Chapter 26 Univentricular Heart
In 1858, Thomas Peacock described hearts in which “The auricular sinuses are separated by a more or less complete septum, and there are generally two auriculo-ventricular apertures, while the ventricle is either wholly undivided or presents only a very rudimentary septum. The arteries which are given off are usually two in number—an aorta and a pulmonary artery.”1
These cases correspond to hearts that consist of two auricles and only one ventricle. A case described by Chemineau in 1699 appears to have been of this description.2
The univentricular heart is unique in its complexity and scope and has sparked intense debate about terminology and embryology.2 Nearly a century and a half after Peacock’s1 description, there is still no consensus about the terminology for hearts with one ventricle.3–6 Single ventricle and univentricular are synonymous (single = uni = one), so these terms are interchangeable and are appropriate when two atria are related entirely or almost entirely to one ventricular compartment that qualifies on purely morphologic grounds as a left, right, or indeterminate ventricle.
Univentricular atrioventricular connection or double inlet ventricle is characterized according to gross morphologic features of the ventricular mass and according to the atrioventricular connections to that mass.4,5,7 Clinically undetectable and clinically irrelevant developmental considerations are important to the morphologist4 but should not determine clinical terminology. It is best to avoid inherently contradictory terms such as ventricular septal defect8 and interventricular communication that imply the presence of two anatomically definitive ventricles divided by a septum. To say that a ventricular septal defect exists in a heart with a single ventricle and “not a trace of an inter-ventricular septum”1 strikes most readers as contradictory irrespective of theoretic arguments to the contrary. The term “functionally” univentricular as applied to the hypoplastic left heart should be regarded as a separate category.9 In this chapter, univentricular and single ventricle refer to hearts in which one ventricular chamber receives the entire flow from the right atrium and the left atrium, both of which together with the entire atrioventricular junction are related to the single ventricle.
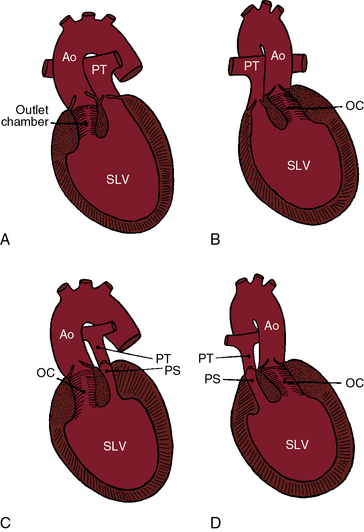
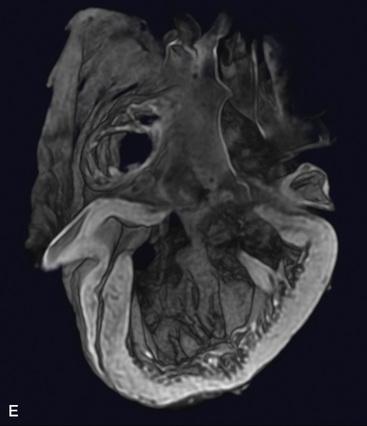
In 80% to 90% of cases, the ventricular chamber that receives the atrioventricular connections is a morphologic left ventricle that incorporates at its base an outlet chamber that is devoid of a sinus or inlet component, that is devoid or virtually devoid of trabeculae, and that is remote from the crux of the heart (Figures 26-1 through 26-4).7 In 10% to 25% of cases, the ventricular chamber that receives the atrioventricular connections has right ventricular morphologic features and incorporates within its mass a rudimentary compartment—a left ventricular remnant or trabecular pouch—that varies in size from well-formed to microscopic (see Figures 26-31 and 26-32).3,7 The trabecular pouch occupies a posterior, inferior, or lateral position within the ventricular mass and may or may not communicate with the cavity. In less than 10% of cases, the univentricular heart has indeterminate morphologic features and incorporates neither an outlet chamber nor a trabecular pouch. Because an indeterminate ventricle does not contain remnants of either a rudimentary morphologic right ventricle or a rudimentary morphologic left ventricle, the term univentricular heart or single ventricle is unassailable on morphologic grounds.
The atrioventricular connections that guard the inlet of a univentricular heart consist of either two separate valves, one patent valve with atresia of the other valve, or a common atrioventricular valve.10,11 It is customary to refer to right atrioventricular or left atrioventricular valves, rather than tricuspid and mitral valves, because tricuspid and mitral morphologic features are not necessarily evident.8 An atrioventricular (AV) valve is likely to be abnormal when it is concordant with the ventricular loop (right AV valve with noninverted outlet chamber, left AV valve with inverted outlet chamber).8,10,12 When the outlet chamber is inverted, the left AV valve tends to be stenotic, and when the outlet chamber is noninverted, the right atrioventricular valve tends to be incompetent.8 A common atrioventricular valve is usually equipped with four leaflets,11 and right atrial isomerism is the usual pattern.11 Straddling of a right or a left AV valve or a common AV valve refers to attachments of tensor apparatus to both sides of an outlet foramen or to both sides of a trabecular pouch.7,8,10,12,13
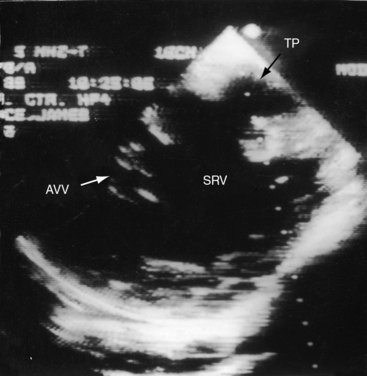
In univentricular hearts that are morphologically left ventricular, the outlet chamber is anterosuperior and either to the right or left of midline. Noninverted applies to a right anterosuperior position of the outlet chamber, and inverted applies to a left anterosuperior position (see Figures 26-1 through 26-4). The outlet chamber is either smooth-walled and devoid of trabeculations (see Figure 26-4) or contains scanty ill-defined trabeculations (see previous). The aorta arises discordantly from the outlet chamber, and the pulmonary trunk arises discordantly from the single morphologic left ventricle (see Figures 26-1 through 26-4),7 so the great arteries are transposed (see Chapter 27).
