9 Unwanted effects and adverse drug reactions
Definitions
The term adverse drug reaction (ADR) should be confined to harmful or seriously unpleasant effects occurring at doses intended for therapeutic (including prophylactic or diagnostic) effect and which call for reduction of dose or withdrawal of the drug and/or forecast hazard from future administration; it is effects of this order that are of importance in evaluating drug-induced disease in the community. The term adverse ‘reaction’ is almost synonymous with adverse ‘effect’, except that an ‘effect’ relates to the drug and a ‘reaction’ to the patient. Both terms should be distinguished from an adverse ‘event’, which is an adverse happening that occurs during exposure to a drug without any assumption being made about its cause (see Prescription event monitoring, p. 52).
Toxicity
implies a direct action of the drug, often at high dose, damaging cells, e.g. liver damage from paracetamol overdose, eighth cranial nerve damage from gentamicin. All drugs, for practical purposes, are toxic in overdose2 and overdose can be absolute or relative; in the latter case an ordinary dose may be administered but may be toxic due to an underlying abnormality in the patient, e.g. disease of the kidney. Mutagenicity, carcinogenicity and teratogenicity (see Index) are special cases of toxicity.
Attribution and degrees of certainty
The following elements are useful in attributing the cause of an adverse event to a drug:
1. The time sequence in relation to taking the drug. The majority of reactions develop soon after exposure. Anaphylactic reactions (within minutes or hours) and hypersensitivity reactions (within weeks) may readily suggest an association, but delayed effects such as carcinogenesis or tardive dyskinesia (after years or even decades) present more difficulty.
2. The effects of withdrawing or reintroducing the drug. Most reactions subside when the drug is discontinued, unless an autoimmune reaction is established, when effects persist. Planned re-exposing a patient to a drug is rarely indicated unless treatment with it is essential and there is no reliable alternative.
3. The relationship to what is already known about the drug. This of course invites questions about consistency with the established pharmacology and toxicology of the drug or related substances.
Degrees of conviction for attributing adverse reactions to drugs may be ascribed as3:
• Definite: time sequence from taking the drug is reasonable; event corresponds to what is known of the drug and is not explicable by concurrent disease or drugs; event ceases on stopping the drug; event returns on restarting the drug (rarely advisable).
• Probable: time sequence is reasonable; event corresponds to what is known of the drug; event ceases on stopping the drug; event not reasonably explained by patient’s disease or other drugs.
• Possible: time sequence is reasonable; event corresponds to what is known of the drug; uncertain relationship to effect of stopping the drug; event could readily have been result of the patient’s disease or other therapy.
• Conditional: time sequence is reasonable; event does not correspond to what is known of the drug; event could not reasonably be explained by the patient’s disease or other drugs.
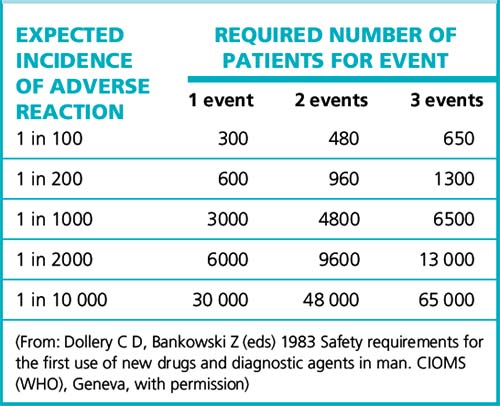
Practicalities of detecting rare adverse reactions
For reactions with no background incidence, the number of patients required to give a good (95%) chance of detecting the effect appears in Table 9.1. Assuming that three events are required before any regulatory or other action should be taken, it shows the large number of patients that must be monitored to detect even a relatively high-incidence adverse effect. The problem can be many orders of magnitude worse if the adverse reactions closely resemble spontaneous disease with a background incidence in the population.
Pharmacovigilance and pharmacoepidemiology
The principal methods of collecting data on ADRs (pharmacovigilance) are:
• Experimental studies, i.e. formal therapeutic trials of Phases 1–3. These provide reliable data on only the commoner events as they involve relatively small numbers of patients (hundreds); they detect an incidence of up to about 1 in 200.
• Observational studies, where the drug is observed epidemiologically under conditions of normal use in the community, i.e. pharmacoepidemiology and pharmacovigilance. Techniques used for post-marketing (Phase 4) studies include the observational cohort study and the case–control study. The surveillance systems are described on pages 52–53.
