CHAPTER 52 Unlinked Arthroplasty
PART A Distal Humeral Hemiarthroplasty
INTRODUCTION
Distal humeral hemiarthroplasty (DHH) was first described in 1947, yet over the past 60 years there have been only a few small series reporting on this technique.1,14,17,25–28 Early series reported treatment of a variety of pathologic conditions, including trauma, inflammatory arthritis, and tumor. Prostheses included both custom-made (of various materials) and nonanatomic humeral components from total elbow systems (capitellocondyllar, kudo) and anatomic components (Street). These early surgeries met with mixed results. The past decade has witnessed renewed interest in DHH with advancements in our understanding of elbow biomechanics and with growing experience in the difficulty of achieving effective outcomes in treating complex distal humerus fractures. This technique provides a treatment alternative that bridges the gap between internal fixation and total elbow arthroplasty (TEA) in unsalvageable distal humeral articular surface pathologies.
INDICATIONS AND CONTRAINDICATIONS
The indications for DHH are evolving, both in terms of what is achievable and what is appropriate (Table 52-1). Initial experience has been in the younger patient or the physically active older patient with a comminuted intra-articular distal humeral fracture (sometimes combined with a column fracture) that involves both trochlea and capitellum, where an adequate reduction and internal fixation cannot be achieved. Open reduction internal fixation (ORIF) remains the gold standard of operative treatment; however, patient factors and bone architecture may limit the surgeon’s ability to reconstruct the elbow. Series reporting on ORIF of comminuted intra-articular fractures indicate a substantial rate of unsatisfactory outcomes and a significant complication rate in complex cases.1,14,17,23,24
TABLE 52-1 Indications and Contraindications for Humeral Hemiarthroplasty
| Indications | Contraindications |
|---|---|
ORIF, open reduction and internal fixation.
Total elbow arthroplasty has gained acceptance as a primary treatment option for intra-articular distal humeral fractures in lower demand, elderly patients with osteopenic bone.4,9–11,20,22 Its application to younger active patients is attendant with the risk of early prosthesis failure.
PRINCIPLES OF DISTAL HUMERAL HEMIARTHROPLASTY
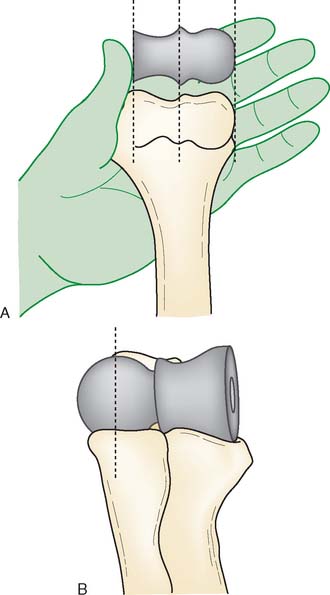
Stability of the intact elbow joint is provided by ulnohumeral congruity,18 a competent radiocapitellar articulation, and collateral ligament integrity.5 Because DHH is an “unlinked” reconstruction, the prosthesis must have a high degree of congruency with the radius and ulna to provide stable force transmission through both medial and lateral articular columns; therefore, sizing is important. For the DHH to function optimally, the prosthesis must be oriented such that the elbow rotation axis relative to the insertions of the anterior band of the medial collateral ligament (MCL) and lateral ulnar collateral ligament (LUCL) is restored.3 This ensures that the ligaments remain isometric through a functional range of motion. The proper joint rotation axis can be determined by surface landmarks, medially at the anterior inferior surface of the medial epicondyle and laterally at the center of a circle formed by the articular surface of the capitellum.2,5–8,19,21
IMPLANT CONSIDERATIONS
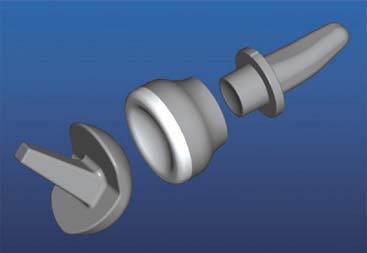
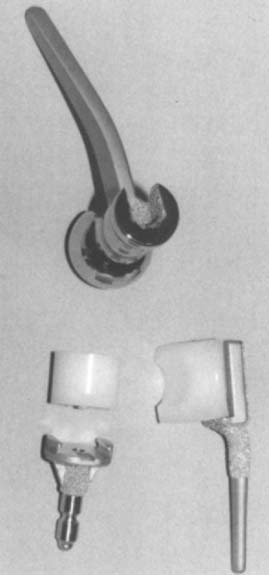
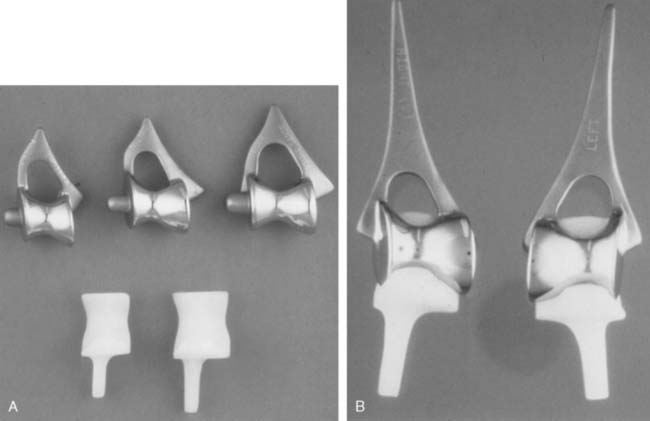
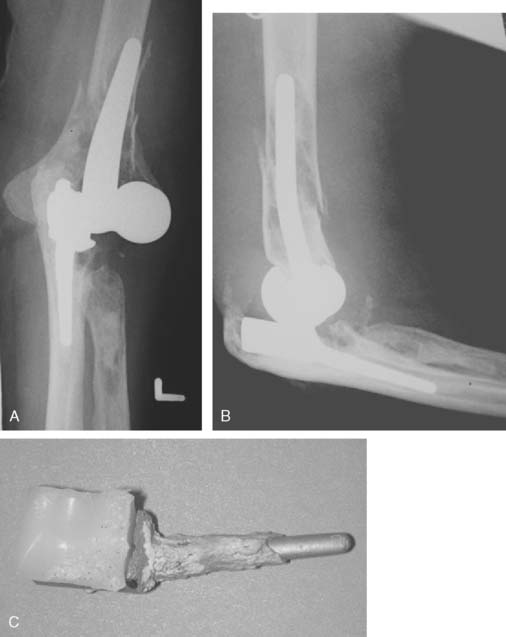
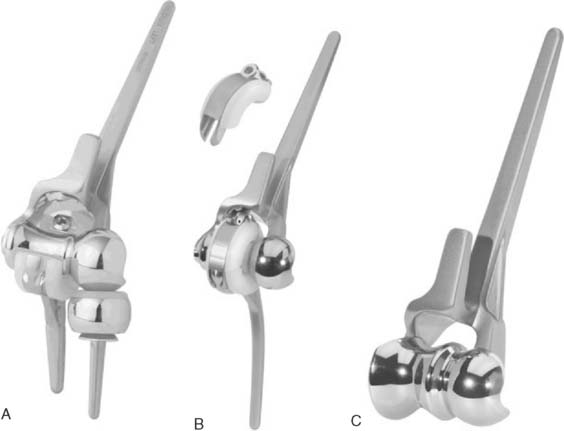
There are currently two systems available (and an additional one, Coonrad/Morrey [Zimmer, Warsaw, IN] under development) that allow DHH with a prosthesis based on normal articular geometry (Fig. 52-1): (1) the Sorbie Questor elbow system (Wright Medical Technologies, Arlington, TN) and (2) the Latitude elbow system (Tornier, Stafford, TX). All require cement fixation and, because they may be subjected to significant forces, optimal cementing techniques should be used.7 The Sorbie Questor humeral component is a monoblock anatomic prosthesis with three sizes (small, medium, and large) that allow a “best fit” in 95% of all elbows.29 It allows fixation of comminuted bone fragments of either supracondylar column. There is no anterior flange or long-stem option to augment humeral fixation. This system allows conversion only to an unconstrained TEA, which may not address future instability issues. Mechanical studies of the Sorbie humeral component indicate that an intact radial head is necessary for elbow stability despite an intact MCL.13 It is attractive because it removes little bone and is relatively easy to insert.
PREOPERATIVE PLANNING
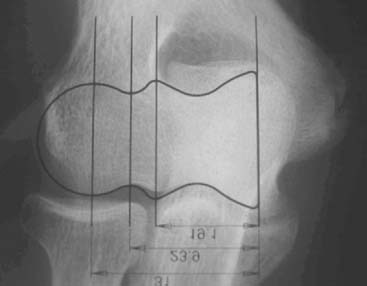
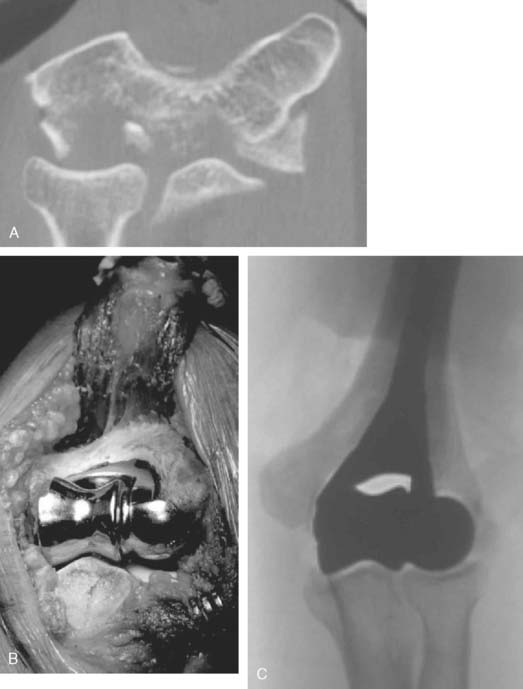
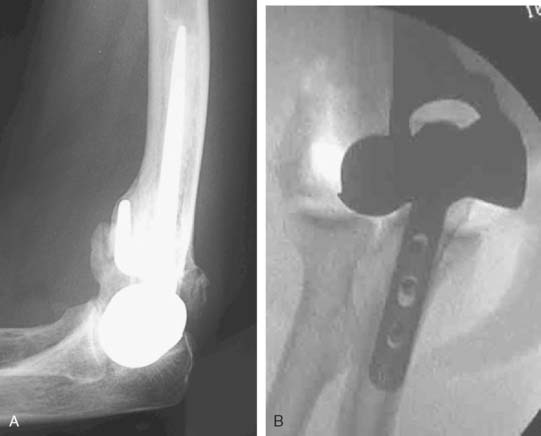
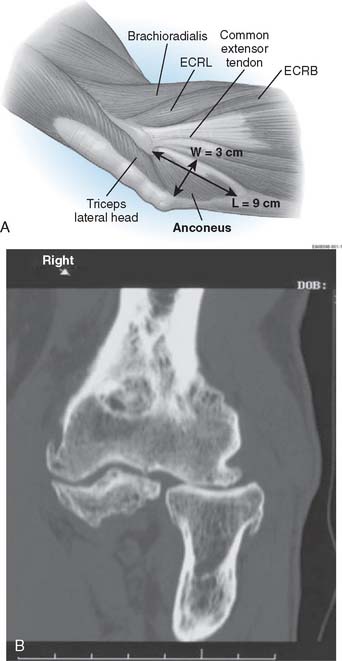
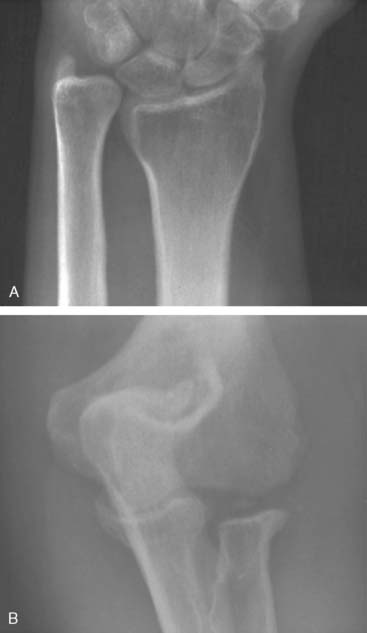
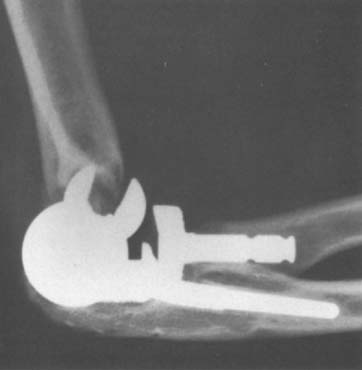
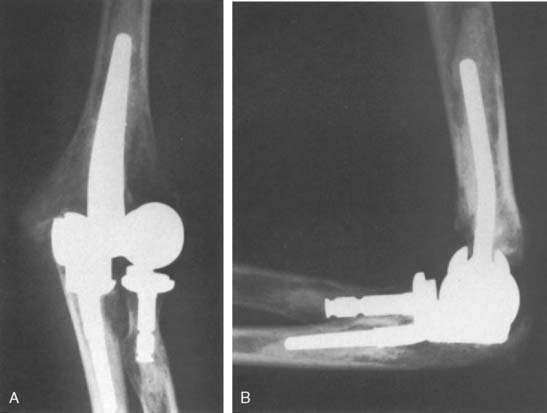
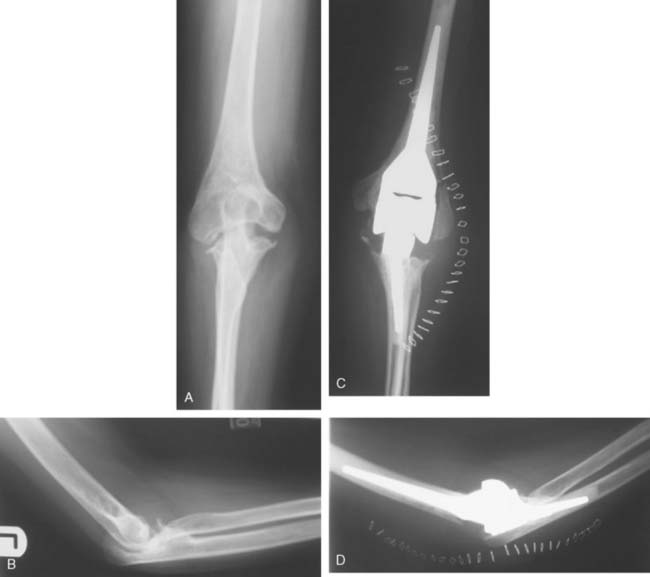
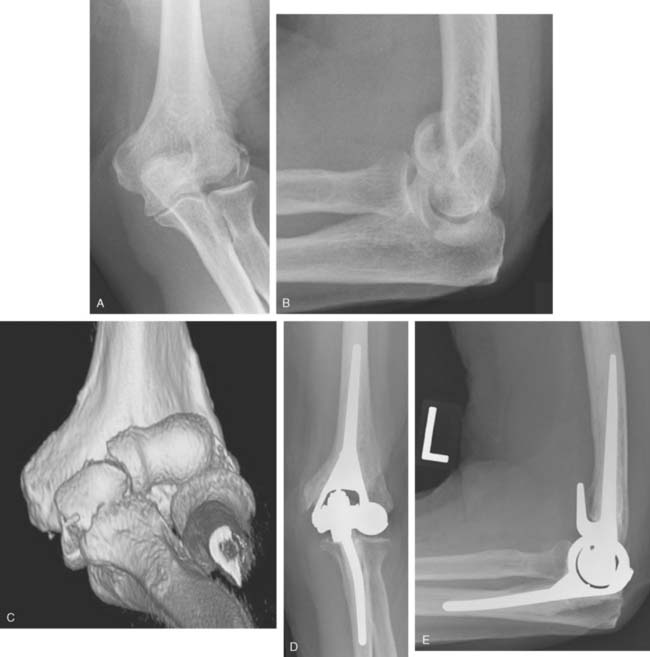
Preoperative imaging studies should include anteroposterior (AP) and lateral views of the elbows. Plain radiographs of the contralateral elbow permit additional templating of the distal humerus for appropriate prosthesis sizing. Sizing is based on the direct correlation between the AP dimensions of the normal articular surface and the radii of the capitellum and trochlea (Fig. 52-2).26 Preoperative assessment might also include a fine-cut computed tomography (CT) scan to evaluate articular comminution, column involvement, and associated injuries to the radial head and coronoid (Fig. 52-3).
