Ultrasound-guided vascular access: Trends and perspectives
Many randomized controlled trials and meta-analyses have associated UGVA with a considerable reduction in complications and increased first-attempt success when compared with standard landmark techniques.1–3 As 1 of their 11 practices to improve patient care, the Agency for Healthcare Research and Quality (AHRQ) recommends the use of ultrasound for placement of a central venous catheter (CVC).4 The National Institute for Clinical Excellence (NICE), as part of their NICE technology guidelines, advocated the routine use of UGVA.5 Most recently, an international, evidence-based consensus statement was developed to assist clinicians in performing UGVA and as a reference for future clinical research. The group advised that ultrasound guidance can be used not only for central venous cannulation but also for peripheral and arterial cannulation. In addition to guidance, the group recommended the use of ultrasound to check for immediate and life-threatening complications, as well as catheter positioning.6
Patient and technical considerations
The implementation of a simple three-step UGVA technique (pre-procedural scanning, real-time ultrasound-guided cannulation, post-procedural scanning) maximizes success rates and decreases complications in the intensive care unit (ICU).6 A pre-procedural scan is recommended to guide the selection of an optimal target vessel.4–6 Usual criteria for the selection of an optimal vein are:
Normal venous patency (venous collapse during breathing or compression without signs of thrombosis)
Easy accessibility (relatively short distance from the skin surface to the vessel wall)
Adequate size (vessel size less than three times the caliber of catheter may carry a greater risk for thrombosis)7
Absence of anatomical variation
Intended purpose of cannulation (i.e., neck surgery often requires infraclavicular vascular access)
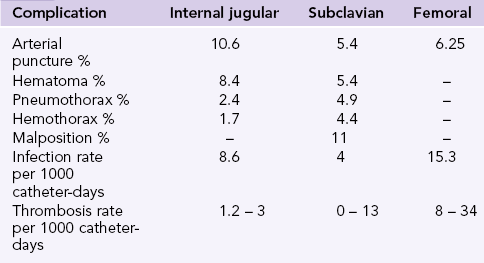
Complication rates after the implementation of landmark techniques differ between sites (Chapter 10). The internal jugular vein carries the highest risk of accidental arterial puncture and hematoma, the subclavian carries the highest risk for pneumothorax, hemothorax, and catheter malposition, while the femoral vein carries the highest risk for thrombosis and infection (Table 11-1). The subclavian vein is considered advantageous in the ICU (compared to the internal jugular or the femoral vein) as it carries the lowest infection risk. However, recent studies show that Chlorhexidine gluconate -impregnated sponge dressing when used with the standard care decreases the incidence of major catheter-related infections from 1.4 to 0.6 per 1000 catheter-days.8 The American Society of Anesthesiologists Task Force on Central Venous Access recommends the use of a central line insertion work and safety checklist and bundling of required equipment to minimize errors, risk of infection, and complications.9 These measures have shown reduction (up to 66%) in central line-associated bloodstream infections.10
Preprocedural tips
• The implementation of a strict sterilization process including use of a sterile probe cover and gel (Figure 11-1)
• Avoid applying extreme probe pressure on the vessel as normal veins are collapsible vessels
• Optimize the two-dimensional image: center the image on the screen and adjust depth, gain and focus, while obtaining the proper orientation of anatomy with standardization of the dot on the left (Chapter 1).
