18 NEUROENDOCRINE SYSTEM
HYPOPHYSIS
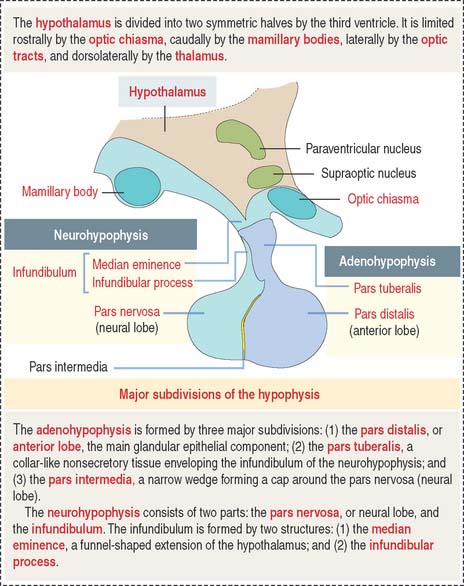
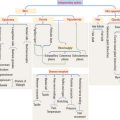
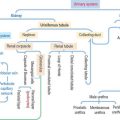
The hypophysis (Greek hypo, under; physis, growth) consists of two embryo-logically distinct tissues (Figure 18-1): (1) the adenohypophysis, the glandular epithelial portion; and (2) the neurohypophysis, the neural portion.
Embryologic origin of the hypophysis
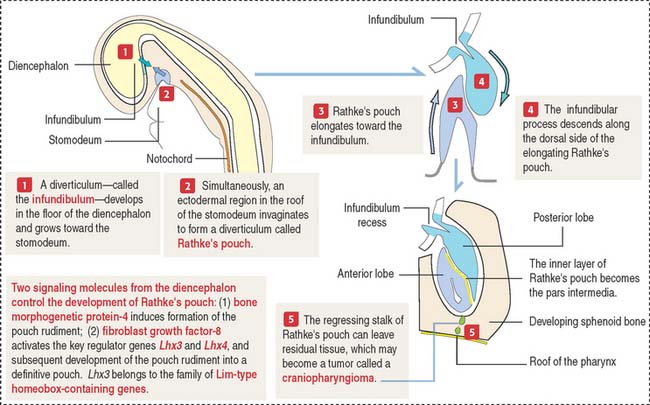
The anterior hypophysis and neurohypophysis have different embryologic origins (Figure 18-2). The anterior hypophysis derives from an evagination (pouch of Rathke) of the ectodermal lining of the future oral cavity extending upward toward the developing neurohypophysis. The neurohypophysis develops from an infundibular downgrowth from the floor of the diencephalon. The connecting stem attached to the pouch of Rathke disappears. However, the connecting stem of the neurohypophysis remains as the core of the infundibular stem, or stalk.
Blood supply of the hypophysis: Hypothalamohypophysial portal circulation
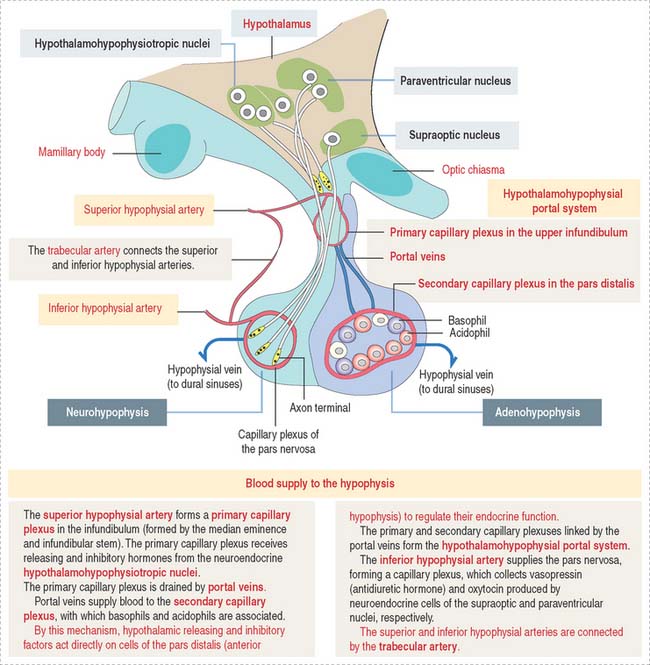
The superior hypophysial artery (derived from the internal carotid arteries) (Figure 18-3) enters the median eminence and upper part of the infundibular stem and forms the first sinusoidal capillary plexus (primary capillary plexus), which receives the secretion of the neuroendocrine cells grouped in the hypothalamic hypophysiotropic nuclei of the hypothalamus.
