Ultrasound in the neonatal and pediatric intensive care unit
Overview
Children and neonates are ideal candidates for the implementation of the holistic approach (HOLA) critical care ultrasound concept (see Chapter 1). This is mainly due to their small body size, which permits the fast application of all possible ultrasound techniques from head to toe. However, in the individual pediatric patient, only a part of the available ultrasound techniques will be clinically indicated, and many sites of generic scanning will need to be omitted. Ultrasound is safe in imaging pediatric patients if used when clinically indicated and with the minimally necessary energy exposures, based on the ALARA (as low as reasonably achievable) principle. In the pediatric intensive care unit (PICU), HOLA ultrasound is easily scaled down to specific application profiles; some of those require expert input to interpret findings that should be processed under the light of clinical judgment or require special expertise. Elective and repetitive ultrasound scanning is often necessary for diagnostic and monitoring purposes, whereas goal-directed therapies can be guided by means of ultrasonography.1,2 This chapter briefly outlines the role of ultrasound in the neonatal intensive care unit (NICU) and PICU.
Hemodynamic monitoring
Ultrasound is an essential tool in the evaluation of nontraumatic, symptomatic, undifferentiated hypotension in NICU and PICU patients. It is a useful adjunct to the physical examination, which may be inconclusive, and aids in bedside hemodynamic monitoring and management of pediatric patients. Cardiovascular morphology and function as well as volume status are basic parameters in the hemodynamic monitoring of both adults and children (see Chapter 36).3,4 Bedside ultrasound can facilitate the assessment of the above-mentioned parameters and guide therapeutic interventions (e.g., administration of fluids or vasoactive agents).
Echocardiography is an essential tool of pediatric hemodynamic monitoring. The assessment of left ventricular (LV) global systolic function is mainly performed by means of LV fraction shortening (FS) and ejection fraction (EF), either by global visual assessment or objective numerical measurement. In pediatric patients, global eye assessment of LVEF is usually an easily applied and reproducible method. When the LV empties more than half of its volume, the EF is considered to be normal (EF > 55%). Mild (EF = 45%-55%), moderate (EF = 30%-44%), and severe (EF < 30%) impairment can be estimated accordingly. Color Doppler imaging and tissue Doppler indexes of transmitral flow, reflecting LV end-diastolic pressure (and thus left atrial pressure), can be also applied in the evaluation of cardiac function as analyzed in the echocardiographic section of this book. Echocardiography can be used in almost all emergency situations, including cardiac arrests.5
Vena cava and global ventricular volume analysis, along with lung ultrasound, can be used in the evaluation of volume status in pediatric patients. By implementing the HOLA ultrasound concept in hemodynamic monitoring (see Chapter 36) and using echocardiography as a core tool, a simple four-step algorithm can be applied in the evaluation of pediatric patients in shock states.
1. Gross ruling out of preexisting cardiac disease (dilatation or hypertrophy of the left and right ventricle or valvular abnormalities) or genetic cardiovascular abnormalities (e.g., ventricular septal defect, aortic coarctation, right-sided aneurysmal aorta).
2. Vena cava analysis.6 A small inferior vena cava (IVC) with spontaneous collapse suggests hypovolemia (e.g., inspiratory collapse > 50% in spontaneous ventilation). Hypovolemia can be considered as “absolute” (hemorrhage) or “relative” (e.g., sepsis, anaphylaxis, third spacing). A distended and fixed IVC may suggest pulmonary hypertension or tamponade when associated with pertinent right ventricular (RV) echocardiographic signs (see step 3). The presence of an A-line profile in lung ultrasound usually rules out pulmonary edema at this stage. Cautious volume loading therapy can therefore be attempted.
