Chapter 8 Ultrasound of Motor Neuron Disease
ALS (also called Charcot’s or Lou Gehrig’s disease) is the most commonly encountered motor neuron disease in modern clinical practice, with an incidence between 1.5 and 2.5 per 100,000 per year.1 It is a neurodegenerative condition in which upper and lower motor neurons are progressively lost, and a disease for which there is no cure. There are variants of ALS in which only upper (primary lateral sclerosis) or only lower (progressive muscular atrophy) motor neurons are affected. SMA is also a neurodegenerative disease, but in contrast to ALS it is inherited (autosomal recessive) and typically affects children. There are four subtypes of SMA, and the divisions are based on the age at onset.
Imaging in Motor Neuron Disease
Historically motor neuron diseases have been diagnosed clinically, with electrodiagnostic studies used to support the diagnosis. Imaging studies are rarely supportive in this regard, although magnetic resonance imaging (MRI) of the brain and cervical spine is often used to rule out ALS mimics.2 More recently, advanced central nervous system imaging techniques, such as functional MRI, MR tractography, and positron-emission tomography (PET) have demonstrated changes in those with ALS.3–5 Although these techniques have provided insight into the pathophysiology of motor neuron disease, they are not well suited for diagnostic applications. Neuromuscular imaging has been explored in motor neuron disease, but the field is not well developed. In theory, peripheral nervous system imaging could be beneficial in motor neuron disease because it could aid in diagnosis, advance our understanding of the pathophysiology, and serve as a surrogate marker of disease progression in therapeutic trials and clinical practice. Some of these applications have been preliminarily explored using neuromuscular ultrasound.
Nerve Ultrasound in Motor Neuron Disease
Imaging studies focusing on peripheral nerves in individuals with motor neuron disease are exceedingly rare. Autopsy studies have shown that nerve roots are atrophic in those with ALS,6 but there is a surprising lack of reports confirming root atrophy through imaging in living individuals. There is also a dearth of studies in which specific emphasis is placed on imaging peripheral nerves in those with motor neuron disease.
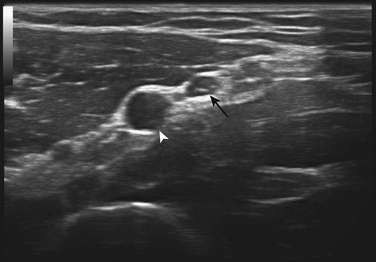
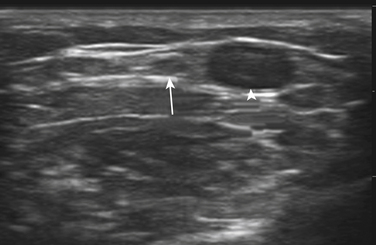
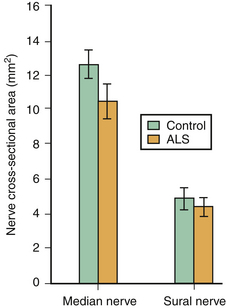
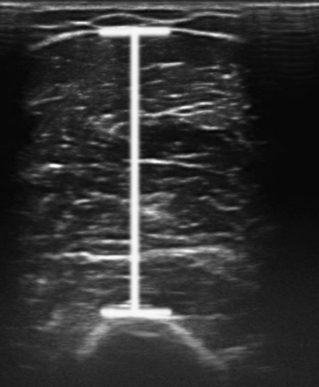
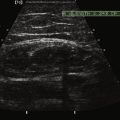
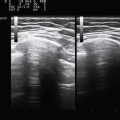
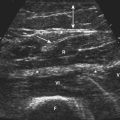
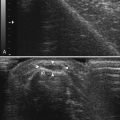
There is one study in which neuromuscular ultrasound was used to examine the nerves of those with ALS.7 In this report, high-resolution ultrasound (Biosound MyLab 25, Esaote Group, Genoa, Italy; with 18MHz transducer) was used to image the median nerve at the mid-arm (Fig. 8.1) and the sural nerve above the lateral malleolus (Fig. 8.2) in 20 individuals with ALS and 20 age- and gender-matched controls. The mean cross-sectional area of the median nerve was slightly smaller in the ALS patients than the controls, whereas the sural nerve area did not differ between these two groups (Fig. 8.3). However, the absolute difference in mean median nerve area between the ALS patients (10.5 mm2) and controls (12.7 mm2) was not large, and the mean median nerve area in the ALS patients was actually slightly larger than that seen in previous studies of healthy controls. The larger median nerve cross-sectional area in controls in this study may be a result of a higher mean age compared with previous control populations, because nerve area does increase slightly with increasing age. This study was limited in that only two nerves were examined, no nerve root imaging was included, and a distal motor nerve was not assessed. However, this report did demonstrate a subtle difference, with the median nerve cross-sectional area being smaller in those with ALS than in an age- and gender-matched control groups.