The hypothalamohypophysial portal system enables (1) the transport of hypothalamic releasing and inhibitory hormones from the primary capillary plexus to the hormone-producing epithelial cells of the anterior hypophysis; (2) the secretion of hormones from the anterior hypophysis into the secondary capillary plexus and to the general circulation; and (3) the functional integration of the hypothalamus with the anterior hypophysis, provided by the portal veins.
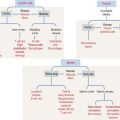
Histology of the pars distalis (anterior lobe)
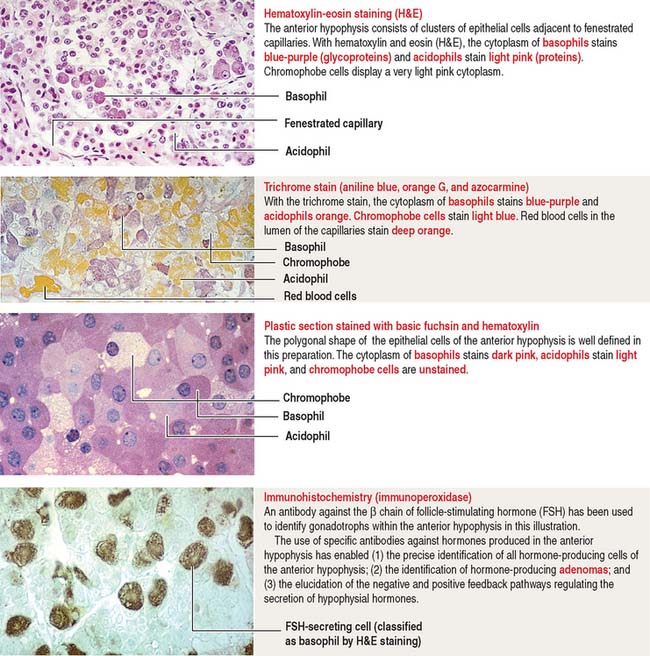
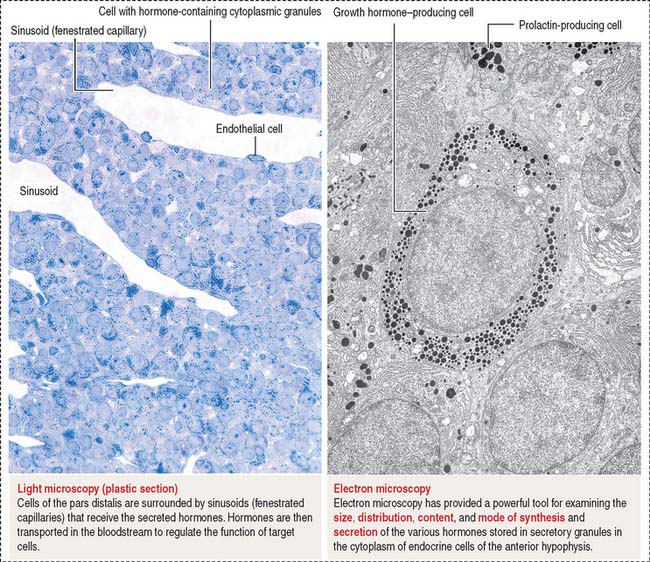
The pars distalis is formed by three components: (1) cords of epithelial cells (Figure 18-4); (2) minimal supporting connective tissue stroma; and (3) fenestrated capillaries (or sinusoids) (Figure 18-5), which are parts of the secondary capillary plexus.
There is no blood-brain barrier in the anterior hypophysis.