Drug-induced illness
The discovery of drug-induced illness can be analysed as follows4:
• A drug commonly induces an otherwise rare illness: this effect is likely to be discovered by clinical observation in the licensing (pre-marketing) formal therapeutic trials and the drug will almost always be abandoned; but some patients are normally excluded from such trials, e.g. pregnant women, and detection will then occur later.
• A drug rarely or uncommonly induces an otherwise common illness: this effect is likely to remain undiscovered. Cardiovascular risk from coxibs (e.g. rofecoxib, Vioxx) approximates as an example, but the degree of increased risk did become apparent after meta-analysis of several clinical trials and observational studies.
• A drug rarely induces an otherwise rare illness: this effect is likely to remain undiscovered before the drug is released for general prescribing. The effect could be detected by informal clinical observation or during any special post-registration surveillance and confirmed by a case–control study (see p. 52); aplastic anaemia with chloramphenicol5 and the oculomucocutaneous syndrome with practolol were uncovered in this way.
• A drug commonly induces an otherwise common illness: this effect will not be discovered by informal clinical observation. If very common, it may be discovered in formal therapeutic trials and in case–control studies, but if only moderately common it may require observational cohort studies, e.g. pro-arrhythmic effects of anti-arrhythmic drugs.
• Drug adverse effects and illness incidence in an intermediate range: both case–control and cohort studies may be needed.
Some impression of the features of drug-induced illness can be gained from the following statistics:
• In a large UK study, the prevalence of ADRs as a cause of admission to hospital was 6.5%, with a median bed stay of 8 days (4% of hospital bed capacity); most reactions were definitely or possibly avoidable; the commonest drugs were: low-dose aspirin, diuretics, warfarin, non-steroidal anti-inflammatory drugs (other than aspirin); the commonest adverse reaction was gastrointestinal bleeding.6
• Overall incidence in hospital inpatients is 10–20%, with possible prolongation of hospital stay in 2–10% of patients in acute medical wards.
• ADRs cause 2–3% of consultations in general practice.
• A study of 661 ambulatory patients found that 25% experienced adverse events, of which 13% were serious and 11% were preventable.7
• Predisposing factors for ADRs are: age over 60 years or under 1 month, female sex, previous history of adverse reaction, hepatic or renal disease, number of medications taken.
• A review of records of coroner’s inquests for a (UK) district with a population of 1.19 million during the period 1986–1991 found that, of 3277 inquests on deaths, 10 were due to errors of prescribing and 36 were caused by adverse drug reactions.8 Nevertheless, 17 doctors in the UK were charged with manslaughter in the 1990s, compared with two in each of the preceding decades, a reflection of ‘a greater readiness to call the police or to prosecute’.9
Sir Anthony Carlisle,10 in the first half of the 19th century, said that ‘medicine is an art founded on conjecture and improved by murder’. Although medicine has advanced rapidly, there is still a ring of truth in that statement, as witness anyone who follows the introduction of new drugs and observes how, after the early enthusiasm, there follow reports of serious toxic effects, and withdrawal of the drug may then follow. The challenge is to find and avoid these, and, indeed, the present systems for detecting adverse reactions came into being largely in the wake of the thalidomide, practolol and benoxaprofen disasters (see p. 63); they are now an increasingly sophisticated and effective part of medicines development.
Drugs and skilled tasks
Many medicines affect performance, and it is relevant to review here some examples with their mechanisms of action. As might be expected, centrally acting and psychotropic drugs are prominent, e.g. the sedative antidepressants, benzodiazepines, non-benzodiazepine and other hypnotics, and antipsychotics (the ‘classical’ type more so than the ‘atypicals’; see p. 322). Many drugs possess anticholinergic activity either directly (atropine, oxybutynin) or indirectly (tricyclic antidepressants, antipsychotics), the central effects of which cause confusion and impaired ability to process information. The first-generation H1-receptor antihistamines (chlorphenamine, diphenhydramine) are notably sedating and impair alertness and concentration, which features the recipient may not recognise. Drugs may also affect performance through cerebral depression (antiepileptics, opioids), hypoglycaemia (antidiabetics) and hypotension (antihypertensives). For alcohol and cannabis, see pp. 142 and 155.
