Chapter 7 Ultrasound of Polyneuropathies
Peripheral nerve ultrasonography is in evolution and its uses are expanding. Nearly two decades ago, the first papers were published on ultrasonographic abnormalities in carpal tunnel syndrome.1,2 After initial skepticism and numerous studies, ultrasonography is becoming widely accepted as a means of evaluating structural abnormalities of the nerve including entrapment, tumors, and transection. Used in conjunction with electrodiagnostic studies, it has the ability to improve patient care. The role of ultrasonography in the assessment of polyneuropathy remains less well defined and parallels the beginnings of research on nerve entrapment syndromes. More rigorous studies are needed, but case reports and case series provide ample hope for the future. In this chapter, the existing literature on ultrasonography in neuropathy is presented along with its implications for diagnosis, treatment, and prognosis.
A Few Words on Getting Started
Standards of Measurement
Optimal sites for measurement of the nerves in polyneuropathy have not been established. A commonly accepted method is to scan the length of the nerve prior to making any measurements. The median and ulnar nerves are most helpful because it is easy to visualize their entire course. By scanning the entire length of the nerve, areas of abnormality are quickly identified and measurements can be made at those sites. Routine measurements are then made at predesignated anatomic landmarks for each nerve (e.g., the antecubital fossa for the median nerve). Cross-sectional area is considered the most reliable measure of nerve size,3 but longitudinal views of the nerve are also useful for demonstrating areas of focal enlargement or compression. All measurements listed in this chapter provide the cross-sectional area in mm2, unless otherwise indicated.
It is necessary that each laboratory establish its own standards and normal values for measuring nerve area and/or diameter. This is no different than the existing recommendations for laboratories performing nerve conduction studies. Published values serve as helpful guideposts but may have been obtained using different techniques, different equipment, and different patient populations. If published values are to be used, clinicians should use the same methods as listed in the relevant publication and select a sample control group that might be similar to their own patients to ensure its validity.
Although not specifically mentioned in many articles or texts, it is important to consider age, gender, height, and weight when defining abnormalities in peripheral nerve size. Some studies have suggested relationships between these factors and nerve cross-sectional area.4,5 Although there is currently no well-established method to adjust for these factors, it is worth considering that male gender, increasing height, and increasing body mass index may be associated with larger nerve cross-sectional area (although the influence of gender may be a function of height and weight). The role of age is less clear at this time and there are conflicting opinions, although there has been some suggestion that increasing age correlates with increasing nerve size.6,7
In addition to nerve size, other aspects of nerve appearance should be documented. Echogenicity and vascularity can be easily assessed in a qualitative manner. Quantitative measures of echogenicity have been described, but a standard means of reporting these findings has not been established at this time.8–10
Immune-Mediated Neuropathies
Chronic Inflammatory Demyelinating Polyneuropathy
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an autoimmune disorder of the peripheral nervous system and results in sensory and motor impairment that may have a fluctuating course. It is typically responsive to treatment with corticosteroids, intravenous immunoglobulin, or plasmapheresis.11,12 However, the degree of recovery is often limited by the extent of secondary axonal loss. Early treatment is crucial in preventing long-term disability, but accurate identification of the disorder in its earliest stages may be difficult. The diagnosis is based on a combination of clinical and electrodiagnostic findings. Published research criteria for definite diagnosis of CIDP require rather stringent electrodiagnostic abnormalities (e.g., rigorously defined conduction block) in at least two motor nerves.13,14 These abnormalities are often absent in some stages of disease, and any technology that permits earlier diagnosis and treatment stands to significantly improve patient care.15,16
The first description of nerve ultrasonography findings in CIDP was published in 2000.17 The authors examined a single patient with a 3-year history of CIDP. Her diagnosis was supported by the clinical presentation, an elevated cerebrospinal fluid (CSF) protein level, abnormal nerve conduction studies, and nerve biopsy. She was experiencing recurrent weakness at the time of the examination and happened to undergo ultrasonography using a 7-MHz linear array transducer as part of an evaluation for goiter. No goiter was found, but marked enlargement of the bilateral brachial plexus was noted. This finding led to further examination of the peripheral nerves. Several, but not all, were noted to be enlarged. Cross-sectional areas were not provided in the study, but it is mentioned that the median nerve was 5 mm in diameter on longitudinal images. The nerve hypertrophy was not unexpected and had previously been reported with magnetic resonance imaging (MRI),18–20 and correlates with the classic histologic “onion bulb” appearance seen in nerves affected by CIDP.21 The onion bulb results from episodes of repeated demyelination and remyelination, creating areas of nerve enlargement.
Following this initial publication, several years passed before the topic was again addressed. In 2004, Matsuoka and colleagues published a study examining the cervical nerve roots in 13 patients with CIDP and 35 control subjects using a 7.5-MHz linear array transducer.22 The C5-7 nerve roots were examined in both groups, and normal values for diameter were established using the group of healthy controls. The authors demonstrated the presence of cervical root hypertrophy in 9 of 13 CIDP patients and noted the enlargement had a direct correlation with CSF protein levels. Based on this evidence, the authors postulated that ultrasonography screening for nerve root hypertrophy might play a role in the diagnosis of CIDP.
A more comprehensive look at the ultrasonography findings in CIDP was recently published in 2009. Zaidman and colleagues included 36 individuals with CIDP as part of a larger study on ultrasonography and neuropathy.4 The median and ulnar nerves were imaged and cross-sectional area measured at predetermined sites in the upper extremities. Patients with CIDP were found to have diffuse nerve enlargement, estimated to be 2.3-fold larger than that seen in the control group. There was a direct correlation between duration of CIDP and nerve size, and an inverse correlation was noted between cross-sectional area and conduction velocity.