OPERATIVE TECHNIQUE
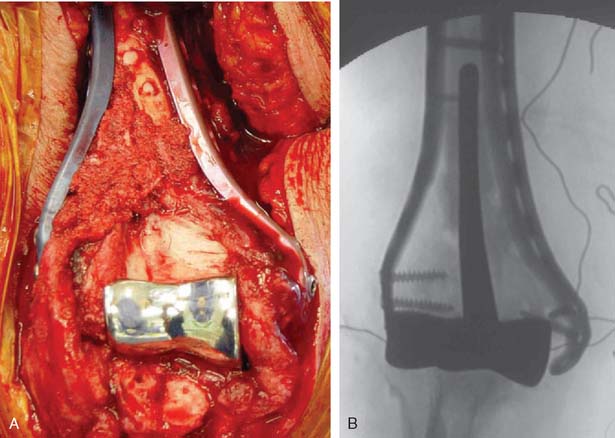
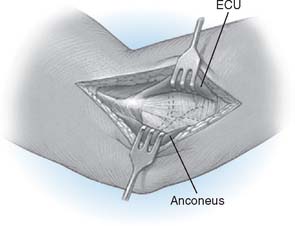
The patient is positioned in the lateral decubitus position. A tourniquet is applied and the arm secured to a short arm support. This allows flexion of the arm to 120 degrees, which facilitates insertion of the component. This also places the posterior aspect of the distal humerus parallel to the floor, when the elbow is flexed 90 degrees. A large sterile basin is placed beneath the arm to catch any bone fragments or instruments. The elbow is approached through an extensile posterior incision, and thick subcutaneous flaps are developed to provide access to both epicondyles and columns. The ulna nerve is exposed and protected. A Chevron osteotomy of the olecranon is performed at the level of the sigmoid fossa bare area, and the triceps mechanism is reflected sufficiently proximal to expose any supracondylar fracture extension (see Chapter 7). “Triceps-on” exposures provide poorer exposure and are associated with increased joint instability. During exposure, the humeral insertions and origins of the MCL and LUCL must be preserved.
Once exposed, the articular surface can be inspected. If the combination of fracture comminution, osteopenia, and patient considerations favors DHH then trial components should be templated with the radial head and anterior sigmoid fossa to confirm proper fit and to avoid overstuffing of the joint. Injury to the radial head, coronoid, and collateral ligaments must be identified to proceed with this option and to ensure a stable reconstruction (see Fig. 52-3).
Next, the bone cuts must be made so that the prosthesis can be inserted to the proper depth and rotation to recreate the flexion axis. Free-hand cuts are generally preferred because bone loss from fracture comminution complicates accurate placement of the cutting jigs.
The trial prosthesis of appropriate size is inserted. Strict attention should be paid to the depth of insertion and rotation when the trial is seated because this determines ligament tension. Once the trial is in place, the ulna is reduced over its articular surface and the joint taken through a range of motion. Difficulty reducing the ulna suggests that the flexion axis is too distal, and the humeral cuts should be revised to prevent an overly tight reconstruction. The tracking of the anterior half of the olecranon during elbow flexion and extension can also provide a sense of the correct prosthesis rotation (Table 52-2).
| Problem | Cause | Solution |
|---|---|---|
| Flexion contracture (intraoperative) |
EUA, examination under anesthesia; LCL, lateral collateral ligament; ORIF, open reduction and internal fixation; PLRI, posterolateral rotatory instability; TEA, total elbow arthroplasty.
A cement restrictor should be used to provide good pressurization of the antibiotic cement. The final prosthesis is then inserted to the correct position. The ulna is reduced to the distal humeral prosthesis, and the elbow is extended (not cycled) until the cement is cured. The olecranon osteotomy is fixed using a tension band or a periarticular olecranon plate and always grafted with autologous bone from the trochlea (Fig. 52-4). Although the plate provides the best fixation (and therefore allows more aggressive early mobilization), there are times when soft tissue considerations prevent its use. The elbow should then be taken through a range of motion to ensure the following: (1) motion has not been constrained by an overly tight reconstuction; (2) the olecranon osteotomy remains reduced as tension develops in flexion; and (3) the ulna nerve does not develop undue tension or encroachment by neighboring hardware. The nerve is transposed as necessary.
TREATMENT OF NONACUTE INJURIES
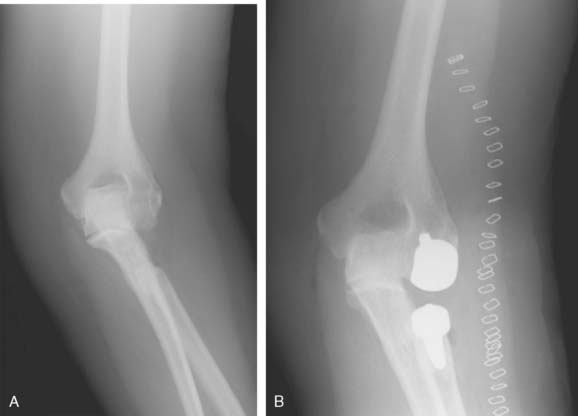
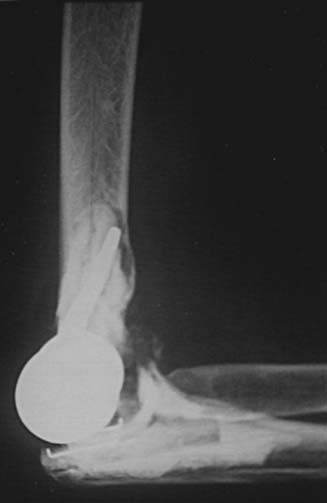
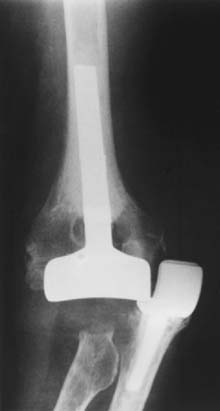
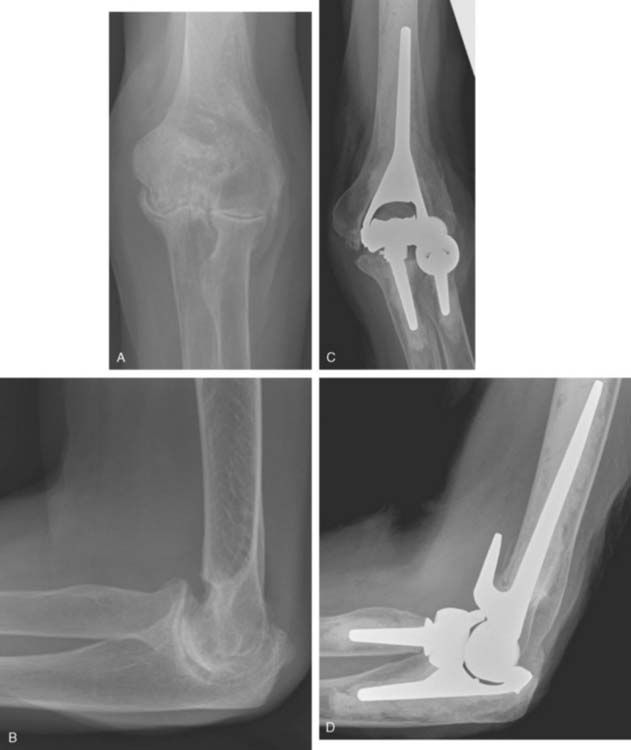
Hemiarthroplasty may also be used as a salvage reconstruction in cases of malunion, fixation failure, or nonunion after open reduction internal fixation. In these instances, the principles of the reconstruction are the same in terms of restoring the column architecture, preserving the collateral ligaments and recreating the rotation axis (Fig. 52-5). A thorough surgical débridement is essential, and in the case of nonunion, tissue should be sent for histopathology and microbiology assessment to rule out infection. Once tissue is obtained, regional infusion of antibiotics is performed to provide optimal tissue levels in already compromised soft tissues. If supracondylar bone loss is present following débridement, a shortening osteotomy may be required, along with medial and lateral plate fixation. Autologous bone graft is routinely used to promote bony union (Fig. 52-6). The ability to achieve a stable reconstruction should be carefully considered in these complex cases in which bony and soft tissue anatomy may have been significantly altered by prior injury and treatment. If concern exists about this ability then a linked TEA may offer a more reliable reconstructive option. Articular cartilage damage of the radial head and sigmoid fossa due to arthrofibrosis or inflammatory arthropathy may lead to inadequate pain relief, reduced functional outcomes, and uncertain longevity. This represents a relative contraindication that may favor a linked TEA. Thus, if a DHH is planned, it probably should be undertaken early rather than late in a failed ORIF.
PITFALLS AND COMPLICATIONS
Potential pitfalls and corresponding considerations and solutions are listed in Table 52-2. Many of these pitfalls can be addressed by meticulous surgical technique, a thorough understanding of elbow biomechanics, and knowledge of the prosthesis systems as they relate to restoring articular geometry and elbow stability.
