Ultrasound-guided regional anesthesia in the intensive care unit
BETTINA U. SCHMITZ, MARIA MATUSZCZAK, MARCO A. FONDI, SARA GUZMAN-REYES, LUDWIG H. LIN and SAMER NAROUZE
Overview
Peripheral regional anesthesia and neuraxial anesthesia are well established in postoperative pain management following major orthopedic, thoracic, and abdominal procedures. Benefits include pain control superior to that achieved with systemic opioids, improved lung function after thoracic and abdominal procedures, and decreased stress/inflammatory responses.1–6 Ultrasound (US) guidance for regional anesthesia has been used increasingly over the last decade because it allows precise positioning of the catheter and local anesthetic (LA) and thus decreases the amount of LA required to achieve a complete block. In addition, the incidence of vascular puncture is lower with US guidance, thereby increasing the safety of the procedure. Fewer needles passes and replacement of nerve stimulation and subsequent muscle contraction with US visualization minimize procedural pain in patients.
In critical care patients, pain can originate from multiple sites, and even though regional anesthesia will not eliminate the need for systemic opioids in most patients, inclusion of regional anesthesia in the pain management regimen can nonetheless result in a significant decrease in overall opioid and sedative requirements. Additionally, these advantages are attractive in the context of the recent paradigm shift toward less sedation in ICU patients.7
Advantages of ultrasound guidance for regional anesthesia
Recent data suggest that US guidance improves the success rate and quality of regional anesthesia procedures.8–11 It allows visualization of the neural and surrounding structures in the region of interest (ROI), and real-time US guidance can demonstrate and be used to optimize spread of LA. This is presumably why US-guided nerve block techniques shorten procedure time, hasten onset of the block, and result in fewer needle passes and a decreased incidence of vascular puncture.12 Moreover, the volume (dose) of LA required to achieve a complete block is decreased under US guidance.13,14
Benefits of ultrasound-guided regional anesthesia in patients in the intensive care unit
1. Better pain control. Multiple studies have documented the superior pain control in patients receiving epidural analgesia or paravertebral analgesia after rib fractures, thoracotomy, and major abdominal surgery. Superior pain control is also achieved by the implementation of regional anesthesia in patients undergoing surgery on the extremities.4,6,15
In the ICU, trauma patients often have multiple injuries. Use of regional anesthesia techniques for all injuries at the same time might be not feasible in most trauma cases. Sequential regional anesthesia, according to the surgical interventions, can reduce baseline and procedural pain and the need for opioids in this population.16 Bolus injections of LA instead of continuous infusion, as well as a lower concentration and volume of the LA solution, can reduce the overall amount of LA used per day and thereby enable performance of regional anesthesia in more than one location in the same patient.
2. Lower requirement for systemic opioids and sedation. Multiple studies have documented better outcomes and decreased levels of sedation in ICU patients. The American College of Critical Care Medicine addressed these results in practice guidelines published in 2013 in which lighter sedation was recommended for most ICU patients.7 However, decreasing the level of sedation in ICU patients without sacrificing good pain control can be challenging. Regional anesthesia provides excellent pain control without affecting the mental status of patients.
3. Improved pulmonary function after thoracic or abdominal surgery and rib fractures. Nishimori et al documented in a Cochrane review that epidural anesthesia improves pulmonary function and reduces the duration of mechanical ventilation in patients undergoing abdominal aortic surgery.15
4. Possible decrease in the development of chronic pain through better management of acute pain. Chronic pain as a sequela of severe undertreated acute pain is well described. However, data supporting the fact that regional anesthesia for the treatment of acute pain can reduce the incidence or severity of chronic pain are still scant. In a retrospective study, Salengros et al documented a higher incidence of allodynia and chronic pain in patients undergoing thoracotomy with high-dose remifentanil anesthesia than in those with epidural anesthesia and low-dose remifentanil.17–19
5. Sympathicolysis. Sympathicolysis is a well-described effect of regional anesthesia that can be beneficial for patients with impaired perfusion of the extremities because of vascular disease or trauma.19
6. Possible impact on recurrence of cancer. Surgical stress and opioids are known to suppress the immune system. Some retrospective studies have shown a lower recurrence rate of cancer in patients treated with regional anesthesia and propofol infusion than in those undergoing anesthesia with opioids and inhalational anesthetics. However, other studies could not confirm these statements, and prospective studies are in progress. Whether better postoperative pain control with regional anesthesia and less use of opioids postoperatively have an influence on tumor recurrence is not certain.20,21
7. Impact of regional anesthesia on mortality and duration of hospital stay. Currently, the overall evidence showing improved mortality and reduced duration of hospital stay after regional or neuraxial anesthesia is insufficient (Box 53-1).