In 1824, Andrew Fernando Holmes, who would later become the first Dean of the Medical Faculty at McGill University, published autopsy observations on a 21-year-old man who died with chronic cyanosis and congestive failure. In the uncommon Holmes heart, the aorta arises concordantly from the morphologic left ventricle and the pulmonary trunk arises concordantly from the outlet chamber (see Figure 26-20). William Osler urged Maude Abbott to republish the case, which was included in her seminal atlas of 1936.14,15 Andrew Fernando Holmes was Canadian, but his 1824 publication on single ventricle was published in a Scottish journal16 because he trained at the University of Edinburgh. Rarely, the outlet chamber gives rise to both great arteries, to neither great artery, or to a common arterial trunk. In univentricular hearts characterized by a single morphologic right ventricle, both great arteries originate from the right ventricle, an arrangement that is a form of double outlet right ventricle (see Chapter 19). Occasionally, the pulmonary trunk originates concordantly from a single right ventricle, and the aorta originates concordantly from the trabecular pouch, which is a left ventricular remnant. A morphologically indeterminate single ventricle incorporates neither an outlet chamber nor a trabecular pouch (see previous), so both great arteries necessarily arise from the indeterminate single ventricle.
The orifice that joins a single left ventricle to an outlet chamber has been variously referred to as a bulboventricular foramen, a ventricular septal defect,8 and an interventricular communication. Bulboventricular foramen assumes that the embryologic foramen is the communication that exists in the univentricular heart, which is not necessarily the case. The terms ventricular septal defect and interventricular communication are discouraged as inherently contradictory (see previous). Outlet foramen is a simple descriptive term that is used herein to refer to the orifice between a single left ventricle and the outlet chamber. A restrictive outlet foramen is a form of subaortic stenosis that can be acquired8 or present at birth7 and tends to coexist with coarctation of the aorta.7
When the pulmonary trunk originates from a single morphologic left ventricle, the accompanying pulmonary stenosis is either subpulmonary or in a bicuspid pulmonary valve (see Figures 26-1, 26-2C, and 26-3).7 Pulmonary stenosis is a feature of the Holmes heart (see Figure 26-20)16 and usually results from obstruction of the outlet foramen of the concordant subpulmonary outlet chamber.14,15,17,18 The degree of stenosis ranges from mild to severe (see Figures 26-2 and 26-3) to atresia (Figure 26-5), a spectrum recognized by Peacock:
“The case of Fleischmann differed in some degree … as though the heart consisted of three cavities, the ventricle only gave rise to one vessel, the orifice of the pulmonary artery being impervious. The child had lived twenty one weeks.”1
Coronary artery origins in univentricular hearts of left ventricular morphology depend on the location of the outlet chamber (see Chapters 6 and 32). A major branch of each coronary artery usually outlines or delimits the surface boundaries of the outlet chamber.
The morphogenesis of univentricular hearts is believed to reside in an abnormality of the ventricular trabecular components of the developing heart.7,19 The left ventricular trabecular component is normally derived from the inlet portion of the embryonic heart tube, and the right ventricular trabecular component is derived from the outlet portion. As the ventricular mass develops, the atrioventricular junction is shared between the left ventricular trabecular component and the right ventricular trabecular component. When the atrioventricular junction retains its connection to the left ventricular trabecular component, the result is double inlet to a morphologic left ventricle. When the atrioventricular junction retains its connection to the right ventricular trabecular component, the result is double inlet to a morphologic right ventricle. When right and left ventricular trabecular components fail to develop, the result is double inlet to an indeterminate ventricle.
The physiologic derangements associated with univentricular hearts are related to six variables: (1) the inherent mechanics of a single ventricle20–23; (2) the mechanics of a morphologic right ventricle versus a morphologic left ventricle24; (3) the morphology and functional state of the atrioventricular valves that guard the inlet to a single ventricle; (4) the degree of mixing within the single ventricle; (5) the pulmonary vascular resistance; and (6) the presence and degree of pulmonary stenosis or subaortic stenosis.18,25
In hearts with two ventricles, each ventricle augments the function of the other ventricle.20,21 Ventricular-ventricular interaction is an integral part of cardiac mechanics and results from coupling of the two ventricles through the interventricular septum and through an anatomic continuum that joins the mural myocardium of the two ventricles.20,21 Ventricular interdependence does not occur unless a right ventricle contributes to left ventricular function and a left ventricle contributes to right ventricular function. Accordingly, ventricular interdependence does not exist in univentricular hearts. The result is abnormal systolic and diastolic function, irrespective of the morphology of the single ventricle.26,27 Because a single ventricle is the pump that serves both the systemic and the pulmonary circulations, the volume handled by a univentricular heart is increased and provokes an adaptive increase in ventricular mass.26–28 In univentricular hearts of right ventricular morphology, the indices that reflect an adaptive increase in ventricular mass are significantly reduced, including mass per se, wall thickness, ratio of wall thickness to transverse ventricular diameter, and ratio of ventricular mass to end-diastolic volume.28 Inadequate mass relative to chamber volume reflects poor adaptation of univentricular hearts of right ventricular morphology.24,28
The physiology of the circulation in univentricular hearts is materially influenced by atrioventricular valve structure and function. Incompetence, stenosis, or atresia of an atrioventricular valve affects flow into the single ventricle and modifies its loading conditions. Atrioventricular valve regurgitation adds to the volume overload of the single ventricle. Atresia of the right or left atrioventricular valve results in a single inlet that does not disturb the circulation, provided there is free access to the single ventricle via a nonrestrictive interatrial communication and across the contralateral atrioventricular valve. However, when the right atrioventricular valve is atretic and the interatrial communication is restrictive, the right atrium is obstructed.29 Similarly, the left atrium is obstructed when the left atrioventricular valve is atretic and the interatrial communication is restrictive.
Right atrial venous blood and left atrial arterialized blood remain remarkably separate within the single ventricular chamber.25 Separation of the streams is greater when pulmonary resistance is low and when the outlet chamber is inverted.25 Unoxygenated blood from the systemic venous atrium selectively finds its way into the pulmonary trunk, and oxygenated blood from the pulmonary venous atrium selectively finds its way into the aorta. Subaortic stenosis diverts even more blood into the pulmonary circulation, so cyanosis is mild and occasionally absent. However, the benefits of increased pulmonary blood flow are achieved at the price of volume overload of the single ventricle.