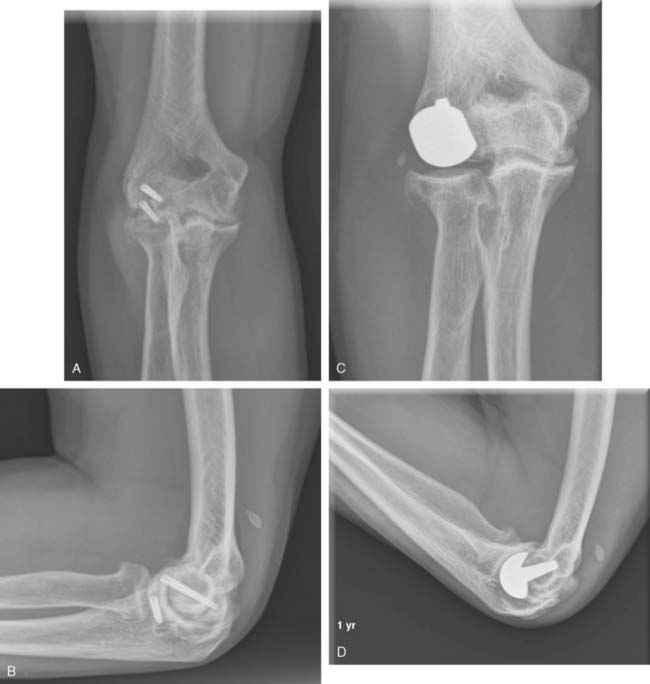
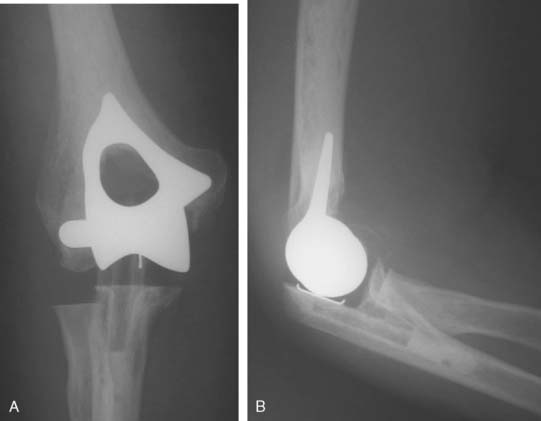
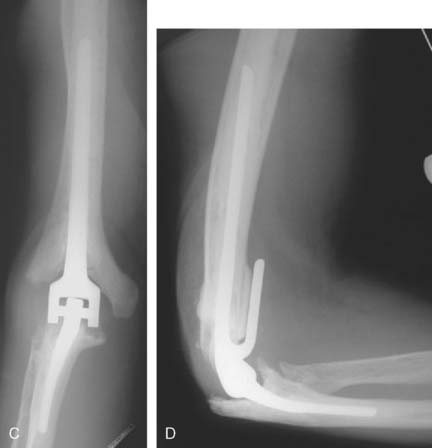
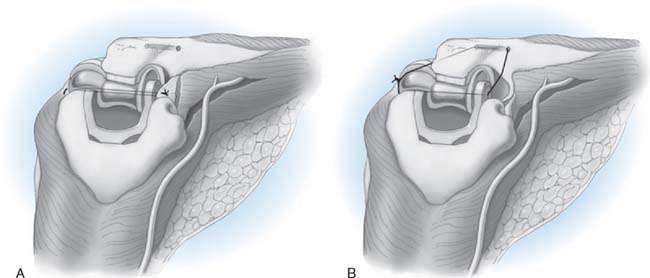
Joint stiffness may occur despite a properly performed reconstruction and early mobilization. If stiffness remains unresponsive to static night splinting, then dynamic splinting can be considered at 2 to 3 months. Persistent stiffness despite these efforts can be managed with contracture release by a column approach at 6 to 12 months postoperatively. Heterotopic ossification, although not common, may occur in the setting of severe elbow trauma (Fig. 52-7A). This is managed by excision and contracture release when the bone is radiographically mature. Instability appears to be related to triceps on exposures, epicondyle comminution, and incorrect collateral ligament reconstruction (see Fig. 52-7B).
RESULTS
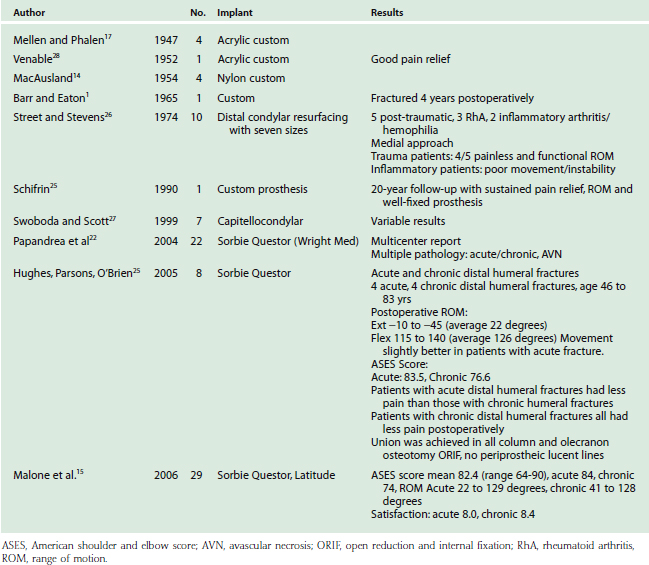
Table 52-3 lists the series of DHH to date. Although long-term prospective data are lacking, except in a few anecdotal case reports, early experience has yielded several observations. First, DHH can lead to substantial pain relief and improvement in function after complex distal humerus fractures. Second, treatment of acute fractures generally results in more favorable outcomes than salvage reconstruction of nonunion, malunion and hardware failure. Third, DHH has proven less effective in chronic conditions such as inflammatory arthritis and hemophilia. Repeat procedures are usually related to hardware removal for fracture or osteotomy fixation. Revision of components is relatively simple especially with the modular systems that can be converted to linked TEA. Future prospective evaluation will better define indications, technique, restrictions and the long-term effectiveness of DHH.
CONCLUSION
Treatment of complex distal humerus fractures or loss of trochlea architecture can be demanding. Although internal fixation and TEA have proven effective in many cases, there are circumstances for which neither represents an ideal treatment. These circumstances include younger or physically active patients. DHH offers an alternative that can provide immediate stability and early mobilization with less risk of implant failure compared with a linked TEA. This is a technically demanding procedure that requires experience with both distal humeral fracture fixation and TEA. Combined techniques may be necessary to restore column architecture and resurface the joint. All supplemental fixation must be rigid enough to allow early motion so that the postoperative recovery from DHH can be reduced to the management of the olecranon osteotomy. Based on early encouraging results, we believe that this is a valuable technique that merits continued refinement through surgical experience and ongoing prospective studies. Our experience indicates a more reliable outcome in the acute (80%) than in the delayed, reconstructed (50%) elbow.16
1 Barr J.S., Eaton R.G. Elbow reconstruction with a new prosthesis to replace the distal end of the humerus: A case report. J. Bone Joint Surg. Am. 1965;47:1408.
2 Beckett K.S., McConnell P., Lagopoulos M., Newman R.J. Variations in the normal anatomy of the collateral ligaments of the human elbow joint. J. Anat. 2000;197:507.
3 Blewitt N., Pooley J. An anatomic study of the axis of elbow movement in the coronal plane: relevance to component alignment in elbow arthroplasty. J. Shoulder Elbow Surg. 1994;3:151.
4 Cobb T.K., Morrey B.F. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. J. Bone Joint Surg. Am. 1997;79:826.
5 Cohen M.S., Bruno R.J. The collateral ligaments of the elbow: anatomy and clinical correlation. Clin. Orthop. Rel. Res. 2001;383:108.
6 Cohen M.S., Hastings H.N. Rotatory instability of the elbow. The anatomy and role of the lateral stabilizers. J. Bone Joint Surg. Am. 1997;79:225.
7 Faber K.J., Cordy M.E., Milne A.D., Chess D.G., King G.J., Johnson J.A. Advanced cement technique improves fixation in elbow arthroplasty. Clin. Orthop. Rel. Res. 1997;334:150.
8 Floris S., Olsen B.S., Dalstra M., Sojbjerg J.O., Sneppen O. The medial collateral ligament of the elbow joint: anatomy and kinematics. J. Shoulder Elbow Surg. 1998;7:345.
9 Frankle M.A., Herscovici D., DiPasquale T.G., Vasey M.B., Sanders R.W. A comparison of open reduction and internal fixation and primary total elbow arthroplasty in the treatment of intraarticular distal humerus fractures in women older than age 66. J. Orthop. Trauma. 2003;17:473.
10 Gambirasio R., Riand N., Stern R., Hoffmeyer P. Total elbow replacement for complex fractures of the distal humerus. An option for the elderly patient. J. Bone Joint Surg. Br. 2001;83:974.
11 Garcia J.A., Mykula R., Stanley D. Complex fractures of the distal humerus in the elderly. The role of total elbow replacement as primary treatment. J. Bone Joint Surg. Br. 2002;84:812.
12 Gramstad G.D., King G.J., O’Driscoll S.W., Yamaguchi K. Elbow arthroplasty using a convertible implant. Tech. Hand Up. Extrem. Surg. 2005;9:153.
13 Inagaki K., O’Driscoll S.W., Neale P.G., Uchiyama E., Morrey B.F., An K.N. Importance of the radial head component in Sorbie unlinked total elbow arthroplasty. Clin. Orthop. Rel. Res. 2002;400:123.
14 MacAusland W.R. Replacement of the lower end of the humerus with a prosthesis: A report of four cases. Western J. Surg. Gynec. Obstet. 1954;62:557.
15 Malone, A. A., Zarkadas, P. C., Hughes, J., and Jansen, S.: American Shoulder and Elbow Surgeons Open Meeting on Biologics in Shoulder Surgery, November 2006.
16 Malone, A., and Hughes, J.: Hemiarthroplasty for the treatment of distal humeral pathology. (Submitted for publication, 2008).
17 Mellen R.H., Phalen G.S. Arthroplasty of the elbow by replacement of the distal portion of the humerus with an acrylic prosthesis. J. Bone Joint Surg. Am. 1947;29:348.
18 Morrey B.F., An K.N. Stability of the elbow: osseous constraints. J. Shoulder Elbow Surg. 2005;14(suppl S):174S.
19 O’Driscoll S.W., Jaloszynski R., Morrey B.F., An K.N. Origin of the medial ulnar collateral ligament. J. Hand Surg. 1992;17:164.
20 Obremskey W.T., Bhandari M., Dirschl D.R., Shemitsch E. Internal fixation versus arthroplasty of comminuted fractures of the distal humerus. J. Orthop. Trauma. 2003;17:463.
21 Ochi N., Ogura T., Hashizume H., Shigeyama Y., Senda M., Inoue H. Anatomic relation between the medial collateral ligament of the elbow and the humero-ulnar joint axis. J. Shoulder Elbow Surg. 1999;8:6.
22 Papandrea, R., Hughes, J., Sorbie, C., et al: Prosthetic Hemiarthroplasty of the Distal Humerus. ASES Meeting, November 2004.
23 Parsons M., O’Brien R., Jason H., Jeffery S. Elbow hemiarthroplasty for acute and salvage reconstruction of intra-articular distal humerus fractures. Tech Shoulder Elbow Surg. 2005;6:87.
24 Ray P.S., Kakarlapudi K., Rajsekhar C., Bhamra M.S. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. Injury. 2000;31:687.
25 Shifrin P.G., Johnson D.P. Elbow hemiarthroplasty with 20-year follow-up study. A case report and literature review. Clin. Orthop. Rel. Res. 1990;254:128.
26 Street D.M., Stevens P.S. A humeral replacement prosthesis for the elbow. Results in ten elbows. J. Bone Joint Surg. 1974;56A:1147.
27 Swoboda B., Scott R.D. Humeral hemiarthroplasty of the elbow joint in young patients with rheumatoid arthritis: a report on 7 arthroplasties. J. Arthroplasty. 1999;14:553.
28 Venable C.S. An elbow and an elbow prosthesis. Am. J. Surg. 1952;51:1590.
29 Wevers H.W., Siu D.W., Broekhoven L.H., Sorbie C. Resurfacing elbow prosthesis: shape and sizing of the humeral component. J. Biomed Eng. 1985;7:241.
PART B Radiohumeral Arthrosis: Anconeus Arthroplasty and Capitellar Prosthetic Replacement
ETIOLOGY
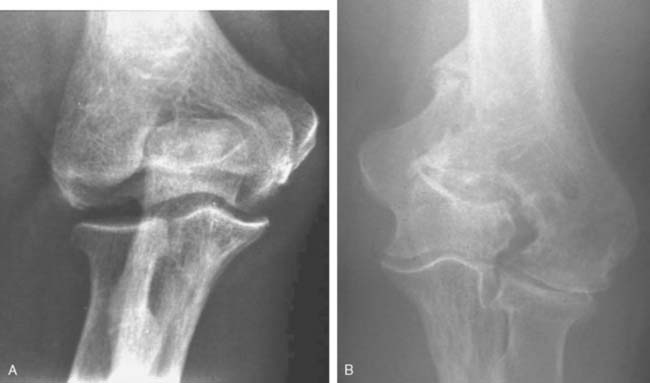
Like so many other pathologic conditions, it seems as though the process is more frequent once we are attuned to investigate for its presence. In general, as a condition, primarily osteoarthritis at the elbow, generally considered uncommon in the past, is today well recognized in the orthopedic community.3,6,10,11 Pathology of the radiohumeral joint occurs from primary osteoarthritis, as a sequelae to osteochondritis dissecans, following capitellar fracture, or secondarily to radial head fracture either ignored, treated by open reduction and internal fixation, or by prosthetic replacement. Finally, the condition is seen after split T and Y type of fractures of the distal humerus resulting in malalignment (Fig. 52-8).
The frequency and impact of these various conditions is not readily available in today’s literature. There has been some interesting studies referable to primary osteoarthritis of the radiohumeral joint that are worth noting. Ortner13 examined the elbow of a population of Eskimos and South American Indians. As a result of this investigation, the authors describe several forms of osteoarthritis at the radiohumeral joint including (1) hypertrophic bone formation peripheral to the articular surface; (2) hypertrophic bone formation in the radial fossa; (3) increased porosity and hypertrophy of the lateral condylar ridge; (4) porosity of the capitellum and; (5) eburnation of the capitellum. These authors note interestingly and accurately that the narrowing of the ulnohumeral joint is not a characteristic of primary osteoarthritis but is more frequently seen at the radiohumeral joint. On the other hand, the osteophyte formation is more commonly observed in the margin of the trochlea and at the coronoid and tip of the olecranon. Subsequently, Goodfellow et al11 documented the increasing incidence of osteoarthritis of the radiohumeral joint with aging and emphasized the early development of the process at the posterior medial ridge separating the trochlea and the capitellum (Fig. 52-9).4 Occupation risks in foundry workers was documented. In general, heavy, repetitive use of the extremity places the joint at risk.