Concerns and problems related to neuraxial and peripheral regional anesthesia in patients in the intensive care unit
Patient-related concerns and problems
1. Coagulopathy and systemic infection. Coagulopathy and systemic and local infection are contraindications to regional anesthesia; moreover, these conditions are prevalent in ICU patients. The American Society of Regional Anesthesia (ASRA) guidelines regarding patients receiving antithrombotic or thrombolytic therapy should be followed. When regional anesthesia procedures are performed in patients with borderline coagulation status, more peripheral approaches (axillary, femoral, fascia iliaca compartment, and popliteal sciatic) should be considered. However, finding a window of appropriate coagulation to safely remove indwelling catheters can be challenging.22
2. Regional anesthesia under deep sedation or anesthesia. Decreasing sedation to facilitate communication with patients during regional anesthesia procedures is not always possible. Deep sedation or anesthesia abolishes the ability of the patient to report symptoms pertinent to LA toxicity or nerve damage. Accordingly, the ASRA recommendations (2008) do not support the routine performance of neuraxial or interscalene blocks in anesthetized or heavily sedated patients. However, the group acknowledged that the risk-benefit ratio can be favorable for regional anesthesia under the aforementioned conditions in selected cases. Neuraxial and peripheral regional anesthesia in pediatric patients under heavy sedation or anesthesia is considered more applicable, mainly because of the inability of pediatric patients to communicate symptoms of toxicity or nerve injury and the potential harm associated with patient movement.
Systemic LA toxicity and nerve damage have been reported even after US-guided peripheral nerve blocks. Meticulous needle control, observation of LA spread, verification of the position of the tip of the catheter with hydrodissection, avoidance of intraneural injection or catheter placement, use of a test dose, and slow injection of LA in small increments can reduce risk for the aforementioned complications.23
3. Risk for compartment syndrome. Compartment syndrome is a complication of extremity trauma. Regional anesthesia itself does not increase the risk for compartment syndrome, but it could mask the associated pain and delay detection. Thus, the lowest effective concentration of LA solution should be infused. Whether this consideration is relevant for an intubated and sedated ICU patient can be questioned. However, because compartment syndrome is a limb-threatening complication, regional anesthesia in patients with high-risk injuries such as tibial and distal radial fractures should be discussed with the orthopedic surgeon, and measurement of compartment pressure should be considered.24,25
4. Positioning. Positioning of traumatized patients for regional anesthesia can be challenging.
Organization-related concerns and problems
1. Training. ICU personnel caring for patients with neuraxial or peripheral regional anesthesia catheters in place need to be adequately educated regarding regional anesthesia and its benefits, possible adverse effects, and complications.
2. Implementation of care protocols. Detailed protocols for the care and follow-up of patients who receive neuraxial or peripheral regional anesthesia should be implemented.
3. Color marking of different access lines. Adding another line to the already numerous lines inserted and attached to patients in the ICU increases the risk for misidentification of neuraxial/regional infusion and intravenous infusion lines. Judicious color marking of the different lines and their connections can reduce this risk.
4. Infection. The risk for infection with an indwelling catheter is higher in ICU patients. The insertion site of the neuraxial or regional anesthesia catheter should be inspected at least once daily for signs of infection and the findings documented.
5. Catheter dislocation. Accidental dislocation or removal of neuraxial or regional anesthesia catheters during mobilization and transportation is a potential problem. The integrity of all indwelling catheters should be checked routinely after mobilization and transport of patients.
6. The future of LA catheters. Extended-release LA formulations might decrease the need for insertion of catheters for continuous LA infusion in the near future.
Table 53-1 lists common indications for neuraxial and peripheral regional anesthesia, whereas Box 53-2 outlines barriers and contraindications to US-guided regional anesthesia in critical care patients.
TABLE 53-1
Indications for Neuraxial and Peripheral Regional Anesthesia in ICU and non-ICU patients
| Indication | Non-ICU patient | ICU Patient |
| Upper extremity analgesia | Joint replacements (shoulder, elbow, hand) Fractures Tendon and muscle repair Vascular surgery Arteriovenous grafts Reimplantation of the finger, hand, and arm |
Joint replacements (shoulder, elbow, hand) Fractures Tendon and muscle repair Vascular surgery Arteriovenous grafts Reimplantation of the finger, hand, and arm |
| Upper extremity sympathicolysis | Ischemia Reimplantation Vascular surgery |
Ischemia Reimplantation Vascular surgery |
| Lower extremity analgesia | Joint replacements (hip, knee, ankle) Fractures Tendon and muscle repair Vascular surgery |
Joint replacements (hip, knee, ankle) Fractures Tendon and muscle repair Vascular surgery |
| Lower extremity sympathicolysis | Ischemia Vascular surgery |
Ischemia Vascular surgery |
| Trunk | Thoracotomy/thoracoscopy Rib fractures Abdominal wall incision |
Thoracotomy/thoracoscopy Rib fractures Abdominal wall incision |
| Neuro-axial | Rib fractures Thoracic surgery Abdominal surgery |
Rib fractures Thoracic surgery Abdominal surgery Ileus Pancreatitis |
Ultrasound technique for regional anesthesia
Ultrasound scan
According to the HOLA concept of US scanning, a preprocedural scan facilitates the visualization of anatomic structures in an ROI, such as nerves, vessels, bones, muscles, and fascia layers (see Chapters 1 and 51). Nerves have a fascicular hyperechoic appearance and are obviously the primary targets during guided nerve blocks. Transverse and longitudinal US views can identify the nerve and confirm spread of the LA along single nerves. Transverse views are less technically complex and used mainly during real-time LA injection. Two-dimensional scanning can be extended (proximally and distally) to identify the location and track the course of neural structures. Such tracking may optimize identification of the target nerve by visualization of adjacent anatomic structures or landmarks (e.g., musculoskeletal, vascular) and their two-dimensional alignment in relation to the former in an ROI.12
Instrumentation
High-frequency transducers are used for superficially located neural structures and low-frequency transducers for deeper structures (see Chapters 1 and 53). Enhanced needle visualization software, echogenic material (needles, catheters), or needle guidance systems may be used for optimization of the procedure.