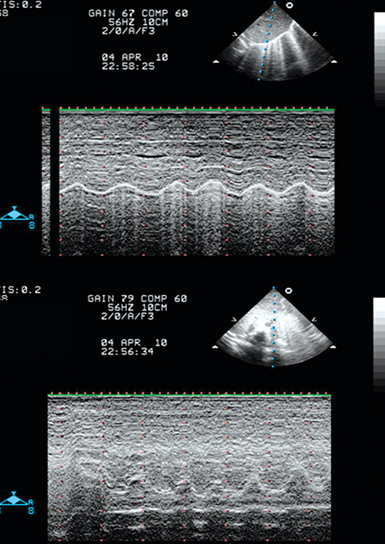
3. Evaluation of RV function. In the presence of a fixed and distended IVC, a small and hyperkinetic right ventricle is suggestive of tamponade (especially if combined with the rapid accumulation of pericardial fluid), whereas a dilatated and hypokinetic right ventricle may signify pulmonary hypertension. The assessment of RV volume is rather difficult by usual two-dimensional echocardiography. However, RV end-diastolic area (EDA) can be easily assessed. Thus we should underline the effect of positive end-expiratory pressure (PEEP) in the hemodynamic equation. With increasing PEEP, RV EDA progressively increases, and thus this phenomenon should be co-evaluated when assessing RV function in a shocked mechanically ventilated pediatric patient (Figure 47-1). Additional echocardiographic signs suggestive of tamponade are right atrial inversion/collapse in late systole, RV inversion/collapse in early diastole, swinging heart (clockwise rotation), and presence of fluids or clots around the heart. Additional Doppler echocardiographic signs suggestive of pulmonary hypertension are analyzed in detail in Chapter 33. At this stage, other causes of obstructive shock, such as a pneumothorax, can be ruled out by lung ultrasound (see Chapters 19–21).
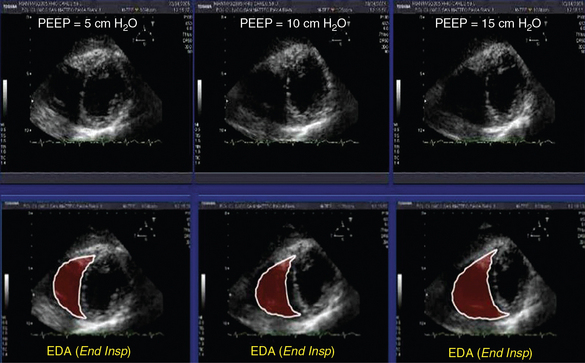
Figure 47-1 Effects of positive end-expiratory pressure (PEEP) of 5, 10, and 15 cm H2O, respectively, on the right ventricular end-diastolic area (EDA) in a mechanically ventilated pediatric patient (bottom, measurements performed at end inspiration).
4. Assessment of LV function. A hypokinetic LV and a B-line profile depicted by lung ultrasound in a hypotensive pediatric patient with low blood oxygen saturation are suggestive of cardiogenic pulmonary edema, indicating thus the administration of diuretics and inotropes. Of note, the typical appearance of adult lung ultrasound signs, such as A-lines and B-lines may differ in pediatric patients, especially in neonates. In general, fluid administration in such cases should be done extremely cautiously.
Lung-pleural ultrasound and mechanical ventilation
Lung ultrasound pediatric applications are similar when compared with the applications of the method in adult patients. Hence diagnosis and management of lung disorders, especially lung interstitial syndrome, pneumothorax, lung consolidation, and pleural effusions, by means of lung ultrasound are routinely performed in the NICU and PICU. Regular follow-up lung ultrasound examinations facilitate the early detection of lung and pleural disorders and enhance their therapeutic management. When using lung ultrasound as a routine diagnostic and monitoring tool, children and neonates are largely spared the detrimental ionizing effect of repetitive chest radiography or computed tomography scans.7 Such ultrasonography also may be used in periendotracheal intubation procedures.8
Sometimes, lung ultrasonographic findings may be difficult to interpret in the NICU because their appearance is slightly different compared with the usual one known in adults.9 For example, the hyperechoic B-lines in neonates and infants may be confluent (not comet tail–like), which makes counting them impossible. A global assessment of the percentage of white (hyperechoic)/black (hypoechoic) areas in the ultrasound screen divided by 10 usually gives a correspondent number of B-lines. This may aid in guiding diuretic or positive-pressure ventilation (PPV) therapy in cardiogenic pulmonary edema.
