Upper Airway Disease
Upper airway disease is a common problem in the pediatric age group. Affected children usually present with acute respiratory distress associated with stridor, apnea, or even acute pulmonary edema. Some children may present with a more chronic course of clinical symptoms characterized by recurrent chest infections or obstructive sleep apnea, which can lead to growth retardation, chronic respiratory failure, cor pulmonale, and even death.1
Children with upper airway obstruction usually present with stridor, a harsh respiratory noise caused by turbulent air passing through a narrowed airway. Timing of stridor in relation to the respiratory cycle can indicate the location of the narrowing. Inspiratory stridor usually results from obstruction above the level of the glottis, whereas expiratory stridor is characteristic of intrathoracic obstructions. Biphasic stridor suggests obstruction in the area between the glottis and subglottis or fixed/critical obstruction at any level.2
Although a thorough history and physical examination may narrow differential diagnostic considerations of upper airway disease in the pediatric population, imaging evaluation plays an important role in establishing the diagnosis, localizing the anatomic area of abnormality, and defining the extent of disease. Frontal and lateral radiographs of the airway and fluoroscopy have been mainstays in upper airway evaluation. Advances in cross-sectional imaging techniques approaching near endoscopic detail have been developed to more accurately demonstrate airway anatomy.3 For practical purposes, the discussion of pathology in this chapter is divided into the supraglottic, glottic, and subglottic regions.
Supraglottic Abnormalities
Etiology: Laryngomalacia is abnormal laxity of the pharyngeal tissues that causes the epiglottis, arytenoids, and aryepiglottic folds to involute and partially obstruct breathing during inspiration. The underlying cause of laryngomalacia is attributed to the immaturity of the laryngeal cartilages and muscles, which allows the larynx and the supralaryngeal structures to collapse.1–4 Laryngomalacia accounts for more than 75% of cases of congenital stridor and is the most common cause of symptomatic partial upper airway obstruction in infants.5 The stridor typically improves when the infant is agitated or active and becomes worse when the infant is at rest.
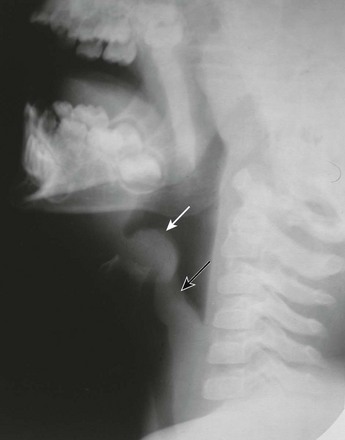
Imaging: The diagnosis of laryngomalacia can be established with use of airway fluoroscopy, although laryngoscopy is the diagnostic procedure of choice.6 The characteristic imaging finding in patients with laryngomalacia consists of downward and posterior bending of the epiglottis and anterior buckling of the aryepiglottic folds, which narrow and eventually obliterate the upper airway (Fig. 51-1). Although airway fluoroscopy appears to be reliable because of its high specificity, it has low sensitivity, and thus negative findings of a fluoroscopic study require further diagnostic evaluation in the setting of a high clinical suspicion of laryngomalacia.7
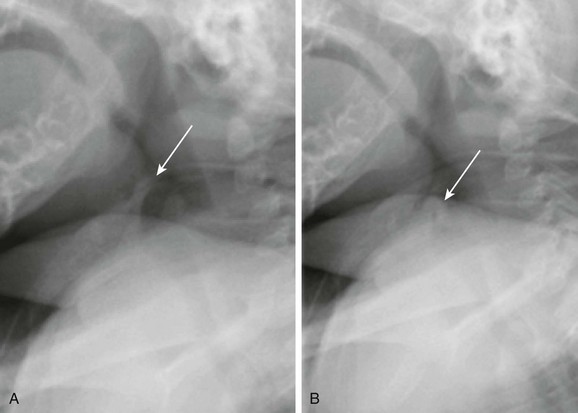
Figure 51-1 Laryngomalacia in a 3-month-old boy with stridor.
A, Still image from an airway fluoroscopic study demonstrates the normal position of the epiglottis (arrow). B, Still image from an airway fluoroscopic study shows laxity of the epiglottis with posterior, downward movement obstructing the airway (arrow) consistent with laryngomalacia.
Treatment and Follow-up: In most infants with laryngomalacia, stridor resolves during the first year of life. However, potentially serious complications, including airway obstruction and sudden death, also may occur. In severe or life-threatening cases, the following surgical treatment options currently are available: supraglottoplasty, an incision in the aryepiglottic folds, epiglottopexy, and tracheostomy.8
Epiglottitis
Etiology: Acute bacterial epiglottitis is a potentially serious cause of upper airway obstruction in children. It is characterized by inflammation and swelling of the epiglottis and the aryepiglottic folds but also can affect the false cords and the subglottic region.9 Epiglottitis usually is attributed to Haemophilus influenzae, although other bacteria including Streptococcus, Staphylococcus, Moraxella, and Pseudomonas also have been implicated.10 Because Haemophilus influenzae is now preventable by immunization, the incidence of epiglottitis has substantially diminished, although vaccine failures do occur.11 Epiglottitis typically occurs in preschool children between 3 and 6 years of age, but it also can occur in adults.12 Epiglottitis usually has a sudden onset with no history of a preceding upper respiratory tract infection. Affected children typically appear toxic with acute stridor, dysphagia, fever, restlessness, drooling, and increased respiratory distress in the recumbent position. Epiglottitis is a life-threatening disease that requires potential emergent intubation.