Muscle Ultrasound in Motor Neuron Disease
Diagnosis in SMA
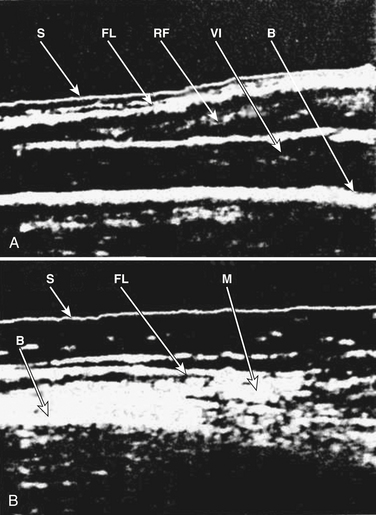
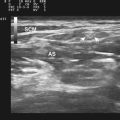
Some of the first studies in the field of neuromuscular ultrasound were performed to assess the diagnostic accuracy of the technique in pediatric neuromuscular disorders. In 1982, Heckmatt and associates subjectively compared muscle ultrasound findings between 60 children with neuromuscular disorders and 60 healthy controls. A clear difference was noted between the two groups, and those with SMA were found to have increased muscle echogenicity, muscle atrophy, and increased depth of subcutaneous tissue (Fig. 8.4).8 This study was followed by a comparison between 20 boys with Duchenne’s muscular dystrophy and 10 children with SMA, and it was found that ultrasonographic muscle changes correlated with changes seen on muscle biopsy, and even those with minimal clinical findings had easily detectable changes of increased muscle echogenicity9 (Box 8.1 lists the neuromuscular ultrasound findings in SMA).
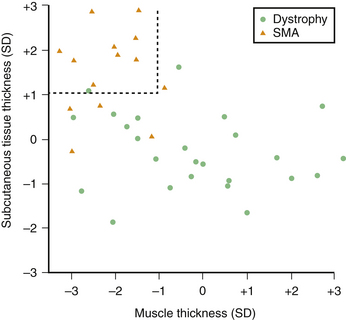
These subjective studies of muscle ultrasound in SMA were then followed by more objective assessments. In the late 1980s, Heckmatt and associates used muscle and subcutaneous tissue thickness measurements as a means of differentiating SMA from muscular dystrophies. Individuals with SMA were found to have measurable muscle atrophy and an increase in subcutaneous tissue that was independent of obesity, whereas muscular dystrophies did not demonstrate atrophy nor changes in subcutaneous tissue thickness (Fig. 8.5).10,11 Reimers and colleagues studied 350 patients with different neuromuscular disorders and showed that calf hypertrophy and pseudohypertrophy were commonly seen in juvenile proximal SMA type 3, but this finding was nonspecific because it was seen in multiple other neuromuscular conditions.12
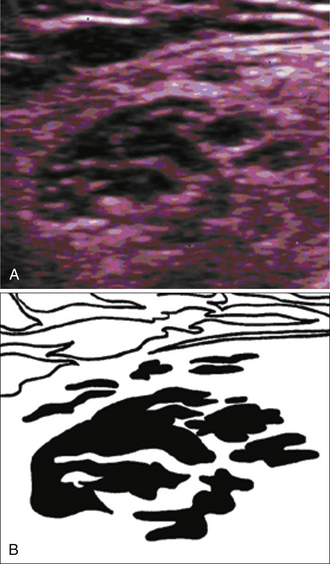
These measurements of muscle size eventually led to even more objective neuromuscular ultrasound studies of SMA. In 2000, Pohle and coworkers used computer-assisted muscle texture analysis, and showed that this technique (which is based on assessments of brightness and muscle micro- and macrostructure) could differentiate SMA from muscular dystrophy, inflammatory myopathy, and inherited sensorimotor neuropathy with a sensitivity of 77% to 94% and a specificity of 81% to 98%.13 These objective measures were advanced even further by Pillen and associates in the late 2000s. This group demonstrated that in SMA, muscle ultrasound shows a heterogeneous increase in echogenicity with associated muscle atrophy, and that computer-assisted gray scale analysis of specific muscle groups could differentiate neurogenic changes (mainly in those with SMA) from myopathic changes with a positive predictive value of 86% and a negative predictive value of 84%.14 They were also able to demonstrate that computer-assisted gray scale analysis improved sensitivity in the diagnosis of SMA to 87% compared with 71% with visual assessment using the Heckmatt grading system, and that computer-assisted analyses improved interrater reliability compared with visual assessment.15 (Note—The visual grading system developed by Heckmatt for assessing muscle