Another development is the possible use of ultrasonography for identification of conduction blocks in CIDP. Conduction blocks are often difficult to demonstrate when present in proximal nerve segments, a factor that may lead to delays in diagnosis and treatment. Three reports describe focal nerve enlargement at the site of electrodiagnostic conduction blocks in two patients with CIDP.23–25 Two of the reports provide images of upper extremity nerves (median, ulnar), with nerve enlargement clearly demonstrated in longitudinal section.24,25 Although this seems to be a promising development, it is worth noting that Zaidman and colleagues did not describe a similar phenomenon.4 Additionally, one of the reports notes that the focal nerve enlargement did not resolve when the patient clinically improved.25 Given prior data that nerve size correlates with duration of CIDP,4 it may be that areas of focal enlargement persist indefinitely. This factor may limit the use of ultrasonography in monitoring the response to therapy.
Clinical Application—CIDP, Case 1
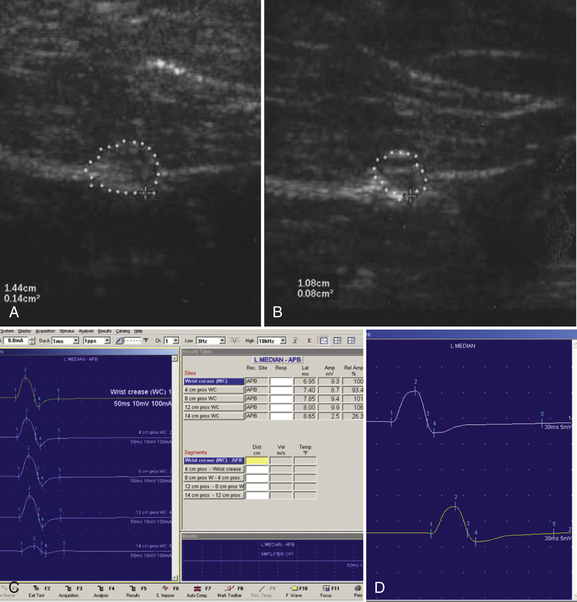
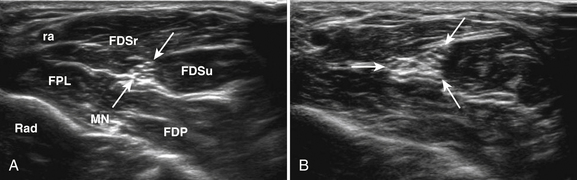
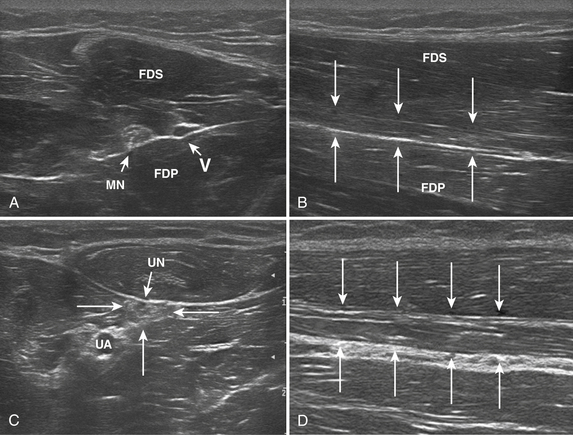
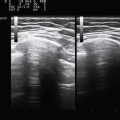
A 20-year-old woman presented with a 6-week history of paresthesias in right digits IV and V, associated with a decline in grip strength. Clinical examination revealed Medical Research Council (MRC) grade 4/5 weakness of the first dorsal interosseous and abductor digiti minimi. There was no sensory loss. Reflexes were absent throughout the upper and lower extremities. Electrodiagnostic studies demonstrated right ulnar neuropathy at the elbow, along with conduction block in the left median nerve (Fig. 7.1C).
Ultrasonography of both upper extremities revealed focal enlargement of the right ulnar nerve at the elbow that could not be differentiated from an entrapment neuropathy. The right median and left ulnar nerves were normal. The left median nerve demonstrated focal enlargement 14 cm proximal to the distal wrist crease—the site of electrodiagnostic conduction block (Fig. 7.1A). She was treated with intravenous immunoglobulin (IVIG) and returned 4 weeks later. There was resolution of the left median conduction block and resolution of the ultrasonographic changes (Fig. 7.1B and D). In this patient, ultrasonography has subsequently been used to monitor response to treatment.
Clinical Application—CIDP, Case 2
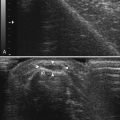
Ultrasonography of the right median nerve showed cross-sectional area of 8 mm2 from the wrist to just above the antecubital fossa (Fig. 7.2A). At 7 cm proximal to the elbow crease the median nerve enlarged to 21 mm2 (Fig. 7.2B and C) and then returned to its previous size of 9 mm2 over a length of 3 cm. This area of focal enlargement provided supportive evidence of CIDP in a case in which NCS were inconclusive. The patient was treated with IVIG and noted rapid improvement in lower extremity strength. He did not return to the laboratory for further testing.