Car driving is a complex multifunction task that includes: visual search and recognition, vigilance, information processing under variable demand, decision-making and risk-taking, and sensorimotor control. It is plain that prescribers have a major responsibility here, both to warn patients and, in the case of those who need to drive for their work, to choose medicines with a minimal liability to cause impairment.11 Patients who must drive when taking a drug of known risk, e.g. benzodiazepine, should be specially warned of times of peak impairment.12
Sources of adverse drug reactions
• The patient may be predisposed to an ADR by age, sex, genetic constitution, known tendency to allergy, disease of drug eliminating organs (see Ch. 8), or social habits, e.g. use of tobacco, alcohol, other recreational drugs (see Ch. 11).
• The known nature of the drug may forewarn. Some drugs, e.g. digoxin, have steep dose–response curves and small increments of dose are more likely to induce adverse or toxic reactions (see p. 92). The capacity of the body to eliminate certain drugs, e.g. phenytoin, may saturate within the therapeutic dose range so that standard increases cause a disproportionate rise in plasma concentration, risking toxic effects (see p. 356). Some drugs, e.g. antimicrobials and particularly penicillins, have a tendency to cause allergy. Anticancer agents warrant special care as they are by their nature cytotoxic (see Ch. 31). Use of these and other drugs may raise longer-term issues of mutagenicity, carcinogenicity and teratogenicity. Ingredients of a formulation, rather than the active drug, may also cause adverse reactions. Examples include the high sodium content of some antacids, and colouring and flavouring agents. The latter are designated in the list of contents by E numbers; tartrazine (E102) may cause allergic reactions.
• The prescriber needs to be aware that adverse reactions may occur after a drug has been used for a long time, at a critical phase in pregnancy, is abruptly discontinued (see p. 99) or given with other drugs (see Drug interactions, Ch. 8).
Aspects of the above appear throughout the book as is indicated. Selected topics are:
Age
The very old and the very young are liable to be intolerant of many drugs, largely because the mechanisms for disposing of them in the body are less efficient. The young are not simply ‘small adults’ and ‘respect for their pharmacokinetic variability should be added to the list of our senior citizens’ rights’.13 Multiple drug therapy is commonly found in the old, which further predisposes to adverse effects (see Prescribing for the elderly, p. 105).
Genetic constitution
Inherited factors that influence response to drugs appear in general under Pharmacogenomics (see p. 101). For convenience, we describe here the porphyrias,14 a specific group of disorders for which careful prescribing in a subgroup, the acute porphyrias, is vital.
Great care in prescribing for these patients is required if serious illness is to be avoided and it is therefore essential that patients and their clinicians have access to information concerning the safe use of prescription medication. Drug lists should be reviewed regularly, and a recent initiative in Europe has made a consensus-based list of safe drugs (available at http://www.porphyria-europe.org) as well as details of common prescribing problems and a link to a searchable drug safety database (http://www.drugs-porphyria.org).
If no recognised safe option is available, use of a drug about which there is uncertainty may be justified. Dr M. Badminton15 writes: ‘Essential treatment should never be withheld, especially for a condition that is serious or life threatening. The clinician should assess the severity of the condition and the activity of the porphyria and make a risk versus benefit assessment.’ In these circumstances the clinician may wish to contact an expert centre for advice (see the list at http://www.porphyria-europe.com), which is likely to recommend that the patient be monitored as follows:
1. Measure porphyrin and porphobilinogen before starting treatment.
2. Repeat the measurement at regular intervals or if the patient has symptoms in keeping with an acute attack. If there is an increase in the precursor levels, stop the treatment and consider giving haem arginate for acute attack (see below).
Allergy in response to drugs
Type III reactions: immune complex-mediated type
These reactions include serum sickness, glomerulonephritis, vasculitis and pulmonary disease.
Cross-allergy
The distinctive features of allergic reactions are16:
• Lack of correlation with known pharmacological properties of the drug.
• Lack of linear relation with drug dose (very small doses may cause very severe effects).
• Rashes, angioedema, serum sickness syndrome, anaphylaxis or asthma; characteristics of classic protein allergy.
• Requirement of an induction period on primary exposure, but not on re-exposure.
• Disappearance on cessation of administration and reappearance on re-exposure to a small dose.