Typically, this process appears to be relatively asymptomatic, but with enhanced awareness and closer scrutiny, the presence of radiohumeral joint symptoms is being increasingly appreciated. These observations have been confirmed somewhat in the recent documentation of intervention for primary osteoarthritis of the elbow. Kelly et al7 described radiohumeral narrowing and instances in which the ulnohumeral joint was débrided arthroscopically. They noted that the radial humeral involvement often typically appears to be well tolerated and need not be directly assessed. Others emphasize the technical ability to débride the ulnohumeral joint but do not comment to any extent on the radiohumeral joint. Although both closed7–9 and open procedures1,14,16 are well-accepted means of treating primary osteoarthritis of the elbow, few make reference specifically to the management of the radial head.7,9 Radiographically, however, the radial head involvement has been documented by Dalal.2 In this study of 50 patients with primary osteoarthritis, radial head involvement was present in approximately 85% but was not typically symptomatic. Some narrowing of the joint was present in approximately 60% compared with only 15% narrowing of the ulnohumeral joint. Savoie’s group is one group that has addressed the radiohumeral component of primary osteoarthritis. These authors perform radial head resection through the arthroscope and, in so doing, document improved motion in both flexion and extension and pronation and supination.9 The long-term impact, however, of removing the radial head in the presence of ulnohumeral joint arthrosis is unknown. This is one of the major issues that prompts one to consider possibly replacing this joint if it is symptomatic enough to deserve treatment on its own merits.
TREATMENT OPTIONS
Experience to date on the treatment of isolated radiohumeral arthrosis or particularly a capitellar arthritis is limited. In general, the treatment options include débridement versus reconstruction. The reconstructive options have relatively little data, but these consist of resecting the capitellum, analogous to isolated resection of the radial head but with little data regarding its outcome. Débridement is effective in those with symptomatic osteochondritis dissecans, particularly when the fragment is loose and causing mechanical symptoms (see Chapters 19 and 38).
Biologic interposition arthroplasty or prosthetic replacement of the capitellum and/or the capitellum and radial head is a consideration. When the capitellum has been destroyed as a result of fracture, often with resorption, a unique problem exists for which there is no good solution. We have performed allograft replacement with or without interposition arthroplasty in a few of these patients. We have also performed an anconeus rotational plasty in patients with deficient or arthritic capitellum and this does seem to be an effective modality. This procedure does not, however, provide axial stability.12 Without question, one of the greatest problems with the radiohumeral joint relates to problems at the articulation after the radial head has been fractured and treated by fixation or prosthetic replacement. Under these circumstances, the radial head is typically excised or the prosthesis is removed. If the medial collateral ligament is stable, this is adequate treatment. If, however, the medial collateral ligament is unstable, then the major indication for consideration of a prosthetic implant exists. This is intended to satisfy the need to stabilize the “lateral column” in instances in which there is deficient medial collateral ligament or axial instability such as an Essex-Lopresti lesion. There is some, but limited, experience with prosthetic replacement to date (Pooley J, personal communication).
ANCONEUS ARTHROPLASTY
Indications
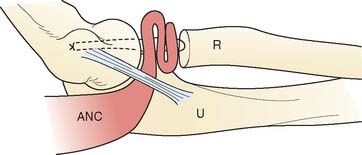
In general, this technique is employed in instances when the proximal radial ulnar or ulnohumeral dysfunction occurs following trauma or resection, or after primary osteoarthrosis. This procedure was designed for two clinical circumstances: (1) radiohumeral impingement, in which the goal is to buffer the proximal radiocapitellar articulation; and (2) rotatory radioulnar impingement.
Anatomy
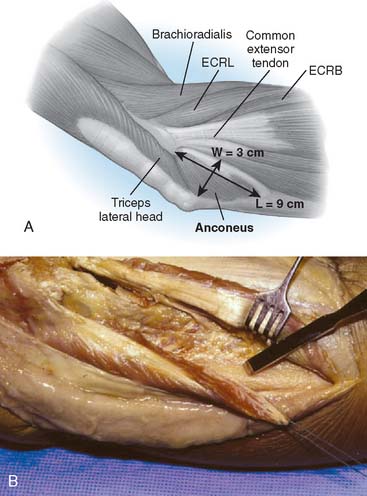
It is important to recognize that the mean length of the anconeus is rather significant, averaging 9 to 10 cm, with a mean width of 3 cm (Fig. 52-10).12,15 The neurovascular survival is by way of a continuation of a branch of the distal radial nerve.
The Surgery
Outcomes
We have reported this technique and our experience in 14 patients who had been treated with one of these types of anconeus interposition arthroplasty with surveillance of at least 2 years (mean, 6.1 years). Twelve of 14 rated their outcome as a success. This included 4 of 5 with Essex-Lopresti injuries (Fig. 52-13).12
CAPITELLAR PROSTHETIC REPLACEMENT
Indications
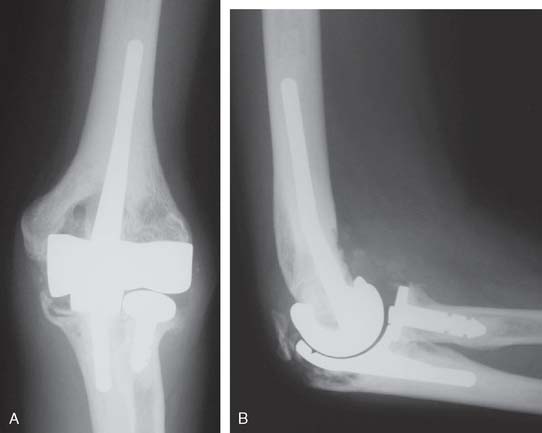
Specific indications are those instances in which the radiohumeral joint is painful and arthritic and when simple excision of the radial head is not adequate owing to the need to stabilize the lateral joint. The most compelling indications are (1) a need to stabilize the lateral column due to deficiency of the medial collateral ligament or (2) in those in whom there is axial radial instability in the face of capitellar arthrosis or deficiency. The capitellum may be replaced to articulate with the native radial head (Fig. 52-14) or in conjunction with a radial head replacement (Fig. 52-15). The latter requires a polyethylene articulation to articulate with the metallic capitellar device (Fig. 52-16).
Surgical Technique
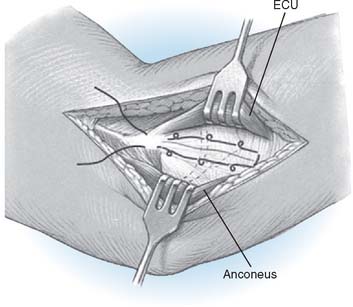
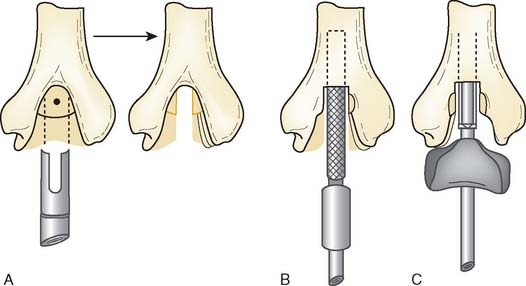
Step 2: Exposure. If the elbow is stable, the capsule is exposed by elevating a portion of the extensor carpi ulnaris sufficiently to allow identification of the lateral collateral ligament complex (Fig. 52-17). Alternatively, the extensor carpi ulnaris may be split longitudinally, with its fibers staying anterior to the humeral attachment of the lateral collateral ligament. The lateral collateral ligament can be refleced off the lateral epicondyle to expose the capitellum. If the ligament has been disrupted, then the exposure progresses through the site of disruption to expose the radiohumeral joint. The common extensor tendon and elbow joint capsule are retracted as needed to maximize exposure.
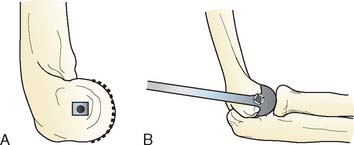
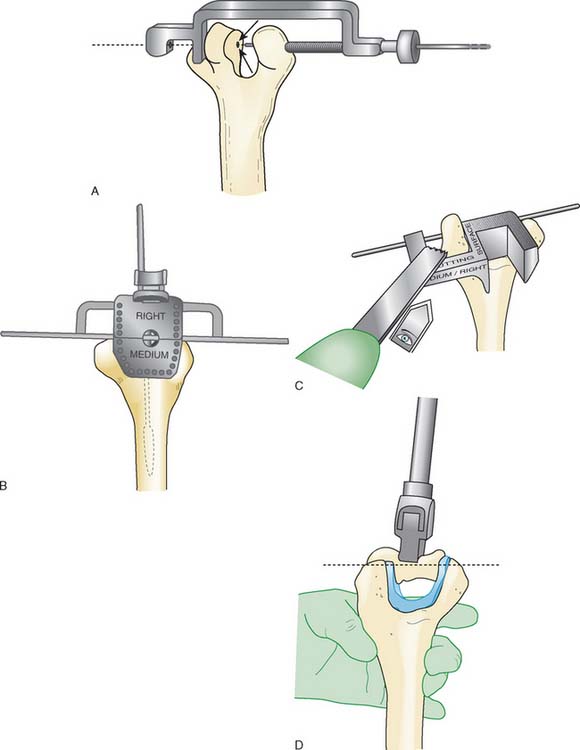
Step 3. Axis of Rotation. Attach the appropriate sized capitellum template to the drill guide, and align the template to the curvature of the capitellum (Fig. 52-18). The curvature of the edge of the template should align with the most distal articular surface of the original capitellum. Once alignment is achieved, insert a 0.062 K-wire through the drill guide, and advance medially to the midline of the distal humerus (Fig. 52-19). Leaving the K-wire in place, remove the axis of rotation locator clamp and template.
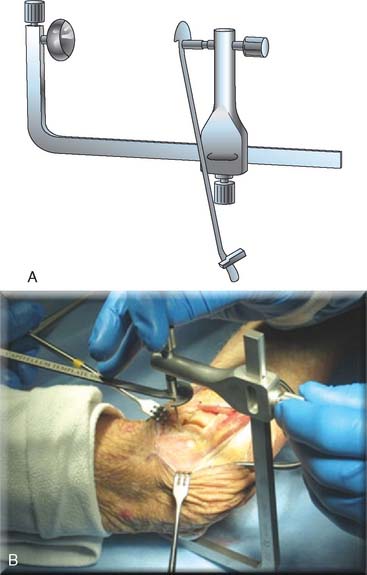
FIGURE 52-19 (A and B) The axis of rotation is replicated by a .062 K-wire, which is inserted by the use of a targeting guide.
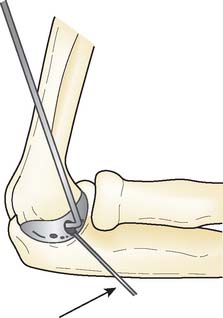
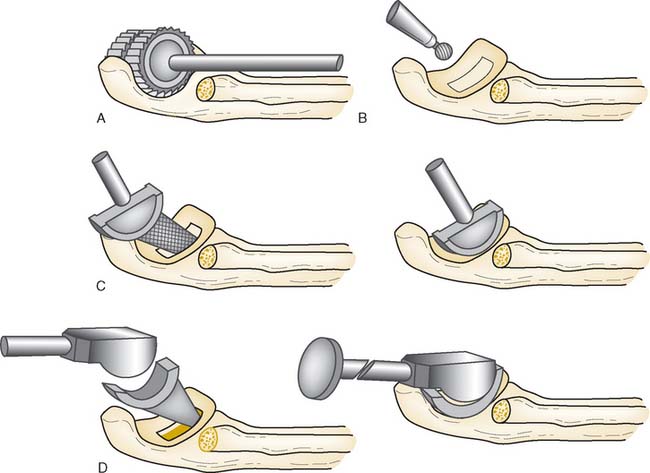
Step 4: Capitellar Resection. There are two resection guide sizes: small and large. Based on the size of the capitellum, place the appropriate resection guide. Align the resection guide handle so that it is parallel with the long axis of the humerus (Fig. 52-20).
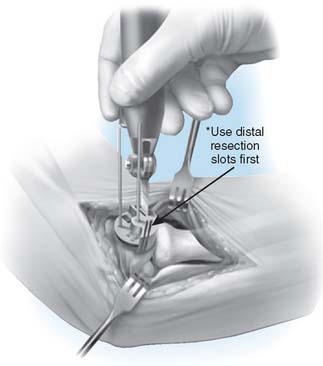
FIGURE 52-20 The resection is of a Chevron type. A distal guide is employed initially to avoid excessive resection.
Step 5: Capitellum Trial. Place and stabilize the capitellum trial flush against the resected surfaces (Fig. 52-21). Insert the reference K-wire, which serves as the alignment pin for subsequent preparation.
Step 6: Broaching of the Distal Humerus. Remove the trial capitellum leaving the reference K-wire. Pass the cannulated 0.35-mm drill over the center K-wire to create the broach pilot hole into the distal humerus. Align the broach with the apex of the Chevron cut, insert broach into the pilot hole and advance until the teeth are at the same level as the capitellum osteotomy (Fig. 52-22). Care should be taken to ensure proper alignment and not to overbroach the distal humerus.