Acute Inflammatory Polyradiculoneuropathy
Acute inflammatory demyelinating polyradiculoneuropathy, or Guillain-Barré syndrome, is the most common cause of nontraumatic paralysis worldwide, affecting 1 to 2 persons per 100,000 each year.26 An immune-mediated disorder, it results in acute onset of ascending paresthesias, weakness, and loss of reflexes. It is one of the few neuromuscular emergencies, and early identification is important because respiratory weakness may ensue. The diagnosis is primarily based on the clinical presentation because electrodiagnostic changes, and CSF cytoalbuminologic dissociation may not be present within the first few days of illness.27
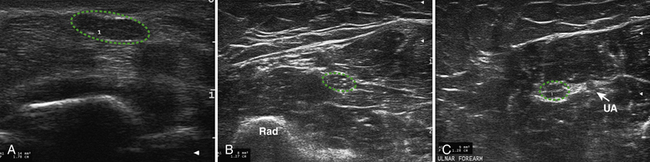
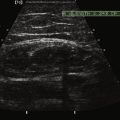
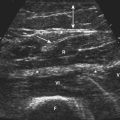
Little is known regarding the ultrasonography changes of acute inflammatory demyelinating polyradiculoneuropathy. Zaidman provides the available published information.4 Seventeen subjects with acute inflammatory demyelinating polyradiculoneuropathy were imaged, with 13 of 17 being imaged within 4 weeks of onset. The ulnar and median nerves were imaged in all and found to be 1.4-fold enlarged as compared with controls. There was no correlation between this enlargement and any electrodiagnostic parameter. Figure 7.3 shows diffuse enlargement of the ulnar nerve in a patient with acute inflammatory demyelinating polyradiculoneuropathy at approximately 2 weeks into the illness. Median and radial nerves were also found to be diffusely enlarged, but are not pictured.
Multifocal Motor Neuropathy
Multifocal motor neuropathy is a demyelinating polyneuropathy that affects only motor nerves. It is associated with the presence of anti-GM1 antibodies, but the diagnosis rests on the clinical examination and electrodiagnostic findings. As with CIDP, conduction block is a sentinel feature, but often difficult to demonstrate when present in the proximal segment of nerves. Histologic evaluation of affected multifocal motor neuropathy nerves has demonstrated demyelination, along with edema and rare “onion bulb” formation.28–31 Nerve enlargement/hypertrophy is not an unexpected finding. As with CIDP, ultrasonography may have a promising role in both the diagnosis and monitoring response to therapy.
Beekman and coworkers published the only existing article on ultrasonography of multifocal motor neuropathy.32 In this study ultrasonography images of the brachial plexus and median, ulnar, and radial nerves were recorded in 21 patients with known multifocal motor neuropathy using a 12-5 MHz linear array transducer. The duration of multifocal motor neuropathy ranged from 2 to 39 years, so patients early in the disease course were not represented. Nerve area was assessed in all patients, and found to be increased as compared with controls in all but two patients. The enlargement was not diffuse, but rather multifocal, and was present in nerves with and without evidence of electrodiagnostic abnormalities. However, it is important to note that the electrodiagnostic studies and ultrasonography were not performed during the same visit, with a mean interval of 1.1 years between the two evaluations.
Clinical Application—Multifocal Motor Neuropathy
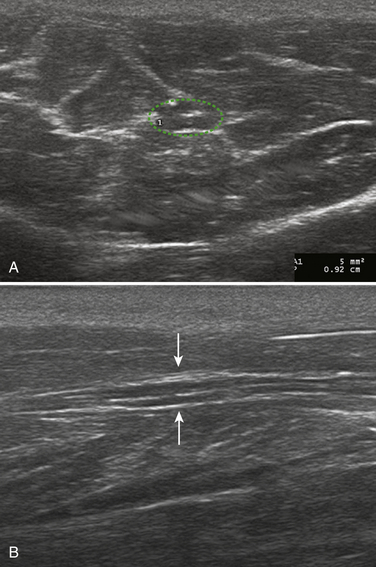
NCS demonstrated a left median conduction block in the forearm. His median nerve was diffusely enlarged from the wrist to the elbow (13 to 17 mm2). His IVIG schedule was increased from 1 g/kg every 4 weeks to 1 g/kg every 2 weeks and the conduction block resolved within 1 month. Follow-up ultrasonography demonstrated resolution of the enlargement noted in the forearm, but then it was noted that the median nerve was markedly enlarged 4 cm above the elbow (25 mm2). Figure 7.4 illustrates enlargement above the elbow with resolution of the previous enlargement in the forearm. Two months later he returned and a conduction block was noted proximal to the elbow with continued enlargement of the median nerve at this site (21 mm2).
Hereditary Neuropathies
Hereditary motor and sensory neuropathies present a unique set of challenges in electrodiagnosis. They are fairly common neuromuscular disorders, affecting 1 in 2500 individuals.33 Although genetic testing is available for the most common subtypes of hereditary motor and sensory neuropathies, electrodiagnosis still plays a role in guiding appropriate testing and in the diagnosis of subtypes without commercially available genetic testing.34 Despite the classic subdivisions of demyelinating and axonal neuropathies, many forms of Charcot-Marie-Tooth disease appear to fall in between, and ultrasonography may make this distinction a bit more clear. Additionally, the presence of underlying neuropathy can complicate diagnosis of superimposed entrapment neuropathies. However, with ultrasonography, these entrapments may be easily visualized; whereas the changes of hereditary motor and sensory neuropathies are diffuse, entrapment neuropathy will manifest as an area of superimposed focal enlargement.
Charcot-Marie-Tooth Disease Type 1
Charcot-Marie-Tooth disease type 1 (CMT1) or hereditary motor and sensory neuropathy is characterized by widespread demyelinating motor and sensory polyneuropathy. The most common form, CMT1a, results from duplication of PMP22 on chromosome 17 and displays autosomal dominant inheritance. Individuals typically present in the first or second decade of life with high-arched feet, thin distal lower extremities, and some degree of distal weakness and sensory loss.35
On nerve biopsy CMT1 is associated with onion bulb hypertrophy.36 This finding is the result of repeated attempts at remyelination and results in pathologic enlargement of nerves. At times the nerves may be large enough to palpate on clinical examination. Based on this knowledge, peripheral nerves are expected to be larger than average in patients with CMT1.