Regional anesthesia of the upper extremity
Anesthesia of the brachial plexus can be performed in the interscalene groove (interscalene approach), directly above the clavicle (supraclavicular approach), under the clavicle (infraclavicular approach), and in the axilla (axillary approach). The different approaches are not equally suitable for ICU patients. An interscalene brachial plexus block provides good analgesia for the shoulder, arm, and forearm, but the phrenic nerve is usually blocked as well, which results in paralysis of the ipsilateral diaphragm (Figure 53-1). This effect can aggravate respiratory problems and lead to respiratory failure in compromised, spontaneously breathing patients. Severe complications (i.e., injection of LA into the spinal cord) have been reported after interscalene blocks performed under deep sedation or general anesthesia, and the ASRA guidelines strongly recommend against performing this block under these conditions.22 Hematoma formation in this area can lead to airway compromise and compression of the neck vessels. Paralysis of the ipsilateral diaphragm is less common with the supraclavicular approach. The supraclavicular approach offers good analgesia to the entire arm, forearm, and hand (Figure 53-2). US-guided techniques can reduce the risk for pneumothorax by meticulous control of passage of the needle and observation of the anatomic structures: artery, rib, and pleura. However, US-guided techniques cannot entirely prevent the typical complications of these two blocks (i.e., pneumothorax, intraneural injection).27–30 With both approaches (interscalene and supraclavicular), the plexus is close to the skin, and dislocation of a regional anesthesia catheter occurs easily with movement. Tunneling of the catheter can reduce this risk.
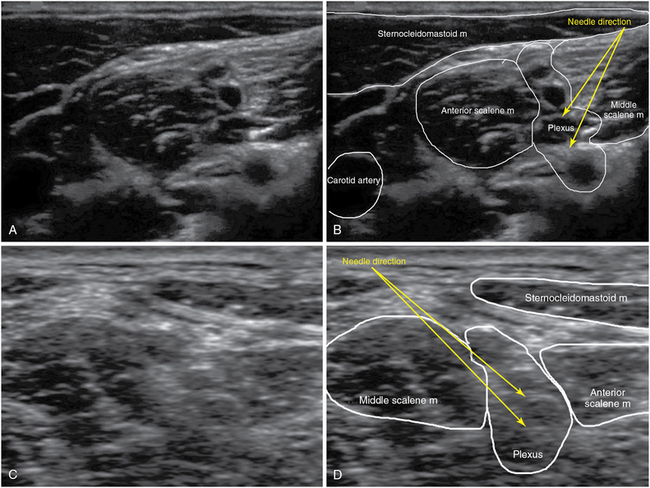
Interscalene brachial plexus block
The classic interscalene approach is performed at the level of the cricoid cartilage, in the groove between the anterior and middle scalene muscles, and it provides excellent anesthesia of the shoulder and arm. The transducer is placed lateral to the cricoid cartilage (transverse plane), along the sternocleidomastoid muscle toward the interscalene groove. At this level the plexus is visualized as either roots or trunks with three or more hypoechoic structures in a “traffic light configuration” between the scalene muscles and under the end of the tapering sternocleidomastoid muscle, no deeper than 1 inch under the skin. Anatomic variations may be observed in individual cases.10
A 50-mm needle is advanced in plane in a posterior-to-anterior direction; a small roll placed under the shoulder and thorax facilitates this approach. An out-of-plane approach with the tip of the needle aimed cranially is not advisable because of the possibility of malposition of the needle and delivery of LA into the spinal canal. With the tip of the needle positioned in the center of the plexus (between the C5/6, C6/7, or C7/8 roots or the superior and middle trunks), an LA volume of 10 to 20 mL is usually injected after negative aspiration. Because the lower roots or trunk of the brachial plexus may be spared with the interscalene technique, an approach at or below the clavicle is generally recommended for anesthesia or analgesia in the distribution of the ulnar nerve. Though not always predictable in individual patients, lower LA volumes may reduce the incidence of the typical adverse effects associated with the interscalene approach: Horner syndrome, recurrent laryngeal nerve paresis, and phrenic nerve paralysis (Figure 53-1).14
Tips for pediatric patients
Pediatric patients are rarely seen with shoulder injuries, and although the approach is similar, this type of block is seldom performed in small children. Because the distance between the interscalene and the supraclavicular approach in a small child can be less than half an inch, LA volumes of 0.2 to 0.3 mL/kg or a maximum of 10 to 20 mL for children over 40 kg may provide sufficient anesthesia of the shoulder and entire arm irrespective of the approach used.