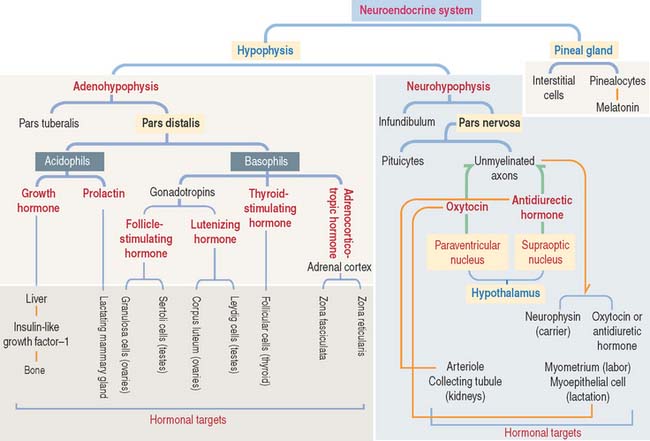
There are three distinct types of endocrine cell in the anterior hypophysis (see Figure 18-4): (1) acidophils (cells that stain with an acidic dye), which are prevalent at the sides of the gland; (2) basophils (cells that stain with a basic dye and are periodic acid-Schiff [PAS]-positive), which are predominant in the middle of the gland; and (3) chromophobes (cells lacking cytoplasmic staining).
Acidophils secrete two major peptide hormones: growth hormone and prolactin. Basophils secrete glycoprotein hormones: the gonadotropin folliclestimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and adrenocorticotropic hormone (ACTH), or corticotropin. Chromophobes include cells that have depleted their hormone content and lost the staining affinity typical of acidophils and basophils.
The precise identification of the endocrine cells of the anterior hypophysis is by immunohistochemistry, which demonstrates their hormone content using specific antibodies (see Figure 18-4).
Growth hormone
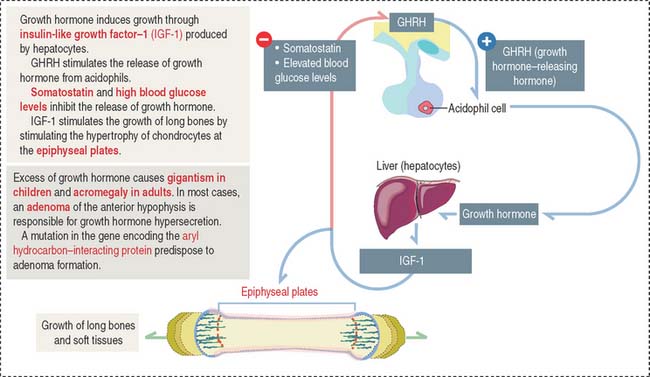
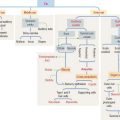
Growth hormone is a peptide of 191 amino acids in length (22 kd). It has the following characteristics (Figure 18-6): (1) Growth hormone has structural homology similar to prolactin and human placental lactogen. There is some overlap in the activity of these three hormones. (2) It is released into the blood circulation in the form of pulses throughout a 24-hour sleep-wake period, with peak secretion occurring during the first two hours of sleep. (3) Despite its name, growth hormone does not directly induce growth; rather, it acts by stimulating in hepatocytes the production of insulin-like growth factor-1 (IGF-1), also known as somatomedin C. The cell receptor for IGF-1 is similar to that for insulin (formed by dimers of two glycoproteins with integral cytoplasmic protein tyrosine kinase domains). (4) The release of growth hormone is regulated by two neuropeptides.
A stimulatory effect is caused by growth hormone–releasing hormone (GHRH), a peptide of 44 amino acids. An inhibitory effect is produced by somatostatin (a peptide of 14 amino acids) and by elevated blood glucose levels. Both GHRH and somatostatin derive from the hypothalamus. Somatostatin is also produced in the islet of Langerhans (pancreas).
Clinical significance: Gigantism (in children) and acromegaly (in adults)
Excessive secretion of growth hormone can occur in the presence of a benign tumor called an adenoma.
When the growth hormone-secreting tumor occurs during childhood and puberty at a time when the epiphyseal plates are still active, gigantism (Greek gigas, giant; extremely tall stature) is observed. If excessive growth hormone secretion occurs in the adult, when the epiphyseal plates are inactive, acromegaly (Greek akron, end or extremity; megas, large) develops. In acromegaly, the hands, feet, jaw, and soft tissues become enlarged. Long bones do not grow in length, but cartilage (nose, ears) and membranous bones (mandible and calvarium) continue to grow, leading to gross deformities.