Pulmonary vascular disease and pulmonary stenosis curtail pulmonary blood flow and adversely affect streaming within the single ventricle. When pulmonary stenosis or pulmonary vascular disease are severe, cyanosis is conspicuous because a smaller volume of oxygenated blood reaches the left atrium and because there is greater mixing of unoxygenated and oxygenated blood within the single ventricle.18
History
The male:female ratio in univentricular hearts is between 2:1 and 4:1.30 Recurrence in siblings is rare.31 Neonates or infants come to attention because of congestive heart failure, cyanosis, or a murmur. The type of presentation and the survival patterns depend on the pulmonary vascular resistance, the presence and degree of pulmonary stenosis, the morphology of the single ventricle, and the presence and degree of subaortic stenosis. Fifty percent of patients with univentricular hearts of left ventricular morphology die within 14 years, with an annual attrition rate of 4.8%.32 Fifty percent of patients with univentricular hearts of right ventricular morphology are dead within 4 years.32
Infants with increased pulmonary blood flow present with congestive heart failure, mild cyanosis, and poor growth and development.18 When subaortic stenosis augments already excessive pulmonary blood flow, congestive failure is refractory. Pulmonary vascular resistance seldom achieves satisfactory regulation of pulmonary flow. The oldest reported survivor with a single morphologic left ventricle and pulmonary vascular disease was a 59-year-old man.33 An exceptional case similar, if not identical, to the 24-year-old man (referred to in Figure 26-18), was described by Peacock1 in a 24-year-old man.
Pulmonary stenosis is more effective than pulmonary vascular resistance in regulating pulmonary blood flow (see Figures 26-2 and 26-3). Peacock described a patient with single ventricle and pulmonary stenosis who suffered from morbus caeruleus but lived to age 11 years: “The heart was found to have two auricles and one ventricle, and from the latter cavity the aorta and pulmonary artery arose.”1 Survival into adolescence and early adulthood is not rare. Longevity occasionally extends into the fourth or fifth decade (see Figures 26-15 and 26-24).14,16,17,34–38 One patient reached 56 years of age, and another reached age 73 years of age and endured three pregnancies despite mild hypoxia.39 Moderate pulmonary stenosis is physiologically desirable, but severe pulmonary stenosis or atresia results in deep even profound cyanosis (see Figure 26-5). Squatting may attenuate dyspnea. Hypoxic spells are rare.
Subaortic stenosis caused by a restrictive outlet foramen has an adverse effect on longevity by augmenting pulmonary blood flow and augmenting volume overload of the single ventricle. Restriction of the outlet foramen can be progressive.8
The multisystem systemic disorders associated with cyanotic congenital heart disease and Eisenmenger’s syndrome are important features in the history of patients with univentricular hearts (see Chapter 17).34
Jugular venous pulse
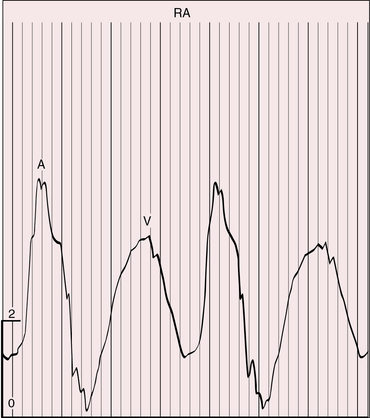
The height and waveform of the jugular pulse are normal when moderate pulmonary stenosis curtails excessive pulmonary blood flow (Figure 26-6). Incompetence of the right atrioventricular valve increases the V wave. Atresia of the right atrioventricular valve increases the A wave, provided the interatrial communication is restrictive. Atresia of the left atrioventricular valve results in a large jugular venous A wave, provided the atrial septal defect is nonrestrictive.
Precordial movement and palpation
Single ventricle of left ventricular morphology generates a precordial impulse analogous to a morphologic left ventricle in a biventricular heart. When pulmonary blood flow is increased, the impulse of the volume-overloaded single left ventricle is hyperdynamic. A visible and palpable systolic impulse in the third left intercostal space is a result of the leftward and anterior position of an inverted outlet chamber (see Figure 26-2A,B). The second heart sound is loud and palpable because the aorta is anterior whether the outlet chamber is inverted or noninverted (Figure 26-7B). A systolic thrill at the mid left sternal border is evidence of subaortic stenosis caused by a restrictive outlet foramen. Potential pulmonary stenotic thrills are attenuated by the posterior position of the pulmonary trunk. A single morphologic right ventricle imparts an impulse at the mid to lower left sternal border and subzyphoid area analogous to a morphologic right ventricular impulse in a biventricular heart. There is no impulse in the third left intercostal space because there is no underlying outlet chamber.
Auscultation
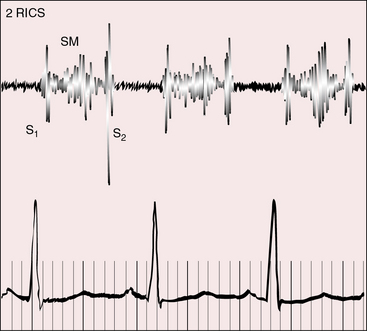
A pulmonary ejection sound that might originate in a mobile stenotic pulmonary valve (see Figure 26-2C) is attenuated because of the posterior position of the pulmonary trunk, and pulmonary ejection sounds do not occur with subpulmonary stenosis (see Figure 26-3). An aortic ejection sound is generated in the dilated anterior ascending aorta in patients with pulmonary atresia (see Figures 26-5 and 26-25).