Principal clinical manifestations and treatment
2a. Non-urticarial rashes (types I, II, IV)
These occur in great variety; frequently they are weeping exudative lesions. It is often difficult to be sure when a rash is due to a drug. Apart from stopping the drug, treatment is non-specific; in severe cases an adrenal steroid should be used. Skin sensitisation to antimicrobials may be very troublesome, especially among those who handle them (see Ch. 17 for more detail).
3. Anaphylactic shock (type I)
Treatment is urgent. The following account combines advice from the UK Resuscitation Council with comment on the action of the drugs used. Advice on the management of anaphylactic shock is altered periodically and the reader should check the relevant website (http://www.resus.org.uk) for the latest information.
• In adults, 500 micrograms of adrenaline/epinephrine injection (0.5 mL of the 1 in 1000 solution) should be given intramuscularly to raise the blood pressure and dilate the bronchi (vasoconstriction renders the subcutaneous route less effective). If there is no clinical improvement, further intramuscular injections of adrenaline/epinephrine 500 micrograms should be given at 5-min intervals according to blood pressure, pulse and respiration. (See website for doses in those <12 years.)
• If shock is profound, cardiopulmonary resuscitation/advanced life support are necessary. Consider also giving adrenaline/epinephrine 1: 10 000 by slow intravenous infusion, at a rate of 100 micrograms/min (1 mL/min of the dilute 1 in 10 000 solution over 5 min), preferably with continuous ECG monitoring, stopping when a response has been obtained. This procedure is hazardous and should be undertaken only by an experienced practitioner who can obtain immediate intravenous access and where other resuscitation facilities are available.
• The adrenaline/epinephrine should be accompanied by an H1-receptor antihistamine, e.g. chlorphenamine 10–20 mg intramuscularly or by slow intravenous injection, and by hydrocortisone 200–500 mg intramuscularly or by slow intravenous injection. The adrenal steroid acts by reducing vascular permeability and by suppressing further response to the antigen–antibody reaction. Benefit from an adrenal steroid is not immediate; it is unlikely to begin for 30 min and takes hours to reach its maximum.
• In severe anaphylaxis, hypotension is due to vasodilatation and loss of circulating volume through leaky capillaries. Thus, when there is no response to drug treatment, 1–2 L of plasma substitute should be infused rapidly. Crystalloid may be safer than colloid, which causes more allergic reactions.
• Where bronchospasm is severe and does not respond rapidly to other treatment, a β2-adrenoceptor agonist is a useful adjunctive measure. Noradrenaline/norepinephrine lacks any useful bronchodilator action (β effect) (see Adrenaline, Ch. 24).
• Where susceptibility to anaphylaxis is known, e.g. in patients with allergy to bee or wasp stings, preventive self-management is feasible. The patient is taught to administer adrenaline/epinephrine intramuscularly from a pre-filled syringe (EpiPen Auto-injector, delivering adrenaline/epinephrine 300 micrograms per dose).
• Half of the above doses of adrenaline/epinephrine may be safer for patients who are receiving amitriptyline or imipramine (increased effect; see p. 318).
Any hospital ward or other place where anaphylaxis may be anticipated should have all the drugs and equipment necessary to deal with it in one convenient kit, for when they are needed there is little time to think and none to run about from place to place (see also Pseudo-allergic reactions, p. 118).
6b. Granulocytopenia (type II, but also pseudo-allergic),
The value of precautionary leucocyte counts for drugs having special risk remains uncertain.18 Weekly counts may detect presymptomatic granulocytopenia from antithyroid drugs, but onset can be sudden and an alternative view is to monitor only with drugs having special risk, e.g. clozapine, where it is mandatory. The chief clinical manifestation of agranulocytosis is sore throat or mouth ulcers, and patients should be warned to report such events immediately and to stop taking the drug, but they should not be frightened into non-compliance with essential therapy. Treatment of the agranulocytosis involves both stopping the drug responsible and giving a bactericidal drug, e.g. a penicillin, to prevent or treat infection.
6d. Haemolysis of all kinds
is included here for convenience. There are three principal categories:
• Allergy (type II) occurs with penicillins, methyldopa, levodopa, quinine, quinidine, sulfasalazine and organic antimony. In some of these cases a drug–protein–antigen/antibody interaction may involve erythrocytes only casually, i.e. a true ‘innocent bystander’ phenomenon.