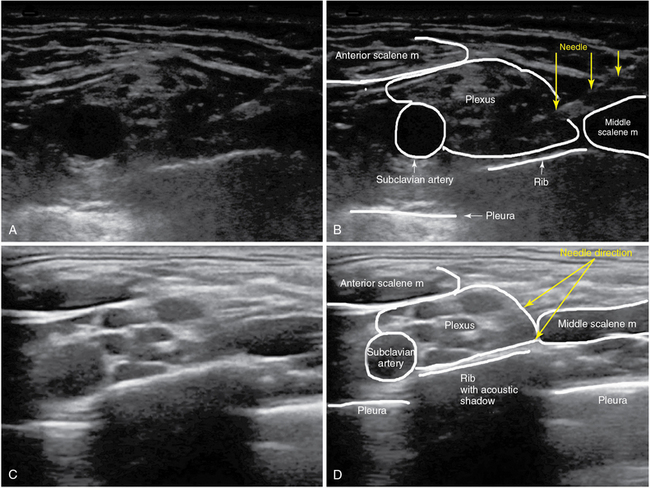
Supraclavicular brachial plexus block
The supraclavicular approach has experienced a renaissance since the introduction of US-guided techniques. In the supraclavicular area the plexus nerves are organized in trunks or divisions and can be visualized as a cluster of hypoechoic structures in a corner posterolateral to the subclavian artery, just above the first rib. The subclavian artery, the first rib, and the pleural line should be identified to perform this block safely. A 50-mm needle can be advanced in either a lateromedial or mediolateral direction. The needle is advanced in plane in the corner between the subclavian artery and the first rib. An LA volume between 15 and 25 mL is generally injected. The same adverse effects of an interscalene block can occur with the supraclavicular approach, but the incidence rate is significantly lower (Figure 53-2).
Tips for pediatric patients
In small children the brachial plexus at the supraclavicular level lies immediately under the skin, and thus the smallest available needles should be used (LA volumes are adjusted to 0.1 to 0.2 mL/kg). A block at this level provides excellent anesthesia for the entire arm.
Infraclavicular brachial plexus block
An infraclavicular block provides anesthesia and analgesia below the midhumerus region. In the infraclavicular area the plexus is organized in hyperechoic cords around the axillary artery, usually with the lateral cord at the 8- to 10-o’clock position, the posterior cord (under the artery) at the 6-o’clock position, and the medial cord at the 2- to 3-o’clock position. Because the brachial plexus lies under both pectoral muscles, the infraclavicular approach is rather deeper (from the skin surface) than the other two previously discussed approaches (Figure 53-3 A,B).
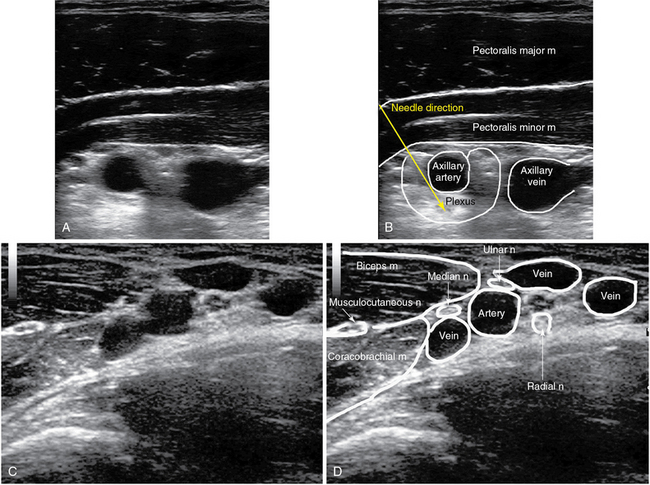
Figure 53-3 Infraclavicular (top row) and axillary (bottom row) approaches to a brachial plexus block.
The transducer is placed in a parasagittal plane to the coracoid process under the clavicle. The pectoral muscles, axillary artery and vein, and the cords (if seen) are identified. An 80-mm needle is inserted in plane below the inferior clavicular border. The tip of the needle is advanced under the artery toward the location of the posterior cord, and an LA volume of 20 to 30 mL is typically injected.31
Axillary brachial plexus block
The axillary approach provides sufficient anesthesia and analgesia for the elbow and the entire lower part of the arm. The US-guided technique provides better block qualities than do the traditional transarterial or nerve stimulator–guided approaches. Specifically, the musculocutaneous nerve, often missed with the traditional approaches, can readily be visualized and anesthetized under US guidance. With the arm abducted, externally rotated, and flexed 90 degrees at the elbow, the transducer is placed in a sagittal/oblique plane across the axilla. The axillary artery is usually visible at a depth of 1 to 2 cm. The hyperechoic nerves are located around the artery and can be identified in the majority of patients: the median nerve at 10 o’clock; the ulnar nerve between 1 and 3 o’clock; and the radial nerve, posterior to the artery, between 3 and 6 o’clock.28 The musculocutaneous nerve is located between the biceps and coracobrachial muscles. A 50-mm needle is inserted in plane, with the axillary artery used as a landmark in the ROI. The musculocutaneous nerve can then be approached anterior to the artery, the median nerve adjacent to the artery, and the radial nerve posterior to the artery in a continuous procedure by making sequential adjustments in the trajectory of the needle. The ulnar nerve can be injected above or below the artery. An LA volume of 5 to 7 mL is sufficient for each nerve. Alternatively, a volume of 10 to 12 mL can be injected both above and below the artery in an effort to achieve a circumferential LA spread pattern around the vessel (Figure 53-3 C,D).