Imaging: Epiglottitis usually is diagnosed with radiography, and endoscopy is obtained for confirmation. A lateral radiograph reveals swelling and marked enlargement of the epiglottis that resembles the shape of a thumb (Fig. 51-2). Associated thickening of the aryepiglottic folds also occurs. Inflammation extends into the glottis and subglottic regions, causing a steeple or funnel configuration of the airway on frontal views. Because of the potentially rapid progression of the condition, which can lead to sudden and complete obstruction of the upper airway, radiographs should be obtained expeditiously with minimal manipulation of the neck. When undergoing imaging, patients should remain upright in their position of maximal comfort. Conditions that mimic bacterial epiglottitis in children include caustic ingestion, angioneurotic edema, chemical or thermal injury, an abscess, and epithelial cyst, among others. The omega epiglottis with prominent lateral folds is a normal variant and should not be misinterpreted as epiglottitis. In such cases, the aryepiglottic folds remain thin.13
Glottic Abnormalities
Etiology: Laryngeal atresia, a rare congenital malformation, is usually fatal. The malformation is caused by nondevelopment of the sixth branchial arch during embryogenesis, resulting in failure of the larynx and the trachea to recanalize. Typical presentation of affected patients with laryngeal atresia at birth is severe respiratory distress despite strong respiratory effort. Associated anomalies include a tracheoesophageal fistula, esophageal atresia, urinary tract abnormalities, limb defects, and low-set ears.14
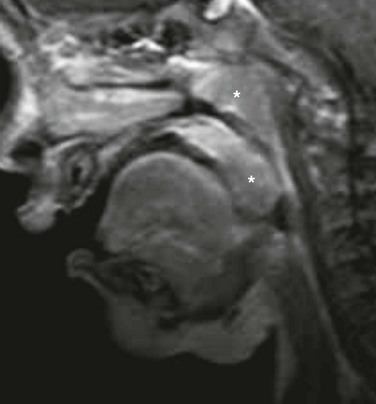
Imaging: Laryngeal atresia can be diagnosed with use of prenatal ultrasound by identifying the signs of congenital high airway obstruction syndrome, such as hyperechogenic lungs, a flattened or inverted diaphragm, a dilated and fluid-filled trachea, fetal hydrops, and polyhydramnios.15 Fetal magnetic resonance imaging (MRI), which correlates highly with prenatal ultrasound, can detect laryngeal atresia with the advantage of having a capability to identify the level of obstruction in most cases (Fig. 51-3).16
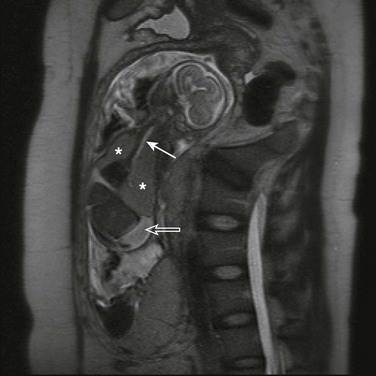
Figure 51-3 Congenital high airway obstruction syndrome in a 20-week-gestation fetus.
A half Fourier acquisition single-shot turbo spin echo sequence of a fetal magnetic resonance imaging scan demonstrates a dilated, fluid-filled trachea up to the mid neck (solid arrow), enlarged hyperintense lungs (asterisks) with inversion of both hemidiaphragms, and ascites (open arrow). (Courtesy of Andrew Mong, MD, Philadelphia, PA.)
Treatment and Follow-up: The treatment of laryngeal atresia focuses on prompt airway intervention at delivery after an accurate prenatal diagnosis, which may allow survival of infants with this otherwise fatal condition. An emergent tracheostomy is required immediately upon birth to secure an airway. Repair of laryngeal atresia requires laryngotracheal reconstruction.
Laryngeal Web
Etiology: A congenital laryngeal web is an uncommon condition caused by faulty embryogenesis of the laryngotracheal groove. This web is a band of varying thickness, usually located anteriorly at the level of the cords, which obliterates the anterior commissure. A congenital laryngeal web occasionally occurs just below the true cords. Affected infants present with a weak or absent cry, varying degrees of stridor, and respiratory distress, and they often may have concomitant abnormalities such as subglottic stenosis.1
Imaging: A definitive diagnosis of laryngeal web can be provided by direct laryngoscopy, but imaging evaluation plays a role in diagnosis. Plain radiography and fluoroscopy can demonstrate that the abnormality is in the glottis region, but no definitive radiographic findings exist.5 Computed tomography (CT) with multiplanar imaging and three-dimensional reconstructions can provide precise information with respect to the exact location of the web, its thickness, and its extent (Fig. 51-4). Furthermore, CT is able to visualize the structures beyond the area of obstruction, which is a limitation of laryngoscopy.17
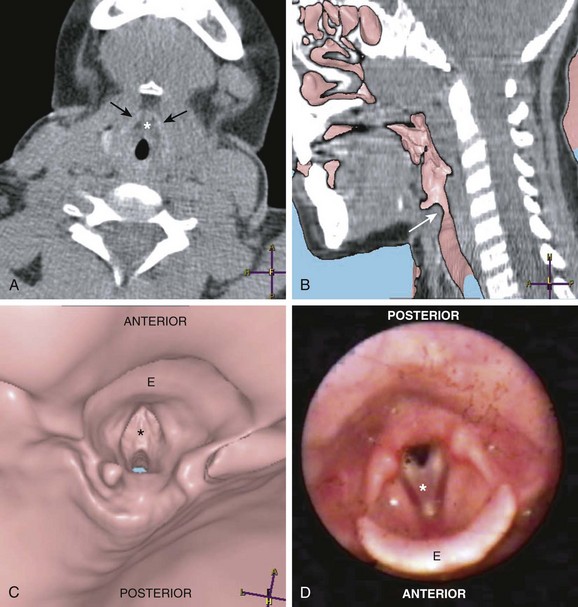
Figure 51-4 Laryngeal web.
A, An axial computed tomography (CT) scan of the neck at the level of the larynx and the thyroid cartilage (black arrows) demonstrates the laryngeal web (asterisk). B, A sagittal virtual endoscopic view superimposed on a sagittal reformatted CT image shows the laryngeal web (arrow). A virtual endocsopic view (C) and corresponding larygoscopy (D) demonstrate the laryngeal web (asterisk). The epiglottis (E) is well demonstrated on these images. (Courtesy of Suleymen Men, MD, Izmir, Turkey.)
Treatment and Follow-up: The primary goals of treatment for a congenital laryngeal web are to provide a patent airway and achieve a sufficient voice quality. Treatment depends on the thickness and extent of the abnormality. Surgical procedures include laryngotracheal reconstruction, laryngofissure and placement of a stent or keel, and endoscopic lysis with application of mitomycin C.18,19
Laryngocele
Etiology: A laryngocele is an abnormal dilatation of the laryngeal saccule, which is a narrow blind pouch arising from the anterior end of the laryngeal ventricle, extending superiorly into the paralaryngeal space, and bounded laterally by the thyroid cartilage.20 A laryngocele may be congenital or acquired. Laryngoceles occur more often in males than in females. In neonates and young children, a laryngocele is likely congenital and is contingent upon the presence of a saccular dilatation of the ventricular appendage. Increased laryngeal pressure may cause the sac to distend, at times extending into the aryepiglottic fold.21
A laryngocele is classified as internal if it lies within the larynx and external if it protrudes through the thyrohyoid membrane. The most common type is mixed. As the laryngocele communicates with the larynx, it normally contains air but may be filled with mucus or fluid.22 An acquired laryngocele may develop in older children or adults who experience increased intralaryngeal pressure, such as glass blowers, persons who play wind instruments, or persons with a chronic cough.
