Chapter 9 Ultrasound of Inflammatory Myopathies
B-Mode Ultrasound Dynamic Evaluation of Muscle Weakness in Myositis
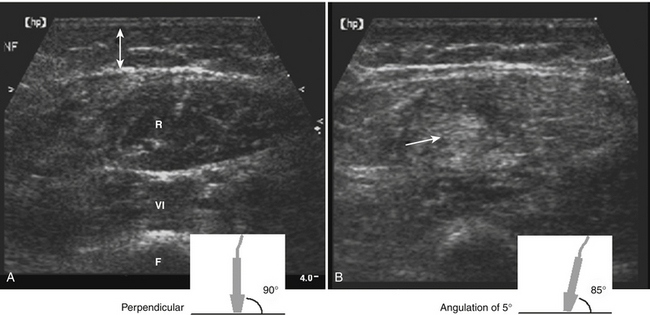
Although the dimensions of normal muscle have been described in a few case series,1,2 the volumes obtained in practice are rather variable, and depend in part on the age and gender of the patient as well as the level of experience of the ultrasonographer.3,4 The reliability and validity of large muscle cross-sectional area measurements were demonstrated in a small study of six normal adults, when used by experienced examiners to monitor muscle thickness over time.5 When properly standardized with regard to location within the muscle, definition of muscle borders, and the angle of incidence (as outlined in Chapter 3), side-to-side comparison of a muscle in a particular patient and changes in muscle thickness can be detected serially over time.
The ability to distinguish between normal and weak muscle with ultrasound in the setting of muscle inflammation has been examined in one small study. Changes in rectus femoris muscle diameter (both horizontally and vertically) in the relaxed and contracted state were compared among nine patients with either “stable active or inactive myositis” (including six with idiopathic polymyositis, two with dermatomyositis, and one with inclusion body myositis, and nine normal patients).6 Horizontal diameter decreased and vertical diameter increased during isometric contraction in all patients. However, the average change in diameter was greater in control patients, and correlated with muscle force measured by dynamometry. These findings suggest that dynamic ultrasound may provide a quantitative adjunct to manual muscle testing in patients with myositis.
Standard B-Mode Ultrasound in Inflammatory Myopathy
Several abnormal ultrasonographic morphologies have been described in the setting of inflammatory myopathy, including “edema-like,” “lipomatous,” and “chronic/atrophic” patterns. None of these patterns is disease specific, and a range of ultrasonographic findings has been described for all inflammatory myopathies.3,7,8
Muscle Edema
In contrast to the normal appearance of muscle (hypoechoic fascicles separated by the hyperechoic perimysial connective tissue), edematous muscle increases in size and echogenicity. This pattern can be observed transiently in the postexercise state,3 which underscores the importance of examining a patient at rest and not immediately after activation of the muscle of interest. This pattern is the least specific, as a multitude of posttraumatic, inflammatory, and infectious etiologies has been associated with muscle edema. Moreover, early infiltration by fat and fibrous tissue can have a similar ultrasonographic appearance, further reducing the diagnostic utility of increased muscle echogenicity.