In 1999, Heinemeyer and Reimers examined the ultrasonographic findings of radial, ulnar, median, and sciatic nerves in 10 patients with hereditary motor and sensory neuropathies.37 Six of the subjects had genetically confirmed CMT1a. These subjects were compared with 50 healthy controls. Nerve diameter was recorded. Despite the expected findings, only a tendency toward larger nerves was noted in the CMT group. The lack of a significant difference between the two groups may have stemmed from the inclusion of pediatric patients, patients with other forms of CMT, or the use of a rather low-frequency ultrasonography transducer (7.5 MHz). The role of the transducer should not be dismissed because the authors were able to image the median nerve at the forearm in only 20 of 50 controls and 7 of 10 patients. The distal median nerve was not visualized in any of the 10 patients imaged.
A follow-up study by Martinoli and associates in 2002 had better success in demonstrating the hypertrophy that would be expected in CMT1.38 A 12-5 MHz transducer was used to examine the mid-forearm median nerve of 12 patients with CMT1a. The control group consisted of 50 healthy individuals. The median nerve measured 5.5 ± 1.2 mm2 in controls and 18.4 ± 4.3 mm2 in those with CMT1a. The median nerves imaged were diffusely enlarged throughout the upper extremity. Enlargement of individual nerve fascicles was also observed, and in general, the larger the nerve, the larger the fascicles. No differences in nerve shape or echogenicity were noted as compared with controls.
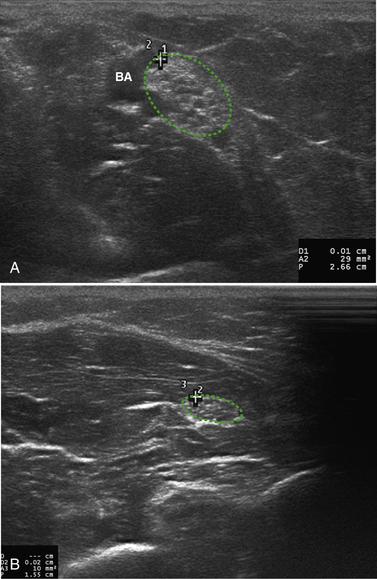
In 2009, two other published manuscripts confirmed Martinoli’s earlier findings. The first, by Cartwright and coworkers, reported the ultrasonography findings of CMT1b in a family possessing the same genetic mutation.39 CMT1b is an autosomal dominant demyelinating polyneuropathy that results from mutations in the myelin protein zero gene. Affected individuals are clinically similar to those with CMT1a, although the family studied in this case also displayed cranial neuropathies. Twelve patients and 24 controls were imaged and had significantly larger median and vagus nerves than controls with the median measuring 20.75 mm2 in the forearm and 13.5 mm2 at the wrist. Of note, sural nerve size was actually smaller in the patient group—attributed to possible length-dependent axonal loss or older age of the patient group as compared with controls. Sample images from this study are provided in Figure 7.5.
Zaidman and colleagues in 2009 provided additional information on ultrasonography and CMT1a—11 patients were included in his study on ultrasonography and polyneuropathy.4 Although exact area measurements were not provided, a nerve size index was calculated. The average nerve size index of median and ulnar nerves was found to be 3.5-fold enlarged in CMT1a as compared with controls. Nerves were diffusely enlarged and no significant tapering was reported, as with the prior study on CMT1b patients.39
CMT2
CMT2 is defined by the presence of hereditary axonal motor and sensory polyneuropathy. It is much less common than CMT1. Patients have a similar presentation as those with CMT1, but may have onset later in life. The exact phenotype can be variable, with some patients even having brisk reflexes. As a result of these differences and the variability reported in nerve conduction studies, the diagnosis can be more difficult. There are more than ten recognized subtypes of CMT2 in the literature.34
The nerve pathology present in cases of CMT2 is dependent on the exact subtype and genotype, but axonal loss is generally seen. Onion bulbs are rare but have been reported. Giant axons may be present in type 2E.40 As a result of the wide spectrum of abnormalities seen on nerve biopsy, diffuse enlargement of the nerves is not expected in all types of CMT2.
Perhaps because of the relative rarity of CMT2, there is little literature on the ultrasonography appearance of affected nerves. Martinoli and associates in 2002 included seven patients with CMT2 as part of their previously described study on the ultrasonography of the median nerve in CMT.38 The subtype of CMT2 was not determined in the seven patients imaged, but they were found to have modestly enlarged median nerve area at the forearm (8.40 ± 1.1 mm2) as compared with controls (5.5 ± 1.2 mm2). There was not a robust difference in fascicular diameter between patients and healthy controls.
Clinical Application—CMT2
A 40-year-old right-handed woman with a family history of CMT2 (subtype unknown) presented for evaluation of numbness in digits I through III. The paresthesias awoke her from sleep on a nightly basis. Physical examination demonstrated normal strength and appearance of the hands. Pinprick sensation was reduced in a bilateral median nerve distribution. Tinel’s sign was present over the median nerve at the wrist. Nerve conduction studies and EMG were performed, demonstrating a pattern consistent with CMT2. Unfortunately, this made it difficult for her referring neurologist to determine if there was superimposed median neuropathy at the wrist.
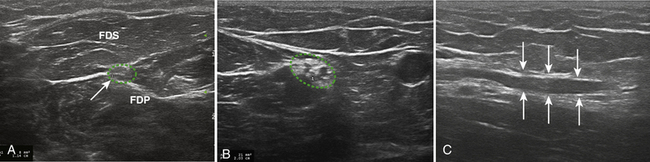
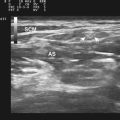
The patient was referred for median nerve ultrasonography to further clarify her diagnosis. Both median nerves measured 14 mm2 at the wrist and 8 mm2 at the forearm, consistent with focal entrapment at the wrist (Fig. 7.6A and B). The right ulnar nerve measured a normal 6 mm2 at the wrist and 8 to 9 mm2 in the forearm (Fig. 7.6C). Based on these findings and the clinical history, bilateral carpal tunnel syndrome was diagnosed and the patient was referred for appropriate treatment. Of note, other hereditary disorders of myelination, such as metachromatic leukodystrophy, may not be associated with nerve hypertrophy either (Fig. 7.7).