Imaging: In the past, anteroposterior (AP) and lateral soft tissue neck radiographs, linear tomography, and contrast laryngoscopy were used to identify laryngoceles.21 However, CT and/or MRI have replaced those older imaging techniques in recent years.20 On CT or MRI, a laryngocele typically is a well-defined structure of air or near-water density value in a characteristic location, with a smooth surface and lack of mucosal abnormality (Fig. 51-5). The exact location and extent of upper airway narrowing due to a laryngocele also can be well evaluated with CT or MRI.
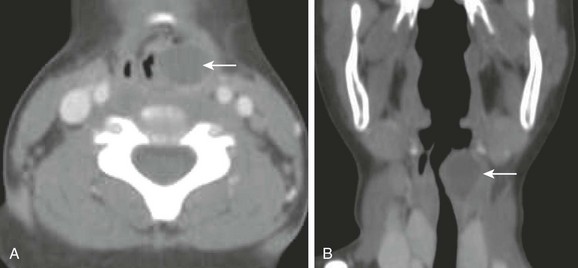
Figure 51-5 A laryngocele in a 5-year-old boy with chronic stridor.
An axial computed tomography scan (A) with coronal reconstruction (B) of the upper airway reveals a well-demarcated fluid-filled rounded structure (arrow) adjacent to the left laryngeal ventricle compatible with a laryngocele. Also noted is a marked narrowing of the upper airway.
Recurrent Respiratory Papillomatosis
Etiology: Recurrent respiratory papillomatosis (RRP) is the most common benign neoplasm to affect the larynx in children.23 It is the proliferation of benign squamous papillomas in the aerodigestive tract, usually involving the larynx. RRP tends to recur, spreads throughout the aerodigestive tract, and can even undergo malignant transformation. The etiology of RRP is infection of the upper airway with human papillomavirus types 6 and 11.24 Vertical transmission occurring during delivery through an infected birth canal is presumed to be the major mode of transmission in children.25
Change in voice (e.g., hoarseness, weak cry, and aphonia) and stridor are the most common symptoms in patients with RRP. Less common presenting symptoms include chronic cough, recurrent pneumonia, failure to thrive, dyspnea, dysphagia, and acute respiratory distress, especially in infants with an upper respiratory tract infection.26 The age of symptom onset typically ranges from neonates to 6 years. RRP can mimic other diseases including croup, tracheomalacia, or asthma. This diagnosis should be considered in children when other common pediatric airway diseases either do not follow the expected natural history or do not respond to treatment.27
Imaging: On plain radiographs, RRP typically appears as an irregular filling defect in the glottis (Fig. 51-6). It also can extend into the subglottic region and seed peripherally in the respiratory tract, resulting in pulmonary nodules or nonspecific lung parenchymal changes.1 A confirmatory diagnosis of RRP is made by direct laryngoscopy and biopsy for tissue diagnosis.27
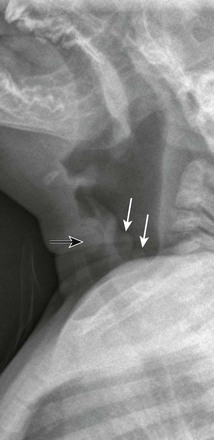
Figure 51-6 Recurrent respiratory papillomatosis in a 4-year-old boy.
A lateral soft tissue radiograph demonstrates a rounded soft tissue mass in the laryngeal region (black arrow). Nodular soft tissue densities also are noted along the aryepiglottic folds (white arrows). (Courtesy Elizabeth H. Ey, MD, Dayton, OH.)
Subglottic Abnormalities
Etiology: Subglottic stenosis is a narrowing of the subglottic airway that can be either congenital or acquired. In pediatric patients, congenital subglottic stenosis is due to faulty recanalization of the fetal laryngeal lumen, whereas the acquired type usually is the result of prolonged endotracheal intubation.1 Because of improved management of neonates who require ventilatory support, the incidence of neonatal subglottic stenosis has diminished substantially.28 Stridor is the most common presentation. However, an infant or child with a mild degree of congenital or acquired subglottic stenosis may remain asymptomatic until an upper respiratory tract infection results in aggravation of the subglottic airway narrowing.4
Imaging: Endoscopy is the reference standard for diagnosis, but imaging plays an important role in the evaluation of subglottic stenosis. Plain radiograph and fluoroscopy, which show fixed narrowing of the subglottic airway, usually enable diagnosis. However, CT with virtual bronchoscopy and MRI can be useful in assessing the exact site and length of stenosis and the airway distal to the area of narrowing (Fig. 51-7).4,29
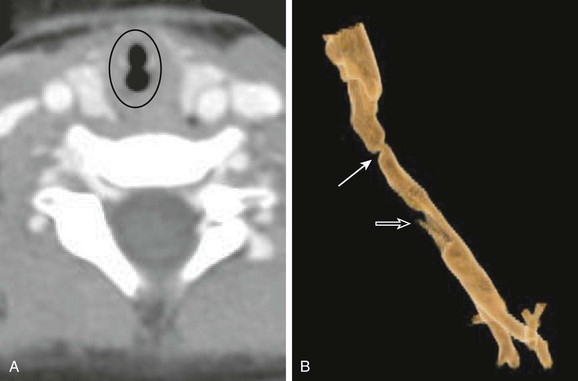
Figure 51-7 Subglottic stenosis in a 2-year-old girl, a former premature infant who had undergone chronic intubation.
A, Axial neck computed tomography demonstrates severe transverse narrowing of the subglottic trachea (circled). B, An external surface volume-rendered reconstruction shows severe subglottic stenosis (solid arrow) and a defect from the tracheostomy tube (open arrow).
