Chapter 37 Upper Cervical and Craniocervical Decompression
Pathology Overview
The pathologies possible at the craniocervical junction are varied.1 Most lesions requiring surgery are approached dorsally through a posterior fossa craniectomy and C1 laminectomy. Table 37-1 lists the pathologies encountered by category. In a more basic organization, the abnormalities can be divided into congenital abnormalities and developmental/acquired pathologies. In Box 37-1 the material is reorganized in an empirical fashion.
BOX 37-1 Origin of Pathology at Craniocervical Junction
Developmental/Acquired
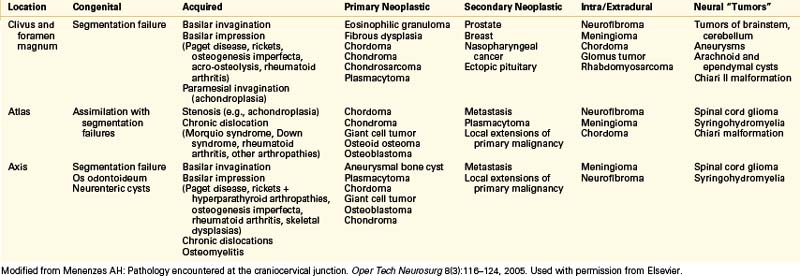
Modified from Menenzes AH: Pathology encountered at the craniocervical junction. Oper Tech Neurosurg 8:116–124, 2005. Used with permission from Elsevier.
Ventral Approaches
Several routes to the ventral clivus and upper cervical spine are accepted. Standard approaches to the ventral cervical spine are limited by the mandible and oropharynx.2–4 Transoral routes to the lower clivus and upper cervical spine are acceptable and safe for addressing pathologies that cause craniocervical instability and that compress neurovascular structures. In 1917 Kanavel5 described the first transoral procedure, which he performed to remove a bullet lodged between the clivus and ventral atlas. In 1962 Fang and Ong6 reported the first series of six patients who underwent transoral decompression for atlantoaxial instability or congenital anomalies. Their high complication rate contributed to the slow acceptance of this approach.
A resurgent interest in the transoral approach led to the development of better techniques and instrumentation.7 Current techniques and practices have lowered complication rates considerably.8 Modern antibiotics and instruments have revolutionized and revitalized this operation. An extrapharyngeal approach for exposing the high cervical region is technically difficult but a reasonable alternative when the transoral approach cannot be used.9 The transoral approach remains an excellent tool in the surgeon’s armamentarium for ventral decompression. It is also versatile in that the upper clivus rostrally and C3 vertebral body caudally can be accessed. The authors prefer this route for extradural pathology. Dural closure is difficult in the transoral setting, and a lateral approach is preferable for intradural lesions. The far-lateral approach is quite robust for this purpose.
As minimally invasive surgery becomes more prevalent and patient demand for it increases, approaches to the upper cervical spine and clivus have become the target of efforts to minimize incisions and complications. In 2008 an endoscopic endonasal approach to odontoid resection was reported.10
Surgical Technique
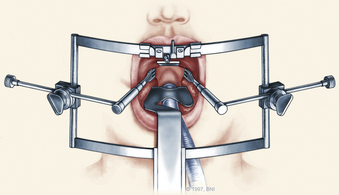
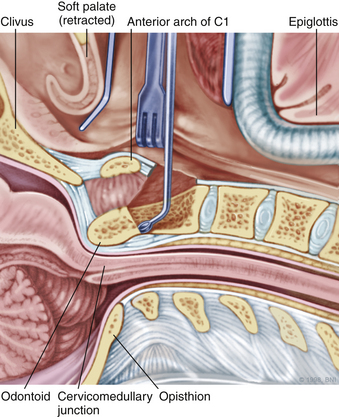
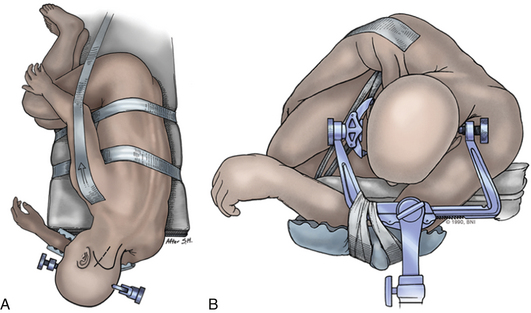
The patient is placed in the supine position on a standard operating room table with the head fixated in a Mayfield three-pin head holder (Codman, Inc., Randolph, Mass.) or a halo-ring adapter. A Spetzler-Sonntag transoral retractor (Aesculap, San Francisco) is positioned over the mouth. Retractors are used to form a rectangle of exposure. The palate is elevated cephalad, and the tongue and endotracheal tube are retracted caudally. The tonsils and lateral oropharyngeal walls are covered with moist gauze and retracted outward (Figs. 37-1 and 37-2). Transnasal catheters and palatal retraction sutures can be used in lieu of the Spetzler-Sonntag retractor. If the mouth cannot be opened sufficiently, a mandible-splitting approach can be used11 and a tracheostomy performed before the surgical approach begins.