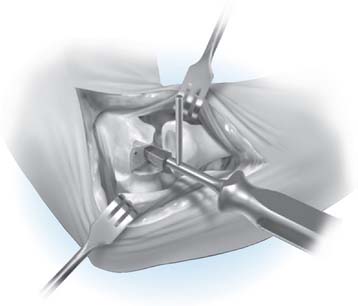
Step 7: Implanting the Final Component. Once broaching is complete, the definitive implant can be inserted (Fig. 52-23). Distraction of the proximal radius as well as flexion of the elbow may be necessary to allow sufficient access for capitellum insertion.
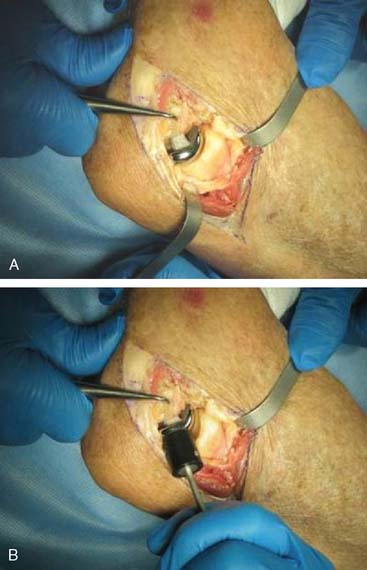
FIGURE 52-23 The capitellum is inserted from distal laterally (A) and is impacted proximal medially (B).
Note: The capitellum may be press-fit or cemented according to surgeon preference.
Step 8: C Closure. A No. 5 absorbable suture is placed at the humeral origin of the lateral collateral ligament, and a running locked stitch proceeds distally through the ulnar attachment, then back proximally, ending at the humeral origin of the ligament (Fig. 52-24). The forearm is placed in full or partial pronation, a valgus stress is applied, and the suture tied.
CLINICAL RESULTS
There is limited data regarding the use of capitellar prosthetic replacements. In our case series,5 we reported effective treatment of chronic Essex-Lopresti lesions in three patients. Otherwise, we have employed a capitellar replacement only in eight additional patients as an isolated replacement, and in four with a radial head replacement as well. To date, there have been no capitellar implant revisions.
1 Antuna S.A., Morrey B.F., Adams R.A., O’Driscoll S.W. Ulnohumeral arthroplasty for primary degenerative arthritis of the elbow: Long-term outcome and complications. J. Bone Joint Surg. 2002;84A:2168.
2 Dalal S., Bull M., Stanley D. Radiographic changes at the elbow in primary osteoarthritis: A comparison with normal aging of the elbow joint. J. Shoulder Elbow Surg. 2007;16(3):358-361.
3 Doherty M., Watt I., Dieppe P.A. Influence of primary generalized osteoarthritis on development of secondary osteoarthritis. Lancet. 1983;2:8.
4 Goodfellow J.W., Bullough P.G. The pattern of aging of the articular cartilage of the elbow joint. J. Bone Joint Surg. 1967;49B:175.
5 Heijink A., Morrey B.F., van Riet R.P., O’Driscoll W., Cooney W.P. Delayed treatment of elbow pain and dysfunction following Essex-Lopresti injury with metallic radial head replacement. Submitted for publication. J. Bone Joint Surg. Am. 2008.
6 Kashiwagi D. Intra-articular changes of the osteoarthritic elbow, especially about the fossa olecrani. Jpn. Orthop. Assoc. 1978;52:1367.
7 Kelly E.W., Bryce R., Coghlan J., Bell S. Arthroscopic debridement without radial head excision of the osteoarthritic elbow. Arthroscopy. 2007;23:151.
8 Krishnan S.G., Harkins D.C., Pennington S.D., Harrison D.K., Burkhead W.Z. Arthroscopic ulnohumeral arthroplasty for degenerative arthritis of the elbow in patients under fifty years of age. J. Shoulder Elbow Surg. 2007;16:443.
9 McLaughlin R.E.2nd, Savoie F.H.3rd, Field L.D., Ramsey J.R. Arthroscopic treatment of the arthritic elbow due to primary radiocapitellar arthritis. Arthroscopy. 2006;22:63.
10 Minami N.M. Roentgenological studies of osteoarthritis of the elbow joint. Jpn. Orthop. Assoc. 1977;51:1223.
11 Mintz G., Fraga A. Severe osteoarthritis of the elbow in foundry workers. Arch. Environ. Health. 1973;27:78.
12 Morrey B.F., Schneeberger A.G. Anconeus arthroplasty: A new technique for reconstruction of the radiocapitellar and/or proximal radioulnar joint. J. Bone Joint Surg. 2002;84A:1960.
13 Ortner D.J. Description and classification of degenerative bone changes in the distal joint surface of the humerus. Am. J. Phys. Anthrop. 1968;28:139.
14 Phillips N.J., Ali A., Stanley D. Treatment of primary degenerative arthritis of the elbow by ulnohumeral arthroplasty. A long-term follow-up. J. Bone Joint Surg. 2003;85B:347.
15 Schmidt C.C., Kohut G.N., Greenberg J.A., Kann S.E., Idler R.S., Kiefhaber T.R. The anconeus muscle flap: its anatomy and clinical application. J. Hand Surg. 1999;24A:359.
16 Wada T., Isogai S., Ishii S., Yamashita T. Debridement arthroplasty for primary osteoarthritis of the elbow. Surgical Technique. J. Bone Joint Surg. 2005;87A:95.
PART C Unlinked Total Elbow Arthroplasty
DESIGN CONSIDERATIONS
Polyethylene wear and aseptic loosening of linked total elbow arthroplasties is a significant problem with longer follow-up, particularly in younger and more active patients.11 When a linked arthroplasty reaches the limits of its intrinsic laxity, forces are transmitted to the linkage mechanism and thereby to the stems and the bone implant interface, contributing to bearing wear and implant loosening.30 The concept of unlinked total elbow arthroplasty is that there is a sharing of load between the prosthesis and the capsule, ligaments, and muscles in an effort to decrease the stresses in the articulation and the stems. A necessary prerequisite for an unlinked arthroplasty is the presence of adequate ligaments and bone stock with which the implant can be stabilized after insertion. Unlinked total elbows were initially referred to as resurfacing devices primarily because most were designed without a humeral stem. Owing to the high incidence of implant loosening with these early designs, most unlinked prostheses now incorporate stems on the components in an effort to improve implant longevity (Fig. 52-25).
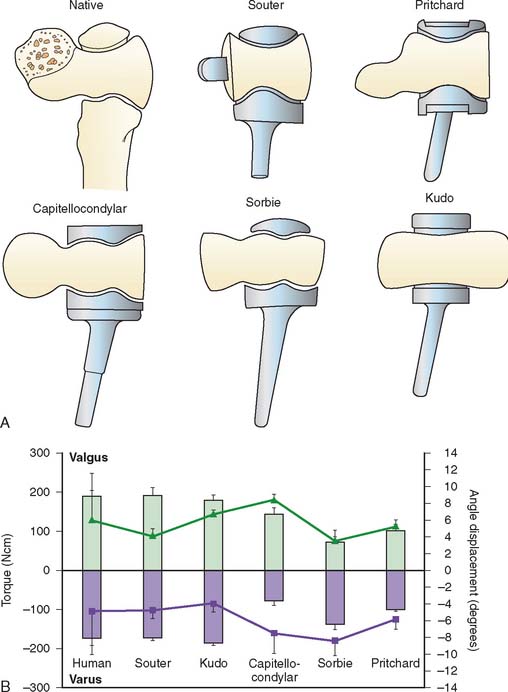
Unlinked total elbow arthroplasties are sometimes referred to as unconstrained devices, whereas loose-hinge linked devices are often referred to as being semiconstrained; however, this is a misnomer. All joint arthroplasties have some element of intrinsic constraint by virtue of the shape and interaction of their articular surfaces. The intrinsic constraint of the ulnotrochlear joint of five unlinked total elbow arthroplasty designs were compared and found to vary markedly between implant types.15 Surprisingly, the implants that more closely resembled the articular geometry of the native articulation tended to less accurately replicate the intrinsic constraint of the human elbow (Fig. 52-26). In a series of cadaveric studies, the kinematics and stability of unlinked and linked total elbow arthroplasties have been evaluated.1,13,14,18,30,35,43 The mechanical behavior of these prostheses has been found to vary markedly depending on their articular design.
In an effort to improve their stability and load transfer, some unlinked designs have incorporated a radial head replacement (Fig. 52-27). The radial head component has been shown to improve the stability of both the Sorbie and Pritchard ERS prostheses in in vitro biomechanical studies.13,35 Although theoretically improving soft tissue balance and load transfer across the elbow, the experience with radial head components in total elbow arthroplasty has been variable. For example, the radial head component of the capitellocondylar prosthesis was abandoned owing to concerns about radiolucent lines around the implant stem, but a subsequent study reported that the outcome was better if a radial head component was used.56 One possible explanation of the variable results with radial head prostheses in unlinked implants to date may be that, in many cases, their designs have been suboptimal. Furthermore, the addition of a third component complicates the surgical technique, making it more challenging to perform correctly, particularly because the instrumentation available to position unlinked total elbows has been lacking relative to other joints such as the hip and knee. Incorrect positioning of a radial head component will result in eccentric motions during prosupination and therefore shear forces across the radiocapitellar articulation. These abnormal kinematics and loads may cause polyethylene wear and early loosening of the radial component. A bipolar radial head component may be more forgiving in this setting because it will compensate for some of the eccentric motions through the bipolar articulation.
Functional and balanced collateral ligaments are a key feature of a successful unlinked total elbow arthroplasty. Incompetent ligaments result in articular maltracking, joint subluxation, or dislocation. This has been demonstrated in an in-vitro cadaveric study and observed clinically.14,18 Maltracking of the arthroplasty leads to excessive polyethylene wear, osteolysis, and aseptic loosening. During the insertion of most unlinked designs, one or both of the collateral ligaments are usually released to achieve adequate exposure. Careful repair and successful ligament healing are needed if an unlinked arthroplasty is to be stable and articulate normally.
Optimal component positioning improves the outcome of an unlinked total elbow arthroplasty.44,54 Replication of the axis of motion to the native state restores the muscle moment arms and the soft tissue tension of the collateral ligaments, which ensures more normal articular tracking and implant loading. Malpositioning of the humeral component has been demonstrated to alter the in vitro kinematics and stability of the capitellocondylar arthroplasty.14 It has also been reported that correct medial-lateral and proximal-distal positioning of the Souter implant had a lower incidence of loosening.44 Another study evaluating the same implant, however, was unable to demonstrate a relationship between humeral component position and aseptic loosening.58 Current techniques to identify and replicate the flexion-extension axis are prone to error. In one study, the surgeon’s ability to select the flexion-extension axis resulted in errors in axis orientation of up to 10 degrees in normal humerii. These errors are likely greater in clinical practice, where the anatomy is more distorted and the exposure of the landmarks is compromised.2 Perhaps the use of computer or image-assisted surgery will improve the accuracy of elbow component placement in the future, such as has been reported for the hip and knee.
In summary, the premise of unlinked designs is that much of their stability is afforded by the geometry of the implant and the surrounding soft tissues rather than the intrinsic constraint of the articulation.1 The goal of an unlinked arthroplasty is to divert the forces that are transferred across the articulation away from the bone implant interface to the surrounding soft tissues. By virtue of their need for adequate ligaments and bone stock, the indications for unlinked designs are narrower than linked arthroplasties and the instability rate is greater. Although the loosening rate should theoretically be lower with unlinked verses linked prostheses, this has yet to be proven in clinical studies.
INDICATIONS
The indications for an unlinked total elbow arthroplasty are similar to those for any design of elbow arthroplasty. The necessary prerequisites to choose an unlinked implant are the presence of sufficient bone stock to support the implant and intact medial and lateral collateral ligaments. The presence of functioning elbow flexor and extensor muscles are particularly important with an unlinked design, which requires muscle balance to maintain implant stability. In the absence of sufficient bone stock or ligamentous constraints, a linked arthroplasty should be used. Typical indications include inflammatory arthritis (rheumatoid, psoriatic, hemophilic), primary or post-traumatic osteoarthritis, osteonecrosis, periarticular tumors and comminuted distal humeral articular fractures in elderly patients.
SURGICAL CONSIDERATIONS OF UNLINKED DESIGNS
There are a number of unlinked designs that have been developed and employed. The design features and surgical considerations of a few of the more commonly used devices will be reviewed. The capitellocondylar implant designed by Ewald consists of a cobalt-chrome humeral component with 5-, 10-, and 15-degree valgus angulations8 (Fig. 52-28) (Johnson and Johnson, Raynham, MA). The ulnar component has two polyethylene thicknesses available. A radial head implant was developed for this system but was abandoned owing to concerns about radiolucent lines around the stems.56 The ulnohumeral joint has a low magnitude of intrinsic constraint, making it prone to maltracking and dislocation.15,17 The implant is most commonly inserted through an extended Kocher approach, with elevation of the triceps off the olecranon from lateral to medial. The medial collateral ligament is preserved, whereas the lateral collateral ligament is cut and repaired during closure. Although excellent results were reported by the inventor, the experience of others has been less favorable owing to problems with instability9,31,40,61 (Fig. 52-29).
The Pritchard ERS arthroplasty (DePuy, Warsaw, IN) consists of humeral, ulnar, and radial components (Fig. 52-30).34 It can be inserted through either an extended Kocher or a Bryan-Morrey triceps reflecting approach.3,28,32 One or both collateral ligaments are divided during the approach and then carefully repaired during closure. The radial head component is important for elbow stability.35 The clinical outcome of this prosthesis is largely unknown, with little available information provided in the literature34 (Fig. 52-31).