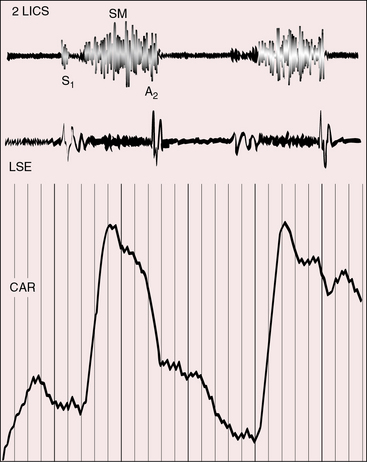
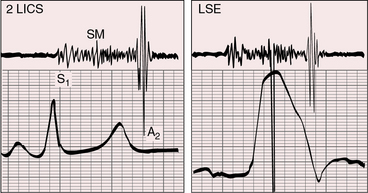
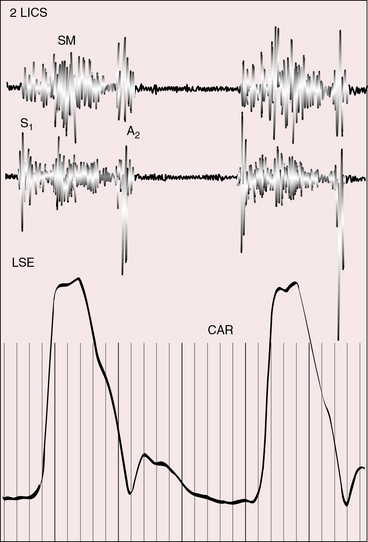
Pulmonary stenotic murmurs are prominent at the mid or lower left sternal border when the stenosis is subpulmonary, and vary inversely in length and loudness according to the degree of stenosis (Figures 26-8, 26-9, and 26-10). As stenosis increases, more blood is diverted into the aorta and less blood enters the pulmonary trunk, so the stenotic murmur softens and shortens. The murmur is damped still further because of the posterior position of the pulmonary trunk (see previous).
The murmur of subaortic stenosis caused by a restrctive outlet foramen is midsystolic and radiates from the mid left sternal border to the left or right base depending on whether the outlet chamber is inverted or noninverted (Figure 26-11). Ventricular failure decreases the gradient across the outlet foramen and decreases the subaortic murmur. Because coarctation of the aorta tends to coexist with subaortic stenosis, posterior auscultation should include the interscapular area over the spine in search of the systolic murmur of coarctation (see Chapter 8).
The second heart sound splits normally. A rise in pulmonary vascular resistance abolishes the split. The aortic component is loud because the aorta is anterior. Audibility of the pulmonary component improves when the posterior pulmonary trunk is hypertensive and dilated. In the presence of pulmonary stenosis, the loud single second heart sound is aortic. In the presence of subaortic stenosis, the second heart sound tends to be single because the aortic component is attenuated and a prominent pulmonary component originates in the hypertensive posterior pulmonary trunk (see Figure 26-11). In a single morphologic right ventricle, the aortic component of the second sound is loud because the aorta is anterior to the pulmonary trunk or side-by-side.
Electrocardiogram
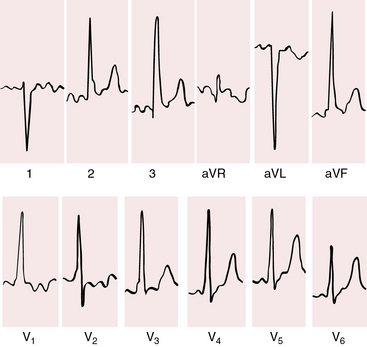
With a single morphologic left ventricle, a ventricular septal structure is lacking at the inlet portion of the ventricular mass. The QRS axis is directed inferior and to the right, away from the inverted outlet chamber and toward the main ventricular mass (Figures 26-12 and 26-13).40
The posterior AV node is hypoplastic and does not form a His bundle or establish a ventricular connection.41–43 Instead, a well-developed anterior accessory AV node gives rise to the His bundle and establishes atrioventricular connections.
When the outlet chamber is noninverted, a long nonbranching penetrating bundle runs down the right parietal wall of the single ventricle toward the outlet foramen before bifurcating into right and left bundle branches.41 When the outlet chamber is inverted, the penetrating bundle encircles the outflow tract of the single ventricle before branching at the outlet foramen. The left bundle branch is concordant with left ventricular morphology of the single left ventricle, and the right bundle branch is concordant with the outlet chamber.41 The QRS axis is directed inferior and to the right, away from the inverted outlet chamber and toward the main ventricular mass (see Figures 26-12 and 26-13).40
An inlet septum is also lacking in univentricular hearts with a morphologic right ventricle and a rudimentary posterior trabecular pouch. However, the ventricular segment between the morphologic right ventricle and the trabecular pouch extends to the crux where a regular posterior AV node and His bundle are formed.44 Distribution of the bundle branches apparently depends on the right/left orientation of the trabecular pouch.
Diversity in the electrocardiogram reflects the diversity of the anatomic variations of univentricular hearts.45–47 Electrocardiographic interpretations become clearer when related to specific morphologic types of single ventricles and their physiologic derangements.
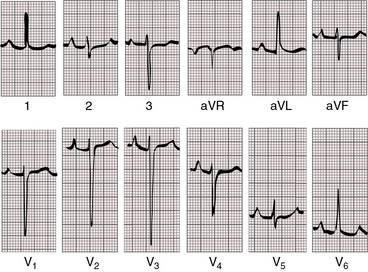
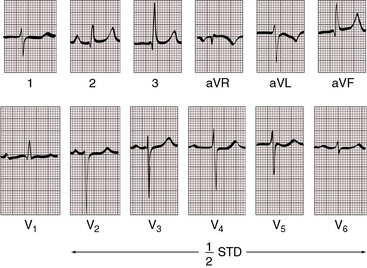
When pulmonary blood flow is increased (Figure 26-14), P waves show left atrial or biatrial abnormalities. When pulmonary blood flow is reduced (Figure 26-15), P waves show right atrial abnormalities. The PR interval tends to be normal with normal atrioventricular conduction despite an elongated nonbranching penetrating bundle (Figure 26-16).
When the outlet chamber is noninverted and the single ventricle is a morphologic left ventricle, the QRS axis tends to be directed leftward and superior—left axis deviation axis deviation (see Figures 26-14 and 26-15).40 Initial depolarization is anterior and leftward, so small Q waves occasionally appear in left precordial leads (see Figure 26-14).40 Left ventricular hypertrophy is noteworthy and is especially striking when pulmonary blood flow is increased and the single ventricle is volume overloaded (see Figure 26-14). Precordial QRS complexes then exhibit voltages of remarkably great amplitude (see Figures 26-14 and 26-16) and patterns that are stereotyped (see Figures 26-14 and 26-16).40,45
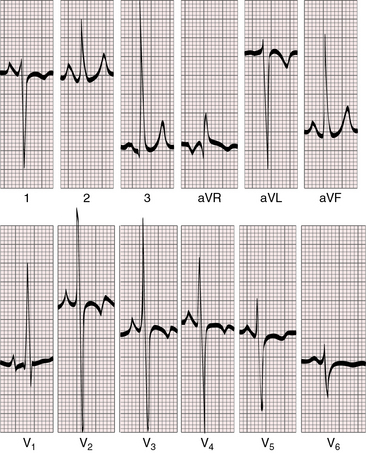
When the outlet chamber is inverted, the QRS axis is inferior and to the right, directed away from the inverted outlet chamber toward the main ventricular mass (see Figures 26-12 and 26-13).40
Conduction is triventricular and often abnormal. PR interval prolongation occasionally culminates in complete heart block.41 The P wave axis shifts to the left, so tall peaked right atrial P waves appear in mid and left precordial leads (see Figure 26-13). This pattern also occurs with noninversion of the outlet chamber (see Figure 26-15).