Hereditary Neuropathy with Liability to Pressure Palsies
Hereditary neuropathy with liability to pressure palsies is an autosomal dominant disorder that results from deletion of PMP22 on chromosome 17. It is estimated to affect 1 in 100,000 individuals. Those affected develop painless mononeuropathies after seemingly incidental trauma, particularly at or near common sites of entrapment.41 Hereditary neuropathy with liability to pressure palsies is often overlooked, and patients may undergo carpal tunnel release or ulnar nerve transposition prior to being correctly diagnosed.
In addition to focal nerve injury, physical examination may reveal hyporeflexia in hereditary neuropathy with liability to pressure palsies. A family history may not be present or simply be unrecognized by the patient. On electrodiagnostic studies, distal slowing with superimposed entrapment neuropathies may be present.41,42 Challenges arise, however, if patients are seen at a time when no focal deficits are present or if only one nerve entrapment syndrome has occurred.
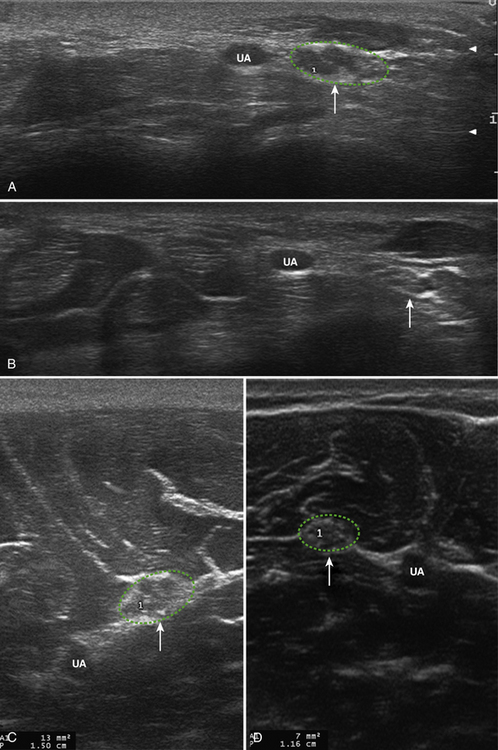
Little is known about the ultrasonography findings of hereditary neuropathy with liability to pressure palsies. Beekman and Visser in 2002 published a case report on this topic, imaging the ulnar nerve with a 12-5 MHz transducer.43 The affected patient had a prior diagnosis of hereditary neuropathy with liability to pressure palsies and presented with sudden onset of ulnar neuropathy symptoms. As expected, the ulnar nerve was markedly enlarged at the ulnar groove (31 mm2). Curious as to the findings, the authors then imaged nerves that were not clinically affected and found them to be enlarged as well (1.5- to 2-fold higher than normal). The enlargement was attributed to the myelin sheath swellings seen on pathologic examination of nerves affected by hereditary neuropathy with liability to pressure palsies and may predispose the nerves to entrapment injury. Sample images of hereditary neuropathies with liability to pressure palsies are presented in Figure 7.8.
Other Acquired Neuropathies
Diabetic Neuropathy
Diabetes mellitus is the most common cause of peripheral neuropathy worldwide. In 2006, 16.8 million individuals were living with diabetes mellitus in the United States, with the number having tripled since 1980.44 The World Health Organization (WHO) estimates that 180 million are affected worldwide and expects this number to double by 2030.45 It is difficult to determine how many persons with diabetes mellitus have neuropathy, but estimates range from a very conservative 6.8 per 1000 (Centers for Disease Control and Prevention [CDC] rate based on 66,000 hospital discharges for the diagnosis in 2003) to as high as 50% (WHO estimates).44,45 Patients may present with a wide spectrum of symptoms: painful paresthesias, ulceration of the lower extremities, or proximal pain and weakness. In addition to the common presentations of length-dependent peripheral sensorimotor polyneuropathy and diabetic amyotrophy, patients are at increased risk for entrapment neuropathies.
Diabetic peripheral neuropathy is typically diagnosed by clinical examination because electrodiagnostic testing is often not readily available in primary care or endocrinology settings. Over the past few years, primary care physicians have been encouraged to routinely screen for sensory impairment using monofilament testing. The sensitivity of this instrument for detecting neuropathy has been reported to range from 57% to 93% when compared with nerve conduction studies.46 Early detection of neuropathy is essential in preventing further complications of disease, including nonhealing ulcers and limb amputation.
To date, there has been little published on ultrasonography and diabetic neuropathy. Lee and Dauphinée addressed this question by recording the cross-sectional area of the tibial nerve in 24 diabetic patients with symptoms of neuropathy.47 The tibial nerve was imaged just proximal and distal to the tarsal tunnel. The mean cross-sectional area was found to be 12 mm2 in patients without diabetes mellitus and in diabetic patients without symptoms of neuropathy. The mean cross-sectional area in those with diabetic neuropathy symptoms was 24 mm2. Although this difference is not subtle, it must be noted that the nerve was imaged at a potential site of entrapment. Because entrapment neuropathies are characterized by focal nerve enlargement, it is difficult to say if the recorded difference was attributable to diabetic neuropathy, tarsal tunnel syndrome, or a combination of both.