• When a clear two-dimensional image of the vein is obtained check its patency by applying probe pressure to exclude thrombosis (Video 11-1).![]()
Notably, a thorough preprocedural scan at a prospective region of interest (ROI) should be performed because of the frequent finding of venous and arterial asymmetry between symmetric sites. In that sense, anatomic diversities may exist as well in occasional patients (e.g., duplicated femoral vein). Venous compressibility and patency should be examined because exclusion of thrombosis is mandatory (Chapter 9). The latter is more commonly observed at the common femoral vein site than at other cannulation sites, and ultrasound monitoring of a central line may reveal cases of catheter-related thrombosis (Figure 11-2). Occasionally, trauma to the venous wall, trapped air, hematomas, arteriovenous fistulas, or injuries to nonvascular adjacent structures may be identified after a “clumsy” blind attempt or multiple blind penetrations, which could produce local trauma (Figure 11-3). Estimating vessel size is equally important, as previously mentioned. The size of the internal jugular vein can be evaluated before and after a Valsalva maneuver, which may make a relatively small-caliber vessel that is seemingly difficult to catheterize become robust and easy to puncture.11 Moreover, the diameter of the internal jugular vein is usually found to be larger when the vessel is depicted in the lower neck area (scanning caudally toward the clavicle) than in the upper neck area (scanning cranially toward the mandible). When planning on catheterizing the internal jugular or subclavian vein, the pleura should be assessed for a sliding lung while identifying the vasculature.
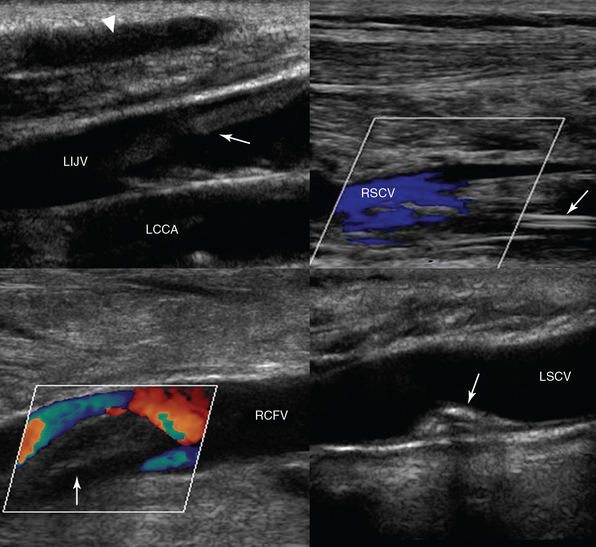
Figure 11-2 (Top) Depiction of a superficial hematoma (arrowhead) and a fresh floating thrombus in the left internal jugular vein (LIJV), which is overlying the left common carotid artery (LCCA), on a longitudinal plane (left). A longitudinal view of the right subclavian vein (RSCV) shows partial flow from central line–associated thrombosis (arrow). (Bottom) Fresh thrombus (arrow) obstructing flow in the right common femoral vein (RCFV) on a longitudinal plane (left). A longitudinal view of the left subclavian vein (LSCV) shows a calcified remnant of a thrombus (following treatment with an anticoagulant) attached to its posterior wall that is not obstructing the vessel’s lumen (right).
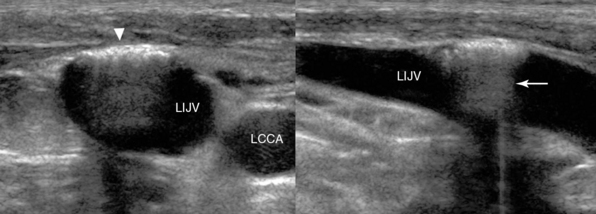
Figure 11-3 Sequelae of a blind attempt at cannulation of the left internal jugular vein (LIJV). LCCA, left common carotid artery. Transverse (left) and longitudinal (right) views show trauma to the anterior wall (arrowhead) of the LIJV with trapped air that is causing enhanced posterior acoustic shadowing (arrow).
Visualization of the vasculature is most often done with B-mode ultrasound. When compared with B-mode, use of the color Doppler mode for vascular access has been associated with a longer learning curve, longer insertion time, and higher cost.12 Although in the vast majority of cases the absence of Doppler capability does not preclude safe catheterization, it can be of assistance when identification of the target vasculature is difficult. Color Doppler and pulsed wave Doppler are useful techniques that facilitate the differentiation between arterial and venous pulsation. The use of ultrasound for vascular access has been shown to decrease the need for correction of coagulopathy before insertion of the catheter. In the hands of experienced operators, when favorable vasculature is visualized, UGVA has been demonstrated to be safe and successful, with a low rate of complications.13,14
Variations in technique
One-person (two-hand) and two-person (three-hand) techniques have been described for USGV. In the one-person technique, the operator controls the transducer with the nondominant hand and the needle with the dominant hand. An assistant, also in full sterile barrier precautions, is required to hold the probe for the two-person technique. The one-person technique is reported to be learned easily and is associated with improved first-pass and overall success of CVC placement when compared with the two-person technique.2,11 When first learning the technique, many operators find the two-person technique easier. Eventually, most operators gravitate toward the one-person technique.