In general, PPV supports the function of an impaired LV by reducing the transmural pressure across the LV free wall (LV afterload is reduced). In contrast, PPV is usually a functional burden on an already impaired RV function because of the reduction of preload and increase of afterload, respectively (see Figure 47-1). Ultrasonography can help in optimizing PPV to achieve the maximal benefit in oxygenation, while avoiding its side effects on cardiac function. An advanced HOLA protocol would be combined lung and cardiac ultrasound to estimate the optimum level of PEEP. Optimization of PEEP could facilitate the recruitment of collapsed alveoli, and the improvement in pulmonary functional residual capacity thus reduces the pulmonary vascular resistance. In turn, this could minimize the burden on RV function. Thus general chest ultrasound (lung and cardiac ultrasound) evaluation could guide both ventilatory and circulatory support. The optimization of heart-lung interaction could enhance the therapeutic effect of mechanical ventilation and also facilitate the weaning process (see Chapter 34).
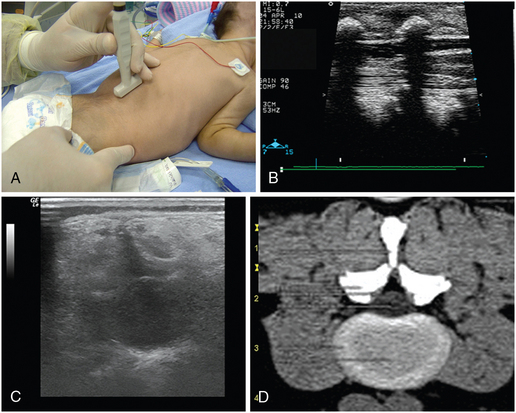
In pediatric patients, diaphragmatic and vocal cord paralysis can lead to extubation failure. Ultrasound can easily detect the above disorders in the PICU. The ultrasound examination of the vocal cords is performed while the child is breathing spontaneously, in a supine position with minimal neck extension, to allow a space for the transducer to be placed and gently manipulated on the cricoid cartilage and trachea.10 A high-frequency linear or curvilinear probe is used. The diaphragm normally moves downward on inspiration and upward on expiration. When paralyzed, the diaphragm either remains immobile in a fixed position (upward) or may exhibit paradoxic movement (moves upward during inspiration). When paretic, the diaphragm is moving in the right direction; however, the excursion is limited. Fluoroscopic examination is the gold standard in assessing diaphragmatic motion. Recently, ultrasound examination has been successfully integrated in the study of diaphragmatic motion. Both hemidiaphragms of the child should be ultrasonographically assessed (on both longitudinal and transverse planes) during quiet respiration by pleural ultrasound. High transverse scans must be undertaken, with both diaphragms in view, for movement comparison. Important technical points follow.
1. In children older than 2 years, a 3- to 5-MHz frequency transducer is preferred, whereas a 5- to 8-MHz frequency curvilinear transducer is used for children younger than 2 years.
2. Subcostal transverse views with the transducer pointer oriented at 9:00 o’clock are not ideal for the visualization of the ipsilateral hemidiaphragm but do aid in a general preview (the pointer should be oriented in the right side of the screen, the posterior aspect of the diaphragm is usually visualized, stomach air prevents the clear visualization of the hemidiaphragm).
3. Longitudinal views with the transducer pointer oriented at 12:00 o’clock (cephalic) are usually applied for visualizing the ipsilateral hemidiaphragm (coronal plane at the midaxillary line (diaphragmatic level) and axial plane at the subcostal position).
4. Next, M-mode recordings (Figure 47-2) are performed when the cursor is almost vertical on the hemidiaphragm (while moving with a maximum deviation of 20 degrees from the vertical position). M-mode tracings are obtained, in the axial subcostal or in the coronal midaxillary views on either side with the transducer pointer oriented at 12:00 o’clock (cephalic).11 The amplitude of the movement of the hemidiaphragmatic copula should be greater than 4 mm and oriented toward the transducer (during inspiration).