Ventricular depolarization is clockwise, so Q waves appear in leads 2, 3, and aVF. Because initial forces of ventricular depolarization are posterior and leftward, Q waves may be present in right precordial leads but not in left precordial leads (see Figures 26-12 and 26-13).40,45 Even though the univentricular heart is morphologically a left ventricle, precordial leads may show a dominant R wave in lead V1 and large equidisphasic RS complexes in midprecordial leads (see Figure 26-13).
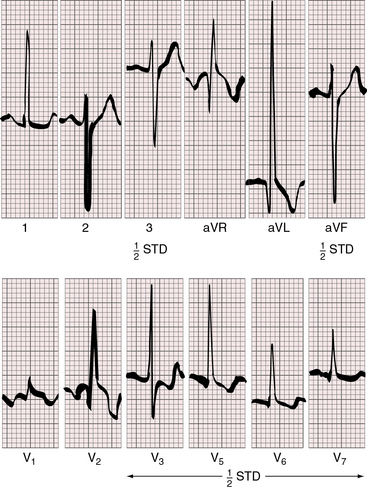
In univentricular hearts with a morphologic right ventricle and a trabecular pouch, atrioventricular conduction is normal because a regular posterior AV node and His bundle are formed at the crux.44 Right axis deviation and tall stereotyped precordial R waves are also features of single morphologic right ventricle (Figure 26-17). The QRS axis is usually rightward (see Figure 26-17) but occasionally is leftward and superior.
X-ray
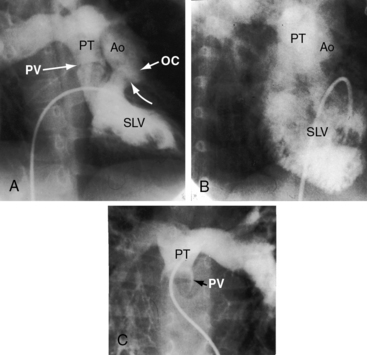
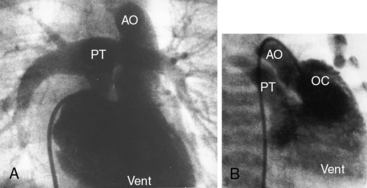
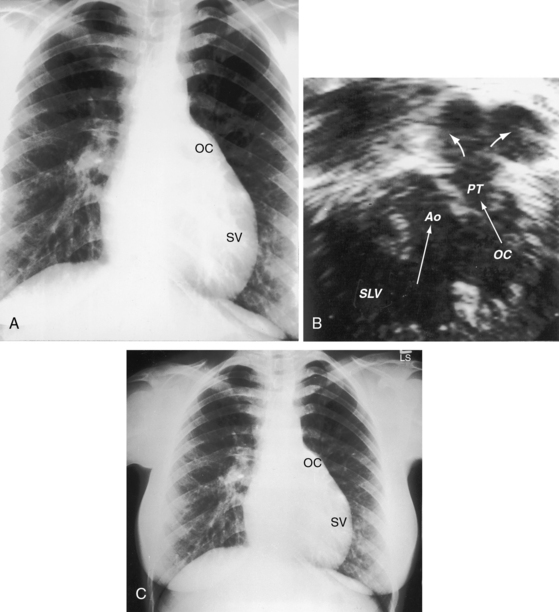
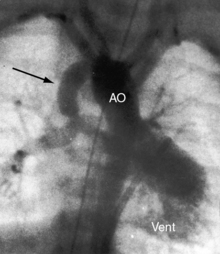
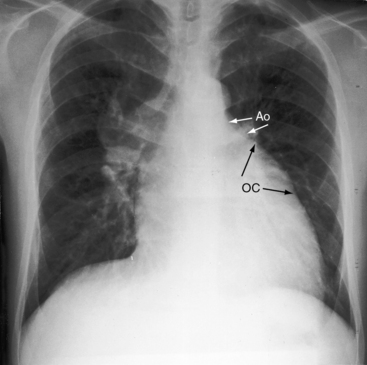
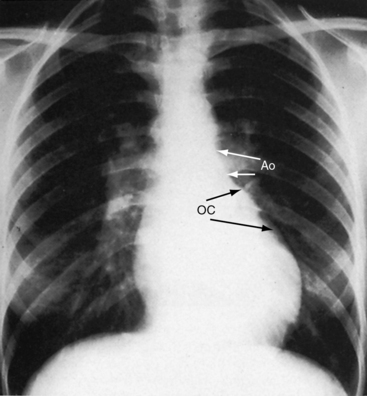
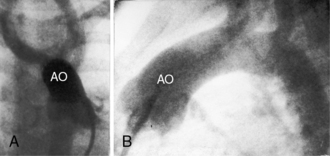
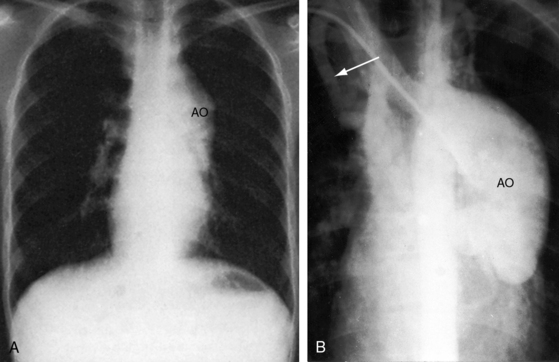
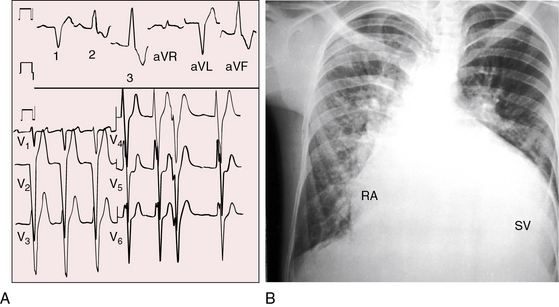
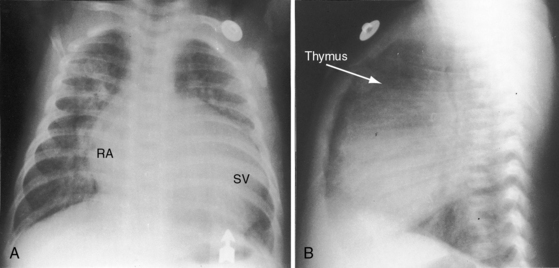
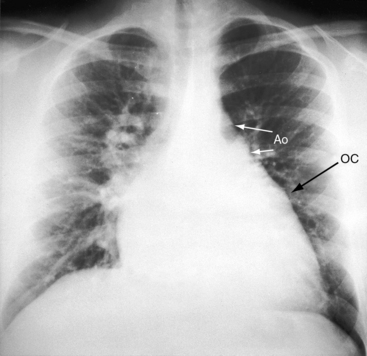
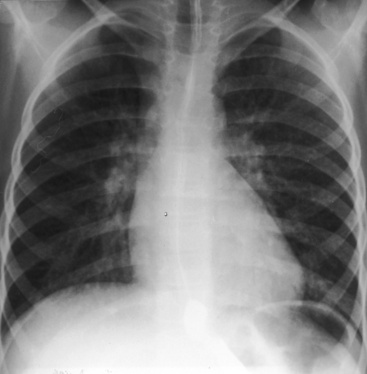
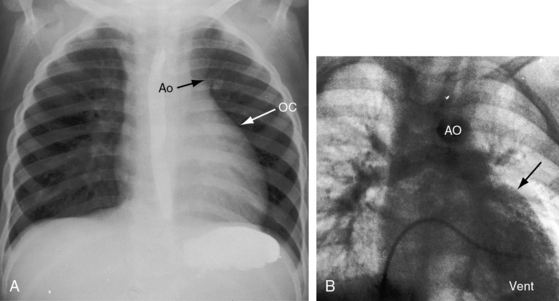
The location of the outlet chamber is a key diagnostic feature of the x-ray. An inverted outlet chamber forms a localized convexity at the upper left cardiac border and gives rise to an aorta that is convex to the left or rises vertically (Figures 26-2A, 26-3, 26-18, and 26-19),48,49 as in congenitally corrected transposition of the great arteries (see Chapter 6).49 In the Holmes heart, the inverted outlet chamber is distinctively convex and, by definition, gives rise to a concordant pulmonary trunk (Figure 26-20 and Video 26-1).18 A noninverted outlet chamber gives rise to an aorta that is convex to the right but is not border-forming (Figure 26-1A), as is the case in complete transposition of the great arteries (see Chapter 27).49 With the exception of the Holmes heart (see Figure 26-20),16,18,40,49 the great arteries are