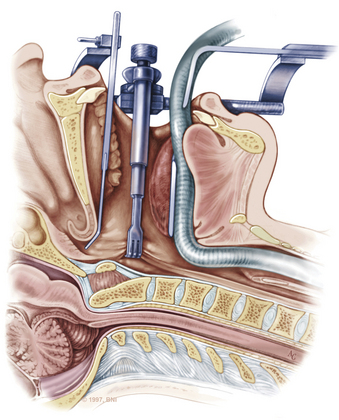
After the retractor is placed in its final position, the oral cavity is cleansed with povidone-iodine (Betadine). A preoperative dose of antibiotics that includes coverage for oral flora should be administered. The authors prefer cefepime and metronidazole. The surgeon sits at the patient’s head, necessitating an adjustment in orientation for the duration of the case. An operating microscope is brought into the field. Stereotactic guidance may be used to guide the angle of approach and to confirm the location before the palatal incision is made.12 Alternatively, fluoroscopy can be used for confirmation. Electrocauterization is used, and the incision proceeds down to bone. Subperiosteal dissection is used to expose the lower clivus, atlas, and axis. The authors seldom find it necessary to divide the soft palate unless the upper clivus is the target. Self-retaining retractors are placed to maintain exposure.
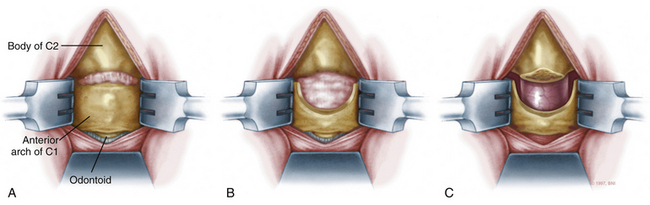
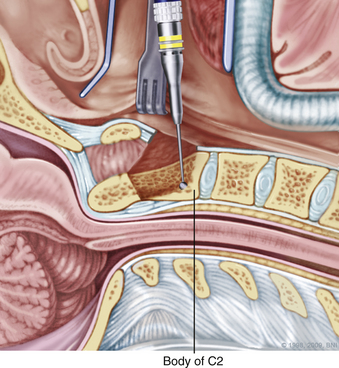
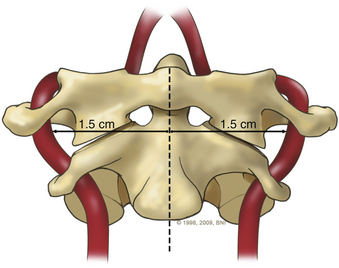
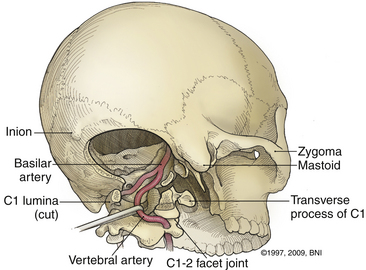
A high-speed air drill is used to remove the ventral arch of C1. The ventral arch of the atlas is the load-bearing portion of the bone.13 An effort should be made to preserve a portion of the arch to prevent spread of the lateral mass and to maintain orientation relative to midline. Approximately 70% of patients undergoing ventral decompression require supplemental internal fixation. This number increases to 90% in patients with rheumatoid arthritis.14 The remaining anterior tubercle of C1 is left as a landmark for posterior screw fixation (Fig. 37-3A–C). Stereotactic navigation may be useful in cases requiring hardware fixation, especially if a large ventral portion of C1 and surrounding bone must be removed. Various instruments can be used to remove bone and tissue carefully. Long-handled curettes and rongeurs, as well as bipolar cautery, are useful adjuncts (Figs. 37-4 to 37-6). The average distance between the vertebral arteries is approximately 3 cm. The anatomic “safe zone” is 1.5 cm lateral to midline in both directions (Fig. 37-7). Preoperative radiographs must be evaluated carefully. In many cases, CT angiography can delineate aberrant or asymmetrical vasculature. Further lateral dissection places the hypoglossal nerve, vertebral artery, and cervical neurovascular bundle at risk.15
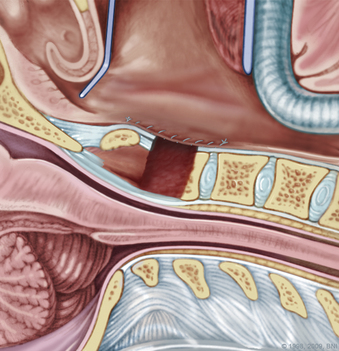
During a transoral odontoidectomy, cautious dissection and a methodical approach are essential to minimize the risk of cerebrospinal fluid (CSF) fistula. The superior ligamentous complex (consisting of the apical and paired alar ligaments) must be sectioned to remove the dens. This step should be performed first with curved curettes. This region is often adherent to the dura, and great care must be taken to avoid durotomy.16,17 A thin shell of bone can be left to avoid a dural tear. Once the last of the odontoid is removed, the frameless guidance or a lateral radiograph can be checked with a radiopaque instrument in the field.18
Pitfalls and Complication Avoidance
Preoperatively, many patients requiring ventral decompression may be severely debilitated. Postoperatively, they may need a course of parenteral nutrition, long-term rehabilitation or skilled-nursing care placement, and a long convalescent period. The surgeon should make it a priority to ensure that patients and their loved ones have appropriate and realistic expectations about outcomes and that they understand the possible risks and complications associated with the procedure.