The Souter-Strathclyde arthroplasty (Stryker, Mahwah, NJ) consists of a cobalt-chrome humeral and an all polyethylene ulnar component48 (Fig. 52-32). The humeral component aims to replicate the shape of the native trochlea and maintain the integrity of the supracondylar ridges and their attached collateral ligaments.47The ulnar component is closely congruous with the humeral component, making the implant relatively constrained when loaded.15,43 The short-stem humeral component has a fin, which is carefully fitted into the medial supracondylar ridge, and a peg that is inserted into the capitellum (Fig. 52-33). The system has long-stem humeral components and long-stem metal-backed ulnar components available to manage bone loss and revision situations. A snap-fit ulnar component is also available that converts the implant into a linked device. The prosthesis is inserted using a posterior approach, typically using a triceps turndown. The medial collateral ligament is preserved, but the lateral ligament is typically divided to obtain sufficient exposure to allow dislocation and radial head excision. Careful repair of the triceps tendon and lateral collateral ligament is essential. Instability of this implant has been less frequent than other unlinked designs; however, loosening of the short-stemmed humeral component has been a common clinical problem.54
The Kudo total elbow arthroplasty (Biomet, Warsaw, IN) has evolved significantly since it was first introduced.21 Initially, the prosthesis had an all-polyethylene ulnar component and an unstemmed humeral component with a cylindrical articulation. Owing to a high incidence of early loosening, both the humeral and ulnar components were redesigned (Fig. 52-34). The current Kudo type 5 prosthesis consists of a 5-degree valgus cobalt-chrome humeral component with a titanium porous coating that is typically inserted uncemented and a metal-backed cemented ulnar component (Fig. 52-35). The humeral articulation has a saddle-shaped design that allows for medial-lateral translation of the ulnar component. The prosthesis is implanted with sectioning of the collateral ligaments and excision of the radial head. The reported results with the current design are favorable, although instability has been problematic owing to the low intrinsic stability relative to other designs19,20,29,33,50–52 (Fig. 52-36).
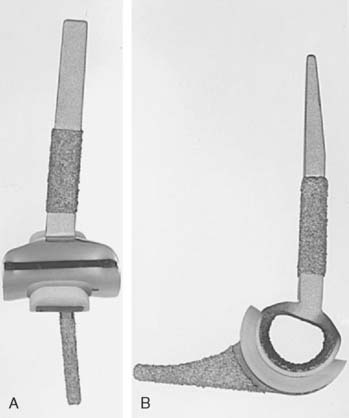
FIGURE 52-35 The current Kudo type 5 total elbow arthroplasty has a stemmed component and a metal-backed ulnar component.
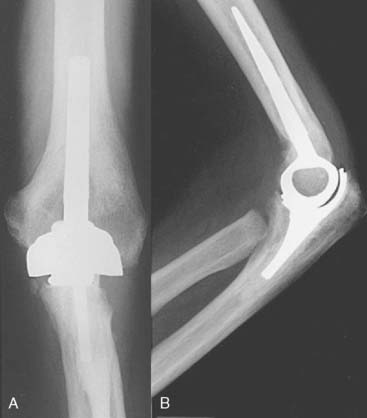
FIGURE 52-36 Anteroposterior (A) and lateral (B) radiographs of a well functioning Kudo type 5 total elbow arthroplasty.
The Sorbie-Questor total elbow arthroplasty (Wright Medical Technology, Arlington, TN) was designed to replicate the normal anatomy of the elbow (Fig. 52-37).45,46 The humeral component has an angled cobalt-chrome stem. A metal-backed ulnar component is available with two polyethylene thicknesses. The radial head component is a stemmed monoblock design. Originally designed without a humeral stem, loosening was a problem until the stem was added. The implant is inserted though an extended Kocher approach with sectioning of the lateral collateral ligament. There are no published results of the outcome of this device (Fig. 52-38).
SURGICAL TECHNIQUE
The management of the triceps is based on surgeon preference. A distally based triceps flap is recommended by the designer. Both the lateral and medial collateral ligaments are divided from their humeral insertions, and the elbow is dislocated. The radial head is excised. The distal humerus is prepared primarily freehand with minimal bone resection (Fig. 52-39). The ulna is prepared using a barrel reamer, burr, and rasps (Fig. 52-40). A trial reduction of the prosthesis is performed to evaluate soft tissue balance and articular tracking. Repositioning of one or both components should be performed, if necessary, to achieve soft tissue tensioning and optimal articular congruency. The humeral component is inserted uncemented if a good press fit is achieved, whereas the ulnar component is routinely cemented. After reduction of the elbow, the triceps and the fascial layers are meticulously repaired. The collateral ligaments are not routinely repaired.
RESULTS
The reported outcome of unlinked total elbow arthroplasty consists primarily of retrospective case series. Typical complications of elbow arthroplasty such as ulnar neuropathy, infection, triceps failure and wound healing problems are discussed in Chapter 61 and will not be highlighted in this chapter. There are no prospective randomized trials comparing the outcome of different unlinked arthroplasties.24 Little et al25 compared the outcome of the Souter, Kudo, and Coonrad-Morrey designs in a nonrandomized prospective cohort study design. The authors were unable to demonstrate a significant difference between the Kudo and Souter designs with respect to outcome or complications. The linked Coonrad-Morrey implant had outcome that was similar to the unlinked designs, with a lower incidence of instability. The loosening rate did not differ between the three designs at an average of 5 years postoperatively.
There have been a number of case series reporting the outcome of the capitellocondylar prosthesis.4,6–10,31,40,42,55,56,61 Collectively, these studies demonstrate a reliable improvement in pain and function, with a modest improvement in motion. The incidence of loosening has been low in most series; however, instability has been a problem, particularly in the hands of surgeons other than the inventor (Fig. 52-41).
Ewald, the surgeon inventor, reported a 3% revision rate out of 202 elbows at an average of 6.7 years.9 Only 1.5% of the elbows had sufficient instability to require revision, and 1.5% developed loosening. Schemitsch et al42 reported that the incidence of instability was higher and pain relief was lower in a matched cohort of patients with a prior radial head excision and synovectomy. Trepman and coworkers,56 also from the same center, reported that the presence of a radial head implant lowered the incidence of instability of this design.
The experience with the capitellocondylar prosthesis reported by other investigators is less favorable owing to a higher incidence of instability. Weiland et al61 reported a malarticulation rate of 29% in 40 prostheses followed for 7.2 years. Although most of these elbows remained functional, maltracking of the articulation was a cause for concern due to polyethylene wear at longer follow-up. Similarly, studies by Tranik et al55 and Davis et al6 reported instability rates of 9% and 13%, respectively, although most could be managed without revision. Ring et al38 demonstrated that ligament reconstruction was unreliable in restoring stability of this unlinked arthroplasty, whereas revision to a linked prosthesis had a high complication rate. Collectively, the aseptic loosening rates for the capitellocondylar prosthesis in these studies have been low. In the most recent reported experience with this implant, Ovesen et al31 reported that 83% of 51 prostheses were functional at an average of 6.9 years postoperatively. Seven prostheses required revision, with instability and infection being the most frequent cause of failure.
The reported experience with the Pritchard ERS total elbow prosthesis has been limited to date.34 The experience at the Mayo Clinic has not been favorable, with a 10-year Kaplan Meier survival of only 54% out of 46 prostheses. Instability, wear, and aseptic loosening were the most common causes for failure (Fig. 52-42). Ulnohumeral component malpositioning was thought to be the most common reason for instability. The failure rate was much greater if the radial head was not replaced; however, technical issues with positioning the radial head component were common. Preservation of the native radial head provided the longest functioning implants.
The results of the Souter total elbow arthroplasty have been widely reported from multiple centers other than that of the surgeon inventor.* The outcome has been uniformly favorable, with a high incidence of improvement in pain and function with a modest improvement in elbow flexion but not extension. In general, instability has been less common than reports of other unlinked designs. Humeral component loosening has been more common than the ulnar component when the short-stem humeral stems were employed53 (Fig. 52-43). The most recent series was reported by Landor et al,23 with 58 elbows followed for an average of 9.5 years. The Mayo elbow performance index improved from 30 to 82 points. The revision rate was 22%, with loosening being the most common cause. Only one elbow was revised for instability. The Kaplan-Meier survival was 70% at 10 years and 53% at 16 years.
Van Der Lugt et al59 reported the results of a prospective study of 204 Souter prostheses inserted for rheumatoid arthritis and followed for an average of 6.4 years. Twelve percent of the prostheses were revised, with loosening of the humeral component being the most common indication. The Kaplan-Meier survival was 77% at 10 years and 65% at 18 years. Trail and coworkers53 evaluated the outcome of 186 prostheses and reported a 12-year survival rate of 87%. Seventy-five percent of the revisions were due to humeral loosening. The use of a long-stem humeral component was subsequently reported to have a much lower incidence of loosening than the short-stem design.54 The use of a snap-fit ulnar component that converts the prosthesis from an unlinked to a linked design was shown to result in a higher incidence of aseptic loosening.
The outcome of the Kudo total elbow arthroplasty has largely been reported from the surgeon-inventor, and has reflected the evolution of the prosthesis design.† The early type 1 and 2 prostheses used an unstemmed humeral component, which had a high incidence of loosening.22 Experience with the later designs has been more promising with a low incidence of instability and loosening. The uncemented humeral component has been more reliable than the ulnar component, in which cement and metal backing has improved the success. Thillemann and coworkers52 reported 67% survival of 17 Kudo three elbows followed for an average of 9.5 years. Two had been revised due to loosening of the ulnar component and one for instability. Progressive valgus tilting of the elbow at longer follow-up raised concerns regarding polyethylene wear of the ulnar component at longer follow-up (Fig. 52-44).
Professor Kudo and his colleagues have reported excellent results, particularly with the current type 5 prosthesis. Tanaka et al demonstrated that the outcome of a metal-backed cemented ulnar component was superior to an all polyethylene design.51 Mori and coworkers29 reported successful outcomes of the type 5 unlinked design at an average of 8 years, even in the presence of mutilating rheumatoid arthritis with bone loss. Tanaka et al50 reported a 90% survival rate at 13 years using the type 3 prosthesis. Preservation of the medial collateral ligament at surgery was not found to influence the long-term outcome.
Potter et al33 reported on the outcome of 35 Kudo type 5 prostheses at an average of 6 years. Although none of the prostheses had been revised, two were loose at follow-up with a Kaplan-Meier survival of 89% at 5 years. One postoperative dislocation was successfully treated with a closed reduction. Willems et al62 reported on the outcome of 36 Kudo prostheses at an average of 5 years. Although the clinical outcome was good or excellent in the majority of patients, 17% had been revised, most commonly for aseptic loosening at longer follow-up.
There are no peer review studies documenting the outcome of the Sorbie total elbow arthroplasty to date. In a prospective study of 48 elbows, infection has been the most common complication in the experience of the surgeon inventor (15%) (Personal communication, Dr. Charles Sorbie). The high initial infection rate was attributed to the use of unsterile tourniquets because there have been no further infections since these were replaced by sterile tourniquets in the latter part of the series. Six percent of the patients have needed a revision for loosening and 4% for instability at an average follow-up of 6.8 years (Fig. 52-45).
In summary, the outcome of unlinked total elbow arthroplasties are similar to those reported for linked prostheses.25,63 Although the loosening rates of the more unconstrained devices are low, the incidence of instability has been problematic in some series. It is likely that patient selection and surgeon experience plays a critical role in the outcome of elbow replacement in general and unlinked devices in particular. Unlinked prostheses are less forgiving than linked designs owing to the need to accurately position the components, balance the soft tissues, and achieve ligament healing. Randomized clinical trials are needed to accurately compare the complications and longevity of elbow prostheses.
1 An K.N. Kinematics and constraint of total elbow arthroplasty. J. Shoulder Elbow Surg. 2005;14(1 Suppl S):168S.
2 Brownhill J.R., Furukawa K., Faber K.J., Johnson J.A., King G.J. Surgeon accuracy in the selection of the flexion-extension axis of the elbow: an in vitro study. J. Shoulder Elbow Surg. 2006;15:451.
3 Bryan R.S., Morrey B.F. Extensive posterior exposure of the elbow. A triceps-sparing approach. Clin. Orthop. Rel. Res. 1982;166:188.
4 Chiodo C.P., Terry C.L., Koris M.J. Reconstruction of the medial collateral ligament with flexor carpi radialis tendon graft for instability after capitellocondylar total elbow arthroplasty. J. Shoulder Elbow Surg. 1999;8:284.
5 Dainton J.N., Hutchins P.M. A medium-term follow-up study of 44 Souter-Strathclyde elbow arthroplasties carried out for rheumatoid arthritis. J. Shoulder Elbow Surg. 2002;11:486.
6 Davis R.F., Weiland A.J., Hungerford D.S., Moore J.R., Volenec-Dowling S. Nonconstrained total elbow arthroplasty. Clin. Orthop. Relat. Res. 1982;171:156.
7 Dennis D.A., Clayton M.L., Ferlic D.C., Stringer E.A., Bramlett K.W. Capitello-condylar total elbow arthroplasty for rheumatoid arthritis. J. Arthroplasty. 1990;5(suppl):S83.