transposed, with the aorta originating discordantly from the outlet chamber and the pulmonary trunk originating discordantly from the morphologic left ventricle (see Figure 26-1). A transposed posteromedial pulmonary trunk may lift its dilated right branch and create a waterfall appearance (see Figure 26-18).48,49 Absence of a thymic shadow is an important radiologic feature of complete transposition of the great arteries in biventricular hearts (see Chapter 27) but is not a feature of complete transposition with univentricular hearts (Figure 26-21B).
The size of the cardiac silhouette increases in response to excessive pulmonary blood flow and volume overload of the single ventricle (see Figures 26-16B, and 26-31A).10,30,48 Left atrial enlargement is best seen in lateral films or with a barium esophagram48 because what appears to be left a atrial appendage in the posteroanterior projection is likely to represent an inverted outlet chamber (Figures 26-18 and 26-19). Right atrial dilation accompanies congestive heart failure, which is reinforced by subaortic stenosis (Figures 26-16B, and 26-21A).
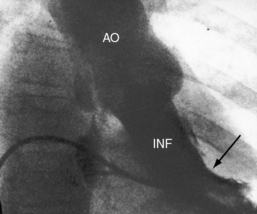
The size of the heart is norma,l or nearly so, in single morphologic left ventricle with severe pulmonary stenosis, but an inverted outlet chamber reveals itself as a bulge at the left upper cardiac border (Figures 26-22, 26-23, and 26-24). Also distinctive is a dilated aorta that arises from an inverted outlet chamber and presents as a convexity to the left (see Figure 26-20) or that ascends vertically and is not border-forming on either side (see Figure 26-24). Pulmonary atresia with an inverted outlet chamber has a box-like cardiac silhouette, with the dilated ascending aorta forming the left upper border that merges with a small underfilled ventricle below and the vertebral column forming the straight right border (Figure 26-25).
In univentricular hearts of right ventricular morphology, both great arteries necessarily arise from the single right ventricle, so the malformation is a form of double outlet right ventricle (see Chapter 19). The vascular pedicle is narrow because the aorta is anterior and the pulmonary trunk is posterior or side-by-side. Pulmonary stenosis is common, so pulmonary vascularity is normal or reduced and the heart size is not significantly increased.