Lateral Approaches
The lateral craniocervical region is rarely involved with bony compressive lesions. Most of these lesions are strongly associated with assimilation of the atlas into the occiput when segmentation fails between the last (fourth) occipital and first cervical sclerotomes. The sclerotomes fail to separate and remain fused, resulting in an incompletely separated atlas and sometimes a bifid lamina. This defect is associated with various syndromes (e.g., achondroplasia, Morquio syndrome) and usually occurs in conjunction with other occipitocervical bony anomalies. Astute clinicians should be wary of such entities, which may be common, such as Chiari I malformations and Klippel-Feil syndrome. Rarer but no less significant entities such as basilar invagination and congenital fusion of C2 and C3 also occur.19,20 Thus lateral approaches may need to be part of a larger operative plan and used in conjunction with other techniques to completely address the pathologies present.
Lateral compression can be unilateral or bilateral. Stenosis of the foramen magnum leads to pain (usually occipital or high cervical) and possibly myelopathy. Instability may be present, and a fusion procedure may be necessary in addition to decompression. Other lesions located ventrally in the foramen magnum such as vertebrobasilar aneurysms and meningiomas can be approached laterally. A lateral approach allows adequate exposure while protecting delicate neural structures.
Lateral approaches to the upper cervical spine have been in development for decades. These surgeries eventually resulted in the techniques commonly used today. Henry described a technique for gaining access to the V2 and V3 segments of the vertebral artery through a similar incision.21 Whitesides and Kelly further developed lateral approaches for spinal fusion using a similar incision.22 Their technique was based on dividing the sternocleidomastoid muscle at its origin in the mastoid and reflecting it dorsally. Since then, numerous authors have modified, refined, and expanded this approach to the lateral upper cervical spine and foramen magnum for both extradural and intradural lesions.23–26 Some authors have reported more aggressive approaches involving mobilization of the vertebral artery and sectioning of the sigmoid sinus when exposure needs to be increased.27 Approaches have been modified to allow resection of lesions traversing the foramen magnum and into the upper cervical spine—the so-called “extreme lateral” approaches.28 Either a laterally or medially based incision can be used with the muscle flap directed medially or laterally, respectively. The authors’ preferred method is based on a dorsal midline approach.
Surgical Technique for Far-Lateral Approach
Intraoperative stereotactic navigation can be helpful with the far-lateral approach. During resection of tumors it can be useful to monitor the lower cranial nerves in addition to SSEPs. The patient is placed in the three-pin head holder with one pin on the forehead in the supraorbital area of the ipsilateral forehead. The two-pin portion is placed low on the contralateral occipital bone, above the level of the inion, leaving the retroauricular area to the midline open (Fig. 37-8A).
The patient is placed in a modified park-bench position.29 The lower arm is slung off the table and cradled with the arm padded and the sling taped to the Mayfield adapter. The head is flexed and rotated contralaterally to bring the lesion to the highest point in the field. The surgeon should guard against the tendency for the patient’s head to translate forward during positioning. Bending the head away from the ipsilateral shoulder opens the angle between the upper cervical spine and occiput. The upper shoulder is pulled caudally and taped to the bed. Care is taken not to tape over the clavicle, as doing so puts tension on the upper brachial plexus and can cause Erb palsy. The axilla is well padded. A beanbag may be used. Foam padding should be used generously. The body, especially the upper hip, should be well taped to the bed, permitting full vertical and lateral rotation of the operating table. While the patient is positioned, the evoked potentials should be monitored closely for changes (Fig. 37-8B).