ultrasound, and the computer-assisted gray scale analysis developed by Polhe and coworkers and Pillen and associates are described in greater detail in Chapter 3). van Baalen and Stephani postulated that the heterogeneous muscle signal seen during muscle ultrasound in SMA occurs because of fiber-type grouping of hypertrophic fibers (Fig. 8.6).16 Aydinli and colleagues took this one step further and showed a high concordance between ultrasonographic and electromyographic findings in evaluating hypotonic infants with SMA.17
Diagnosis in ALS
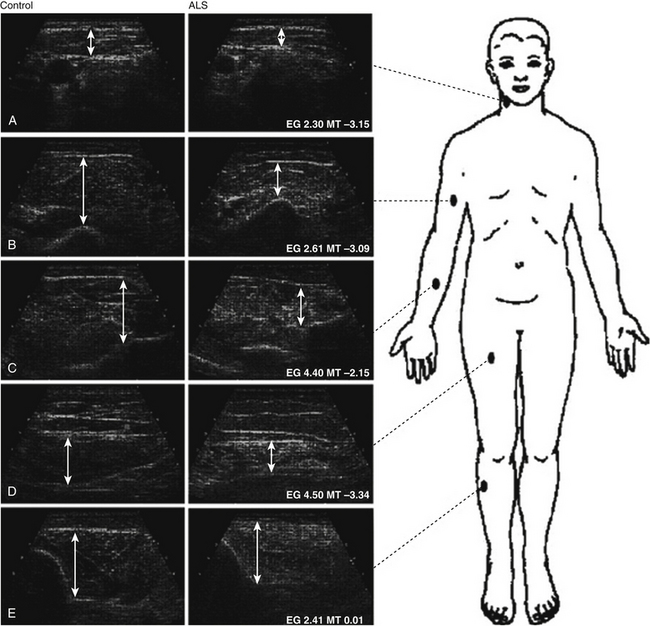
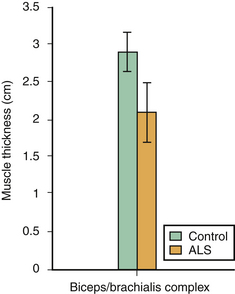
Compared with SMA, there are fewer studies of muscle ultrasound in ALS. Arts and coworkers studied 48 individuals with ALS and performed quantitative ultrasound analyses on five muscle groups. This study demonstrated that those with ALS have significantly increased echogenicity, along with decreased muscle thickness, in the biceps brachii, forearm flexors, and rectus femoris muscles (Fig. 8.7).18 Cartwright and colleagues confirmed that those with ALS had significant biceps brachii/brachialis atrophy compared with age- and gender-matched controls (Fig. 8.8).7
The other interesting finding noted in ALS studies is that fasciculations were readily detected with real-time ultrasound imaging. Fasciculations occur when motor neurons sporadically fire and cause activation of an entire motor unit. This results in contraction of all the muscle fibers of a motor unit, and if it is superficial it can be seen as a quiver of the skin. Ultrasound can readily detect fasciculations, and unlike EMG, ultrasound can quickly and painlessly scan large areas looking for the presence of fasciculations. Arts and coworkers showed that 24 out of 25 patients screened using real-time ultrasound were found to have fasciculations,18 and Cartwright and colleagues noted tongue fasciculations with ultrasound in 50% of those with ALS, including one individual in whom the fasciculations could not be seen with visual inspection (Video 8.1).7 This is of diagnostic importance in ALS, because the presence of fasciculations in muscles proximal to the knee is indicative of muscle involvement in ALS; and the distribution of muscle involvement aids in the diagnosis of ALS.19 From these studies it can be postulated that using ultrasound to rapidly scan muscle segments, such as the paraspinals for example, may assist in the detection of clinically occult fasciculations and help establish the diagnosis of motor neuron disease. It should be noted that fasciculations detected by ultrasound can also be seen in SMA,20,21 and it has even been suggested that ultrasonographic measurement of fasciculation duration can differentiate SMA (longer duration) from more acute neurogenic processes, such as ALS (shorter-duration fasciculations).22