Tips for pediatric patients
In children the axillary approach of the brachial plexus has the shortest learning curve because sonographic detection of the neurovascular bundle is easy. The transducer is positioned perpendicular to the axillary artery with the arm in abduction. The needle is then advanced in plane and redirected accordingly to access each target nerve as previously described, with 1 to 2 mL of LA being delivered at each location.
Regional anesthesia of the lower extremity
The lateral popliteal approach can be performed with the patient in a supine position by elevating the lower part of the leg so that the US probe can be positioned on the posterior aspect of the thigh. The infragluteal approach is usually performed with lateral positioning. Catheters can easily be inserted in both locations. Technical details of the aforementioned blocks are included later (Figure 53-5 and 53-6).
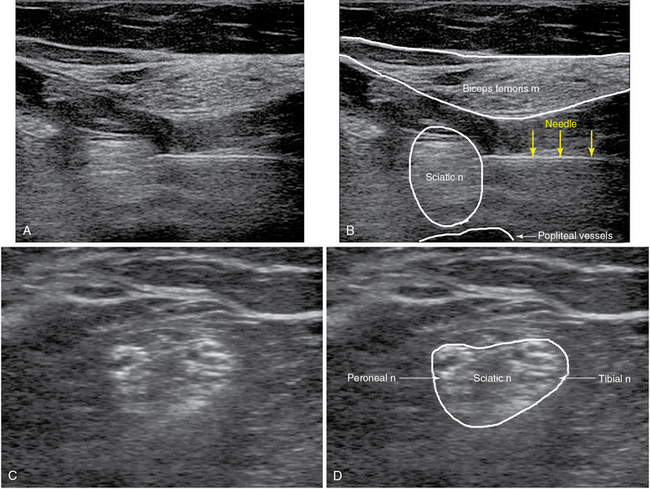
Figure 53-6 Sciatic nerve block: popliteal approach before the bifurcation (top row) and at the bifurcation (bottom row).
Femoral nerve block/fascia iliaca compartment block
Femoral nerve block
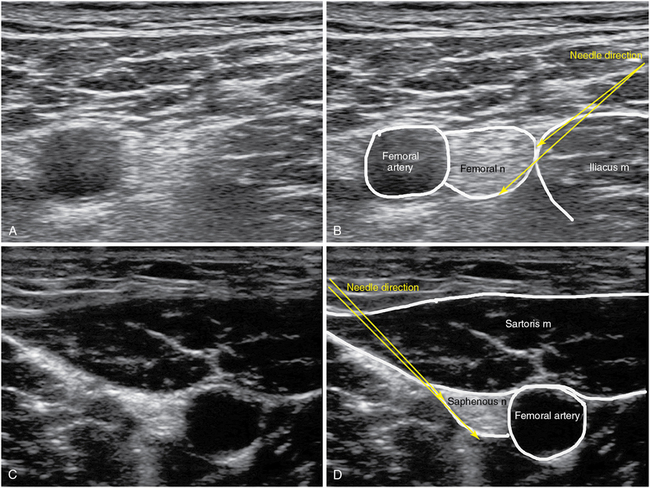
An FN block provides anesthesia to the anterior aspect of the thigh and knee. It is used for postoperative pain management after total knee arthroplasty and other surgical knee procedures. The FN is located under the fascia iliaca lateral to the femoral artery and medial to the iliacus muscle in the inguinal crease. A high-frequency transducer is placed on the inguinal crease to identify the femoral vein, artery, and nerve (in a medial-to-lateral order); the iliacus muscle, fascia lata (above the femoral artery), and fascia iliaca (separating the artery from the nerve) are also visualized. An 80- to 100-mm needle is inserted in plane 1 to 2 cm lateral to the transducer. The needle perforates the fascia lata and then the fascia iliaca and is advanced to the lateral FN border or just below the nerve, and an LA volume of 10 to 20 mL is usually injected. Spread of LA above the fascia iliaca results in incomplete anesthesia/analgesia (Figure 53-4 A,B).26
Fascia iliaca compartment block
This diffusion block provides anesthesia in the distribution of the FN and lateral cutaneous FN. It is performed with the patient in a supine position and provides excellent results either in the emergency department or in the perioperative setting for patients with hip or femoral fractures and after knee surgery.33 The US technique is similar to the FN block technique just described; however, the needle is inserted more lateral to the transducer (in comparison to the FN insertion point), and a higher LA volume of 30 to 40 mL is injected within or right below the fascia iliaca. Thus, a block of both the FN and lateral cutaneous FN can be achieved.