Croup
Etiology: Viral croup or laryngotracheobronchitis is the most common cause of upper airway obstruction in children from 6 months to 3 years of age. Parainfluenza and influenza virus are the two most common etiologic agents for viral croup. They induce a reactive inflammatory response, which results in subglottic edema and subsequent upper airway narrowing. Clinically, viral croup is characterized by a low-grade fever and varying degrees of inspiratory stridor, barking cough, and hoarseness.4,31
Imaging: Although the diagnosis of viral croup is mainly clinical, airway studies often are obtained for diagnostic confirmation and exclusion of other causes of acute stridor, such as epiglottitis. On a frontal radiograph, the lateral walls of the subglottic larynx normally are convex or shouldered. Mucosal edema in viral croup narrows this space, which results in a loss of lateral convexity, creating a “steeple” shape below the vocal cords (Fig. 51-8). The narrowing can extend 5 to 10 mm below the vocal cords. The steeple sign is not specific for croup and may be seen in some children with epiglottitis. Furthermore, it can be absent in children affected with viral croup. On lateral radiographs, the hypopharynx often is overdistended, and the subglottic region is hazy or indistinct as a result of narrowing of the airway by mucosal edema. The epiglottis, aryepiglottic folds, and prevertebral spaces are normal in persons with viral croup.1–4
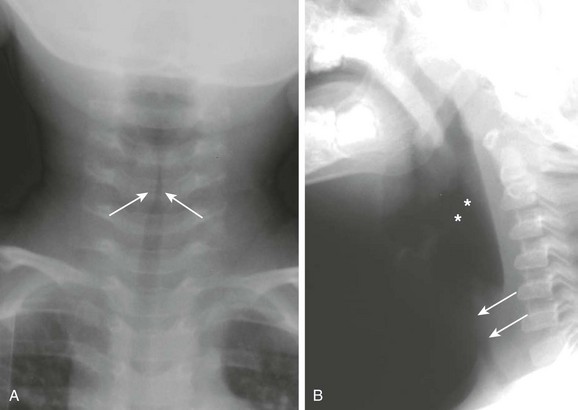
Figure 51-8 Croup in a 1-year-old girl with stridor.
A, A frontal soft tissue neck radiograph demonstrates a “steeple” appearance of the subglotttic trachea (arrows). B, A lateral soft tissue neck radiograph shows a dilated hypopharyngeal region (asterisks), as well as haziness and narrowing of the subglottic region (arrows).
Bacterial Tracheitis
Etiology: Bacterial tracheitis is a potentially life-threatening cause of upper airway obstruction. Bacterial tracheitis also has been referred as membranous croup, pseudomembranous croup, bacterial croup, purulent tracheobronchitis, and membranous laryngotracheobronchitis. The common etiologic bacteria include Staphylococcus, Moraxella, Streptococcus, and Haemophilus species, although it is not unusual for a bacterial infection to coexist with viral disease.32,33 The bacterial infection causes an inflammatory response that produces thick adherent mucopurulent exudates, ulceration, and sloughing of the laryngeal, tracheal, and bronchial mucosa. Such bacterial infection eventually results in various degrees of upper airway obstruction.32,33 Affected children usually are preschool- to school-aged children and are sicker than those affected with viral croup. Affected children usually present with a history of viral respiratory illness, rhinorrhea, cough, fever, and sore throat that may be present up to a week. These clinical symptoms in children with bacterial tracheitis often progress to high fever, a toxic appearance, and severe upper airway obstruction with hoarseness, cough, stridor, and tachypnea.32–34 A definitive diagnosis of bacterial tracheitis is made on the basis of the history and physical examination, combined with findings from laboratory and bronchoscopic evaluation.34
Imaging: Typical radiographic findings of bacterial tracheitis on the frontal radiograph of the cervical airway is narrowing of the subglottic airway. On lateral radiographs of the upper airway, diffuse haziness and marked irregularity of the anterior wall of the trachea may be seen, which is referred to as the “candle-dripping sign” (Fig. 51-9).32,35 Opaque linear streaks or filling defects, membranes, or plaquelike irregularities can be seen within the airway. Chest radiograph abnormalities are common in children with bacterial tracheitis, with at least 50% showing concomitant findings of pneumonia.35
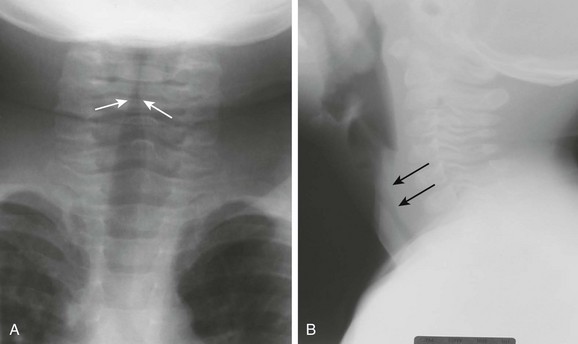
Figure 51-9 Bacterial tracheitis in a 7-year-old girl with a high fever, cough, and stridor.
A, A frontal soft tissue neck radiograph shows subglottic tracheal narrowing (arrows). B, A lateral soft tissue neck radiograph shows haziness of the subglottic tracheal region with irregularity of the posterior tracheal wall (arrows).
Treatment and Follow-up: When managing symptomatic children with bacterial tracheitis, a patent airway should be established to treat acute respiratory failure.33 Antibiotic therapy usually is initiated, and laryngoscopy may be required for endoscopic removal of the obstructing purulent secretions and membranes.
Retropharyngeal Cellulitis/Abscess
Etiology: Retropharyngeal cellulitis/abscess is a serious and occasionally life-threatening pyogenic infection with the potential to encroach on the upper airway. It occurs as a consequence of infections of the nasopharynx, paranasal sinuses, or middle ear. Usual etiologic agents are Streptococcus, Staphylococcus, and Haemophilus, although anaerobes also have been implicated. The infection extends to the lymph nodes located in the space between the posterior pharyngeal wall and prevertebral fascia. The infection occurs predominantly in early childhood; 75% of patients are younger than 5 years.36 In older children and adults, factors leading to retropharyngeal cellulitis/abscess include regional trauma, foreign body ingestion, complication of prior surgical procedures, or immunocompromised states. Typical clinical symptoms are fever, neck stiffness, and dysphagia, but occasionally affected patients will present with signs of upper airway obstruction.1,4 Diagnosis is based on clinical suspicion with supportive imaging studies.