Prolactin
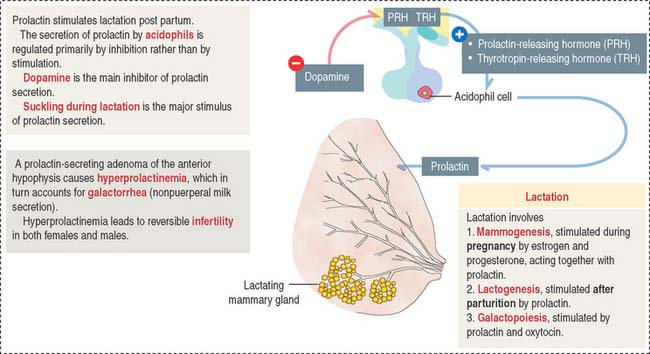
The predominant action of prolactin is to stimulate the initiation and maintenance of lactation post partum (Figure 18-7). Lactation involves the following: (1) Mammogenesis, the growth and development of the mammary gland, is stimulated primarily by estrogen and progesterone in coordination with prolactin and human placental lactogen. (2) Lactogenesis, the initiation of lactation, is triggered by prolactin acting on the developed mammary gland by the actions of estrogens and progesterone. Lactation is inhibited during pregnancy by high levels of estrogen and progesterone, which decline at delivery. Either estradiol or prolactin antagonists are used clinically to stop lactation. (3) Galactopoiesis, the maintenance of milk production, requires both prolactin and oxytocin.
The effects of prolactin, placental lactogen, and steroids on the development of the lactating mammary gland are discussed in Chapter 23, Fertilization, Placentation, and Lactation.
A stimulatory effect on prolactin release is exerted by prolactin-releasing hormone (PRH) and thyrotropin-releasing hormone (TRH). Prolactin is released from acidophils in a pulsatile fashion, coinciding with and following each period of suckling. Intermittent surges of prolactin stimulate milk synthesis.
Gonadotropins: Follicle-stimulating hormone and luteinizing hormone
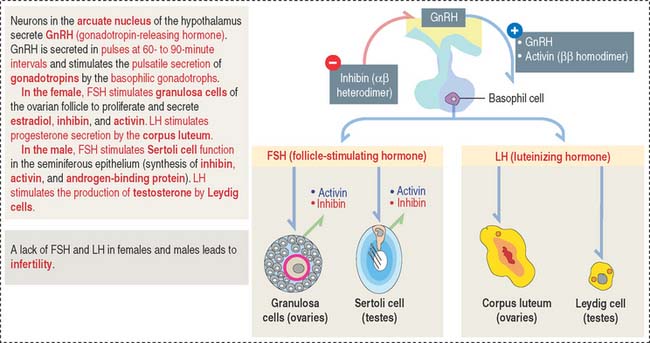
Gonadotrophs (gonadotropin-secreting cells) (Figure 18-8) secrete both FSH and LH. Gonadotrophs constitute about 10% of the total cell population of the anterior hypophysis.
In the female, FSH stimulates the development of the ovarian follicles by a process called folliculogenesis. In the male, FSH acts on Sertoli cells in the testes to stimulate the aromatization of estrogens from androgens and the production of androgen-binding protein, with binding affinity to testosterone.
In the female, LH stimulates steroidogenesis in the ovarian follicle and corpus luteum. In the male, LH controls the rate of testosterone synthesis by Leydig cells in the testis. The function of FSH and LH in the male is analyzed in Chapter 20, Spermatogenesis.
We will discuss in Chapter 20, Spermatogenesis, and Chapter 22, Follicle Development and Menstrual Cycle, the functions of FSH and LH in spermatogenesis, Leydig cell function, folliculogenesis, and luteogenesis.
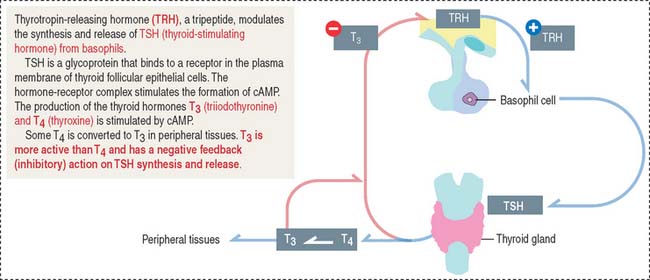
Thyroid-stimulating hormone (thyrotropin)
Thyrotropic cells represent about 5% of the total population of the anterior hypophysis.
TSH is the regulatory hormone of thyroid function (Figure 18-9) and growth. The mechanism of action of TSH on thyroid cell function is discussed in the thyroid gland section of Chapter 19, Endocrine System. Thyrotropin-releasing hormone (TRH), a 3-amino-acid-peptide produced in the hypothalamus, stimulates the synthesis and release of TSH from basophils. TRH also stimulates the release of prolactin. The release of TSH is inhibited by increased concentrations of the thyroid hormones triiodothyronine (T3) and thyroxine (T4).