• Dose-related pharmacodynamic action on normal cells, e.g. lead, benzene, phenylhydrazine, chlorates (weed-killer), methyl chloride (refrigerant), some snake venoms.
• Idiosyncrasy (see Pharmacogenetics). Precipitation of a haemolytic crisis may also occur with the above drugs in the rare genetic haemoglobinopathies. Treatment is to withdraw the drug, and an adrenal steroid is useful in severe cases if the mechanism is immunological. Blood transfusion may be needed.
Effects of prolonged administration: chronic organ toxicity
Lung
Amiodarone may cause pulmonary fibrosis. Sulfasalazine is associated with fibrosing alveolitis.
Liver
Methotrexate may cause liver damage and hepatic fibrosis; amiodarone may induce steatohepatitis (fatty liver) (see also alcohol, p. 142).
Carcinogenesis:
see also Preclinical testing (Ch. 3). Mechanisms of carcinogenesis are complex; prediction from animal tests is uncertain and causal attribution in humans has finally to be based on epidemiological studies. The principal mechanisms are:
• Alteration of DNA (genotoxicity, mutagenicity). Many chemicals or their metabolites act by causing mutations, activating oncogenes; those substances that are used as medicines include griseofulvin and alkylating cytotoxics. Leukaemias and lymphomas are the most common malignancies.
• Immunosuppression. Malignancies develop in immunosuppressed patients, e.g. after organ transplantation and cancer chemotherapy. There is a high incidence of lymphoid neoplasm. Chlorambucil, melphalan and thiotepa present particular high relative risks. The use of immunosuppression in, e.g., rheumatoid arthritis, also increases the incidence of neoplasms.
• Hormonal. Long-term use of oestrogen replacement in postmenopausal women induces endometrial cancer. Combined oestrogen/progestogen oral contraceptives may both suppress and enhance cancers (see Ch. 38). Diethylstilbestrol caused vaginal adenosis and cancer in the offspring of mothers who took it during pregnancy in the hope of preventing miscarriage. It was used for this purpose for decades after its introduction in the 1940s, on purely theoretical grounds. Controlled therapeutic trials were not done and there was no valid evidence of therapeutic efficacy. Male fetuses developed non-malignant genital abnormalities.19
Carcinogenesis due to medicines follows prolonged drug exposure,20 i.e. months or years; the cancers develop most commonly over 3–5 years, but sometimes years after treatment has ceased. There is a higher incidence of secondary cancers in patients treated for a primary cancer.
Adverse effects on reproduction
Testing of new drugs on animals for reproductive effects has been mandatory since the thalidomide disaster, even though the extrapolation of the findings to humans is uncertain (see Preclinical testing, Ch. 3). The placental transfer of drugs from the mother to the fetus is considered on page 86.
Drugs may act on the embryo and fetus:
• Directly (thalidomide, cytotoxic drugs, antithyroid drugs, aromatic retinoids, e.g. isotretinoin): any drug affecting cell division, enzymes, protein synthesis or DNA synthesis is a potential teratogen, e.g. many antimicrobials.
Late pregnancy
For a discussion of anticoagulants in pregnancy, see Chapter 29.
Babies born to mothers dependent on opioids may show a physical withdrawal syndrome.
Detection of teratogens
Aronson J.K. Routes of drug administration: uses and adverse effects. Part 1: intramuscular and subcutaneous injection. Drug Ther. Bull. 2008;253:971–974. (also Part 2: sublingual, buccal, rectal, and some other routes. 254, 975–978)
Aronson J.K., Ferner R.E. Joining the DoTS: new approach to classifying adverse drug reactions. Br. Med. J.. 2003;327:1222–1225.
Baxter K., Sharp J.M. Adverse drug interactions. Drug Ther. Bull.. 2008;248:952–954.
Eigenmann P.A., Haenggeli C.A. Food colourings and preservatives – allergy and hyperactivity. Lancet. 2004;364:823–824.
Ferner R.E., McDowell S.E. Doctors charged with manslaughter in the course of medical practice, 1795–2005: a literature review. J. R. Soc. Med.. 2006;99:309–314.
Gray J. Why can’t a woman be more like a man? Clin. Pharmacol. Ther.. 2007;82:15–17.