Watanabe and colleagues also reported nerve enlargement in patients with diabetes mellitus.48 In this study, 20 diabetic patients and 20 healthy volunteers underwent imaging of the median nerve at the carpal tunnel, 5 cm proximal to the wrist, and at the elbow. Patients with symptoms of carpal tunnel syndrome or a Phalen’s sign on examination were excluded from participation. Diabetic patients were divided into those with and without diabetic peripheral neuropathy on the basis of physical examination. No significant differences were found between the control group and diabetic patients without evidence of neuropathy (n = 26 limbs). In these two groups, cross-sectional area at the carpal tunnel was 9 mm2, 7.1 to 7.4 mm2 at 5 cm proximal to the wrist, and 7.4 to 7.5 mm2 at the elbow. Diabetic patients with neuropathy (n = 14 limbs) had a larger area at the carpal tunnel (13.5 ± 2.8 mm2) and 5 cm proximal to the wrist (9.1 ±2.7 mm2), but not the elbow (7.2 ± 2.6 mm2). Again, these findings should be interpreted cautiously because the nerve enlargement was noted at or near a site of entrapment.
In contrast to the data presented by Lee and Watanabe, Zaidman and coworkers found no difference between nerve size in controls and in those with axonal neuropathy associated with diabetes mellitus.4 Included in this previously described study were 36 patients with axonal neuropathy, some of whom were noted to have neuropathy attributed to diabetes mellitus. Data are not presented on how many of the 36 were diabetic, and further analysis or comment is not possible.
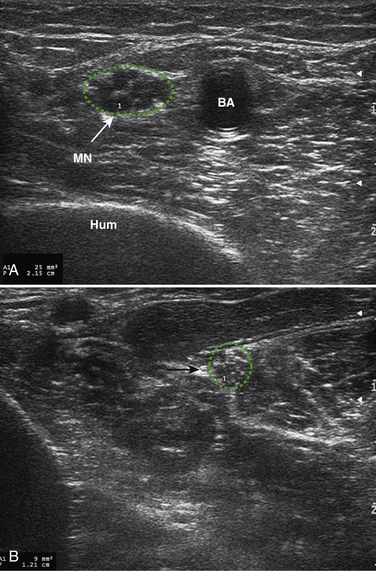
Figure 7.9A through D demonstrates some views of a patient living with an axonal length-dependent polyneuropathy for many years. These findings are similar to those present in long-standing diabetic polyneuropathy.
Vasculitic Neuropathy
Vasculitic neuropathy is one of the most difficult diagnoses in neuromuscular medicine, with nerve biopsy considered to be the gold standard. However, even nerve biopsy does not always provide the answers due to patchy involvement of nerves. The sensitivity of nerve biopsy has been noted to be as low as 60%.49 Additionally, nerve biopsy often leaves the patient with permanent deficits in sensation or strength. Vasculitic neuropathy may be a primary, nonsystemic disorder or it can arise secondary to other inflammatory processes, such as widespread systemic vasculitis (e.g., Churg-Strauss syndrome). Classically, it manifests with rapid onset of painful sensory and motor loss affecting the lower extremities. Mononeuritis multiplex is the textbook presentation of vasculitic neuropathy, but affected patients may also display a length-dependent, symmetric polyneuropathy.50
When positive, nerve biopsy may yield a variety of findings. In addition to perivascular inflammation, perineurial thickening may be seen. Loss of myelinated nerve fibers may also be evident.51 The exact histology depends on many factors that include duration of disease, current disease activity, and the degree of involvement of the tissue being examined. As a result of this heterogeneity, it is difficult to predict what ultrasonography might show, although it is likely that nerves will enlarge during acute disease because of the presence of edema.
Early diagnosis and treatment of vasculitic neuropathy are essential in preventing not only permanent neurologic deficits but also widespread systemic damage and even death. Systemic vasculitic neuropathies are often fatal without treatment.52 Although research is limited, ultrasonography may permit better identification of early vasculitic neuropathy or more accurate selection of nerve biopsy sites.
There have been only two publications on the findings of vasculitic neuropathy. The first, by Nodera and associates in 2006, demonstrates decreased echogenicity and diffuse thickening of the median, ulnar and tibial nerves, as well as the cervical nerve roots.53 Follow-up studies were performed after 2 weeks of corticosteroid therapy and showed marked reduction in the size of the previously imaged ulnar nerve. No comment was made on the appearance of the other nerves previously imaged. Of note, nerve diameter was measured, as opposed to cross-sectional area.
The second publication, by Ito and coworkers in 2007, compares the ultrasonography appearance of the tibial nerves in patients with vasculitic neuropathy with that seen in controls.54 All patients had involvement of the tibial nerve documented on physical and electrodiagnostic examination. The nerves were imaged 2 cm proximal to the top border of the medial malleolus using a 7.5 MHz linear array transducer. Eleven tibial nerves were imaged in 8 patients and compared with 35 tibial nerves in 35 control subjects. The mean cross-sectional area in patients was 13.5 ± 3.7 mm2 as compared with 7.9 ± 1.5 mm2 in controls. The authors postulate that nerve enlargement in vasculitic neuropathy may be secondary to vasculitic-granulomatous lesions in the epineurium as well as edema. There was no nerve biopsy tissue available for correlation with the ultrasonography findings.
Leprosy
Leprosy is a chronic infectious disease that is found throughout warmer areas of the globe, including the southern United States and Europe. The skin and nerves are affected, and nerve involvement primarily manifests as sensory loss. This involvement may occur in both untreated patients or manifest as episodes of acute neuritis in the context of initiating therapy.55 Early recognition of nerve injury is important in guiding treatment and preventing permanent damage.