Perhaps one of the most intriguing findings in all of neuromuscular imaging, and certainly in imaging of motor neuron disease, is that it has recently been suggested that fibrillations can be seen with ultrasound. In 2007, van Baalen and Stephani reported visualizing fibrillations in 10 infants with genetically confirmed SMA, and these were seen using a 15MHz transducer and appeared as chaotic movements within the muscle.23 They argued that the term fibration is more accurate than fibrillation because they were visualizing movements of entire muscle fibers. Pillen and associates subsequently used ultrasound and EMG simultaneously to evaluate eight patients with fibrillations and were able to confirm that small, irregularly oscillating movements seen within muscles were from fibrillations (Video 8.2).24 They found these movements to be less easily detected in cooler limbs, and they noted high interrater reliability (kappa of 0.65) between trained ultrasonographers for the detection of fibrillations. Further investigation is needed, but the potential ability of neuromuscular ultrasound to visualize the small movements generated by denervated muscle fibers is promising.
Two other reported findings observed during muscle ultrasound in those with ALS merit discussion. First, Yoshioka and coworkers used ultrasound to evaluate the diaphragm in patients with ALS and found decreased diaphragmatic excursion and no change in diaphragm thickness during respiration.25 They felt this occurred because of diaphragmatic weakness or paralysis, and that ultrasound of the diaphragm may be useful in the diagnosis of ALS (for further discussion of diaphragm ultrasound, see Chapter 12). Second, Saigusa and colleagues used M-mode color Doppler to assess tongue movements in dysarthric patients with ALS and healthy controls.26 They found ultrasound to be a noninvasive and radiation-free method that accurately quantified abnormal tongue movements.
Surrogate Marker of Disease Progression
In a study of 20 individuals with ALS, the thickness of the biceps brachii/brachialis muscle complex (measuring from most superficial aspect of the biceps to the edge of the humerus, at the mid-arm level) was obtained and compared with standard markers of disease progression, such as the ALS functional rating scale, strength testing, and vital capacity (Fig. 8.9).7 Muscle thickness correlated with biceps brachii strength (r = 0.51, p = 0.02), indicating that muscle ultrasound may be a painless, noninvasive, radiation-free, and sensitive technique for monitoring disease progression in ALS and other motor neuron diseases. Yoshioka and coworkers also suggested that diaphragm ultrasound may serve as a surrogate marker of disease progression in ALS.25 Prospective studies of both methods (ultrasound of biceps brachii/brachialis complex and of the diaphragm) are needed to confirm this possible application.
Conclusion
As noted, neuromuscular ultrasound in individuals with motor neuron disease is a relatively unexplored field, but one with significant potential. Studies of nerve and muscle echotexture, using ultra high-frequency transducers may help further our understanding of disease pathogenesis. Examination of nerve roots and distal motor nerves may demonstrate changes that could aid in the diagnosis of motor neuron diseases. Prospective analyses of muscle thickness (including the diaphragm) using ultrasound may demonstrate that it is an excellent technique for following disease progression, both in therapeutic trials and clinical practice. Finally, quantitative muscle ultrasound combined with visualization of fibrillations, may allow for earlier and more accurate diagnosis of motor neuron disease.