Muscle Atrophy
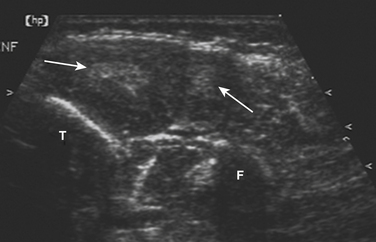
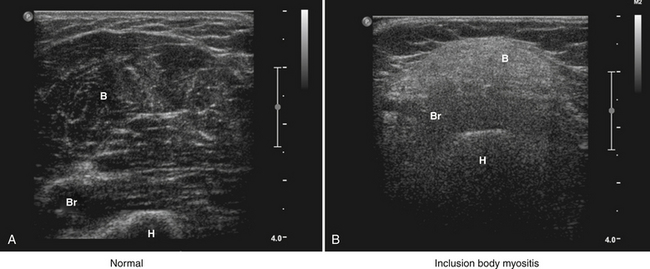
Muscle atrophy can also be identified by ultrasound, and is characterized by increased echogenicity and reduced volume. In contrast to the edematous pattern, in which higher echogenicity is a result of increased water within the muscle, increased density of scattering interfaces and higher acoustic impedance is believed to be the cause in atrophy. Chronic infiltration by fat and fibrous tissue further increases muscle echogenicity. Thus, the combination of reduced muscle volume and increased echogenicity is characteristic of a long-standing process, consistent with either chronic inflammatory myopathy or denervation and disuse.9–11
Echogenicity
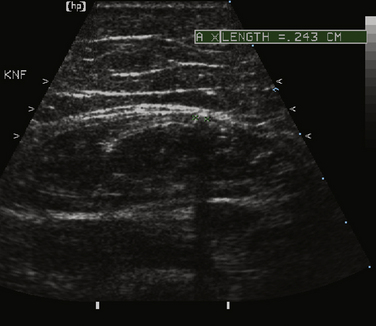
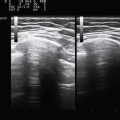
The degree of increased echogenicity, also referred to as echo intensity, can be estimated using either a subjective visual grading scale or quantitative methodology (Fig. 9.1). The most commonly used visual scale was developed by Heckmatt and associates initially for patients with muscular dystrophies.12,13 The Heckmatt score grades muscle echogenicity as grade I: normal echogenicity of muscle with a strong bone echo; grade II: increased muscle echogenicity but with a distinct bone echo; grade III: marked increase in muscle echogenicity, reducing the ability to identify the adjacent bone echo; and grade IV: very strong muscle echogenicity with complete loss of bone echo. Because of concerns regarding low interobserver agreement when using the visual rating scale,14 quantitative methods of measuring echogenicity using computer-assisted gray scale analysis have been developed. To facilitate reproducibility across different imaging platforms, ideal standards for machine configuration, calibration, probe positioning, and selection of the region of interest have been studied.15 As the reproducibility of quantitative gray scale analysis of muscle echogenicity is confirmed and more widespread experience is gained, it will likely come into more common clinical practice (see further discussion of qualitative and quantitative muscle echogenicity assessments in Chapters 3 and 10).
Doppler Ultrasound in Inflammatory Myopathies
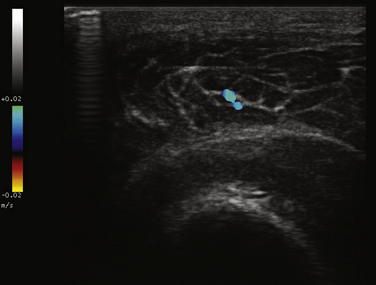
Power Doppler ultrasound provides a noninvasive means of estimating vascular volume and detecting soft tissue hyperemia.16 The technique is neither angle dependent nor subject to aliasing artifact, like conventional color Doppler imaging, which is also used for muscle imaging. However, power Doppler is not capable of measuring capillary flow, and will not differentiate between venous and arterial circulation.3 Doppler signal in muscle is also increased immediately after exercise because of a temporary increase in muscle vascularity, highlighting the importance of interpreting findings in recently exercised muscle with caution.17
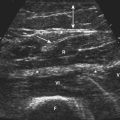
One study designed to assess the utility of combining gray scale and Doppler techniques, examined 73 patients with inflammatory myopathies (including dermatomyositis [17], polymyositis [10], lupus-associated myositis [5], inclusion body myositis [3], focal myositis [1], and sarcoid myositis), and compared ultrasonographic and clinical findings (e.g., muscle power and function) with 6 control subjects. Significant differences between patients and controls were identified using both ultrasound techniques. Specifically, patients with shorter disease duration trended toward increased vascularity on Doppler measurements (Fig. 9.2), correlating with functional and inflammatory scores on clinical assessment. Abnormal findings on gray scale ultrasound (e.g., increased echogenicity and atrophy) were associated with longer disease duration and lower creatine kinase levels.18 The authors concluded that combining the two techniques may increase the ability to differentiate myositis from normal muscle, and help distinguish active from chronic muscle inflammation. A more recent case report highlighted similar findings in a patient with inflammatory myopathy, and resolution of the power Doppler abnormalities after treatment with steroids.19