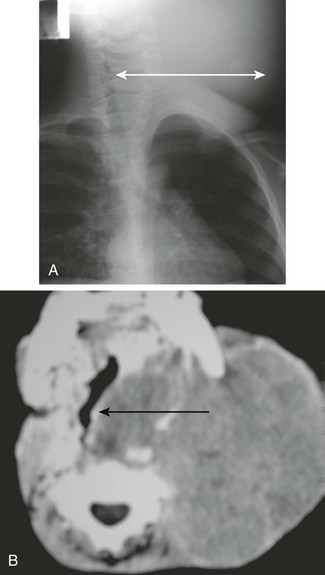
Imaging: The retropharynx, a potential space between the pharynx and the cervical spine, is easily appreciated on the lateral radiograph of the airway. In infants and children, the prevertebral space is wider because the vertebrae are not fully ossified. As a general guide, the thickness of the prevertebral soft tissue space from C1 to C4 is equal to half of the vertebral body width. In the older child, the vertebral bodies become larger and the prevertebral space becomes smaller. Below C4, the thickness of the prevertebral space normally equals the adjacent vertebral body width. If the thickness of the prevertebral space is greater than the adjacent vertebral body width below C3, it usually is abnormal.2 Attention to imaging technique is important when interpreting the lateral neck radiograph because a poorly obtained neck radiograph that is taken obliquely, on expiration, or with the neck flexed can accentuate the thickness of the retropharyngeal space and mimic pathology. Typical findings of retropharyngeal cellulitis/abscess on a lateral neck radiograph include a fixed soft tissue widening of the retropharyngeal soft tissue, anterior displacement of the airway, and often reversal of the cervical lordotic curvature.1,4 A plain radiograph cannot distinguish between retropharyngeal cellulitis and an abscess, but if air is seen in the widened retropharyngeal space, an abscess can be diagnosed with confidence.4 CT is now the preferred imaging modality to confirm the diagnosis and demonstrate the size, extent, and location of the pus collection from a retropharyngeal abscess.1,37,38 Lesions with a low-density center surrounded by an enhancing thick rim are more characteristic of a retropharyngeal abscess rather than cellulitis (Fig. 51-10).
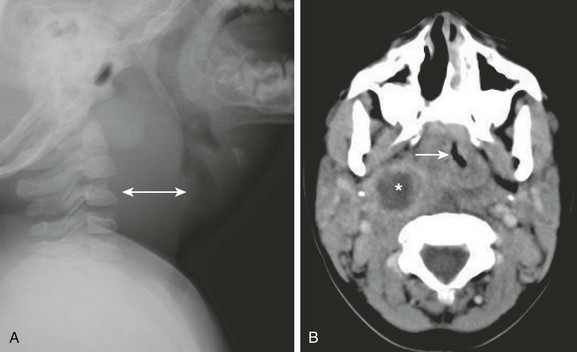
Figure 51-10 A retropharyngeal abscess in a 3-year-old girl.
A, A lateral soft tissue neck radiograph shows markedly widened retropharyngeal soft tissue (double arrow). B, An axial computed tomography scan through the upper airway demonstrates a rounded area of hypoattenuation with enhancing border compatible with abscess (asterisk) and associated soft tissue swelling effacing the airway (arrow).
Treatment and Follow-up: Although retropharyngeal abscesses traditionally have been treated with surgical drainage of the pus collection, some cases may be managed with antibiotics alone. The role of operative drainage varies between studies38,39 and also depends on the appearance and extent of disease. Nonoperative management is evolving, and a trial of antibiotics is considered in cases of retropharyngeal cellulitis and some small retropharyngeal abscesses with no evidence of airway compromise.37
Other Causes of Upper Airway Abnormalities
Etiology: Obstructive sleep apnea syndrome (OSA) is a disorder of breathing during sleep that is characterized by prolonged partial or complete upper airway obstruction that disrupts normal ventilation and sleep patterns. Typical symptoms of OSA include snoring, disturbed sleep, and daytime neurobehavioral problems. Common complications from OSA are neurocognitive impairment, behavioral problems, failure to thrive, and cor pulmonale in severe cases. Risk factors for developing OSA include adenotonsillar hypertrophy, obesity, craniofacial anomalies, and neuromuscular disorders.40 Nocturnal polysomnography (sleep study) is the only diagnostic technique shown to quantify the ventilatory and sleep abnormalities associated with sleep-disordered breathing.
Imaging: For imaging assessment of OSA, a lateral soft tissue neck radiograph can be obtained to evaluate adenoid size.1 The adenoidal-nasopharyngeal ratio derived from the lateral soft tissue neck radiograph is a clinically useful index of pharyngeal patency.41,42 Videofluoroscopic evaluation of the airway while the child is asleep also can be performed to investigate inspiratory collapse of the nasopharynx and oropharynx as a result of proximal airway obstruction acting in concert with sleep-related hypotonia of the pharyngeal musculature.43 More recently, cine MRI has been advocated as the more accurate diagnostic imaging technique because it shows the volumetric dimension of the adenoids and palatine tonsils (Fig. 51-11). Furthermore, it also can demonstrate dynamic motion of the upper airway, thereby allowing evaluation of the relationship between the size of the adenoid and palatine tonsils and the degree of airway motion.44
Treatment and Follow-up: Treatment of mild cases of OSA include lifestyle changes such as weight loss. For moderate to severe OSA, the two most commonly used treatments include a continuous positive airway pressure or automatic positive airway pressure device that can maintain the patency of the patient’s airway during sleep by providing the pressurized air flow into the upper airway. Additionally, several surgical treatments currently are available in patients with anatomic causes of upper airway obstruction, including septoplasty, turbinate surgery, tonsillectomy, and uvulopalatopharyngoplasty.
Foreign Body
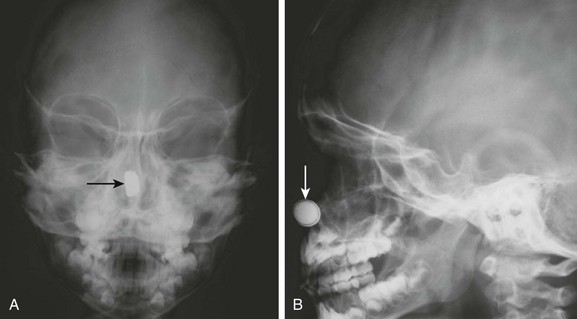
Etiology: Most patients who present with foreign bodies in the upper airway are children between 6 months to 3 years of age. In these patients, the most commonly aspirated foreign bodies are food, plastic toys, and small household items. A diagnosis of foreign body aspiration often is missed or delayed because the causative event is usually unobserved and the symptoms are nonspecific in children.45 Nasal foreign bodies tend to be located on the floor of the nasal passage, just below the inferior turbinate, or in the upper nasal fossa anterior to the middle turbinate (Fig. 51-12).46 Affected pediatric patients often present with unilateral, foul-smelling nasal discharge.