Procedural ultrasound
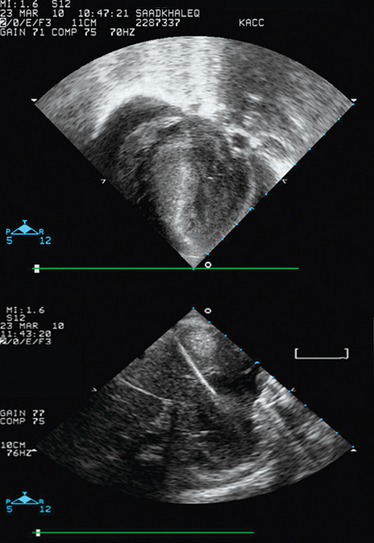
Common procedures (e.g., ultrasound-guided vascular access) are accomplished in the emergency room/ICU with ultrasound guidance.12 Additional ultrasound-guided procedures can be performed in the NICU and PICU, such as lumbar puncture (Figure 47-3), paracentesis of fluid collections, pericardiocentesis (Figure 47-4), and placement of peripherally inserted central venous catheters. Finally, abscess, gall bladder and suprapubic drainage, or the placement of transhepatic lines can also be performed with ultrasound guidance in pediatric patients.
Ultrasonographic evaluation of newborn emergencies
Abdominal surgical emergencies
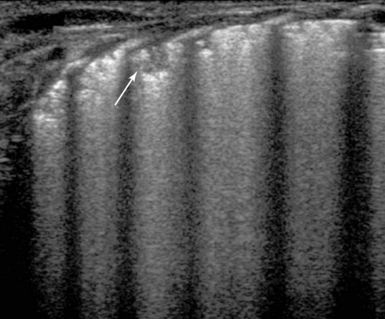
Necrotizing enterocolitis (NEC) is an acquired disorder that is characterized by diffuse necrotic injury of the mucosal and submucosal layers of the bowel wall. It is the most serious gastrointestinal disorder that occurs during the neonatal period. NEC affects mostly the extremely-low-birth-weight (ELBW) infants but can occur in full-term infants as well. The mortality rate for NEC is estimated to be 11.5 to 12.3 per 100,000 infant deaths, greatly exceeding that of other gastrointestinal (surgical) disorders. Radiographic findings are essential in the staging of NEC and determine the need for surgical intervention. Ultrasonography may detect air bubbles moving through the wall of the intestine (pneumatosis intestinalis Figure 47-5). Accordingly, color Doppler imaging may detect hyperemia or decreased blood flow in the intestinal wall.13 Ultrasonographic detection of air in the portal system can also be indicative of NEC (see Chapter 41). The above ultrasound findings, along with other clinical signs, aid in the diagnosis of NEC before bowel perforation and development of peritonitis.
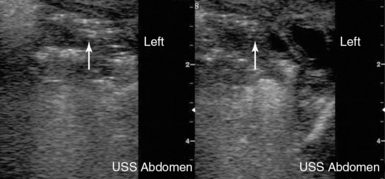
Figure 47-5 Ultrasound detecting hyperechoic punctiform lesions (white arrow) casting acoustic shadows, which are indicative of air bubbles in the intestinal wall (pneumatosis intestinalis).
Neonatal bowel obstruction is a serious disorder that can be complicated by intestinal perforation. Early radiologic studies in cases of neonatal bowel obstruction are needed to rule out intestinal malrotation with midgut volvulus. Up to 40% of patients with malrotation present within the first week of life. It is often difficult to differentiate malrotation from other causes of intestinal obstruction, such as intestinal atresia. Ultrasonography may aid in diagnosing intestinal obstruction (see Chapter 41). The diagnosis of a dilatated intestinal loop with liquid content adjacent to a very narrow atrophic loop may indicate the diagnosis of intestinal atresia. The identification of the superior mesenteric vein to the left of the superior mesenteric artery is highly suggestive of malrotation.
