, to; toward the tail) portion, comprising the distal body and the antrum (see Figure 15-11). During swallowing, the orad region of the stomach and the LES relax to accommodate the ingested material. The tonus of the muscularis adjusts to the volume of the organ without increasing the pressure in the lumen.
Upper Digestive Segment
The gastric gland (present in the fundus and body) has a pit, a neck, and a body. The cell types found in the gastric glands are (1) surface mucous cells (found in the pit); (2) mucous neck cells (located at the junction of the pit with the body); and (3) zymogen-producing chief cells and HCl-producing parietal cells seen in the body region of the gland. Two additional cell types are the stem cells (precursor cells of all glandular cells), and gastroenteroendocrine cells (enterochromaffin cells). Chief cells have a well-developed rough endoplasmic reticulum and apical secretory zymogen granules, and predominate in the lower third of the body of the gland. They produce pepsinogen, which upon release is converted to pepsin. Parietal cells predominate in the upper region of the body of the gland and produce HCl (following stimulation by acetylcholine, gastrin, and histamine) and intrinsic factor. Parietal cells have abundant mitochondria, an intracellular canaliculus, and H+,K+-dependent ATPase–rich tubulovesicular system. Autoantibodies to H+,K+-dependent ATPase and intrinsic factor cause autoimmune gastritis. Destruction of parietal cells reduces HCl in the gastric juice (achlorhydria) and intrinsic factor (required for the transport and uptake of vitamin B12 by enterocytes in the ileum). Vitamin B12 deficiency causes pernicious anemia characterized by a decrease in the production of red blood cells and the release into the blood circulation of large red blood cells (megaloblastic anemia).
Cholecystokinin stimulates the contraction of the gallbladder and relaxes the sphincter of Oddi.