Fornage and Nerot published a case report in 1987 on the use of nerve ultrasonography in the diagnosis of tuberculoid leprosy in one patient, but more recently Martinoli and colleagues in 2000 examined 23 patients with leprosy using peripheral nerve ultrasonography.56,57 Fifty-eight nerves with clinical or electrodiagnostic abnormalities were imaged, including ulnar, median, and posterior tibial nerves; 50 of 58 nerves also were imaged with MRI. Seventeen nerves were normal with both ultrasonography and MR imaging; 30 nerves (52%) displayed areas of marked fusiform enlargement and were hypoechoic. The remainder of the nerves had normal size, but abnormal echogenicity (either hypo- or hyperechoic). Nerves that had reversal reactions were more likely to be enlarged.
Elias and coworkers in 2009 investigated the role of ultrasonography in examination of the ulnar nerve in patients with a new diagnosis of leprosy.58 Twenty-one consecutive patients with leprosy underwent ultrasonography of the ulnar nerve prior to initiating treatment and 19 of 21 also underwent electrodiagnostic studies. The ulnar nerves were scanned from the axilla to hand, and the cross-sectional area was measured at several predetermined points. Ulnar nerve cross-sectional areas were found to be significantly larger in patients as compared with controls. For example, at the cubital tunnel was 6.84 ± 1.92 mm2 in controls and 14.51 ± 6.31 mm2 in patients with leprosy. Nerve enlargement was present in 19 of 21 patients, and NCS abnormalities were present in 16 of 19 patients studied.
A similar study in 2009 examined 20 patients with leprosy and compared them with 30 healthy controls.59 Ulnar, median, lateral popliteal, and posterior tibial nerves were imaged. Patients with leprosy were noted to have larger nerves than the controls. This was true for all nerves imaged, and the differences between the two groups were quite marked— ulnar nerve cross-sectional area measured 8.5 mm2 in controls and 22.7 mm2 in patients with leprosy. Alterations in echogenicity and vascularity were recorded, but the differences between control and patient groups were less clear.
1. Buchberger W., Schön G., Strasser K., et al. High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med. 1991;10:531-537.
2. Buchberger W., Judmaier W., Birbamer G., et al. Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol. 1992;159:793-798.
3. Bartels B.H., Meulstee J., Verhage, et al. Ultrasound imaging of the ulnar nerve: correlation of preoperative and intraoperative dimensions. Clin Neurol Neurosurg. 2008;110:687-690.
4. Zaidman C.M., Al-Lozi M., Pestronk A. Peripheral nerve size in normal and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40:960-966.
5. Visser L.H., Smidt M.H., Lee M.L. High resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 2008;79:63-67.
6. Wiesler E.R., Chloros G.D., Cartwright M.S., et al. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;32:726-732.
7. Hobson-Webb L.D., Padua L. Median nerve ultrasonography in carpal tunnel syndrome: findings from two laboratories. Muscle Nerve. 2009;40:94-97.
8. Arts I.M.P., Pillen S., Schelhasa J., et al. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41:32-41.
9. Gdynia H.J., Müller H.P., Ludolph A.C., et al. Quantitative muscle ultrasound in neuromuscular disorders using the parameters “intensity,” “entropy,” and “fractal dimension,”. Eur J Neurol. 2009;10:1151-1158.
10. Pillen S., van Dijk J.P., Weijers G., et al. Quantitative gray-scale analysis in skeletal muscle ultrasound: a comparison study of two ultrasound devices. Muscle Nerve. 2009;39:781-786.
11. Brannagan T.H. Current treatments of chronic immune-mediated demyelinating polyneuropathies. Muscle Nerve. 2009;39:563-578.
12. Merkies I.S., Bril V., Dalakas M.C., et al. Health-related quality-of-life improvements in CIDP with immune globulin IV 10%: the ICE study. Neurology. 2009;72:1337-1344.
13. Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Neurology. 1991;41:617-618.
14. Nicolas G., Maisonobe T., Le Forestier N., et al. Proposed revised electrophysiological criteria for chronic inflammatory demyelinating polyradiculopathy neuropathy. Muscle Nerve. 2002;25:26-30.
15. De Sousa E.A., Chin R.L., Sander H.W., et al. Demyelinating findings in typical and atypical chronic inflammatory demyelinating polyneuropathy: sensitivity and specificity. J Clin Neuromuscul Dis. 2009;10:163-169.
16. Magda P., Latov N., Brannagan T.H., et al. Comparison of electrodiagnostic abnormalities and criteria in a cohort of patients with chronic inflammatory demyelinating polyneuropathy. Arch Neurol. 2003;60:1755-1759.
17. Taniguchi N., Itoh K., Wang Y., et al. Sonographic detection of diffuse peripheral nerve hypertrophy in chronic inflammatory demyelinating polyneuropathy. J Clin Ultrasound. 2000;28:488-491.
18. Duggins A.J., McLeod J.G., Pollard J.D., et al. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain. 1999;122:1383-1390.
19. Mizuno K., Nagamatsu M., Hattori N., et al. Chronic inflammatory demyelinating polyradiculoneuropathy with diffuse and massive peripheral nerve hypertrophy: distinctive clinical and magnetic resonance imaging features. Muscle Nerve. 1998;21:805.
20. Naganuma M., Doi S., Shima K., et al. Chronic inflammatory demyelinating polyradiculoneuropathy associated with multifocal nerve hypertrophy: report of a case with MRI study. Rinsho Shinkeigaku. 1991;31:1186.
21. Matsuda M., Ikeda S., Sakurai S., et al. Hypertrophic neuritis due to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): a postmortem pathological study. Muscle Nerve. 1996;19:163-169.
22. Matsuoka N., Kohriyama T., Ochi K., et al. Detection of cervical nerve root hypertrophy by ultrasonography in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci. 2004;219:15-21.
23. Smith E.C., Hobson-Webb L.D., Massey E. Nerve ultrasound in motor conduction block: pre- and posttreatment findings. Muscle Nerve. 2008;38:1369.