Central nervous system emergencies
Intraventricular hemorrhage
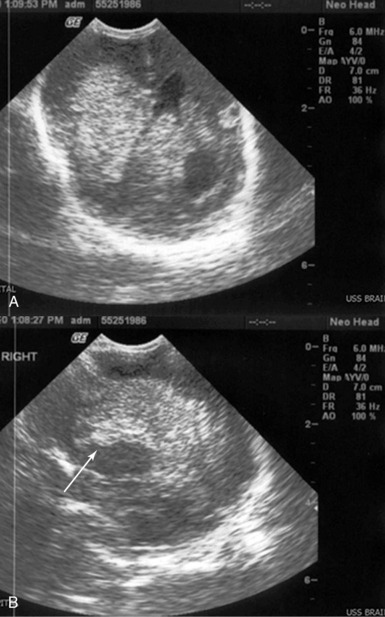
Extremely-low-birth-weight preterm infants are at high risk of many of the complications of prematurity. Sudden deterioration of their clinical status may occur in the first few days of life because of various causes. Severe intraventricular hemorrhage (IVH) is a detrimental complication that occurs in ELBW infants because of impaired cerebral autoregulatory ability. The latter may result in rapid alterations of cerebral blood flow and large changes in cerebral blood pressure. Subsequently, these sudden changes can result in hemorrhage inside the lateral ventricles and hemorrhagic infarction in the adjacent brain parenchyma (grade IV IVH). Grade IV IVH is usually manifested as a neonatal shock state accompanied by metabolic acidosis, hypotension, apnea, and bradycardia (differential diagnosis: pneumothroax, patent ductus arteriosus, etc.). Correct diagnosis would impact decisions for further management and support as well as avoidance of unnecessary therapy. An ultrasound transducer (5-10 MHz) that fits in the infant’s open anterior fontanel can easily yield the diagnosis (Figure 47-6).
Respiratory emergencies
Transient tachypnea of the newborn
1. Delivered by cesarean section (CS)
2. Born to mothers with diabetes
Copetti et al3 described a peculiar lung ultrasound sign, called the double lung point, in newborns with TTN. In the latter case, lung ultrasound showed a difference in lung echogenicity between the upper and lower lung areas. Specifically, coalescent B-lines were observed in the inferior lung fields, whereas those were rare in the superior fields (Figure 47-7). Of note, this ultrasonographic finding was not observed in healthy infants or in infants with respiratory distress syndrome, atelectasis, pneumothorax, pneumonia, or pulmonary hemorrhage. Thus sensitivity and specificity of the double lung point were 100% in the diagnosis of TTN.
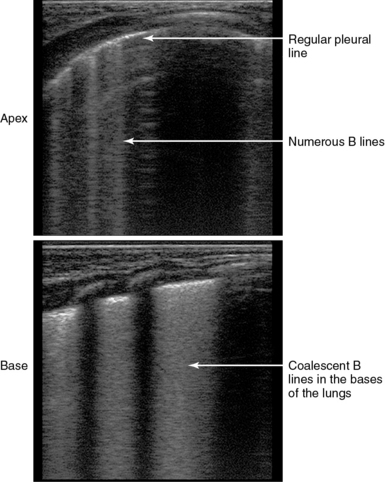
Figure 47-7 The double lung point in transient tachypnea of the newborn (TTN) of the newborn. (Courtesy Dr. R. Copetti.)
Of interest, in normal babies delivered by CS, multiple B-lines can be found soon after birth, bilaterally. These eventually disappear after a few days. Finally, the application of mechanical ventilation in neonates can result in several complications, such as bronchopulmonary dysplasia, which can be ultrasonographically detected as small multiple subpleural consolidations underlining an irregular and rather coarse pleural line (Figure 47-8). Thus it is worth mentioning that the classic “bat sign,” which is formed by the acoustic shadows of the ribs, in lung ultrasound is rather obscure in neonates, because their ribs consist of very soft cartilaginous components.
Cardiovascular emergencies
Analyzing all possible cardiovascular emergencies of the newborn is beyond the scope of this chapter. However, we briefly outline one of the most common causes of neonatal shock—a patent ductus arteriosus (PDA). Neonatal shock may be due to the closure of the ductus arteriosus in some ductal-dependent cardiac lesions, such as severe aortic coarctation, pulmonary atresia, and transposition of great arteries. Ultrasound PDA examination aids in establishing a diagnosis and monitors the effect of prostaglandin infusion. Identification of the blood flow direction (by color Doppler imaging) in the PDA aids in the diagnosis of pulmonary hypertension (right–to-left shunt). Another scenario is a neonate who suffers from heart failure and becomes inevitably ventilator dependent, in the absence of intracardiac lesions, mainly because of the presence of a large PDA with a left-to-right shunt. Intracardiac thrombi can also be the cause of neonatal shock status.14 In all the above clinical scenarios, ultrasound examination of the heart and the PDA is essential in determining the correct diagnosis and monitoring therapy.15
Pearls and highlights
• Children and neonates are ideal candidates for the implementation of the HOLA critical care ultrasound concept. This is mainly due to their small body size, which permits the fast application of all possible ultrasound techniques from head to toe.