8 Ewald F.C., Scheinberg R.D., Poss R., Thomas W.H., Scott R.D., Sledge C.B. Capitellocondylar total elbow arthroplasty. J. Bone Joint Surg. Am. 1980;62:1259.
9 Ewald F.C., Simmons E.D.Jr., Sullivan J.A., Thomas W.H., Scott R.D., Poss R., Thornhill T.S., Sledge C.B. Capitellocondylar total elbow replacement in rheumatoid arthritis. Long-term results. J. Bone Joint Surg. Am. 1993;75:498.
10 Friedman R.J., Lee D.E., Ewald F.C. Nonconstrained total elbow arthroplasty. Development and results in patients with functional class IV rheumatoid arthritis. J. Arthroplasty. 1989;4:31.
11 Hildebrand K.A., Patterson S.D., Regan W.D., MacDermid J.C., King G.J. Functional outcome of semiconstrained total elbow arthroplasty. J. Bone Joint Surg. Am. 2000;82-A:1379.
12 Ikavalko M., Lehto M.U., Repo A., Kautiainen H., Hamalainen M. The Souter-Strathclyde elbow arthroplasty. A clinical and radiological study of 525 consecutive cases. J. Bone Joint Surg. Br. 2002;84:77.
13 Inagaki K., O’Driscoll S.W., Neale P.G., Uchiyama E., Morrey B.F., An K.N.. Importance of a radial head component in Sorbie unlinked total elbow arthroplasty. Clin. Orthop. Relat. Res, 2002;400:123-131.
14 Itoi E., Niebur G.L., Morrey B.F., An K.N. Malrotation of the humeral component of the capitellocondylar total elbow replacement is not the sole cause of dislocation. J. Orthop. Res. 1994;12:665.
15 Kamineni S., O’Driscoll S.W., Urban M., Garg A., Berglund L.J., Morrey B.F., An K.N. Intrinsic constraint of unlinked total elbow replacements—the ulnotrochlear joint. J. Bone Joint Surg. Am. 2005;87:2019.
16 Khatri M., Stirrat A.N. Souter-Strathclyde total elbow arthroplasty in rheumatoid arthritis: medium-term results. J. Bone Joint Surg. Br. 2005;87:950.
17 King G.J., Glauser S.J., Westreich A., Morrey B.F., An K.N. In vitro stability of an unconstrained total elbow prosthesis. Influence of axial loading and joint flexion angle. J. Arthroplasty. 1993;8:291.
18 King G.J., Itoi E., Niebur G.L., Morrey B.F., An K.N. Motion and laxity of the capitellocondylar total elbow prosthesis. J. Bone Joint Surg. Am. 1994;76:1000.
19 Kudo H., Iwano K., Nishino J. Cementless or hybrid total elbow arthroplasty with titanium-alloy implants. A study of interim clinical results and specific complications. J. Arthroplasty. 1994;9:269.
20 Kudo H., Iwano K., Nishino J. Total elbow arthroplasty with use of a nonconstrained humeral component inserted without cement in patients who have rheumatoid arthritis. J. Bone Joint Surg. Am. 1999;81:1268.
21 Kudo H., Iwano K., Watanabe S. Total replacement of the rheumatoid elbow with a hingeless prosthesis. J. Bone Joint Surg. Am. 1980;62:277.
22 Kudo H., Iwano K. Total elbow arthroplasty with a non-constrained surface-replacement prosthesis in patients who have rheumatoid arthritis. A long-term follow-up study. J. Bone Joint Surg. Am. 1990;72:355.
23 Landor I., Vavrik P., Jahoda D., Guttler K., Sosna A. Total elbow replacement with the Souter-Strathclyde prosthesis in rheumatoid arthritis. Long-term follow-up. J. Bone Joint Surg. Br. 2006;88:1460.
24 Little C.P., Graham A.J., Carr A.J. Total elbow arthroplasty: a systematic review of the literature in the English language until the end of 2003. J. Bone Joint Surg. Br. 2005;87:437.
25 Little C.P., Graham A.J., Karatzas G., Woods D.A., Carr A.J. Outcomes of total elbow arthroplasty for rheumatoid arthritis: comparative study of three implants. J. Bone Joint Surg. Am. 2005;87:2439.
26 Lyall H.A., Cohen B., Clatworthy M., Constant C.R. Results of the Souter-Strathclyde total elbow arthroplasty in patients with rheumatoid arthritis. A preliminary report. J. Arthroplasty. 1994;9:279.
27 Malone A.A., Taylor A.J., Fyfe I.S. Successful outcome of the Souter-Strathclyde elbow arthroplasty. J. Shoulder Elbow Surg. 2004;13:548.
28 Mehta J.A., Bain G.I. Surgical approaches to the elbow. Hand Clin. 2004;20:375.
29 Mori T., Kudo H., Iwano K., Juji T. Kudo type-5 total elbow arthroplasty in mutilating rheumatoid arthritis: a 5- to 11-year follow-up. J. Bone Joint Surg. Br. 2006;88:920.
30 O’Driscoll S.W., An K.N., Korinek S., Morrey B.F. Kinematics of semi-constrained total elbow arthroplasty. J. Bone Joint Surg. Br. 1992;74:297.
31 Ovesen J., Olsen B.S., Johannsen H.V., Sojbjerg J.O. Capitellocondylar total elbow replacement in late-stage rheumatoid arthritis. J. Shoulder Elbow Surg. 2005;14:414.
32 Patterson S.D., Bain G.I., Mehta J.A. Surgical approaches to the elbow. Clin. Orthop. Relat. Res. 2000;370:19.
33 Potter D., Claydon P., Stanley D. Total elbow replacement using the Kudo prosthesis. Clinical and radiological review with five- to seven-year follow-up. J. Bone Joint Surg. Br. 2003;85:354.
34 Pritchard R.W. Anatomic surface elbow arthroplasty. A preliminary report. Clin. Orthop. Relat. Res. 1983;179:223.
35 Ramsey M., Neale P.G., Morrey B.F., O’Driscoll S.W., An K.N. Kinematics and functional characteristics of the Pritchard ERS unlinked total elbow arthroplasty. J. Shoulder Elbow Surg. 2003;12:385.
36 Rauhaniemi J., Tiusanen H., Kyro A. Kudo total elbow arthroplasty in rheumatoid arthritis. Clinical and radiological results. J. Hand Surg. Br. 2006;31:162.
37 Reinhard R., van der Hoeven M., de Vos M.J., Eygendaal D. Total elbow arthroplasty with the Kudo prosthesis. Int. Orthop. 2003;27:370.
38 Ring D., Kocher M., Koris M., Thornhill T.S. Revision of unstable capitellocondylar (unlinked) total elbow replacement. J. Bone Joint Surg. Am. 2005;87:1075.
39 Rozing P. Souter-Strathclyde total elbow arthroplasty. J. Bone Joint Surg Br. 2000;82:1129.
40 Ruth J.T., Wilde A.H. Capitellocondylar total elbow replacement. A long-term follow-up study. J. Bone Joint Surg. Am. 1992;74:95.
41 Samijo S.K., Van den Berg M.E., Verburg A.D., Tonino A.J. Souter-Strathclyde total elbow arthroplasty: medium-term results. Acta Orthop. Belg. 2003;69:501.
42 Schemitsch E.H., Ewald F.C., Thornhill T.S. Results of total elbow arthroplasty after excision of the radial head and synovectomy in patients who had rheumatoid arthritis. J. Bone Joint Surg. Am. 1996;78:1541.
43 Schneeberger A.G., King G.J., Song S.W., O’Driscoll S.W., Morrey B.F., An K.N. Kinematics and laxity of the Souter-Strathclyde total elbow prosthesis. J. Shoulder Elbow Surg. 2000;9:127.
44 Shah B.M., Trail I.A., Nuttall D., Stanley J.K. The effect of epidemiologic and intraoperative factors on survival of the standard Souter-Strathclyde total elbow arthroplasty. J. Arthroplasty. 2000;15:994.
45 Shiba R., Sorbie C., Siu D.W., Bryant J.T., Cooke T.D., Wevers H.W. Geometry of the humeroulnar joint. J. Orthop. Res. 1988;6:897.
46 Sorbie C., Shiba R., Siu D., Saunders G., Wevers H. The development of a surface arthroplasty for the elbow. Clin. Orthop. Rel. Res. 1986;208:100.
47 Souter, W. A.: Anatomical Trochlear Stirrup Arthroplasty of the Rheumatoid Elbow. Elbow Joint Proceedings of the International Seminar, Kobe, Japan Editor: Kashiwagi, D., Excerpta Medica, Elsevier Science Publishers 1985; 305.
48 Souter W.A.. Total replacement arthroplasty of the elbow. Joint replacement in the upper limb, 5. Institution of Mechanical Engineers, London, 1977;96.
49 Talwalkar S.C., Givissis P.K., Trail I.A., Nuttall D., Stanley J.K. Survivorship of the Souter-Strathclyde elbow replacement in the young inflammatory arthritis elbow. J. Bone Joint Surg. Br. 2005;87:946.
50 Tanaka N., Kudo H., Iwano K., Sakahashi H., Sato E., Ishii S. Kudo total elbow arthroplasty in patients with rheumatoid arthritis: a long-term follow-up study. J. Bone Joint Surg. Am. 2001;83-A:1506.
51 Tanaka N., Sakahashi H., Ishii S., Kudo H. Comparison of two types of ulnar component in type-5 Kudo total elbow arthroplasty in patients with rheumatoid arthritis: a long-term follow-up. J. Bone Joint Surg. Br. 2006;88:341.
52 Thillemann T.M., Olsen B.S., Johannsen H.V., Sojbjerg J.O. Long-term results with the Kudo type 3 total elbow arthroplasty. J. Shoulder Elbow Surg. 2006;15:495.
53 Trail I.A., Nuttall D., Stanley J.K. Survivorship and radiological analysis of the standard Souter-Strathclyde total elbow arthroplasty. J. Bone Joint Surg. Br. 1999;81:80.
54 Trail L.A., Nuttall D., Stanley J.K. Comparison of survivorship between standard and long-stem Souter-Strathclyde total elbow arthroplasty. J. Shoulder Elbow Surg. 2002;11:373.
55 Trancik T., Wilde A.H., Borden L.S. Capitellocondylar total elbow arthroplasty. Two- to eight-year experience. Clin. Orthop. Relat. Res. 1987;223:175.
56 Trepman E., Vella I.M., Ewald F.C. Radial head replacement in capitellocondylar total elbow arthroplasty. 2- to 6-year follow-up evaluation in rheumatoid arthritis. J. Arthroplasty. 1991;6:67.
57 Valstar E.R., Garling E.H., Rozing P.M. Micromotion of the Souter-Strathclyde total elbow prosthesis in patients with rheumatoid arthritis 21 elbows followed for 2 years. Acta Orthop. Scand. 2002;73:264.
58 van der Lugt J.C., Geskus R.B., Rozing P.M. Limited influence of prosthetic position on aseptic loosening of elbow replacements: 125 elbows followed for an average period of 5.6 years. Acta Orthop.. 2005;76:654.
59 van der Lugt J.C., Geskus R.B., Rozing P.M. Primary Souter-Strathclyde total elbow prosthesis in rheumatoid arthritis. J. Bone Joint Surg. Am. 2004;86-A:465.
60 Verstreken F., De Smet L., Westhovens R., Fabry G. Results of the Kudo elbow prosthesis in patients with rheumatoid arthritis: a preliminary report. Clin. Rheumatol. 1998;17:325.
61 Weiland A.J., Weiss A.P., Wills R.P., Moore J.R. Capitellocondylar total elbow replacement. A long-term follow-up study. J. Bone Joint Surg. Am. 1989;71:217.
62 Willems K., De Smet L. The Kudo total elbow arthroplasty in patients with rheumatoid arthritis. J. Shoulder Elbow Surg. 2004;13:542.
63 Wright T.W., Wong A.M., Jaffe R. Functional outcome comparison of semiconstrained and unconstrained total elbow arthroplasties. J. Shoulder Elbow Surg. 2000;9:524.
PART D Convertible Total Elbow Arthroplasty
DESIGN CONSIDERATIONS
As has been outlined earlier in this chapter, unlinked total elbow arthroplasties have a theoretical advantage over linked devices owing to a sharing of load across the articulation between the prosthesis and the joint capsule, ligaments, and muscles. Although the majority of patients with elbow arthritis can be managed with an unlinked elbow arthroplasty, there are patients who require a linked prosthesis due to a lack of adequate bone stock or functional collateral ligaments. Although typically this can be predicted preoperatively, it is always desirable to have a linkable implant immediately available in the operating room whenever a surgeon is planning to perform an unlinked total elbow arthroplasty. If an unlinked implant is not stable or is not articulating congruously intraoperatively, then it should be converted to a linked design. If this is recognized before the unlinked components are cemented, then a different design of linked arthroplasty can be used, although repeat bony preparation will be needed, which is time consuming. The advantage of a convertible prosthesis is significant in this setting because rather than changing prosthesis designs, a linkage mechanism can simply be applied to the same prosthesis for which the bony resection has been completed. If the stems have already been cemented, then the ability to couple an unlinked prosthesis without removing the stems makes a convertible implant system even more appealing. The advantage of having one implant system to manage the majority of clinical situations is also helpful for hospitals by reducing inventories and cost. For surgeons, a convertible design means that only one implant system needs to be mastered.