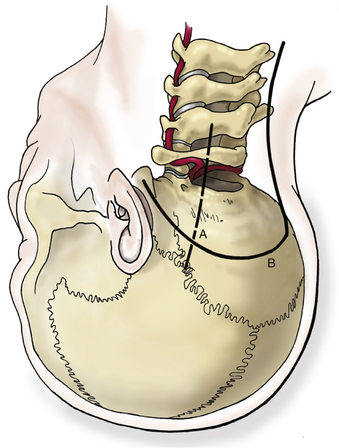
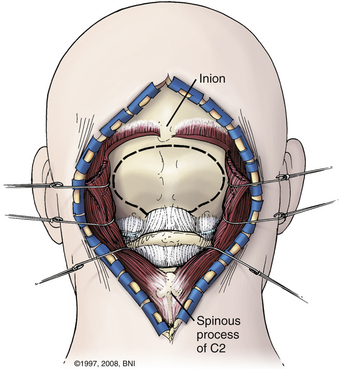
A satisfactory stereotactic film and registration are critical. Either a curvilinear “hockey stick” or paramedian incision can be used. A paramedian incision is simpler, but stereotactic guidance should be used to avoid injury to the vertebral artery (Fig. 37-9). If used, a curvilinear incision is curved rostromedially below the superior nuchal line. The inferior part of the incision extends caudally to about C4. In heavier patients C4 may be difficult to identify. A cuff of muscle and fascia can be left near the superior nuchal line to aid in closure. Using subperiosteal dissection, the surgeon elevates the remaining muscle and fascia in one flap and retracts it laterally. This maneuver exposes the occiput down to the spinous process of C2. Fish hooks or heavy “0” sutures can be used to hold the flap away with a Leyla bar or other retractor system. The C2 ganglion is preserved throughout its course.
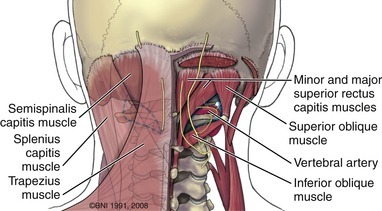
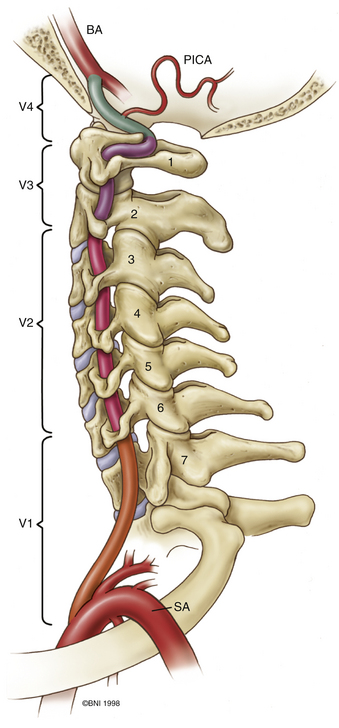
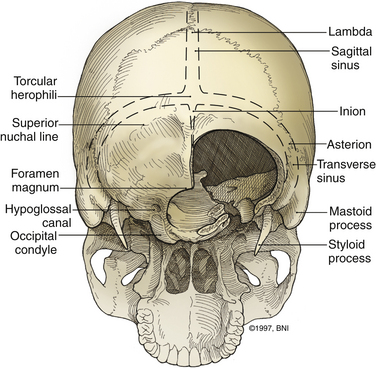
The ring of C1 can usually be palpated deeper to and above the spinous process of C2. Subperiosteal dissection proceeds laterally onto the lateral mass of C1. The suboccipital triangle is composed of the superior oblique, inferior oblique, and splenius capitis muscle. Deep to the triangle is the vertebral artery. The lateral point of the triangle is the C1 tubercle. The vertebral artery usually courses along the upper border of the C1 lamina (Fig. 37-10). Familiarity with the particular patient’s anatomy, especially the vertebral artery and its segments (Fig. 37-11), is important. The V1 (ostial) segment arises from the subclavian artery and courses superiorly to the transverse foramen of C6. The V2 (transverse) segment extends from C6 to the C2 foramen. The V3 (suboccipital) segment courses from the C2 foramen until it penetrates the dura and enters the subarachnoid space. When the artery penetrates the dura, it becomes the V4 (intracranial) segment. V4 courses anteromedially along the medulla and joins its twin segment at the vertebrobasilar junction to form the basilar artery.
During operative exposure, the surrounding venous plexus can cause troublesome bleeding. Bipolar cautery, surgical cellulose (SURGICEL NU-KNIT, Ethicon, Somerville, N.J.), and liquid hemostatic agents (Floseal) usually control any bleeding encountered.