Echocardiogram
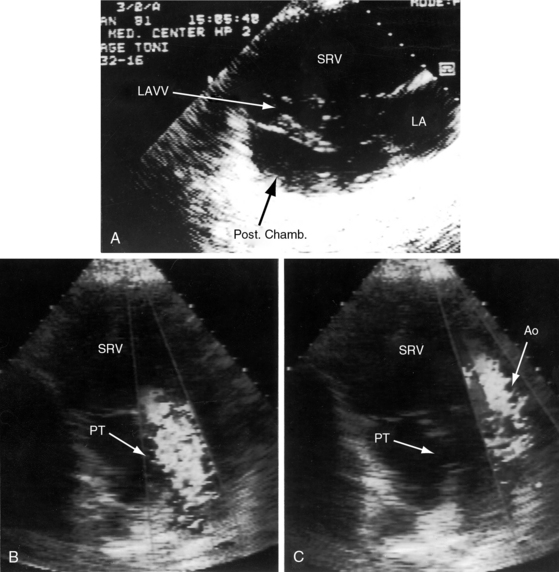
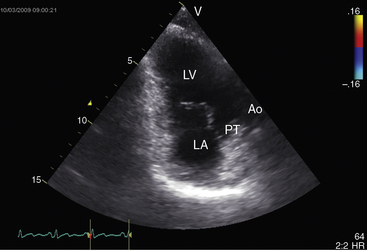
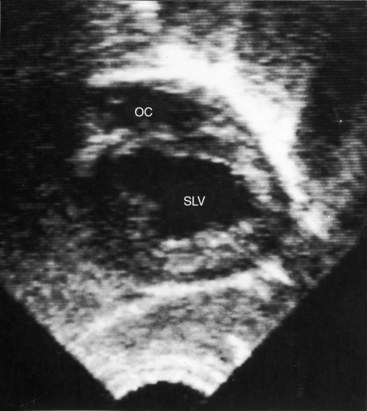
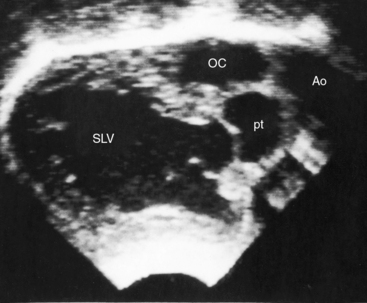
Echocardiography with color flow imaging and Doppler interrogation identifies a single morphologic left ventricle with an outlet chamber at its base.8,10,50 The internal architecture of the single ventricle can be characterized (Figures 26-26 and 26-27) with two separate patent atrioventricular valves that are usually present (Figure 26-28 and Videos 26-2A and 26-2B). Right and left AV valve morphology is concordant with inversion or noninversion of the outlet chamber.8 An atrioventricular valve can be incompetent, stenotic, or imperforate, and part of the tensor apparatus can straddle the outlet foramen.8,13 Color flow imaging establishes the presence and degree of incompetence of the right or left AV valve. A common atrioventricular valve is a feature of atrial isomerism with a univentricular heart (see Chapter 3).
Echocardiography identifies an inverted or noninverted outlet chamber (Figures 26-26 through 26-30) from which the aorta arises discordantly and identifies a single left ventricle from which the pulmonary trunk arises discordantly (see Figures 26-27, 26-29 [and Videos 26-3A and 26-3B], and 26-30). In the Holmes heart, the outlet chamber is concordant with the pulmonary trunk and the aorta is concordant with the single morphologic left ventricle (Figure 26-20B). Color flow imaging with continuous wave Doppler scan establishes the presence and degree of pulmonary stenosis or subaortic stenosis caused by a restrictive outlet foramen. When subaortic stenosis exists from infancy, the aortic arch should be interrogated with two-dimensional imaging and color flow and continuous wave Doppler scan because of the increased incidence of coarctation of the aorta and arch hypoplasia.
A univentricular heart of right ventricular morphology is recognized by the morphologic characteristics of the ventricular endocardium and the posterior trabecular pouch (Figures 26-31 and 26-32A), which may be straddled by the tensor apparatus of the atrioventricular valve. Two-dimensional echocardiography with color flow imaging shows two side-by-side great arteries arising from the single right ventricle (Figures 26-32B,C). Continuous-wave Doppler scan identifies the presence and degree of pulmonary stenosis.
1 Peacock T.B. On malformations of the human heart. London: John Churchill; 1858.
2 Khairy P., Poirier N., Mercier L.-A. Univentricular heart. Circulation. 2007;115:800-812.
3 Thies W.R., Soto B., Diethelm E., Bargeron L.M.Jr, Pacifico A.D. Angiographic anatomy of hearts with one ventricular chamber: the true single ventricle. Am J Cardiol. 1985;55:1363-1366.
4 Cook A.C., Anderson R.H. The anatomy of hearts with double inlet ventricle. Cardiol Young. 2006;16(suppl 1):22-26.
5 Jacobs M.L., Anderson R.H. Nomenclature of the functionally univentricular heart. Cardiol Young. 2006;16(suppl 1):3-8.
6 Cook A.C., Anderson R.H. The functionally univentricular circulation: anatomic substrates as related to function. Cardiol Young. 2005;15(suppl 3):7-16.
7 VanPraagh R., Ongley P.A., Swan H.J. Anatomic types of single or common ventricle in man. Morphologic and geometric aspects of 60 necropsied cases. Am J Cardiol. 1964;13:367-386.
8 Bevilacqua M., Sanders S.P., Van P.S., Colan S.D., Parness I. Double-inlet single left ventricle: echocardiographic anatomy with emphasis on the morphology of the atrioventricular valves and ventricular septal defect. J Am Coll Cardiol. 1991;18:559-568.
9 Jacobs J.P., Anderson R.H., Weinberg P.M., et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young. 2007;17(suppl 2):1-28.
10 Freedom R.M., Picchio F., Duncan W.J., Harder J.R., Moes C.A., Rowe R.D. The atrioventricular junction in the univentricular heart: a two-dimensional echocardiographic analysis. Pediatr Cardiol. 1982;3:105-117.
11 Stein J.I., Smallhorn J.F., Coles J.G., Williams W.G., Trusler G.A., Freedom R.M. Common atrioventricular valve guarding double inlet atrioventricular connexion: natural history and surgical results in 76 cases. Int J Cardiol. 1990;28:7-17.
12 Tandon R., Becker A.E., Moller J.H., Edwards J.E. Double inlet left ventricle. Straddling tricuspid valve. Br Heart J. 1974;36:747-759.
13 Rice M.J., Seward J.B., Edwards W.D., et al. Straddling atrioventricular valve: two-dimensional echocardiographic diagnosis, classification and surgical implications. Am J Cardiol. 1985;55:505-513.
14 Abbott M.E. Atlas of congenital cardiac disease. New York: The American Heart Association; 1936.
15 Jutras L.C. Magnetic resonance of hearts in a jar: breathing new life into old pathological specimens. Cardiol Young. 2010;20:275-283.
16 Holmes A.E. Case of malformation of the heart. Transaction of the Medico-Chirurgical Society of Edinburgh. 1824;1:252.