The ergonomics of UGVA is equally important. Ideally, the ultrasound machine should be placed on the contralateral side of the ROI while the operator stands at the cannulation site (Figures 11-4 and 11-5). Usually, the dominant hand of the operator holds the needle and the nondominant hand holds the transducer. Hence, the latter can be flexed slightly while resting on the body surface of the patient to stabilize the elbow in a comfortable position. Because the elbow of the operator remains stable, it is easier for the operator’s wrist to relax and sweep the transducer accurately while the needle is moved forward gently under the transducer in real time.
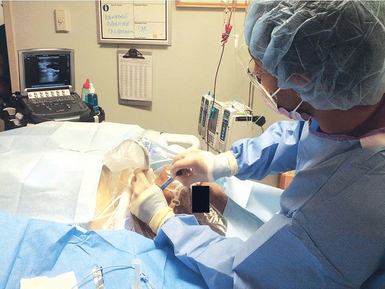
Figure 11-4 Demonstration of proper equipment setup for cannulation of the internal jugular vein. The ultrasound machine is placed downstream of the operator in a direct line of sight.
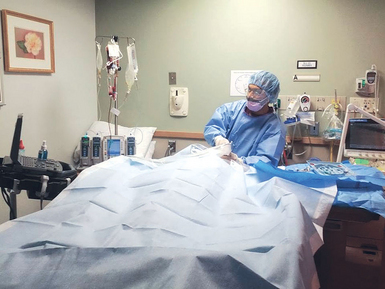
Figure 11-5 Demonstration of proper equipment setup for cannulation of the internal jugular vein. The ultrasound machine is placed downstream of the operator in a direct line of sight.
Variations on needle insertion have been described (Chapter 10). Real-time, dynamic visual guidance (free-hand access) throughout catheterization is always superior to marking a spot with ultrasound and then trying to locate a vessel without guidance (Figures 11-6 and 11-7).2,11,12, 13–16 As described previously, longitudinal visualization of the vessel allows improved and continuous visualization of the advancing needle tip but requires a more skilled operator, especially when attempting to catheterize small-caliber vessels. The transverse approach, though easier to learn, has been associated with an increased incidence of posterior wall perforation, probably because operators are often mistaking the shaft of the needle for the tip since it is easy to advance the tip of the needle past the ultrasound beam.
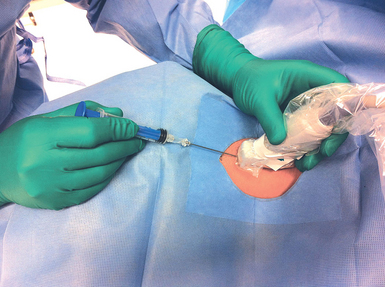
Figure 11-6 Free hand access dynamic cannulation on the longitudinal axis requires fine coordination of movements (note that the plane and trajectory of the needle follows the plane of the probe).
Figure 11-7 Free-hand access dynamic cannulation on the transverse axis (the angle of the penetrating needle is around 45 degrees) is technically less demanding, although the visualization of the needle may be indeed more problematic, compared to the longitudinal axis approach.
1. Localize the target vessel.
2. Measure the distance from the surface of the skin to the wall of the vessel.
3. Superficially insert the needle posterior to the transducer, to the same distance measured in step 2, at a 45-degree angle.
4. Tilt the transducer toward the needle to identify the tip of the needle.
5. Advance the needle into the vessel while simultaneously advancing and tilting the transducer forward in a slow creeping motion and keeping the tip of the needle visualized at all times.