Although most patients who undergo an unlinked total elbow arthroplasty have an excellent outcome, there are some patients who develop elbow instability postoperatively, which is often difficult to manage.3,8,10 When this occurs early, a closed reduction and a period of immobilization may allow the ligaments to heal and the prosthesis to function normally. However, when presenting as a chronic problem, attempts at ligament reconstruction have not been reliable. The concept of having an unlinked elbow arthroplasty that can be linked in the setting of postoperative elbow instability is desirable because removal of a well-fixed unlinked elbow arthroplasty to revise it to a linked arthroplasty is technically difficult with a significant risk of complications.10
SURGICAL CONSIDERATIONS OF CONVERTIBLE DESIGNS
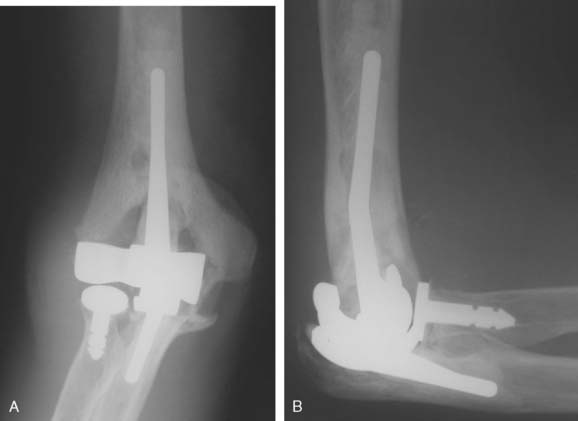
Two convertible total elbow arthroplasties have been developed and used to date. The Acclaim (Depuy, Warsaw, IN) is a cobalt-chrome implant with humeral and ulnar components. (Fig. 52-46) The humeral component has fins to resist rotation. The articulation is centralized to the long axis of the humeral component, which results in a lateral shift of the ulna with respect to the humerus, thereby tightening the medial collateral ligament. This lateral shift is thought to better balance the forces across the implant because it does not incorporate a radial head replacement. The unlinked articulation can be converted to a linked version by partial removal of the epicondyles, exchange of the bobbin to a polyethylene yoke, replacement of the polyethylene ulnar insert with a hinged component, and insertion of a polyethylene pin mechanism.
The Latitude System (Tornier Inc., Stafford, TX) is a modular device with the option to be configured as a humeral hemiarthroplasty and an unlinked or linked total elbow arthroplasty (Fig. 52-47). The cobalt-chrome implant has a modular humeral component, which incorporates fins and an anterior flange to resist axial rotation and posterior displacement. The axis bolt of the humeral component is cannulated to facilitate secure stable collateral ligament repair to the implant and adjacent bone. The ulnar component is metal backed with thick polyethylene and an extended coronoid process to resist dislocation. A bipolar radial head is available for use when the radial head is arthritic and requires excision. The conversion between an unlinked to a linked device can be accomplished easily by adding a locking cap during the initial surgery or subsequently through a minimally invasive approach to manage postoperative instability.
SURGICAL TECHNIQUE
The surgical technique for the Latitude total elbow arthroplasty is outlined here.4 The patient is placed supine, with the arm across the chest. A sterile tourniquet is employed and prophylactic antibiotics administered before tourniquet inflation. A midline posterior elbow incision is placed just medial to the tip of the olecranon, and full-thickness flaps are developed on the deep fascia. The ulnar nerve is identified, mobilized, and transposed anteriorly.
Management of the triceps tendon varies according to the preference of the surgeon. The implant can be placed by elevating the triceps from medial to lateral, as in the Bryan-Morrey approach, from lateral to medial, as in the extended Kochler approach, or through a triceps splitting approach, where the triceps is elevated from the olecranon both medially and laterally.1,6,9,12 The design of the instrumentation also allows for the bony preparation to be performed and the implants to be inserted while preserving the attachment of the triceps to the olecranon. The ‘triceps-on’ approach reduces the risk of triceps insufficiency with postoperative weakness and pain that can occur when the triceps tendon is detached to perform an elbow arthroplasty.2,9 Unfortunately, the triceps-on approach does compromise visualization to some extent, but it can be a useful technique, particularly when there is epicondylar bone loss or when the joint is not too tight. The medial and lateral collateral ligaments and their corresponding common flexor and extensor origins are tagged for later repair and then sharply released from the epicondyles as much as necessary to achieve joint dislocation.
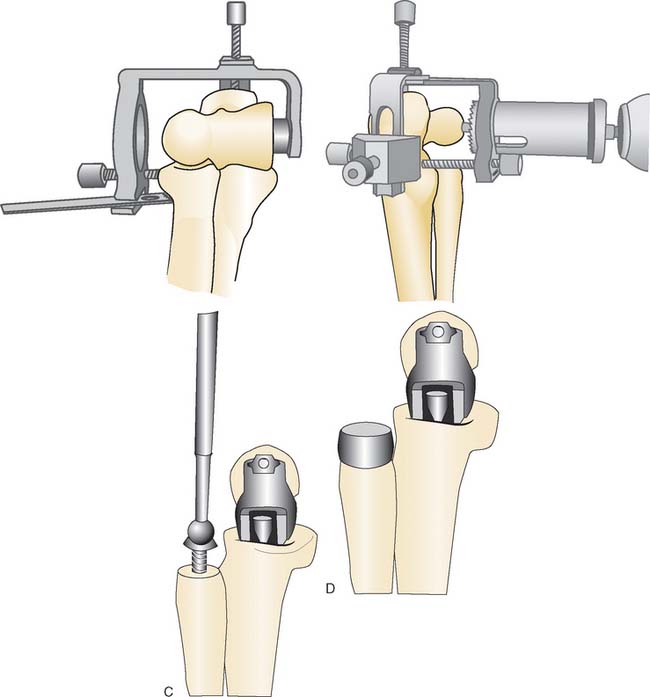
The correct sizing of the implant is best achieved by matching the anatomical spool size to the articular surfaces of the ulna and radius and the distal humerus. The key to sizing the Latitude system is choosing the correct joint width such that with the anatomic spool sitting in the trochlear groove, the radial head articulates congruously with the capitellum of the spool (Fig. 52-48). This approach will ensure that the native radial head (or a radial head component) will articulate congruously with the capitellum of the humeral component. There are four prosthesis sizes available for the total elbow arthroplasty and six sizes available for the anatomic distal humeral hemiarthroplasty.
As for any total elbow arthroplasty, reproduction of the flexion-extension axis is crucial for a successful result.5,11 With the Latitude system, all subsequent steps are based on the accurate determination of this axis. Using a circular guide, the lateral point of isometry is determined at the center of the arc of the capitellum when viewed from the lateral side. The anteroinferior aspect of the medial epicondyle is the medial landmark. The intercondylar portion of the distal humerus is removed, with a saw up to the proximal aspect of the olecranon fossa to facilitate central axis pin placement. An axis guide is used to insert the pin, which marks the flexion-extension axis of the elbow (Fig. 52-49A).
The medullary canal is opened with a burr, and the offset of the axis of the medullary canal with the flexion-extension axis is determined. Anterior, centered, and posterior offset humeral configurations are available. The distal humeral cuts are made using a mechanized series of jigs based off the flexion-extension and medullary axes (see Fig. 52-49B and C). The humeral canal is rasped and trial placement of the humeral component is performed (see Fig. 52-49D).
Preparation of the radius and ulna is accomplished using a cutting guide and the anatomic spool, which is seated into the articular dish of the radial head and the guiding ridge of the olecranon. If the radial head is to be excised, this is done with a sagittal saw and the trochlear cut is made with a bell-shaped saw from lateral to medial (Fig. 52-50A and B). If the native radial head is to be preserved, then the bell-shaped saw is used to cut the ulna from medial to lateral. The ulnar canal is then opened with a burr and prepared using flexible reamers and rasps. After inserting the trial ulnar component, the radius is broached and an appropriate sized radial head trial is placed. The bipolar radial head accommodates for ±10 degrees of angular rotation but cannot substitute for malalignment of the proximal radius to the capitellum (see Fig. 52-50C and D).
The collateral ligaments are repaired back to the epicondyles using nonabsorbable Krackow sutures passed through the cannulated screw, which is located at the axis of rotation of the implant (Fig. 52-51A). When tied on the contralateral side of the elbow, these sutures securely approximate the ligaments to their attachment sites while avoiding problems with sutures pulling through the often weak bone of the epicondyles. An additional suture can be placed through the cannulated screw of the implant and through a drill hole in the proximal ulna to act as a temporary artificial ligament to prevent instability in the postoperative period while the collateral ligaments are healing (see Fig. 52-51B). The triceps is carefully secured to the olecranon if it was detached. Drains are placed if indicated and the elbow is placed in a well-padded splint in relative extension.
COMPLICATIONS
The complications of a convertible total elbow arthroplasty are similar to any arthroplasty and are discussed in Chapter 53. These include infection, ulnar neuropathy, fractures, triceps avulsion, and problems with soft tissue healing. The complications specific to the unlinked configuration would be similar to those for any unlinked device, most importantly being instability, whereas for a linked device, it would presumably be polyethylene wear of the linkage mechanism.
RESULTS
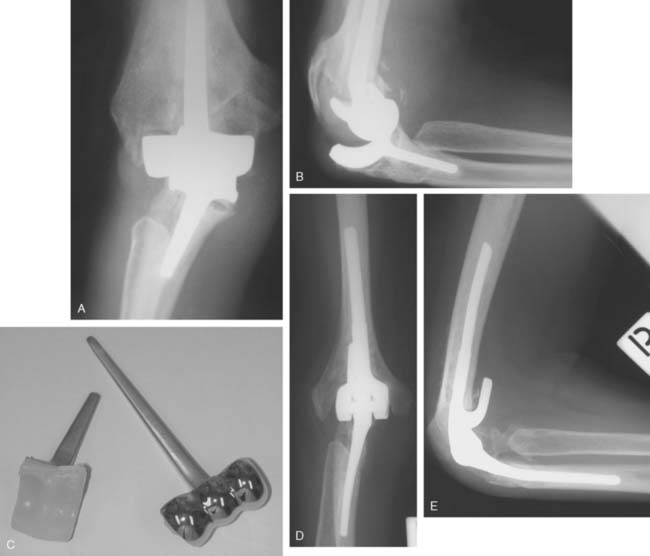
The initial experience with the Acclaim total elbow has been favorable, with a significant improvement in pain and motion7 (Fig. 52-52). The preliminary experience with 65 prostheses reported four intraoperative fractures and one dislocation. There are no studies available as of yet documenting the survivorship of this prosthesis.
There are no reports documenting the results of the Latitude convertible total elbow arthroplasties. The author’s experience with the Latitude system to date has been favorable because the implant has been able to deal with a wide variety of pathologies and patient profiles. The hemiarthroplasty has been used to manage acute distal humeral fractures, nonunions, and avascular necrosis. Patients with relatively well-preserved bone stock and ligaments are typically managed with the unlinked version of the arthroplasty and this is the author’s current preference (Fig. 52-53). Patients with poor bone stock or ligaments, particularly related to previous trauma, are typically managed with a linked arthroplasty (Fig. 52-54). The radial head is replaced if it is damaged by disease, such as in rheumatoid arthritis, but is retained if it is relatively well preserved, for example in post-traumatic arthritis or acute distal humeral fractures.
1 Bryan R.S., Morrey B.F. Extensive posterior exposure of the elbow. A triceps-sparing approach. Clin. Orthop. 1982;166:188.
2 Celli A., Arash A., Adams R.A., Morrey B.F. Triceps insufficiency following total elbow arthroplasty. J. Bone Joint Surg. Am. 2005;87:1957.
3 Chiodo C.P., Terry C.L., Koris M.J. Reconstruction of the medial collateral ligament with flexor carpi radialis tendon graft for instability after capitellocondylar total elbow arthroplasty. J. Shoulder Elbow Surg. 1999;8:284.
4 Gramstad G.D., King G.J., O’Driscoll S.W., Yamaguchi K. Elbow arthroplasty using a convertible implant. Tech. Hand Up. Extrem. Surg. 2005;9:153.
5 Itoi E., Niebur G.L., Morrey B.F., An K.N. Malrotation of the humeral component of the capitellocondylar total elbow replacement is not the sole cause of dislocation. J. Orthop. Res. 1994;12:665.
6 Joshi R.P., Yanni O., Gallannaugh S.C. A modified posterior approach to the elbow for total elbow replacement. J. Shoulder Elbow Surg. 1999;8:606.
7 Lerch K., Tingart M., Trail I., Grifka J. [Total elbow arthroplasty. Indications, operative technique and results after implantation of an Acclaim elbow prosthesis]. Orthopade. 2003;32:730.
8 O’Driscoll S.W., King G.J. Treatment of instability after total elbow arthroplasty. Orthop. Clin. North Am. 2001;32:679. ix
9 Pierce T.D., Herndon J.H. The triceps preserving approach to total elbow arthroplasty. Clin. Orthop. Rel. Res. 1998;354:144.
10 Ring D., Kocher M., Koris M., Thornhill T.S. Revision of unstable capitellocondylar (unlinked) total elbow replacement. J. Bone Joint Surg. Am. 2005;87:1075.
11 Shah B.M., Trail I.A., Nuttall D., Stanley J.K. The effect of epidemiologic and intraoperative factors on survival of the standard Souter-Strathclyde total elbow arthroplasty. J. Arthroplasty. 2000;15:994.
12 Wolfe S.W., Ranawat C.S. The osteo-anconeus flap. An approach for total elbow arthroplasty. J. Bone Joint Surg. Am. 1990;72:684.