Saphenous nerve block
The saphenous nerve (SN) is the major sensory branch of the FN. When used in conjunction with an ScN block, it provides anesthesia of the entire leg below the knee. In the midthigh region, the SN is located adjacent to the femoral artery under the sartorius muscle (“adductor canal”). A high-frequency transducer is placed 10 to 15 cm above the knee in a transverse orientation. The SN is not visible in all cases and adjacent structures can be used as landmarks. The femoral artery is visualized deep under the sartorius muscle, which in turn can be detected sonographically as a triangularly shaped muscle just medially to the quadriceps muscle. A 100-mm needle is inserted in plane 1 to 2 cm anterior to the transducer. The tip of the needle is positioned under the sartorius muscle, adjacent to the artery in the fascial plane, and an LA volume of 5 to 10 mL is injected after negative aspiration. As the femoral artery travels distally, it dives deeper, and its superficial branch (the superficial geniculate artery) accompanies the SN. Hence, this technique should be used for blocking the SN at a level above the bifurcation of the femoral artery (Figure 53-4 C,D).
Sciatic nerve block
Infragluteal approach
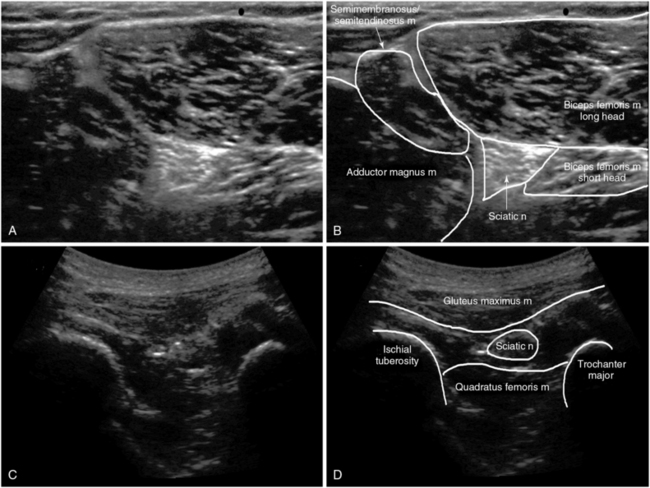
The patient is positioned laterally (hip and knee joints flexed) while the target leg is placed in an upright position. This facilitates palpation and US detection of the bony landmarks used with this block: the greater trochanter and ischial tuberosity. Low-frequency transducers are commonly used for scanning in the infragluteal area (in thin subjects, high-frequency transducers may be used). The ScN is usually located below the midline of an imaginary hammock formed by the greater trochanter and ischial tuberosity. The nerve appears as a triangulated or wedge-shaped hyperechoic structure overlying the quadratus femoris muscle. A 100-mm needle is inserted in plane either lateral or medial (about 1 to 2 cm) to the transducer. When the tip of the needle approaches the nerve, 15 to 20 mL of LA is injected after negative aspiration (Figures 53-5 and 53-6 A,B).34
Popliteal approach
The popliteal approach can be performed with the patient in the prone, lateral, or supine position. The transducer is placed in a transverse plane in the popliteal fossa, between the tendons of the semimembranosus/semitendinosus muscle medially and the biceps femoris muscle laterally. The rather superficially located tibial nerve (TN) can be visualized just adjacent to the popliteal vessels, whereas the peroneal nerve (PN) is not always visible in the fossa. However, when the transducer tracks the TN cephalad, the PN can usually be detected. At a variable distance, usually about 3 to 4 inches, proximal to the fossa the two nerves merge to form the ScN. At the level of the ScN bifurcation, an 80- to 100-mm needle is inserted in plane, and 10 to 20 mL of LA is injected after negative aspiration (Figure 53-6 C,D).
Regional anesthesia of the trunk
Trunk blocks (paravertebral, transversus abdominis plane [TAP], and rectus sheath) provide anesthesia to the thoracic and abdominal wall and offer an alternative analgesic solution in patients in whom a neuraxial block is not recommend or is contraindicated, such as deeply sedated ICU patients. In contrast to neuraxial analgesia, trunk blocks do not provide analgesia to visceral structures. Pain originating from these structures needs to be addressed with systemic pain medication. Nonetheless, trunk blocks can significantly reduce the amount of pain medication required by allowing the use of less systemic analgesia and lighter sedation, thus facilitating spontaneous breathing and weaning from mechanical ventilation.35
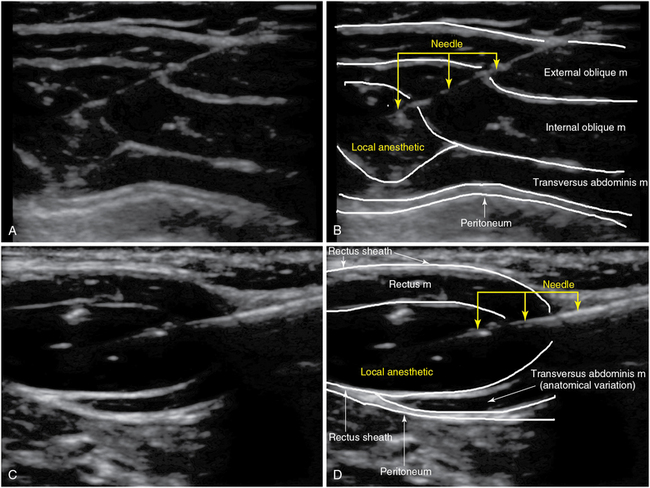
The TAP block and rectus sheath block provide analgesia to the anterior abdominal wall and can reduce the pain after any open abdominal surgery. Performed under US guidance, the fascia layer between the internal oblique and transversus abdominis muscles or the posterior rectus sheath can easily be identified in most patients. Catheters for continuous analgesia can be inserted unilaterally or bilaterally, depending on the location of the incision, under US guidance by using the hydrodissection method. Insertion of two catheters (i.e., one at a lower level and one at a higher level from the incision on each side) might be necessary to provide adequate distribution of LA for long incisions (Figure 53-7).