6. The tip of the needle should be seen indenting and advancing through the anterior vessel wall.
Intraprocedural and postprocedural tips
For the most part, evaluation of target vessels is limited to the insertion point and what can be visualized immediately proximal and distal to the insertion site. Although a well-chosen site increases the success of catheter deployment and positioning, it does not guarantee success. When inserting a CVC into the internal jugular or subclavian vein, the rib cage obscures further evaluation of the vessel downstream. 13–16 Fragou et al demonstrated that the contralateral vasculature could be visualized by an assistant while advancing the guidewire to assess for potentially misdirected catheter deployment.17 If the guidewire is seen in the contralateral vasculature, attempts could be made to redirect the wire. In addition, the guidewire should always be visualized within the vein before any dilation is performed to prevent potential catastrophic arterial damage (Figure 11-8).
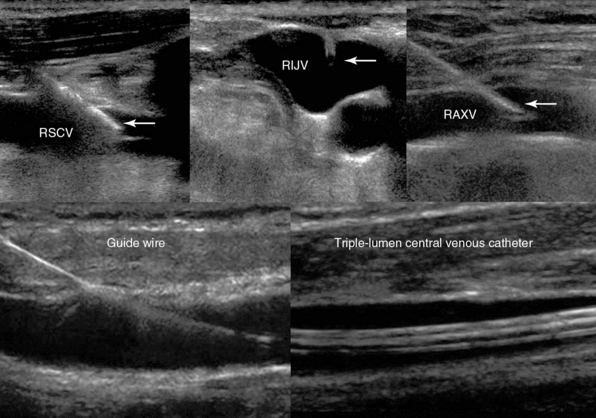
Figure 11-8 (Top) Real-time visualization of an echogenic vascular cannula, regardless of the angle of its insertion, in the right subclavian (RSCV), internal jugular (RIJV), and axillary (RAXV) veins. (Bottom) Depiction of the guidewire (left) and the triple-lumen central venous catheter (right) following successful central venous cannulation of the RIJV.
After the procedure the pleura should once again be visualized and assessed for lung sliding to ensure that pneumothorax is not present (Chapter 19). If difficulty was encountered despite using UGVA, such as an inability to thread the guidewire or unexpected resistance in catheter deployment, the vasculature should be scanned for the potential development of a hematoma, pseudoaneurysm, or arteriovenous fistula (Chapter 8).
Simulation training and echogenic technology
In recent years, implementation of simulation-based training for CVC insertion has been increasing. Such training has resulted in a significant reduction in mechanical and infectious complications and an increase in ultrasound use.18 At the present time, simulation mannequins and vascular task trainers, compatible with ultrasound, can be used to improve the skills of novice and less experienced operators.
Optimal image acquisition can be affected and deteriorated by subcutaneous air, obesity, trauma, and mechanical ventilation in the ICU. Such factors often make continuous visualization of the tip of the needle a challenge. The use of echogenic vascular cannulas, which have the potential to improve image acquisition and thus needle visualization, has been shown to improve success rates in technically difficult cases of vascular access.19,20 Etched reflectors on the tip of the vascular cannula improve visualization of the needle independent of the angle of insertion, in a method analogous to a bicycle reflector (see Figure 11-8). Recent, preliminary data have demonstrated improved continuous needle visibility and decreased access times and technical complexity of UGVA when using echogenic needle technology in either a longitudinal or transverse approach.19,20
Pearls and highlights
• As 1 of their 11 practices to improve patient care, the AHRQ recommends the use of ultrasound for CVC placement.
• The American Society of Anesthesiologists Task Force on Central Venous Access recommends the use of a central line insertion work and safety checklist and bundling of required equipment to minimize errors, risk of infection, and complications
• The implementation of a simple three-step UGVA technique (pre-procedural scanning, real-time ultrasound-guided cannulation, post-procedural scanning) maximizes success rates and decreases complications in the ICU
• A thorough preprocedural scan can familiarize the operator with the sonographic anatomy of the cannulation site (e.g., detection of possible anatomic diversity, venous and arterial asymmetry between symmetric sites) while excluding preexisting thrombosis, which is mandatory.