Greenhalgh T., Kostopoulou O., Harries C. Making decisions about benefits and harms of medicines. Br. Med. J.. 2004;329:47–50.
Peters T.J., Sarkany R. Porphyria for the general physician. Clin. Med. (Northfield Il). 2005;5:275–281.
Strickler B.H.C., Psaty B.M. Detection, verification, and quantification of adverse drug reactions. Br. Med. J.. 2004;329:44–47.
Trontell A. Expecting the unexpected – drug safety, pharmacovigilance and the prepared mind. N. Engl. J. Med.. 2004;351:1385–1387.
Woosley R.L. Discovering adverse reactions: why does it take so long? Clin. Pharmacol. Ther.. 2004;76:287–289.
1 From: The remedy worse than the disease. Matthew Prior (1664–1721).
2 A principle appreciated by Paracelsus 500 years ago, who stated that ‘All things are poisons and there is nothing that is harmless; the dose alone decides that something is no poison’. The physician, alchemist and philosopher is regarded as the founder of chemical therapeutics; he was the first to use carefully measured doses of mercury to treat syphilis.
3 Journal of the American Medical Association 1975 234:1236.
4 After Jick H 1977 The discovery of drug-induced illness. New England Journal of Medicine 296:481–485.
5 Scott J L, Finegold S M, Belkin G A, Lawrence J S 1965 A controlled double-blind study of the hematologic toxicity of chloramphenicol. New England Journal of Medicine 272:1137–1142.
6 Pirmohamed M, James S, Meakin S et al 2004 Adverse drug reactions as a cause of admission to hospital: prospective analysis of 18 820 patients. British Medical Journal 329:15–19.
7 Gandhi T K, Weingart S N, Borus J et al 2003 Adverse events in ambulatory care. New England Journal of Medicine 348:1556–1564.
8 Ferner R E, Whittington R M 1994 Coroner’s cases of death due to errors in prescribing or giving medicines or to adverse drug reactions: Birmingham 1986–1991. Journal of the Royal Society of Medicine 87:145–148.
9 Ferner R E 2000 Medication errors that have led to manslaughter charges. British Medical Journal 321:1212–1216.
10 Noted for his advocacy of the use of ‘the simple carpenter’s saw’ in surgery.
11 Gull D G, Langford N J 2006 Drugs and driving. Adverse Drug Reactions Bulletin 238:911–914.
12 Nordic countries require that medicines liable to impair ability to drive or to operate machinery be labelled with a red triangle on a white background. The scheme covers antidepressants, benzodiazepines, hypnotics, drugs for motion sickness and allergy, cerebral stimulants, antiepileptics and antihypertensive agents. In the UK there are some standard labels that pharmacists are recommended to apply, e.g. ‘Warning. May cause drowsiness. If affected do not drive or operate machinery. Avoid alcoholic drink’.
13 Fogel B S 1983 New England Journal of Medicine 308:1600.
14 The view that King George III suffered from acute porphyria is widely expressed but erroneous: his illness was probably bipolar disorder (Peters T 2011 King George III, bipolar disorder, porphyria and lessons for historians. Clinical Medicine 11:261–264).
15 Department of Medical Biochemistry, University Hospital of Wales, Cardiff, UK. We are grateful to Dr Badminton for contributing the section on porphyria.
16 Assem E-S K 1998 Drug allergy and tests for its detection. In: Davies D M (ed) Davies’s textbook of adverse drug reactions. Chapman and Hall, London, p. 790.
17 Where cells are being destroyed in the periphery and production is normal, transfusion is useless or nearly so, as the transfused cells will be destroyed, though in an emergency even a short cell life (platelets, erythrocytes) may tip the balance usefully. Where the bone marrow is depressed, transfusion is useful and the transfused cells will survive normally.
18 In contrast to the case of a drug causing bone marrow depression as a pharmacodynamic dose-related effect, when blood counts are part of the essential routine monitoring of therapy, e.g. cytotoxics.
19 Herbst A L 1984 Diethylstilboestrol exposure – 1984 [effects of exposure during pregnancy on mother and daughters]. New England Journal of Medicine 311:1433–1435.
20 Carcinogens that are effective as a single dose in animals are known, e.g. nitrosamines.
21 By permission from Smithells R W 1983 In: Hawkins D F (ed) Drugs and pregnancy. Churchill Livingstone, Edinburgh.