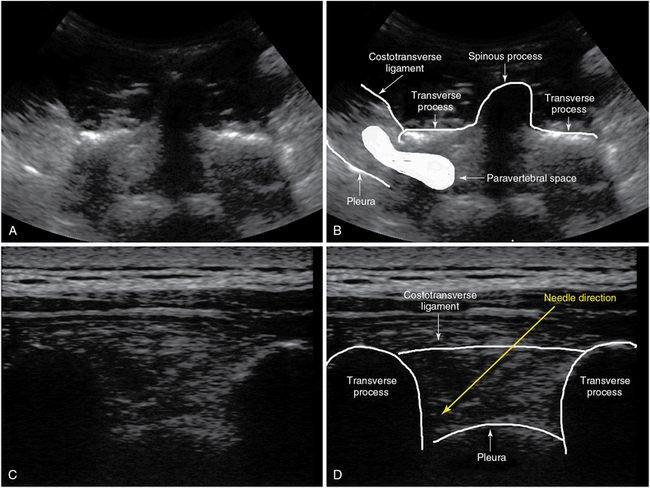
Paravertebral regional anesthesia provides analgesia to the chest, abdominal wall, or both. The pain relief achieved with a paravertebral block is comparable to that with a thoracic epidural in patients with rib fractures or after thoracotomy with the benefit of a superior safety profile. (Figure 53-8).36
The paravertebral space, bordered by the transverse process, the vertebral body, the pleura, and the costotransverse ligament, can be identified with US. An in-plane approach allows good control of the tip of the needle and reduces the risk for accidental perforation of the pleura. Performed unilaterally or bilaterally, a paravertebral block provides pain control after thoracotomy, thoracoscopy, rib fracture, flank incision for nephrectomy, and open cholecystectomy. Catheters can be placed for continuous LA infusion (Figure 53-8).37
Technical details of the aforementioned blocks are included in the following sections.
Trunk blocks
Transversus abdominis plane, rectus sheath, and ilioinguinal/iliohypogastric blocks
Transversus abdominis plane block.
The TAP block reaches the thoracolumbar nerves in the layer of fascia between the internal oblique and transversus abdominis muscles. Different approaches have been described: an approach at the triangle of Petit, subcostal and midaxillary line approaches, and an approach just lateral to the rectus sheath (in children). The triangle of Petit is located at the lateral abdominal wall and is bordered by the costal margin cephalad, the iliac crest caudad, the external oblique muscle anteriorly, and the latissimus dorsi muscle posteriorly (Figure 53-7 A,B).
In all the aforementioned approaches the three muscles of the abdominal wall (external and internal oblique and transversus abdominis) are identified with high-frequency transducers. Next, an 80- to 100-mm needle is advanced (in plane) in the fascia layer, between the internal oblique and transversus abdominis muscles, and 15 to 20 mL of LA is injected after negative aspiration. A TAP block provides sufficient analgesia to the abdominal wall and can be performed unilaterally or bilaterally, depending on the surgical approach. A TAP block can be considered a valuable alternative in patients with a contraindication to neuraxial anesthesia/analgesia and for ambulatory procedures, surgery restricted to the abdominal wall (e.g., hernia), and control of postoperative pain in the ICU (Figure 53-7 A,B).38
Tips for pediatric patients.
In children the three abdominal wall layers are very thin but easily identifiable. The technique can be challenging because of excursion of the abdominal wall with every respiratory cycle when the child is breathing heavily. A TAP block can provide excellent analgesia for many abdominal surgical procedures. However, current experience with pediatric continuous TAP blockade is limited.38
Rectus sheath block.
A rectus sheath block provides good analgesia to the midline or paramedian abdominal wall. The transducer is placed lateral to the midline at the level of the umbilicus (transverse plane). The rectus, external and internal oblique, and transversus abdominis muscles, as well as the aponeurosis, are identified. A 50- to 100-mm needle is advanced (in plane) in the posterior rectus sheath, and delivery of LA produces a hypoechoic lens-shaped space deep to the rectus muscle. The procedure is repeated on the other side of the abdomen; a total LA volume of 30 to 40 mL is generally required. The indication for a rectus sheath block is analgesia for midline incisions above the arcuate line (e.g., umbilical hernia) (Figure 53-7 C,D).