• Ultrasound monitoring of central lines aids in early identification of catheter-associated thrombosis.
• Postprocedural scanning can confirm flawless deployment of the guidewire or catheter (or both) in the vein, avoiding thus the pitfall of dilating an artery, and can facilitate repositioning when the guidewire or catheter is malpositioned.
• Simulation-based UGVA training is a new educational tool that can improve the skills of novice users.
• The use of echogenic vascular cannulas, which enhance real-time visualization of the tip of the needle regardless of its angle of insertion, could prove to be advantageous in technically difficult catheterization scenarios in the ICU.
References
1. Hind, D, Calvert, N, McWilliams, R, et al, Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361.
2. Milling, TJ, Jr., Rose, J, Briggs, WM, et al, Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764–1769.
3. Randolph, AG, Cook, DJ, Gonzales, CA, et al, Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053–2058.
4. Rothschild, JM, Ultrasound guidance of central vein catheterizationShojania KG, Duncan BW, McDonald KM, et al, eds.. Making health care safer: a critical analysis of patient safety practices, Evidence Report/Technology Assessment No. 43. Agency for Healthcare Research and Quality: Rockville, MD, 2001; 245–253.
5. National Institute for Clinical Excellence. Guidance on the use of ultrasound locating devices for placing central venous catheters, National Institute for Clinical Excellence Technology Appraisal Guidance No. 49, 2002. Available at http://www.nice.org.uk/nicemedia/live/11474/32461/32461.pdf, May 22, 2013. [Accessed].
6. Lamperti, M, Bodenham, AR, Pittiruti, M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012; 38(7):1105–1117.
7. Pittiruti, M, Malerba, M, Carriero, C, et al, Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access 2000;1(3):100–107.
8. Timsit, JF, Schwebel, C, Bouadma, L, et al, Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAM. 2009; 301:1231–1241.
9. Rupp, SM, Apfelbaum, JL, Blitt, C, et al, Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiolog. 2012; 116:539–573.
10. Pronovost, P, Needham, D, Berenholtz, S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006; 355:2725–2732.
11. Denys, BG, Uretsky, BF, Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516–1519.
12. Feller-Kopman, D, Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice 2007. Chest. 2007;132(1):302–309.
13. Della Vigna, P, Monfardini, L, Bonomo, G, et al, Coagulation disorders in patients with cancer: nontunneled central venous catheter placement with US guidance—a single-institution retrospective analysis. Radiology. 2009;253(1):249–252.
14. Tercan, F, Ozkan, U, Oguzkurt, L. US-guided placement of central vein catheters in patients with disorders of hemostasis. Eur J Radiol. 2008; 65(2):253–256.
15. Hosokawa, K, Shime, N, Kato, Y, et al. A randomized trial of ultrasound image–based skin surface marking versus real-time ultrasound-guided internal jugular vein catheterization in infants. Anesthesiology. 2007; 107(5):720–724.
16. Mansfield, PF, Hohn, DC, Fornage, BD, et al. Complications and failures of subclavian-vein catheterization. 1994. N Engl J Med. 1994; 331(26):1735–1738.
17. Fragou, M, Gravvanis, A, Dimitriou, V, et al, Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607–1612.
18. Sekiguchi, H, Tokita, JE, Minami, T, et al, A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652–658.
19. Stefanidis, K, Fragou, M, Pentilas, N, et al. Optimization of cannula visibility during ultrasound-guided subclavian vein catheterization, via a longitudinal approach, by implementing echogenic technology. Crit Care Res Pract. 2012; 2012:617149.
20. Stefanidis, K, Pentilas, N, Dimopoulos, S, et al. Echogenic technology improves cannula visibility during ultrasound-guided internal jugular vein catheterization via a transverse approach. Crit Care Res Pract. 2012; 2012:306182.