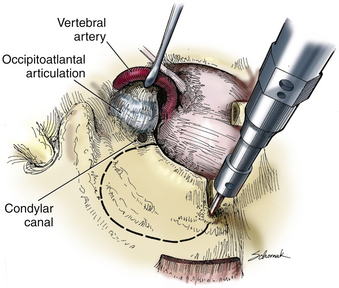
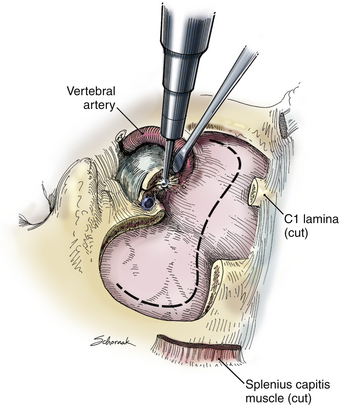
A C1 hemilaminectomy is performed from just beyond midline to the ipsilateral sulcus arteriosus. The artery can be unroofed from the foramen to mobilize it. A lateral suboccipital craniotomy is performed with a foot-plate drill (Fig. 37-12). The craniotomy is enlarged using rongeurs until the lateral mass of C1 and the occipital condyle are exposed. The bony lesion is exposed extradurally. The vertebral artery is kept moist and protected. A high-speed air drill is used to remove the lesion extradurally. A diamond bur is useful, but care should be taken to avoid heating the bit near the artery. Upbiting curettes can be used for lesions that are difficult to reach. If a dural breach is seen, primary closure or a patch graft should be used with fibrin glue. If deemed necessary, a stabilization procedure may be performed. The wound is closed in anatomic layers, and a lumbar drain is placed if the dura is violated.
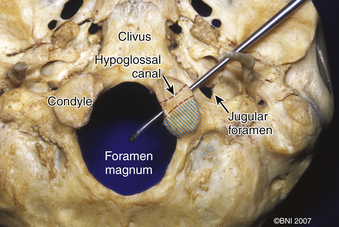
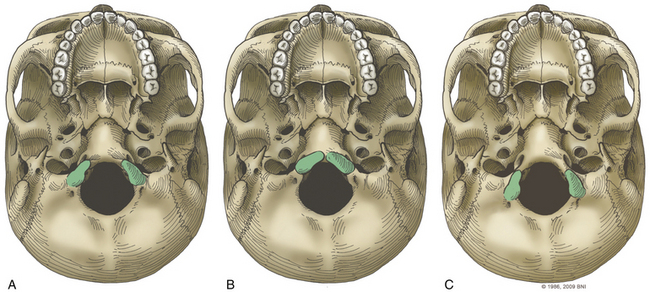
The occipital condyle can be removed to assist further decompression of the foramen magnum (Fig. 37-13). The hypoglossal foramen runs in a posteromedial to anterolateral direction with the occipital condyle (Fig. 37-14). The posterolateral portion of the condyle can be removed to increase access to the anterior portion of the foramen magnum. A wide surgical corridor can be achieved, with access to the anterior portion of the foramen magnum (Figs. 37-15 and 37-16). The relation of the occipital condyles can vary greatly from patient to patient. Some patients have anteriorly or posteriorly displaced configurations (Figs. 37-17A–C). Drilling carefully and gradually can achieve the desired access to the anterior foramen magnum. If brisk bleeding from the dorsal condylar emissary vein is encountered, it can be controlled with NU-KNIT or bone. The anatomy of the hypoglossal canal must be understood to avoid injuring the 12th cranial nerve. The canal runs anterolaterally to posteromedially. The dorsal portion of the condyle can be removed with a high-speed drill. Using navigation at this depth is difficult. In select cases, occipitocervical stabilization should be considered. Biomechanical stability is altered when more than 50% of the condyle is resected.30 Stability is also reduced significantly if the ventral portion of the condyle is removed. Options for fusion include occipitocervical plating with lateral mass screws, Steinmann pin fixation, or sublaminar wires.31,32
Pitfalls and Complication Avoidance
Other complications reported with this technique and its variants25,27,28 include venous air embolism, lower cranial nerve palsies, CSF leakage, hydrocephalus, brainstem edema, and death. Complications are most common with ventral and lateral tumors that require some brain retraction and cranial nerve dissection. If hydrocephalus is present preoperatively, it may be prudent to place an external ventricular or lumbar drain before surgery. Theoretically, doing so may reduce the need for retraction and lessen the likelihood of a CSF fistula.
Dorsal Approaches
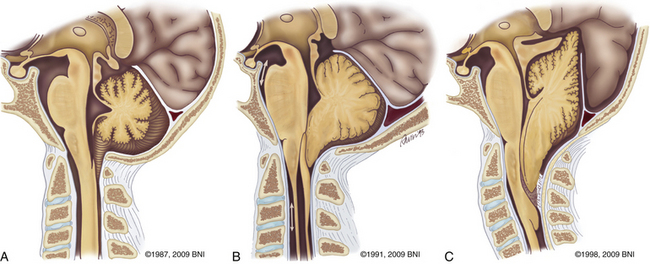
Described by Hans Chiari in 1891,33 Chiari I malformations result from descent of the cerebellar tonsillar below the level of the foramen magnum. Others have also been involved in the elucidation of this neurologic abnormality.34,35 Patients usually suffer from headaches. Cervical myelopathy and hydrocephalus can be a part of the constellation of findings. Myelopathy may result from direct compression from tonsillar herniation or syringomyelia. Hydrocephalus reflects alterations in CSF flow patterns. Both conditions usually resolve after posterior fossa decompression and duraplasty.36 Syringomyelia most often resolves 3 to 6 months after surgery.37 Type II Chiari malformations are often associated with spina bifida and may necessitate decompression if symptoms occur (Fig. 37-18C).