• Echocardiography has a pivotal role in the investigation of cardiovascular structure and function and in the assessment of volume status in PICU and NICU patients.
• Lung and pleural ultrasound can detect and monitor various pulmonary abnormalities as well as mechanical ventilation side effects. However, their findings should be interpreted with caution because the typical appearance of adult lung ultrasound signs may differ in pediatric patients, especially in neonates.
• Ultrasound can evaluate diaphragmatic and vocal cord paralysis, which may lead to extubation failure in PICU and NICU patients.
• Combined lung and cardiac ultrasound can estimate the optimum level of PEEP in mechanically ventilated pediatric patients. The optimization of PEEP could improve the respiratory status while minimizing the burden on RV function in these patients. The subsequent optimization of heart-lung interaction could further enhance the therapeutic effect of mechanical ventilation and also facilitate the weaning process.
• Several newborn emergencies, such as necrotizing enterocolitis, neonatal bowel obstruction, brain intraventricular hemorrhage, transient tachypnea (type II respiratory distress syndrome), and patent ductus arteriosus can be ultrasonographically identified and monitored in the NICU.
References
1. De Bruyn, R, Pediatric ultrasound: how, why and when. Elsevier/Churchill Livingstone, Edinburgh, 2005.
2. Hegenbarth, MA, Bedside ultrasound in the pediatric emergency department: basic skill or passing fancy. Clin Ped Emerg Me. 2004; 5:201–216.
3. Spurney, CF, Sable, CA, Berger, JT, Martin, GR. Use of a hand-carried ultrasound device by critical care physicians for the diagnosis of pericardial effusions, decreased cardiac function, and left ventricular enlargement in pediatric patients. J Am Soc Echocardiogr. 2005; 18:313–319.
5. Vignon, P, Dugard, A, Abraham, J, et al. Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med. 2007; 33:1795–1799.
6. Tsung, JW, Blaivas, M, Feasibility of correlating the pulse check with focused point-of-care echocardiography during pediatric cardiac arrest: a case series. Resuscitatio. 2008; 77:264–269.
7. Barbier, C, Loubieres, Y, Schmit, C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004; 30:1740–1746.
8. Rosenberg, HK. The complementary roles of ultrasound and plain film radiography in differentiating pediatric chest abnormalities. Radiographics. 1986; 6:427–445.
9. Galicinao, J, Bush, AJ, Godambe, SA, Use of bedside ultrasonography for endotracheal tube placement in pediatric patients: a feasibility study. Pediatric. 2007; 120:1297–1303.
10. Copetti, R, Cattarossi, L, Macagno, F, et al, Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis,. Neonatolog. 2008; 94:52–59.
11. Shaath, GA, Jijeh, A, Alkurdi, A, et al. Ultrasonography assessment of vocal cords mobility in children after cardiac surgery, J Saudi Heart Assoc. dx.doi.org/10.1016/j.jsha.2012.02.009, 2012.
12. Epelman, M, Navarro, OM, Daneman, A, Miller, SF, M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radio. 2005; 35:661–667.
13. Froehlich, CD, Rigby, MR, Rosenberg, ES, et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Crit Care Med. 2009; 37:1090–1096.
14. Chandler, JC, Hebra, A. Necrotizing enterocolitis in infants with very low birth weight. Semin Pediatr Surg. 2009; 9:63–72.
15. O’Carroll, C, El-Khuffash, A, Molloy, E, et al. Targeted neonatal echocardiography (TnECHO) and increased detection of intracardiac thrombi and endocarditis in very low birth weight infants. Internet J Pediatr Neonatol. 12, 2010.