Contrast-Enhanced Ultrasound
The sensitivity of ultrasound for measuring muscle perfusion and vascular volume can be enhanced by two to three orders of magnitude with contrast agents.3 Encapsulated microbubbles stabilized by proteins and lipids, and measuring 1 to 5 microns in size are the agents most commonly used. These agents remain intravascular and are either destroyed by high-energy ultrasound pulses or caused to oscillate by lower mechanical index pulses, resulting in a measurable change within muscle tissue. Microbubble detection is neither flow nor velocity dependent, making it a highly sensitive measure of perfusion, including flow within capillaries.7
Perfusion can be measured indirectly or directly using microbubble contrast agents. The indirect method involves placing the probe in a fixed position over a muscle, injecting contrast, and measuring the signal intensity over time. The resulting perfusion curves provide an indirect measure of perfusion, including time to peak and maximum intensity values. Absolute perfusion quantification requires application of a “replenishment kinetics” model in a region of interest after destruction of microbubbles within that region. The feasibility of this technique has been demonstrated in normal volunteers,20 and a detailed protocol with limitations has been published by Weber and colleagues using an ultrasound machine with power Doppler capability, a linear array transducer operating at 7 MHz, and injection of an intravenous contrast agent.7 Of note, contraindications to using this contrast agent protocol include severe cardiac and pulmonary disease, pregnancy, and galactosemia.
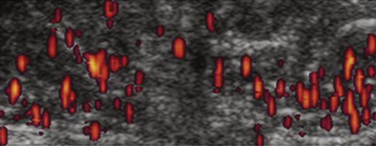
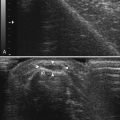
In patients suspected of having dermatomyositis or polymyositis, contrast-enhanced ultrasound has been prospectively studied in 22 patients, and results were compared with 10 healthy volunteers. In patients with histologically confirmed myositis, blood volume, blood flow, and blood flow velocity measurements derived from the contrast-enhanced study were increased when compared with patients who did not have myositis. The authors concluded that the technique could be used to noninvasively identify increased muscle perfusion in clinically involved muscle groups in patients with myositis (Fig. 9.3).21
Contrast-enhanced ultrasound has also been prospectively compared with magnetic resonance imaging (MRI) and muscle biopsy in patients with dermatomyositis or polymyositis. In one study, elevated blood flow measurements yielded a sensitivity of 73%, specificity of 91%, positive predictive value of 80%, and a negative predictive value of 88% for myositis, compared with increased signal intensity on T2-weighted magnetic resonance images to identify muscle inflammation (100%, 88%, 77%, and 100%, respectively). The authors suggested that increased skeletal muscle perfusion measured by ultrasound could serve as a diagnostic marker for inflammatory myopathy, and may augment specificity for inflammation when both ultrasound and MRI are used together.7,22 Moreover, normalization of elevated perfusion values has been associated with a treatment response, suggesting the technique has the potential to assess myositis activity over time and serve as a surrogate marker of disease progression.21
Disease-Specific Findings
Idiopathic Inflammatory Myopathies