1. Logroscino G., Traynor B.J., Hardiman O., et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry. 2008;79:6-11.
2. Shook S.J., Pioro E.P. Racing against the clock: recognizing, differentiating, diagnosing, and referring the amyotrophic lateral sclerosis patient. Ann Neurol. 2009;65(Suppl 1):S10-S16.
3. Senda J., Ito M., Watanabe H., et al. Correlation between pyramidal tract degeneration and widespread white matter involvement in amyotrophic lateral sclerosis: a study with tractography and diffusion-tensor imaging. Amyotroph Lateral Scler. 2009;10:288-294.
4. Abrahams S., Goldstein L.H., Simmons A., et al. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain. 2004;127:1507-1517.
5. Johansson A., Engler H., Blomquist G., et al. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. 2007;255:17-22.
6. Konagaya M., Kato T., Sakai M., et al. A clinical and pathological study of a Japanese case of amyotrophic lateral sclerosis/parkinsonism-dementia complex with family history. J Neurol. 2003;250:164-170.
7. Cartwright M.S., Walker F.O., Caress J.B., Diagnostic ultrasound in amyotrophic lateral sclerosis, 2009, Unpublished Work
8. Heckmatt J.Z., Leeman S., Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656-660.
9. Kamala D., Suresh S., Githa K. Real-time ultrasonography in neuromuscular problems in children. J Clin Ultrasound. 1985;13:465-468.
10. Heckmatt J.Z., Pier N., Dubowitz V. Assessment of quadriceps femoris muscle atrophy and hypertrophy in neuromuscular disease in children. J Clin Ultrasound. 1988;16:177-181.
11. Heckmatt J.Z., Pier N., Dubowitz V. Real-time ultrasound imaging of muscles. Muscle Nerve. 1988;11:56-65.
12. Reimers C.D., Schlotter B., Eicke B.M., Witt T.N. Calf enlargement in neuromuscular diseases: a quantitative ultrasound study in 350 patients and review of the literature. J Neurol Sci. 1996;143:46-56.
13. Pohle R., Fischer D., von Rohden L. Computer-supported tissue characterization in musculoskeletal ultrasonography. Ultraschall Med. 2000;21:245-252.
14. Pillen S., Verrips A., van A.N., et al. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007;17:509-516.
15. Pillen S., van K.M., Nievelstein R.A., et al. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32:1315-1321.
16. van Baalen B.A., Stephani U. Muscle fibre type grouping in high resolution ultrasound. Arch Dis Child. 2005;90:1189.
17. Aydinli N., Baslo B., Caliskan M., et al. Muscle ultrasonography and electromyography correlation for evaluation of floppy infants. Brain Dev. 2003;25:22-24.
18. Arts I.M., van Rooij F.G., Overeem S., et al. Quantitative muscle ultrasonography in amyotrophic lateral sclerosis. Ultrasound Med Biol. 2008;34:354-361.
19. Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
20. Reimers C.D., Muller W., Schmidt-Achert M., et al. Sonographic detection of fasciculations. Ultraschall Med. 1988;9:237-239.
21. Scheel A.K., Toepfer M., Kunkel M., et al. Ultrasonographic assessment of the prevalence of fasciculations in lesions of the peripheral nervous system. J Neuroimaging. 1997;7:23-27.
22. Kohno S., Kawai M. Real time sonographic imaging of fasciculation. Rinsho Shinkeigaku. 1998;38:259-262.
23. van Baalen B.A., Stephani U. Fibration, fibrillation, and fasciculation: say what you see. Clin Neurophysiol. 118, 2007. 1418–1420
24. Pillen S., Nienhuis M., van Dijk J.P., et al. Muscles alive: ultrasound detects fibrillations. Clin Neurophysiol. 2009;120:932-936.
25. Yoshioka Y., Ohwada A., Sekiya M., et al. Ultrasonographic evaluation of the diaphragm in patients with amyotrophic lateral sclerosis. Respirology. 2007;12:304-307.
26. Saigusa H., Saigusa M., Aino I., et al. M-mode color Doppler ultrasonic imaging of vertical tongue movement during articulatory movement. J Voice. 2006;20:38-45.