Clinical significance: Hypothyroidism
A deficiency in the secretion of TSH (observed in rare cases of congenital hypoplasia of the hypophysis) produces hypothyroidism, characterized by reduced cell metabolism and temperature and basal metabolic rate and mental lethargy. Hypothyroidism is also observed in the autoimmune disorder Hashimoto’s disease. Hypothyroidism can also result from a disease of the thyroid gland or a deficiency in dietary iodine. We will discuss hyperthyroidism in the thyroid gland section of Chapter 19, Endocrine System when we describe Graves’ disease.
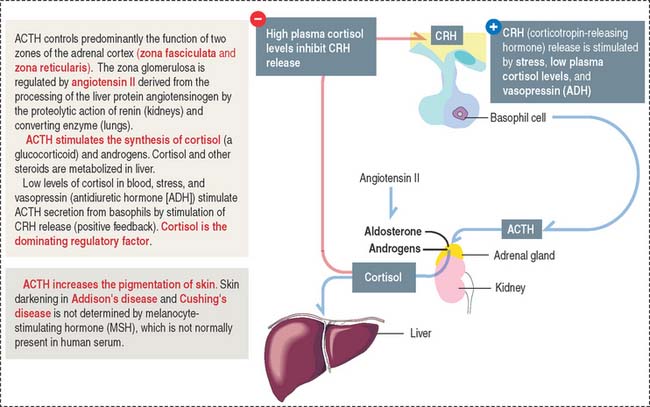
Adrenocorticotropic hormone
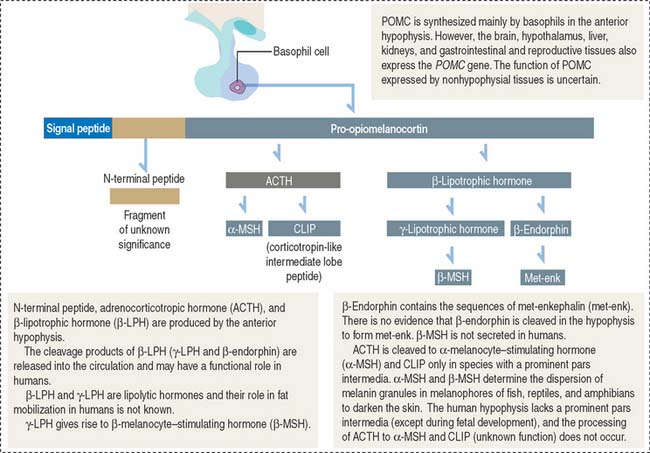
ACTH, or corticotropin, is a single-chain protein, 39 amino acids in length (4.5 kd), with a short circulating time (7 to 12 minutes). Its primary action is to stimulate growth and steroid synthesis in the zonae fasciculata and reticularis of the adrenal cortex. The zona glomerulosa of the adrenal cortex is under the control of angiotensin II (see the adrenal gland section of Chapter 19, Endocrine System). The effects of ACTH on the adrenal cortex are mediated by cyclic adenosine monophosphate (cAMP). ACTH also acts beyond the adrenal gland by increasing skin pigmentation and lipolysis.
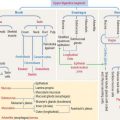
ACTH derives from a large glycosylated precursor of 31 kd called pro-opiomelanocortin (POMC), processed in the anterior hypophysis. The products of POMC are the following (Figure 18-10):
The release of ACTH is controlled by the following (Figure 18-11):
ACTH is secreted in a circadian manner (morning peaks followed by a slow decline afterward).
Clinical significance: Cushing’s disease
An ACTH-secreting adenoma of the hypophysis causes Cushing’s disease. This disease is characterized by an increase in the production of cortisol by the zona fasciculata of the adrenal cortex (see the adrenal gland section in Chapter 19, Endocrine System), obesity, osteoporosis, and muscle wasting. A reduction in the secretion of ACTH results in diminished secretion of cortisol and in hypoglycemia.
A loss of ACTH decreases adrenal androgen secretion. In females, androgen deficiency causes loss of pubic and axillary hair. This effect is not observed in males because it is compensated for by the testicular secretion of androgens.