Polymyositis, dermatomyositis, and inclusion body myositis are a heterogeneous group of autoimmune syndromes characterized by muscle weakness and inflammation. Although they are often referred to collectively as the idiopathic inflammatory myopathies, histopathology underlying these disorders is quite different, albeit incompletely understood. Clinically, polymyositis is characterized by painless, symmetric proximal weakness. Weakness of neck flexion can occur late in polymyositis; ocular or facial weakness is extremely uncommon. Dermatomyositis is additionally characterized by erythematous papules over the interphalangeal joints (Gottron’s papules), violaceous discoloration over the eyelids (heliotrope rash), photosensitivity, a rash over the shoulders (“shawl sign”), and characteristic nailfold changes. A small proportion of patients have amyopathic dermatomyositis (i.e., dermatomyositis “sine myositis”), demonstrating pathognomonic skin findings in the absence of weakness. Inclusion body myositis is characterized by a more gradually progressive course and a distinct pattern of asymmetric muscle weakness most often involving the deep forearm flexors and the proximal muscles in the lower limbs.23,24
Several studies have examined the utility of ultrasound, specifically in patients with idiopathic inflammatory myopathies. One of the largest to date was conducted by Reimers and coworkers examining adult patients with histologically provend polymyositis (30), dermatomyositis (18), and inclusion body myositis (13), as well as a group of patients with granulomatous myositis (9), and comparing them with 102 control patients. A maximum of 21 muscles were examined (median 15 muscles in control, 16 in myopathy patients) with a 3.75 MHz linear array transducer, recording both visual and quantitative gray scale echogenicity measurements. The sensitivity of muscle ultrasound for detecting myositis with combined visual and quantitative assessment (83%) was not significantly different from electromyography (92%) or elevated serum creatine kinase activity (69%). The positive predictive value of ultrasound was 95%, and the negative predictive value was 89%. Overall, ultrasound was found to be a useful tool for identifying inflammatory myopathy, but no single pattern consistently predicted the underlying idiopathic inflammatory myopathy type.25
A longitudinal study of 11 patients with untreated inflammatory myopathies (dermatomyositis [4], scleroderma-polymyositis [4], polymyositis [2], lupus myositis [1]) assessed the utility of serial ultrasound measurements to identify disease activity and response to therapy. Patients were examined with a high-frequency (6-12 MHz) linear ultrasound probe in B-mode. Muscle echogenicity (using a visual grading scale) and perimysial septa counts were measured at the rectus femoris and vastus lateralis/medialis at study entry and at 6 months. All patients received a standardized treatment regimen with a prednisolone taper to a minimum maintenance dose in combination with methotrexate or azathioprine per the treating clinician’s judgment. Fifteen healthy adults were studied as control subjects. Results included significantly higher muscle echogenicity and septa counts in patients compared with controls. Of the seven patients who completed the 6-month follow-up ultrasound assessment, echogenicity and septa counts returned to normal in six of the seven. Correlation between ultrasound findings and various clinical parameters did not reach statistical significance.26
Inclusion Body Myositis
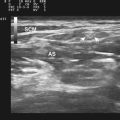
As a consequence of the different speed in disease evolution, inclusion body myositis is typically characterized by more significant muscle atrophy and increased echogenicity because of fatty infiltration, when compared with polymyositis and dermatomyositis. Of note, comparison of ultrasound and histopathologic findings suggests that muscle lipomatosis has a much greater effect on muscular echogenicity than muscle fibrosis in these cases (Fig. 9.4).25 It is also important to note that the ultrasonographic muscle abnormalities follow the clinical distribution of muscle disease, with preferential involvement of the forearm flexors and quadriceps.