24. Granata G., Pazzaglia C., Calandro P., et al. Ultrasound visualization of nerve morphological alteration at the site of conduction block. Muscle Nerve. 2009;40:1068-1070.
25. Imamura K., Tajiri Y., Kowa H., et al. Peripheral nerve hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy detected by ultrasonography. Intern Med. 2009;48:581-582.
26. McGrogan A., Madle G.C., Seaman H.E., et al. The epidemiology of Guillain-Barré syndrome worldwide: a systematic literature review. Neuroepidemiology. 2009;32:150-163.
27. Vucic S., Kiernan M.C., Cornblath D.R. Guillain-Barré syndrome: an update. J Clin Neurosci. 2009;16:733-741.
28. Léger J.M., Behin A. Multifocal motor neuropathy. Curr Opin Neurol. 2005;18:567-573.
29. Van Asseldonk J.T.H., Franssen H., Van den Berg-Vos R.M., et al. Multifocal motor neuropathy. Lancet Neurol. 2005;4:309-319.
30. Taylor B.V., Dyck P.J., Engelstad J., et al. Multifocal motor neuropathy: pathologic alterations at the site of conduction block. J Neuropathol Exp Neurol. 2005;63:129-137.
31. Auer R.N., Bell R.B., Lee M.A. Neuropathy with onion bulb formations and pure motor manifestations. Can J Neurol Sci. 1989;16:194-197.
32. Beekman R., van den Berg L.H., Franssen H., et al. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology. 2005;65:305-307.
33. Martyn C., Hughes R.A.C. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
34. Pareyson C., Marchesi D. Diagnosis, natural history and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009;8:654-667.
35. Thomas P.K. Overview of Charcot-Marie Tooth disease type 1A. Ann N Y Acad Sci. 1999;883:1-5.
36. Sereda M., Griffiths I., Pühlhofer A., et al. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron. 1996;16:1049-1060.
37. Heinemeyer O., Reimers C.D. Ultrasound of radial, ulnar, median and sciatic nerves in healthy subjects and patients with hereditary motor and sensory neuropathies. Ultrasound Med Biol. 1999;25:481-485.
38. Martinoli C., Schenone A., Bianchi S., et al. Sonography of the median nerve in Charcot-Marie-Tooth disease. AJR. 2002;178:1553-1556.
39. Cartwright M.S., Brown M.E., Eulitt P., et al. Diagnostic nerve ultrasound in Charcot-Marie-Tooth disease type 1B. Muscle Nerve. 2009;40:98-102.
40. Fabrizi G.M., Cavallaro T., Angiari C., et al. Giant axon and neurofilament accumulation in Charcot-Marie-Tooth disease type 2E. Neurology. 2004;62:1429-1431.
41. Stögbauer F., Young P., Kuhlenbäumer G., et al. Hereditary recurrent focal neuropathies: clinical and molecular features. Neurology. 2000;54:546-551.
42. Andersson P.B., Yuen E., Parko K., et al. Electrodiagnostic features of hereditary neuropathy with liability to pressure palsies. Neurology. 2000;54:40-44.
43. Beekman R., Visser L.H. Sonographic detection of diffuse peripheral nerve enlargement in hereditary neuropathy with liability to pressure palsies. J Clin Ultrasound. 2002;30:433-436.
44. Centers for Disease Control’s Diabetes Program. Available at www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Accessed November 7, 2009.
45. World Health Organization. Diabetes Fact Sheet. Available at www.who.int/mediacentre/factsheets/fs312/en/print.html. Accessed November 7, 2009.
46. Feng Y., Schlösser F.J., Sumpio B.E. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50:675-682.
47. Lee D., Dauphinée D.M. Morphological and functional changes in the diabetic peripheral nerve. J Am Podiatr Med Assoc. 2005;95:433-437.
48. Watanabe T., Ito H., Morita A., et al. Sonographic evaluation of the median nerve in diabetic patients. J Ultrasound Med. 2009;28:727-734.
49. Collins M.P., Mendell J.R., Periquet M.I., et al. Superficial peroneal nerve/peroneus brevis muscle biopsy in vasculitic neuropathy. Neurology. 2000;55:636-643.
50. Burns T.M., Schaublin G.A., Dyck P.J. Vasculitic neuropathies. Neurol Clin. 2007;25:89-113.
51. Agarwal V., Singh R., Wiclaf, et al. A clinical, electrophysiological, and pathological study of neuropathy in rheumatoid arthritis. Clin Rheumatol. 2008;27:841-844.
52. Dyck P.J., Benstead T.J., Conn D.L., et al. Nonsystemic vasculitic neuropathy. Brain. 1987;110:843-854.
53. Nodera H., Sato K., Terasawa Y., et al. High-resolution sonography detects inflammatory changes in vasculitic neuropathy. Muscle Nerve. 2006;34:380-381.
54. Ito T., Kijima M., Watanabe T., et al. Ultrasonography of the tibial nerve in vasculitic neuropathy. Muscle Nerve. 2007;35:379-382.
55. Pearson J.M.H., Ross W.F. Nerve involvement in leprosy: pathology, differential diagnosis and principles of management. Lepr Rev. 1975;46:199-212.
56. Fornage B.D., Nerot C. Sonographic diagnosis of tuberculoid epilepsy. J Ultrasound Med. 1987;6:105-107.
57. Martinoli C., Derchi L.E., Bertolotto M., et al. ultrasonography and MR imaging of peripheral nerves in leprosy. Skeletal Radiol. 2000;29:142-150.
58. Elias J., Nogueira-Barbosa M.H., Feltrin L.T., et al. Role of ulnar nerve sonography in leprosy neuropathy with electrophysiology correlation. J Ultrasound Med. 2009;28:1201-1209.
59. Jain S., Visser L.H., Praveen T.L.N., et al. High-resolution sonography: a new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3:e498.