The most common form of dwarfism, achondroplasia, is often associated with craniocervical deformities.38 Most cases are sporadic. The foramen magnum becomes narrowed due to craniovertebral abnormalities related to thickening of the occipital bone. A thick epidural band can accompany the bony changes.39 Spinal stenosis leads to spinal cord compression and hydrocephalus.39 The major threat is respiratory compromise and the risk of sudden death.40 Newborns with achondroplasia may benefit from early neurologic and respiratory screening, MRI of the craniocervical junction, and early decompression for severe cases.41,42 Rheumatologic and degenerative diseases can also cause chronic subluxation and basilar settling. Irreducible cervicomedullary compression often results. Dorsal decompression and C1 laminectomy with dorsal stabilization are often used to correct the deformity, decompress neurologic structures, and halt progression.
Surgical Technique for Chiari Decompression
Monitoring SSEPs is a useful adjunct. Baseline readings should be obtained before the patient is positioned. Lower cranial nerve monitoring is not used routinely at our institution. Preoperative halo traction or Gardner-Wells tongs may be placed to assist reduction of the deformity or maintain stability. Often patients undergo further reduction when under general anesthesia. Before the incision is made in patients in traction, a lateral radiograph should be obtained to ensure their optimal alignment.
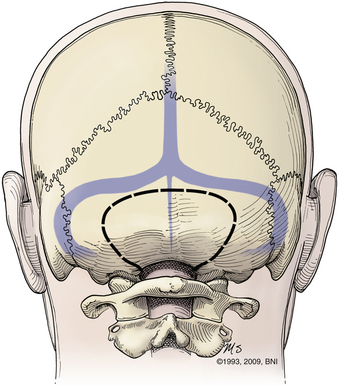
Patients are turned prone onto chest rolls, and the arms are tucked at the sides. The chin is tucked forward, against the neck in a military tuck position (Fig. 37-19). The planned craniectomy borders the venous sinuses on all borders. Care should be taken to avoid an overaggressive lateral decompression to prevent cerebellar sagging (Fig. 37-20). A midline incision is planned from the inion to C4. The nuchal line is an anatomic bloodless plane in the midline. Dissection proceeds in this plane to the occiput and spinous process of C2. The muscle attachments of C2 are left intact, but the top of C2 should be exposed (Fig. 37-21). The region around C1 and the foramen magnum is often the deepest part of the exposure. The occiput is exposed laterally and inferiorly in a subperiosteal fashion. Near the foramen magnum, curettes can be used to expose the foramen magnum carefully. A sharp ventral curve of the occipital bone indicates the margin of the foramen magnum. The C1 lamina is identified. A bifid C1 is often associated with a Chiari I malformation, and the surgeon should be cautious while finding the lamina. On both sides of midline, the exposure is continued laterally onto the ring of C1 using periosteal elevators and curettes. Laterally, the paravertebral venous complex can cause brisk bleeding. Such bleeding can be avoided by the use of bipolar forceps as a periosteal dissector on the lateral portion of the atlas and by use of Metzenbaum scissors to cut the coagulated muscle. SURGICEL and Floseal are helpful hemostatic adjuncts. Small, curved curettes are used to palpate under the ring of C1 and foramen magnum.
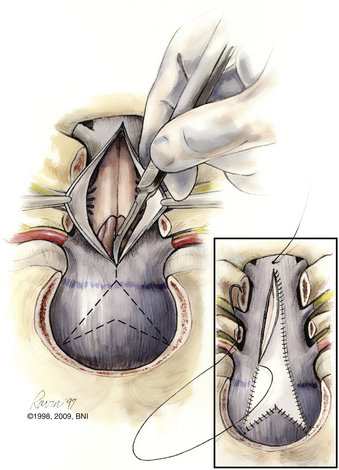
A thick fibrous band, which can be quite vascular, is often present at the foramen magnum, especially in achondroplastic patients. If a duraplasty is planned, the dura is opened in a Y-shaped fashion. This configuration allows hemostatic clipping of the occipital sinus in the posterior fossa midline. Clips are placed sequentially along the dural incision. Arachnoid adhesions obstructing the foramen of Magendie should be lysed. The authors do not use bipolar cautery near the cerebellar tonsils. The practice of plugging the obex has also fallen from favor. A chevron-shaped patch is used for grafting. Historically, autologous fascia lata or skull periosteum is the graft best tolerated. Bovine pericardium, cadaveric dura, or paravertebral fascia are all reasonably reliable substitutes.