NEUROHYPOPHYSIS
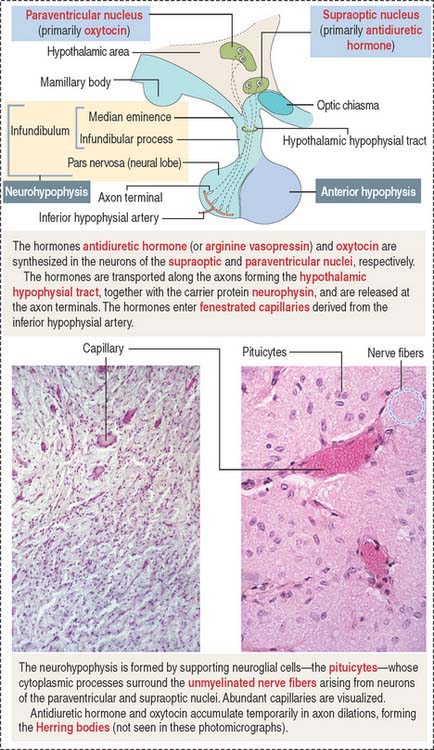
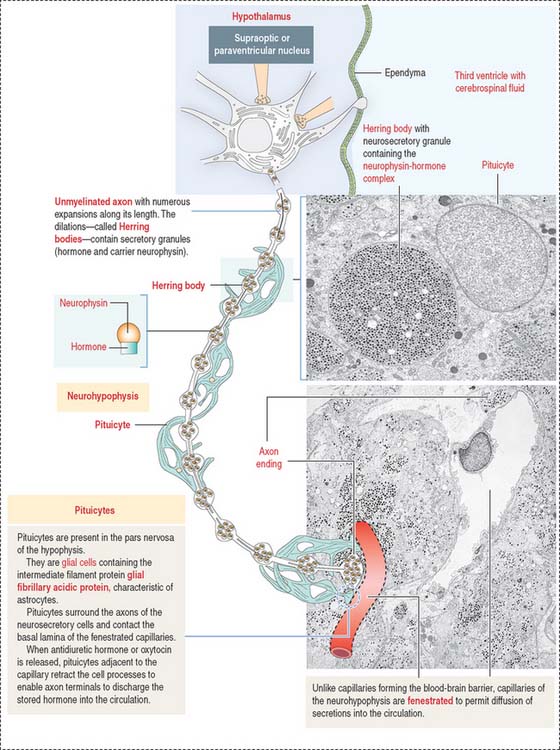
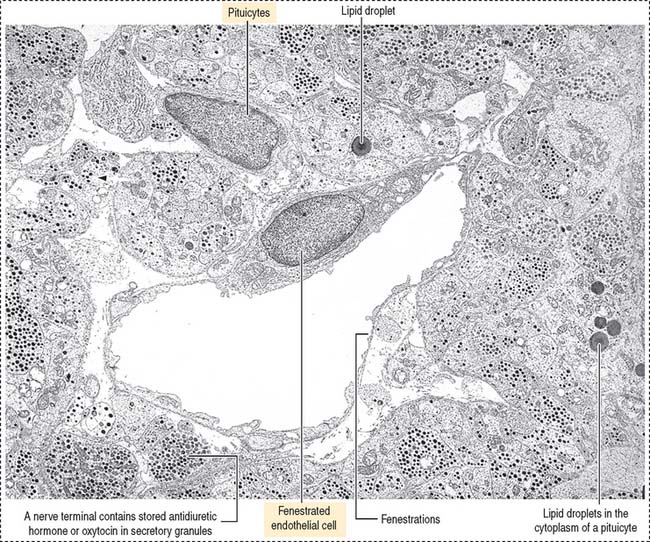
The neurohypophysis consists of three histologic components (Figures 18-12 and 18-13): (1) Pituicytes, resembling astrocytes, provide support to the axons. (2) Unmyelinated axons, derived from neuroendocrine cells (called magnicellular neurons because their cell bodies are large) of the supraoptic and paraventricular nuclei, make up the infundibulum and form the hypothalamohypophysial tract. Axons, with bulging intermittent segments and terminals (called Herring bodies) containing secretory products (the neurophysin-hormone complex), are found in the pars nervosa (neural lobe). Neurophysin is secreted with the hormone and does not have an apparent biologic action other than serving as a hormone carrier during axonal transport. (3) Fenestrated capillaries are derived from the inferior hypophysial artery.