Dermatomyositis
A study of five children with acute untreated dermatomyositis revealed an increase in muscle echogenicity in two, with marked alteration in the relative echogenicity of the rectus femoris and vastus lateralis muscles when the transducer was angulated in the transverse plane (Fig. 9.5). The authors proposed that this finding was relatively specific for dermatomyositis, and speculated that it was caused by underlying perifascicular atrophy, infiltration of the connective tissue surrounding the fascicles by inflammatory cells, and the oblique orientation of the vastus and rectus femoris muscles.27 Other authors have suggested that this finding can be observed in any inflamed muscle, as well as normal muscle, and is thus not a clinically useful finding.28
MRI and ultrasound were also used to evaluate five patients with cutaneous lesions consistent with dermatomyositis, but no clinical evidence of myositis, and compared with studies from both positive and negative control groups. Three of the five patients with dermatomyositis sine myositis had MRI and ultrasound findings consistent with muscle inflammation. In addition, positive controls with known prior muscle involvement but no evidence of active myositis at the time of the study were found to have similar changes on ultrasound and MRI. The authors concluded that both MRI and ultrasound were useful adjunctive means of identifying muscle involvement in these patients, and that although ultrasound appeared to be the more cost-effective test, MRI was more sensitive and specific for inflammation.29
Finally, although generally not seen in adult forms of idiopathic inflammatory myopathy, perifascicular and intramuscular calcifications, which appear as echogenic foci with posterior acoustic shadowing, can be found in children with juvenile dermatomyositis (Fig. 9.6).30
Pyomyositis
Pyomyositis is an intramuscular infection involving one or more muscle groups. This disease is believed to be caused by transient bacteremia in the setting of muscle injury, caused by either intensive exercise, trauma, the presence of concurrent medical conditions (e.g., human immunodeficiency virus [HIV], diabetes), or medications (e.g., zidovudine, steroids) adversely affecting muscle health. Although the most common pathogen is Staphylococcus aureus, other etiologies include streptococci (group A, B, C, G, or Streptococcus pneumoniae), gram-negative organisms, anaerobes, mycobacteria, fungi, and parasites. The stages of pyomyositis include: (1) the “invasive stage” involving infection of the muscle causing edema and pain, (2) the “suppurative phase” occurring 1 to 3 weeks later and characterized by formation of an abscess, and (3) the final stage involving the systemic spread, septicemia, and multifocal abscesses.31
Ultrasound findings in pyomyositis include increased echogenicity of muscle fibers, swelling of the subcutaneous fat, and reduced fibroadipose septa echogenicity. When present, abscesses can be identified as circumscribed areas of low echogenicity. Although an abscess cannot be reliably distinguished from a hematoma or seroma,28 the presence of septations within the collection or increased surrounding vascularity on color Doppler imaging have been proposed as supportive evidence of abscess formation.32 In appropriate cases, ultrasound can also be used to direct placement of a needle for biopsy or therapeutic drainage.
In a retrospective study of 12 children with pyomyositis, the diagnostic utility of ultrasound and MRI was reviewed. In patients with limb muscle involvement, ultrasound successfully identified altered echogenicity in all cases and fluid collection in 5 patients, consistent with the clinical and MRI findings. However, ultrasound only identified inflammatory changes in 1 of the 4 patients with confirmed pelvic involvement. The authors of the study suggested that ultrasound is useful in pyomyositis affecting the limb, but the modality is not sufficiently sensitive to exclude pelvic involvement. These findings are consistent with a prior study suggesting a lower diagnostic yield for pyomyositis affecting the muscles of the pelvis33.
1. Young A., Stokes M., Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5:145-154.
2. Kawakami Y., Abe T., Kanehisa H., Fukunaga T. Human skeletal muscle size and architecture: variability and interdependence. Am J Hum Biol. 2006;18:845-848.
3. Adler R.S., Garofalo G. Ultrasound in the evaluation of the inflammatory myopathies. Curr Rheumatol Rep. 2009;11:302-308.
4. Maurits N.M., Bollen A.E., Windhausen A., et al. Muscle ultrasound analysis: normal values and differentiation between myopathies and neuropathies. Ultrasound Med Biol. 2003;29:215-225.
5. Reeves N.D., Maganaris C.N., Narici M.V. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91:116-118.
6. Chi-Fishman G., Hicks J.E., Cintas H.M., et al. Ultrasound imaging distinguishes between normal and weak muscle. Arch Phys Med Rehabil. 2004;85:980-986.
7. Weber M.A., Krix M., Delorme S. Quantitative evaluation of muscle perfusion with CEUS and with MR. Eur Radiol. 2007;17:2663-2674.
8. Walker U.A. Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Curr Opin Rheumatol. 2008;20:656-661.
9. Sofka C.M., Haddad Z.K., Adler R.S. Detection of muscle atrophy on routine sonography of the shoulder. J Ultrasound Med. 2004;23:1031-1034.