Imaging: Radiography can be helpful in localizing radiopaque foreign bodies such as coins, buttons, and batteries, but because most foreign bodies are radiolucent, management should not be based solely on imaging.47 Plain radiography is sufficient to demonstrate radiopaque upper airway and esophageal foreign bodies. On frontal radiographs, coins in the esophagus are seen en face, whereas coins in the trachea are seen in tangent because of a posterior gap in the tracheal cartilage rings. Food and other nonradiopaque foreign bodies in the upper airway often are identified only with an endoscopy.4
Treatment and Follow-up: Most nasal foreign bodies can be removed by a skilled physician, although attempts at removal may push the nasal foreign body into the pharynx, creating an airway hazard. Pharyngeal and laryngeal foreign bodies are medical emergencies because airway obstruction usually occurs at the time of aspiration and results in immediate respiratory distress. Flexible or rigid endoscopy usually is required to confirm the diagnosis, remove the foreign body, and avoid complications including airway obstruction, laryngeal edema, and pushing the foreign body into the subglottic space or trachea.47
Traumatic Injuries
Etiology: Pediatric laryngotracheal injuries are infrequent because of the softer cartilages and the protection provided by the prominent mandible.48,49 The degree and presentation of blunt and penetrating injuries are diverse. External evidence of trauma often associated with laryngotracheal injuries in children includes bruising, cuts, abrasions, and subcutaneous emphysema. Airway compromise is suggested if dysphonia, dysphagia, or dyspnea is present.50 The most important clinical signs of laryngeal injury include hoarseness and subcutaneous emphysema.49 Injuries may be subtle and present with minimal initial symptoms that, if undiagnosed, may rapidly progress to loss of the airway. Thus a high index of suspicion is needed to promptly and accurately diagnose and manage pediatric laryngotracheal injuries. Fiberoptic laryngoscopy is mandatory when injury to the larynx and trachea is suspected.49,50
Imaging: In any pediatric patient with possible upper airway injury, plain radiographs of the neck and chest may aid in the diagnosis. Radiographs also can be useful to assess the cervical spine. Findings of laryngotracheal injuries in pediatric patients vary considerably according to the mechanism of injury, but radiographic evidence of soft tissue air and wounds opening into the airway are common findings. CT is also useful and can play a primary role in localizing the injury and determining its extent (Fig. 51-13).49–52
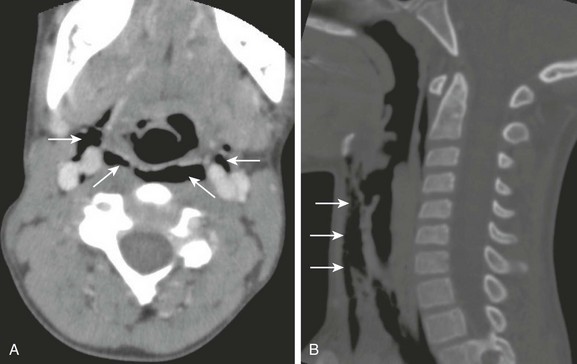
Figure 51-13 Laryngeal trauma in a 10-year-old boy who tripped and struck his anterior neck across a log.
Axial (A) and sagittal reconstruction (B) computed tomography scans of the neck show air dissecting along the parapharyngeal region (arrows) compatible with laryngeal trauma. (Courtesy Elizabeth H. Ey, MD, Dayton, OH.)
Neoplasms
Etiology: Subglottic hemangioma, a congenital vascular tumor, is the most common primary neoplasm affecting the subglottic trachea. Although the tumor is present at birth, patients with subglottic hemangioma usually become symptomatic between 1 and 6 months of age because of the rapid growth of hemangiomas at this stage. Affected infants usually present with the sudden onset of acute respiratory difficulty that varies in severity and includes stridor and crouplike symptoms. The symptoms are intermittent at first, but they eventually become persistent, especially as the hemangioma grows. If the subglottic hemangioma extends superiorly to involve the true cords, hoarseness also may be present. The sudden enlargement of a hemangioma as a result of hemorrhage can cause varying degrees of acute upper airway narrowing, potentially resulting in life-threatening asphyxia. Fifty percent of patients also have concomitant cutaneous hemangiomas. During its initial proliferative phase, the mass grows rapidly during the first 18 months of life, followed by a gradual involution that may last until age 10 years. Generally, a subglottic hemangioma is a self-limiting condition, and the respiratory distress tends to diminish and may disappear. However, the behavior of the individual lesion is unpredictable, and the symptoms are variable in severity.53
Imaging: Plain radiographs of infants and children with subglottic hemangiomas typically show an irregular, small, soft tissue mass below the true vocal cords (Fig. 51-14). These lesions have a characteristic radiographic appearance of a soft tissue shadow bulging into the airway lumen, often with asymmetric subglottic narrowing. However, symmetric narrowing of the trachea can be caused by a hemangioma if it is located central to the plane of projection. CT or MRI can show the mass at the subglottic region with intense enhancement following the administration of contrast material (see Fig. 51-14).53
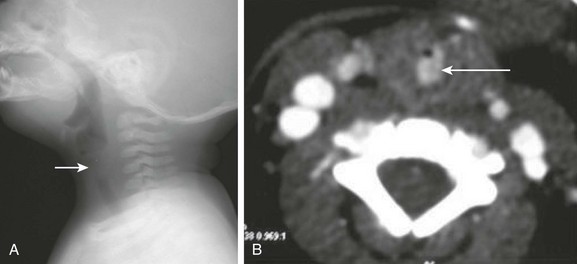
Figure 51-14 Subglottic hemangioma in a 6-month-old boy with stridor.
A, A lateral neck radiograph demonstrates haziness and soft tissue densities in the subglottic tracheal region (arrow). B, An axial computed tomography scan at the subglottic region shows the hemangioma (arrow), an intensely enhancing soft tissue mass causing marked narrowing of the subglottic trachea.
Treatment and Follow-up: The currently available treatment options for subglottic hemangioma include conservative monitoring in mildly symptomatic patients with a small subglottic hemangioma and laser therapy, laryngotracheoplasty, and direct surgical excision in patients with severe airway compromise. It has recently been recognized that propranolol may work for the treatment of hemangiomas.