Pituicytes are astrocyte-like glial cells with abundant glial fibrillary acidic proteins, an intermediate filament protein, and a few lipid droplets in their cytoplasm. The cytoplasmic processes of pituicytes (Figure 18-14) (1) surround the axons derived from the neuroendocrine cells, (2) extend between the axon terminals and the basal lamina surrounding fenestrated capillaries, and (3) retract to enable the release into the blood of secretory granules stored in the axon terminals (see Figure 18-14).
Axons in the neurohypophysis derive from the supraoptic nuclei and the paraventricular nuclei.
Some neurons of the paraventricular nuclei are small and their axons project to the median eminence rather than to the pars nervosa. These neurons, called parvicellular neurons (Latin parvus, small), secrete ADH and oxytocin entering the hypophysial portal blood at the median eminence. Large neurons of the supraoptic and paraventricular nuclei, called magnicellular neurons (Latin magnus, large), give rise to axons forming the hypothalamic hypophysial tract. The terminals of these neurons are located in the pars nervosa. Both the supraoptic and paraventricular nuclei contain neurons synthesizing ADH and oxytocin. However, neurons of the supraoptic nuclei produce primarily ADH and the paraventricular nuclei synthesize primarily oxytocin.
Although the neuroendocrine cells of the supraoptic and paraventricular nuclei are located behind the blood-brain barrier, their products are transported to nerve terminals and released outside the blood-brain barrier into fenestrated capillaries.
Clinical significance: Diabetes insipidus
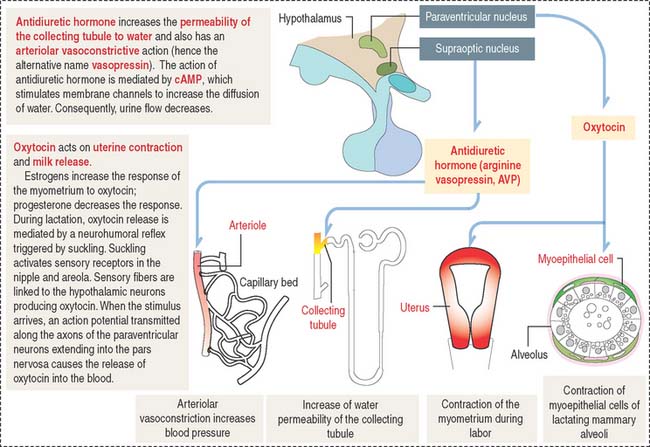
Oxytocin participates in the contraction of smooth muscle, in particular the uterus during labor, and myoepithelial cells lining the secretory acini and lactiferous ducts of the mammary gland to facilitate milk ejection (or letdown of milk) during lactation (Figure 18-15).
Antidiuretic hormone regulates water excretion in the kidneys and is also a potent vasoconstrictor at high doses (see Figure 18-15). This is the basis for its alternative name, vasopressin (arginine vasopressin [AVP]). An increase in osmotic pressure in circulating blood or reduced blood volume triggers the release of ADH. Retention of water reduces plasma osmolality, which acts on hypothalamic osmoreceptors to suppress the secretion of ADH.
Neurogenic diabetes insipidus occurs when the secretion of ADH is reduced or absent. Polyuria is a common clinical finding. Patients with diabetes insipidus can excrete up to 20 L of urine in 24 hours. Neurogenic diabetes insipidus is caused by a head injury, an invasive tumor damaging the hypothalamic hypophysial system, or autoimmune destruction of vasopressin-secreting neurons.
PINEAL GLAND
Development of the pineal gland
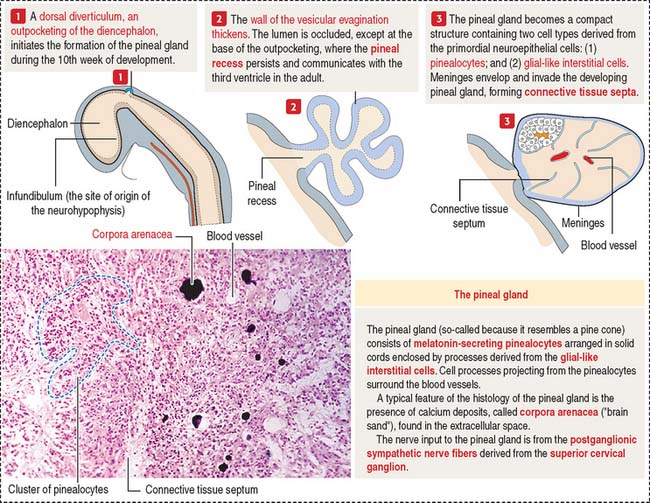
The pineal gland develops from a saccular outpocketing of the posterior diencephalic roof in the midline of the third ventricle (Figure 18-16).