A watertight closure is critical, and special attention should be paid to the inferior corner when the graft is sewn in place (Fig. 37-22). At this stage, CSF leaks can occur from a small dural defect in closure. More critical is the cervical fascial closure. The junction of the cervical fascia and galea at the inion should be closed meticulously. Here, most CSF leaks occur due to inadequate closure, especially in the corners of the durotomy. If allograft is used, some surgeons prefer a short course of low-dose steroids to reduce the rate of postoperative chemical meningitis.
Pitfalls and Complication Avoidance
The most commonly reported complications associated with dorsal approaches are CSF leaks, hydrocephalus, and pseudomeningoceles. Dural closure is therefore paramount to prevent complications. There have been no prospective, randomized studies on preoperative placement of a ventriculostomy, and only class III data are available. Many have reported their experiences with achondroplasia. Aryanpur et al.43 reported four documented CSF leaks in 15 cases; 1 patient developed meningitis. Three of their patients required postoperative shunting. Other studies on this entity have reported low rates of CSF leakage.36,39,41,44
Batzdorf U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J Neurosurg. 1988;68:726-730.
Dickman C.A., Spetzler R.F., Sonntag V.K.H., Apostolides P.J. Transoral approach to the craniovertebral junction. In: Dickman C.A., Spetzler R.F., Sonntag V.K.H., editors. Surgery of the craniovertebral junction. New York: Thieme; 1998:355-370.
Hadley M.N., Spetzler R.F., Sonntag V.K. The transoral approach to the superior cervical spine. A review of 53 cases of extradural cervicomedullary compression. J Neurosurg. 1989;71:16-23.
Klimo P.Jr., Rao G., Brockmeyer D. Congenital anomalies of the cervical spine. Neurosurg Clin North Am. 2007;18:463-478.
Menezes A.H. Complications of surgery at the craniovertebral junction: avoidance and management. Pediatr Neurosurg. 1991;17:254-266.
Menezes A.H. Pathology encountered at the craniocervical junction. Oper Tech Neurosurg. 2005;8:116-123.
Spetzler R.F., Grahm T.W. The far-lateral approach to the inferior clivus and the upper cervical region. BNI Q. 1990;6:35-38.
1. Menezes A.H. Pathology encountered at the craniocervical junction. Oper Tech Neurosurg. 2005;8:116-123.
2. Cloward R. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
3. Smith G.W., Robinson R.A. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-624.
4. Southwick W.O., Robinson R.A. Surgical approaches to the vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg [Am]. 1957;39:631-644.
5. Kanavel A. Bullet located between the atlas and the base of the skull: technique of removal through the mouth. Surg Clin Chicago. 1917;1:361-366.
6. Fang H.S.Y., Ong G.B. Direct anterior approach to the upper cervical spine. J Bone Joint Surg. 1962;44:1588-1604.
7. Hadley M.N., Spetzler R.F., Sonntag V.K. The transoral approach to the superior cervical spine. A review of 53 cases of extradural cervicomedullary compression. J Neurosurg. 1989;71:16-23.
8. Spetzler R.F., Dickman C.A., Sonntag V.K.H. The transoral approach to the anterior cervical spine. Contemp Neurosurg. 1991;13:1-6.
9. Vender J.R., Harrison S.J., McDonnell D.E. Fusion and instrumentation at C1-3 via the high anterior cervical approach. J Neurosurg. 2000;92:24-29.
10. Magrini S., Pasquini E., Mazzatenta D., et al. Endoscopic endonasal odontoidectomy in a patient affected by down syndrome: technical case report. Neurosurgery. 2008;63:E373-E374.
11. Vishteh A.G., Beals S.P., Joganic E.F., et al. Bilateral sagittal split mandibular osteotomies as an adjunct to the transoral approach to the anterior craniovertebral junction. Technical note. J Neurosurg. 1999;90:267-270.
12. Pollack I.F., Welch W., Jacobs G.B., Janecka I.P. Frameless stereotactic guidance. An intraoperative adjunct in the transoral approach for ventral cervicomedullary junction decompression. Spine. 1995;20:216-220.
13. Benzel E. Biomechanically relevant anatomy and material properties of the spine and associated elements. In: Benzel E., editor. Biomechanics of spine stabilization. Rolling Meadows, ILL: American Association of Neurological Surgeons; 2001:1-18.
14. Dickman C.A., Locantro J., Fessler R.G. The influence of transoral odontoid resection on stability of the craniovertebral junction. J Neurosurg. 1992;77:525-530.
15. Ai F., Yin Q., Wang Z., et al. Applied anatomy of transoral atlantoaxial reduction plate internal fixation. Spine. 2006;31:128-132.