Lymphoma
Etiology: Lymphoma is a neoplasm in the lymphatic cells of the immune system. It is the most common malignancy involving the extracranial head and neck in children. Hodgkin and non-Hodgkin lymphoma together account for 10% to 15% of all childhood malignancies in developed countries.54 Most children with head and neck lymphoma present with cervical lymph node involvement. They may present with nodal disease involving Waldeyer’s ring (i.e., lymphatic tissue in the nasopharynx, base of the tongue, tonsils, and soft palate). Hodgkin lymphoma, characterized histologically by the presence of Reed-Sternberg cells, is more commonly seen in adolescents. Non-Hodgkin lymphoma is more common than Hodgkin lymphoma in children younger than 10 years. Lymphomas typically present with enlarged, painless cervical lymph nodes, but other symptoms, including airway obstruction, are dependent on the extent and involvement of surrounding structures.
Imaging: Plain radiographs typically show a masslike opacity in the region of neck (Fig. 51-15). Narrowing and displacement of the upper airway also may be seen on plain radiographs. Compared with plain radiographs, cross-sectional imaging such as CT or MRI can better demonstrate the location, degree, and extent of the upper airway compromise. Both CT and MRI with intravenous contrast can be used for diagnosis, staging, and follow-up.55
Treatment and Follow-up: Pediatric patients with lymphoma compromising the upper airway usually respond well to chemotherapy and do not require emergent tracheostomy placement. With the reduction in size of lymph nodes after chemotherapy, the patency of the upper airway subsequently can be regained.
Rhabdomyosarcoma
Etiology: Rhabdomyosarcoma is the most common soft tissue sarcoma arising from the connective tissues in children younger than 15 years. It accounts for 8% of all childhood malignancies.56 Most rhabdomyosarcomas occur in the first decade of life, with a slight male predominance. The head and neck is the primary site of the disease in 40% of children with rhabdomyosarcoma.57
Imaging: Plain radiographs usually show a large masslike opacity, and ultrasound often demonstrates a heterogeneous mass in patients with cervical rhabdomyosarcoma. However, a complete evaluation, particularly for any upper airway obstruction, requires a cross-sectional imaging study such as CT or MRI. MRI is the imaging modality of choice for rhabdomyosarcoma. Typically it is hyperintense on T2-weighted sequences and isointense or minimally hyperintense on T1-weighted sequences. Moderate to intense enhancement usually is seen after administration of contrast.55 CT is less effective than MRI in determining the extent of the mass but is important in determining bone involvement.
Treatment and Follow-up: Treatment of rhabdomyosarcoma with upper airway obstruction includes chemotherapy, radiation therapy, and sometimes surgical resection. Urgent tracheostomy placement to secure the airway below the level of obstruction and provide sufficient air flow for respiration may be required in pediatric patients who do not respond to nonsurgical treatment.
Bradshaw, K. Imaging the upper airways. Paediatr Respir Rev. 2001;2:46–56.
Goodman, TR, McHugh, K. The role of radiology in the evaluation of stridor. Arch Dis Child. 1999;81:456–459.
John, SD, Swischuk, LE. Stridor and upper airway obstruction in infants and children. Radiographics. 1992;12:625–643.
Laya, BF, Lee, EY. Congenital causes of upper airway obstruction in pediatric patients: updated techniques and review of imaging findings. Semin Roentgenol. 2012;47(2):147–158.
Lee, EY, Restrepo, R, Dillman, JR, et al. Imaging evaluation of trachea and bronchi: systematic review and updates. Semin Roentgenol. 2012;47(2):182–196.
Lloyd, C, Mchugh, K. The role of radiology in head and neck tumors in children. Cancer Imaging. 2010;10:49–61.
Macpherson, RI, Leithiser, RE. Upper airway obstruction in children: an update. Radiographics. 1985;5(3):339–376.
References
1. Macpherson, RI, Leithiser, RE. Upper airway obstruction in children: an update. Radiographics. 1985;5(3):339–376.
2. Bradshaw, K. Imaging the upper airways. Paediatr Respir Rev. 2001;2:46–56.
3. Wiett, GJ, Long, FR, Shiels, IIWE, et al. Advances in pediatric airway radiology. Otolaryngol Clin North Am. 2000;33(1):15–28.
4. John, SD, Swischuk, LE. Stridor and upper airway obstruction in infants and children. Radiographics. 1992;12:625–643.
5. Tucker, GF. Laryngeal development and congenital lesions. Ann Otol Rhino Laryngol. 1980;74:142–145.
6. Sivan, Y, Ben-Ari, J, Soferman, R, et al. Diagnosis of laryngomalacia by fiberoptic endoscopy: awake compared with anesthesia-aided technique. Chest. 2006;130(5):1412–1418.
7. Berg, E, Naseri, I, Sobol, SE. The role of airway fluoroscopy in the evaluation of children with stridor. Arch Otolaryngol Head Neck. 2008;134(4):415–418.
8. Ahmad, SM, Soliman, AMS. Congenital anomalies of the larynx. Otolaryngol Clin North Am. 2007;40:177–191.
9. Shackelford, GD, Siegel, MJ, Mcalister, WH. Subacute edema in acute epiglottitis in children. Am J Roentgenol. 1978;131:603–605.
10. Shah, RK, Roberson, DW, Jones, DT. Epiglottitis in the Haemophilus type B vaccine era: changing trends. Laryngoscope. 2004;114:557–560.
11. McEwan, J, Giridharan, W, Clarke, RW, et al. Paediatric acute epiglottitis: not a disappearing entity. Int J Pediatr Otorhinolaryngol. 2003;67(4):317–321.
12. Dort, JC, Frohlich, AM, Tate, RB. Acute epiglottitis in adults: diagnosis and treatment in 43 patients. J Otolaryngol. 1994;23(4):281–285.
13. Goodman, TR, McHugh, K. The role of radiology in the evaluation of stridor. Arch Dis Child. 1999;81:456–459.
14. Hartnick, CJ, Cotton, RT. Syndromic and other congenital anomalies of the head and neck. Otolaryngol Clin North Am. 2000;33(6):232–238.
15. Onderoglu, L, Karamursel, BS, Bulun, A, et al. Prenatal diagnosis of laryngeal atresia. Prenat Diagn. 2003;23(4):277–280.
16. Mong, A, Johnson, AM, Kramer, SS, et al. Congenital high airway obstruction syndrome: MR/US findings, effect on management, and outcome. Pediatr Radiol. 2008;38(11):1171–1179.
17. Men, S, Ikiz, AO, Topcu, I, et al. CT and virtual endoscopy findings in congenital laryngeal web. Int J Pediatr Otorhinolaryngol. 2006;70(6):202–207.