Histology of the pineal gland
Two cell types form the pineal gland (see Figure 18-16): (1) the pinealocytes and (2) the glial-like interstitial cells.
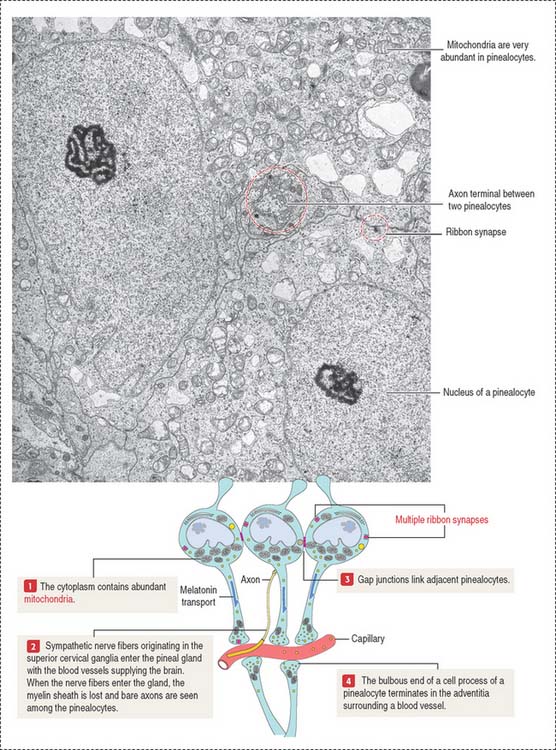
The pinealocytes are secretory cells organized into cords and clusters resting on a basal lamina and surrounded by connective tissue, blood vessels lined by fenestrated endothelial cells, and nerves. The pinealocyte has two or more cell processes ending in bulbous expansions. One of the processes ends near capillaries. The cytoplasm contains abundant mitochondria and multiple synaptic ribbons that are randomly distributed (Figure 18-17). Single ribbon synapses can be seen at the synaptic end of sensory cells of the retina (see Figure 9-18) and inner ear (see Figure 9-28).
Like the anterior hypophysis, the pineal gland lacks a blood-brain barrier.
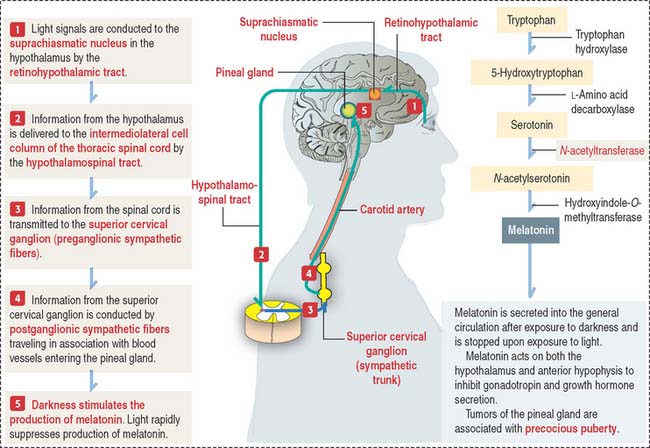
The pineal gland secretes melatonin, the “hormone of darkness”
Melatonin is the major biologically active substance secreted by the pineal gland. Melatonin is synthesized from tryptophan by pinealocytes and immediately secreted (Figure 18-18). During night (with complete darkness), the melatonin content of the pineal gland is highest.
Circadian clock, an endogenous oscillator controlling circadian rhythms
When a suprachiasmatic nucleus is transplanted to a recipient with a damaged suprachiasmatic nucleus, it displays the circadian pacemaker properties of the donor rather than those of the host. The mechanism by which individual neurons of the suprachiasmatic nucleus are recruited to organize a pacemaker that oversees the circadian rhythms is not fully known.
Clinical significance: Precocious puberty
Essential concepts
Corticotropin-releasing hormone (CRH) derived from neuroendocrine neurons of the paraventricular nuclei (which also produce antidiuretic hormone [ADH]), stimulates the release of ACTH. This CRH stimulatory effect is potentiated by ADH and angiotensin II. High levels of cortisol prevent the release of CRH or ACTH.