16. Hadley M.N., Martin N.A., Spetzler R.F., et al. Comparative transoral dural closure techniques: a canine model. Neurosurgery. 1988;22:392-397.
17. Menezes A.H. Complications of surgery at the craniovertebral junction: avoidance and management. Pediatr Neurosurg. 1991;17:254-266.
18. Dickman C.A., Spetzler R.F., Sonntag V.K.H., Apostolides P.J. Transoral approach to the craniovertebral junction. In: Dickman C.A., Spetzler R.F., Sonntag V.K.H., editors. Surgery of the craniovertebral junction. New York: Thieme; 1998:355-370.
19. Hensinger R.N. Osseous anomalies of the craniovertebral junction. Spine. 1986;11:323-333.
20. Menezes A.H., Ryken T.C. Craniovertebral junction abnormalities and the pediatric spine. In: Weinstien S., editor. The pediatric spine—principles and practice. Philadelphia: Lippincott Williams & Wilkins; 2001:219-237.
21. Henry A. Extensile exposure, ed 2. Edinburgh, New York: Churchill Livingstone; 1973.
22. Whitesides T.E.Jr., Kelly R.P. Lateral approach to the upper cervical spine for anterior fusion. South Med J. 1966;59:879-883.
23. al-Mefty O., Borba L.A., Aoki N., et al. The transcondylar approach to extradural nonneoplastic lesions of the craniovertebral junction. J Neurosurg. 1996;84:1-6.
24. Babu R.P., Sekhar L.N., Wright D.C. Extreme lateral transcondylar approach: technical improvements and lessons learned. J Neurosurg. 1994;81:49-59.
25. Bertalanffy H., Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815-821.
26. Dowd G.C., Zeiller S., Awasthi D. Far lateral transcondylar approach: dimensional anatomy. Neurosurgery. 1999;45:95-99.
27. George B., Dematons C., Cophignon J. Lateral approach to the anterior portion of the foramen magnum. Application to surgical removal of 14 benign tumors: technical note. Surg Neurol. 1988;29:484-490.
28. Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
29. Spetzler R.F., Grahm T.W. The far-lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Q. 1990;6:35-38.
30. Vishteh A.G., Crawford N.R., Melton M.S., et al. Stability of the craniovertebral junction after unilateral occipital condyle resection: a biomechanical study. J Neurosurg. 1999;90:91-98.
31. Gantwerker B., Dickman C., Sonntag V. Craniovertebral junction instability after lateral approach of the craniocervical junction. In: Bruneau M., George B., Spetzler R., editors. Pathology and surgery around the vertebral artery. Paris: Springer-Verlag, 2010.
32. Shin H., Barrenechea I.J., Lesser J., et al. Occipitocervical fusion after resection of craniovertebral junction tumors. J Neurosurg Spine. 2006;4:137-144.
33. Chiari H. Über Veränderungen des Kleinhirns infolge von Hydrocephalie des Grosshirns. Dtsch Med Wochenschr. 1891;17:1172-1175.
34. Arnold J. Myelocyste Transposition von Gewebskeimen und Sympodie. Beitr Path Anat. 1894;16:1-28.
35. Cleland F.R.S. Contribution to the study of spina bifida, encephalocele, and anencephalus. J Anat Physiol. 1883;17:257-292.
36. Batzdorf U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J Neurosurg. 1988;68:726-730.
37. Wetjen N.M., Heiss J.D., Oldfield E.H. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J Neurosurg Pediatr. 2008;1:118-123.
38. Klimo P.Jr., Rao G., Brockmeyer D. Congenital anomalies of the cervical spine. Neurosurg Clin North Am. 2007;18:463-478.
39. Ryken T.C., Menezes A.H. Cervicomedullary compression in achondroplasia. J Neurosurg. 1994;81:43-48.
40. Pauli R.M., Scott C.I., Wassman E.R.Jr., et al. Apnea and sudden unexpected death in infants with achondroplasia. J Pediatr. 1984;104:342-348.
41. Keiper G.L.Jr., Koch B., Crone K.R. Achondroplasia and cervicomedullary compression: prospective evaluation and surgical treatment. Pediatr Neurosurg. 1999;31:78-83.
42. Reid C.S., Pyeritz R.E., Kopits S.E., et al. Cervicomedullary compression in young patients with achondroplasia: value of comprehensive neurologic and respiratory evaluation. J Pediatr. 1987;110:522-530.
43. Aryanpur J., Hurko O., Francomano C., et al. Craniocervical decompression for cervicomedullary compression in pediatric patients with achondroplasia. J Neurosurg. 1990;73:375-382.
44. Bagley C.A., Pindrik J.A., Bookland M.J., et al. Cervicomedullary decompression for foramen magnum stenosis in achondroplasia. J Neurosurg. 2006;104:166-172.