10. Strobel K., Hodler J., Meyer D.C., et al. Fatty atrophy of supraspinatus and infraspinatus muscles: accuracy of US. Radiology. 2005;237:584-589.
11. Pillen S., Arts I.M., Zwarts M.J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37:679-693.
12. Heckmatt J.Z., Dubowitz V., Leeman S. Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet. 1980;1:1389-1390.
13. Heckmatt J.Z., Leeman S., Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656-660.
14. Pillen S., van Keimpema M., Nievelstein R.A., et al. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32:1315-1321.
15. Zaidman C.M., Holland M.R., Anderson C.C., Pestronk A. Calibrated quantitative ultrasound imaging of skeletal muscle using backscatter analysis. Muscle Nerve. 2008;38:893-898.
16. Martinoli C., Pretolesi F., Crespi G., et al. Power Doppler sonography: clinical applications, Eur J Radiol. 1998;27(Suppl 2):S133-S140.
17. Newman J.S., Adler R.S., Rubin J.M. Power Doppler sonography: use in measuring alterations in muscle blood volume after exercise. AJR Am J Roentgenol. 1997;168:1525-1530.
18. Meng C., Adler R., Peterson M., Kagen L. Combined use of power Doppler and gray-scale sonography: a new technique for the assessment of inflammatory myopathy. J Rheumatol. 2001;28:1271-1282.
19. Visser L.H. High-resolution power Doppler sonography in inflammatory myopathy. Muscle Nerve. 2009;39:553-554.
20. Krix M., Weber M.A., Krakowski-Roosen H., et al. Assessment of skeletal muscle perfusion using contrast-enhanced ultrasonography. J Ultrasound Med. 2005;24:431-441.
21. Weber M.A., Krix M., Jappe U., et al. Pathologic skeletal muscle perfusion in patients with myositis: detection with quantitative contrast-enhanced US: initial results. Radiology. 2006;238:640-649.
22. Weber M.A., Jappe U., Essig M., et al. Contrast-enhanced ultrasound in dermatomyositis and polymyositis. J Neurol. 2006;253:1625-1632.
23. Limaye V.S., Blumbergs P., Roberts-Thomson P.J. Idiopathic inflammatory myopathies. Intern Med J. 2009;39:179-190.
24. Dimachkie M.M., Barohn R.J. Idiopathic inflammatory myopathies. Front Neurol Neurosci. 2009;26:126-146.
25. Reimers C.D., Fleckenstein J.L., Witt T.N., et al. Muscular ultrasound in idiopathic inflammatory myopathies of adults. J Neurol Sci. 1993;116:82-92.
26. Mittal G.A., Wadhwani R., Shroff M., et al. Ultrasonography in the diagnosis and follow-up of idiopathic inflammatory myopathies: a preliminary study. J Assoc Physicians India. 2003;51:252-256.
27. Heckmatt J.Z., Pier N., Dubowitz V. Real-time ultrasound imaging of muscles. Muscle Nerve. 1988;11:56-65.
28. Reimers C.D., Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol. 1997;9:475-485.
29. Stonecipher M.R., Jorizzo J.L., Monu J., et al. Dermatomyositis with normal muscle enzyme concentrations: a single-blind study of the diagnostic value of magnetic resonance imaging and ultrasound. Arch Dermatol. 1994;130:1294-1299.
30. Fleckenstein J.L., Reimers C.D. Inflammatory myopathies: radiologic evaluation. Radiol Clin North Am. xii, 1996;34:427-439.
31. Crum-Cianflone N.F. Infection and musculoskeletal conditions: infectious myositis. Best Pract Res Clin Rheumatol. 2006;20:1083-1097.
32. Gottlieb R.H., Meyers S.P., Hall C., et al. Pyomyositis: diagnostic value of color Doppler sonography. Pediatr Radiol. 1995;25(Suppl 1):S109-S111.
33. Gubbay A.J., Isaacs D. Pyomyositis in children. Pediatr Infect Dis J. 2000;19:1009-1012.