18. Unal, M. The successful management of congenital laryngeal web with endoscopic lysis and topical mitomycin-C. Int J Pediatr Otorhinolaryngol. 2004;68(2):231–235.
19. Amir, M, Youssef, T. Congenital glottic web: management and anatomical observation. Clin Respir J. 2004;4:202–207.
20. Glazer, HS, Mauro, MA, Aronberg, DJ, et al. Computed tomography of laryngoceles. AJR Am J Roentgenol. 1983;140:549–552.
21. Lindell, MM, Jing, BS, Fischer, EP, et al. Laryngocele. AJR Am J Roentgenol. 1978;131:259–262.
22. Kumar, G, Bradley, PJ, Wastie, ML. Case of the month. What a blow. Br J Radiol. 1998;71:799–800.
23. Bauman, NM, Smith, RJH. Recurrent respiratory papillomatosis. Pediatr Clin North Am. 1996;43:1385–1401.
24. Wiatrak, BJ, Wiatrak, D, Bracken, TR, et al. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114(suppl 104):1–23.
25. Bennett, RS, Powell, KR. Human papillomavirus; association between laryngeal papillomas and genital warts. Pediatr Infect Dis J. 1987;6:229–232.
26. Derkay, CS, Wiatrak, B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118(7):1236–1247.
27. Zacharisen, MC, Conley, SF. Recurrent respiratory papillomatosis in children: masquerader of common respiratory diseases. Pediatrics. 2006;11(5):1925–1931.
28. Walner, DL, Loewen, MS, Kimura, RE. Neonatal subglottic stenosis: incidence and trends. Laryngoscope. 2001;111(1):48–51.
29. Seam, N, Finkkelstein, SE, Gonzales, DA, et al. The work up of stridor: virtual bronchoscopy as a complimentary technique in the diagnosis of subglottic stenosis. Respir Care. 2007;52(3):337–339.
30. Holinger, PH, Kutnick, SL, Schild, JA, et al. Subglottic stenosis in infants and children. Ann Otol Rhinol Laryngol. 1976;85:591–599.
31. Leung, AK, Kellner, JD, Johnson, DW. Viral croup: a current perspective. J Pediatr Health Care. 2004;18(6):297–301.
32. Jones, R, Santos, J, Overall, J. Bacterial tracheitis. JAMA. 1979;242:721–726.
33. Kasian, GF, Bingham, WT, Steinberg, J, et al. Bacterial tracheitis in children. CMAJ. 1980;140:46–50.
34. Al-Mutairi, B, Kirk, V. Bacterial tracheitis in children; approach to diagnosis and treatment. Paediatr Child Health. 2004;9(1):25–30.
35. Han, BK, Dunbar, JS, Striker, TW. Membranous laryngotracheobronchitis (membranous croup). AJR Am J Roentgenol. 1979;133:53–58.
36. Craig, FW, Schunk, JE. Retropharyngeal abscess in children: clinical presentation, utility of imaging, and current management. Pediatrics. 2003;111(6):1394–1398.
37. Craig, FW, Schunk, JE. Retropharyngeal abscess in children: clinical presentation, utility of imaging, and current management. Pediatrics. 2003;111(6):1394–1398.
38. Lalakea, M, Messner, AH. Retropharyngeal abscess management in children: current practices. Otolaryngol Head Neck Surg. 1999;121:398–405.
39. Kirse, DJ, Roberson, DW. Surgical management of retropharyngeal space infections in children. Laryngoscope. 2001;111:1413–1422.
40. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Elk Grove Village, IL: American Academy of Pediatrics; 2000.
41. Fujioka, M, Young, LW, Girdany, BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgnol. 1979;133:401–404.
42. Villorente, FF, Dizon, ML, Laya, BF, et al. Correlation of adenoidal-nasopharyngeal ratio and palatine tonsil size in lateral soft tissue neck radiograph with polysomnogram among children symptomatic for sleep apnea. Philippine College Radiol J. 2010;4(1):26–31.
43. Fernbach, SK, Brouillette, RT, Riggs, TW, et al. Radiologic evaluation of adenoids and tonsils in children with obstructive sleep apnea: plain films and fluoroscopy. Pediatr Radiol. 1983;13(5):258–265.
44. Donnelly, LF, Casper, KA, Chen, B. Correlation on cine MR imaging of size of adenoid and palatine tonsils with degree of upper airway motion in asymptomatic sedated children. AJR Am J Roentgenol. 2002;179(2):503–508.
45. Heim, SW, Maughan, KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76(8):1185–1189.
46. Chan, TC, Ufberg, J, Harrigan, RA, et al. Nasal foreign body removal. J Emerg Med. 2004;26:441–445.
47. Silva, AB, Muntz, HR, Clary, R. Utility of conventional radiography in the diagnosis and management of pediatric airway foreign bodies. Ann Otol Rhinol Laryngol. 1998;107(10 Pt 1):834–838.
48. Shires, CB, Preston, T, Thompson, J. Pediatric laryngeal trauma: a case series at a tertiary children’s hospital. Int J Pediatr Otorhinolaryngol. 2011;75(3):401–408.
49. Granholm, T, Farmer, DL. The surgical airway. Respir Care Clin North Am. 2001;7(1):13–23.
50. Francis, S, Gaspard, DJ, Rogers, N, et al. Diagnosis and management of laryngotracheal trauma. J Natl Med Assoc. 2002;94(1):21–24.
51. Cicala, RS, Kudsk, Ka, Butts, A, et al. Initial evaluation and management of upper airway injuries in trauma patients. J Clin Anesth. 1991;3(2):91–98.
52. Jalisi, S, Zoccoli, M. Management of laryngeal fractures: a 10 year experience. J Voice. 2011;25(4):473–479.
53. Koplewitz, BZ, Springer, C, Slasky, BS. CT of hemangiomas of the upper airways in children. AJR Am J Roentgenol. 2005;184:663–670.
54. Pastore, G, Magnani, C, Verdecchia, A, et al. Survival of childhood lymphomas in Europe, 1978-1992: a report from EUROCARE study. Eur J Cancer. 2001;37:703–710.
55. Lloyd, C, Mchugh, K. The role of radiology in head and neck tumors in children. Cancer Imaging. 2010;10:49–61.
56. Dagher, R, Helman, L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44.
57. McHugh, K, Boothroyd, A. The role of radiology in childhood rhabdomyosarcoma. Clin Radiol. 1999;54:2–10.