17 Klaus A.P., Smith R.M., Schneider A.B., Parker B.M. Single ventricle with normal relationship of the great vessels and pulmonic stenosis. A case report of an adult with the “Holmes heart”. Am Heart J. 1969;78:530-536.
18 Marin-Garcia J., Tandon R., Moller J.H., Edwards J.E. Common (single) ventricle with normally related great vessels. Circulation. 1974;49:565-573.
19 De La Cruz M.V., Markwald R.R., Krug E.L., et al. Living morphogenesis of the ventricles and congenital pathology of their component parts. Cardiol Young. 2001;11:588-600.
20 Fogel M.A., Weinberg P.M., Fellows K.E., Hoffman E.A. A study in ventricular-ventricular interaction. Single right ventricles compared with systemic right ventricles in a dual-chamber circulation. Circulation. 1995;92:219-230.
21 Fogel M.A., Weinberg P.M., Gupta K.B., et al. Mechanics of the single left ventricle: a study in ventricular-ventricular interaction II. Circulation. 1998;98:330-338.
22 Damiano R.J.Jr, La F.P.Jr, Cox J.L., Lowe J.E., Santamore W.P. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol. 1991;261:H1514-H1524.
23 Williams R.V., Ritter S., Tani L.Y., Pagoto L.T., Minich L.L. Quantitative assessment of ventricular function in children with single ventricles using the Doppler myocardial performance index. Am J Cardiol. 2000;86:1106-1110.
24 Piran S., Veldtman G., Siu S., Webb G.D., Liu P.P. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189-1194.
25 Macartney F.J., Partridge J.B., Scott O., Deverall P.B. Common or single ventricle. An angiocardiographic and hemodynamic study of 42 patients. Circulation. 1976;53:543-554.
26 Akagi T., Benson L.N., Green M., et al. Ventricular performance before and after Fontan repair for univentricular atrioventricular connection: angiographic and radionuclide assessment. J Am Coll Cardiol. 1992;20:920-926.
27 Parikh S.R., Hurwitz R.A., Caldwell R.L., Girod D.A. Ventricular function in the single ventricle before and after Fontan surgery. Am J Cardiol. 1991;67:1390-1395.
28 Sano T., Ogawa M., Yabuuchi H., et al. Quantitative cineangiographic analysis of ventricular volume and mass in patients with single ventricle: relation to ventricular morphologies. Circulation. 1988;77:62-69.
29 Cabrera A., Azcuna J.I., Bilbao F. Single primitive ventricle with D-transposition of the great vessels and atresia of the left A-V valve. Am Heart J. 1974;88:225-228.
30 Hallermann F.J., Davis G.D., Ritter D.G., Kincaid O.W. Roentgenographic features of common ventricle. Radiology. 1966;87:409-423.
31 Shapiro S.R., Ruckman R.N., Kapur S., et al. Single ventricle with truncus arteriosus in siblings. Am Heart J. 1981;102:456-459.
32 Moodie D.S., Ritter D.G., Tajik A.J., O’Fallon W.M. Long-term follow-up in the unoperated univentricular heart. Am J Cardiol. 1984;53:1124-1128.
33 Habeck J.O., Reinhardt G., Findeisen V. A case of double inlet left ventricle in a 59-year-old man. Int J Cardiol. 1991;30:119-120.
34 Niwa K., Perloff J.K., Kaplan S., Child J.S., Miner P.D. Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricular heart. J Am Coll Cardiol. 1999;34:223-232.
35 Chambers W.N., Criscitiello M.G., Goodale F. Cor triloculare biatriatum. Survival to adult life. Circulation. 1961;23:91-101.
36 Ammash N.M., Warnes C.A. Survival into adulthood of patients with unoperated single ventricle. Am J Cardiol. 1996;77:542-544.
37 Mehta J.B., Hewlett R.F. Cor triloculare biauriculare: an unusual adult heart. Br Heart J. 1945;7:41-44.
38 Sagar K.B., Mauck H.P. Univentricular heart in adults: report of nine cases with review of the literature. Am Heart J. 1985;110:1059-1062.
39 Chen M.S., Younoszai A., Gurm H.S., Asher C.R. Images in cardiovascular medicine. Univentricular heart. Circulation. 2004;109:2030.
40 Davachi F., Moller J.H. The electrocardiogram and vectorcardiogram in single ventricle. Anatomic correlations. Am J Cardiol. 1969;23:19-31.
41 Anderson R.H., Arnold R., Thapar M.K., Jones R.S., Hamilton D.I. Cardiac specialized tissue in hearts with an apparently single ventricular chamber (double inlet left ventricle). Am J Cardiol. 1974;33:95-106.
42 Bharati S., Lev M. The course of the conduction system in single ventricle with inverted (L-) loop and inverted (L-) transposition. Circulation. 1975;51:723-730.
43 Wenink A.C. Development of the human cardiac conducting system. J Anat. 1976;121:617-631.
44 Essed C.E., Ho S.Y., Hunter S., Anderson R.H. Atrioventricular conduction system in univentricular heart of right ventricular type with right-sided rudimentary chamber. Thorax. 1980;35:123-127.
45 Elliott L.P., Ruttenberg H.D., Eliot R.S., Anderson R.C. Vectorial analysis of the electrocardiogram in common ventricle. Br Heart J. 1964;26:302-311.
46 Freireich A.W., Nicolson G.B. A rare electrocardiographic finding occasionally seen in single ventricle hearts; report of two cases of cor triloculare biatriatum. Am Heart J. 1952;43:526-532.
47 Neill C.A., Brink A.J. Left axis deviation in tricuspid atresia and single ventricle; the electrocardiogram in 36 autopsied cases. Circulation. 1955;12:612-619.
48 Carey L.S., Ruttenberg H.D. Roentgenographic features of common ventricle with inversion of the infundibulum: corrected transpositon with rudimentary left ventricle. Am J Roentgenol Radium Ther Nucl Med. 1964;92:652-668.
49 Elliott L.P., Gedgaudas E. The roentgenologic findings in common ventricle with transposition of the great vessels. Radiology. 1964;82:850-865.
50 Child J.S. Transthoracic and transesophageal echocardiographic imaging: anatomic and hemodynamic assessment. In Perloff J.K., Child J.S., Aboulhosn J., editors: Congenital heart disease in adults, 3rd ed, Philadelphia: Saunders/Elsevier, 2009.