Paravertebral block
This block delivers LA to the spinal nerves just after their exit from the spinal canal. The wedge-shaped paravertebral space is located adjacent to the spinal column bilaterally. It is bordered anterolaterally by the parietal pleura (thorax), anteriorly by the psoas muscle (abdomen), medially by the transverse process and vertebral body, and posteriorly by the costotransverse ligament. The thoracic paravertebral space is a continuum (in the cephalad-caudad orientation), and thus a single LA injection can result in multiple levels of anesthesia. The patient is placed in a sitting or lateral position, and blocks can be attempted at any level of the thoracic and lumbar spine (depending on the level of anesthesia desired). High- or low- frequency transducers are used (depending on block level and patient size). The transducer is placed (cephalad-caudad orientation) over the spinous processes. By sweeping the transducer laterally, deep structures such as the transverse processes and parietal pleura can be visualized delineating the costotransverse ligament. A 100-mm needle is inserted in plane and advanced through the ligament between two consecutive transverse processes. Loss of resistance can be felt when the tip of the needle perforates the ligament. An LA volume of 5 mL per level for multiple injections or 20 mL for a single one is required. Higher LA volumes may cause anterior pleural displacement or spread of LA into the spinal canal (or both). An epidural hematoma occurring after this block is rare in comparison to epidural anesthesia techniques.24 Indications for a paravertebral block include breast, thoracic, and abdominal wall surgery; multiple rib fractures; thoracotomy; and thoracoscopy (Figure 53-8).
Epidural analgesia
The benefits of epidural analgesia after thoracic and abdominal surgery are widely recognized.15 For most patients, preoperative insertion of the epidural catheter in the OR is preferred. Possible indications for epidural catheter placement in the ICU include postoperative analgesia after emergency thoracic and abdominal surgery, unilateral or bilateral rib fractures, and pancreatitis. US-guided epidural catheter placement is an emerging technique that is not widely used yet. The key structures for epidural anesthesia—ligamentum flavum, dura, and epidural space—are partially obscured by the surrounding vertebrae in the US evaluation but can be identified in most patients. Notably, identification of the aforementioned landmarks by US facilitates epidural catheter placement, especially in patients with poorly defined surface landmarks, previous back surgery, and difficult anatomy.32 Most commonly, US is used preprocedurally to determine the midline and depth of the epidural space because the epidural anesthesia itself is performed with loss of resistance technique. Real-time US-guided epidural anesthesia has been described mainly in infants.39
Pearls and highlights
• US-guided neuraxial and regional anesthesia is underused in the ICU setting.
• In selected ICU patients, US-guided neuraxial and regional anesthesia can decrease the need for systemic opioids and sedation.
• Epidural anesthesia provides analgesia after thoracic or abdominal surgery that is superior to that provided by systemic opioids.
• Thoracic epidural analgesia and paravertebral anesthesia achieve better pain control and result in improved pulmonary function after rib fractures.
• US-guided peripheral nerve block techniques shorten the procedure time, hasten the onset of analgesia, result in fewer needle passes, and decrease the incidence of accidental vascular puncture.
• Hydrodissection with small amounts of normal saline facilitates visualization of the needle and tip of the catheter during US-guided nerve blocks.
• Preprocedural US scanning can facilitate epidural anesthesia in patients with poorly defined surface landmarks and anatomic variation of the spine.
• The ASRA guidelines regarding the performance of regional anesthesia in situations in which anticoagulation and antiplatelet agents are being administered should be followed in routine practice.
• The ASRA recommendations concerning regional anesthesia in anesthetized or heavily sedated patients should be followed when regional anesthesia is administered in the ICU.
References
1. Ahlers, O, Nachtigall, I, Lenze, J, et al. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008; 101(6):781–787.
2. Bagry, H, de la Cuadra Fontaine, J-C, Asenjo, JF, et al. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med. 2008; 33(1):17–23.
3. Hanna, MN, Murphy, JD, Kumar, K, Wu, CL, Regional techniques and outcome: what is the evidence. Curr Opin Anaesthesiol. 2009;22(5):672–677.
4. Ho, AMH, Karmakar, MK, Critchley, LAH. Acute pain management of patients with multiple fractured ribs. Curr Opin Critical Care. 2011; 17(4):323–327.
5. Richman, JM, Liu, SS, Courpas, G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006; 102(1):248–257.
6. Werawatganon, T, Charuluxananan, S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 1, 2005. [CD004088].
7. Barr, J, Fraser, GL, Puntillo, K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41(1):278–280.
8. Antonakakis, JA, Ting, PH, Sites, B, Ultrasound-guided regional anesthesia for peripheral nerve blocks: an evidence-based outcome review. Anesthesiol Clin. 2011;29(2):179–191.
9. Gelfand, HJ, Ouanes, J-PP, Lesley, MR, et al, Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth. 2010;23(2):90–96.
10. Martinoli, C, Bianchi, S, Santagroce, E, et al. Brachial plexus sonography. AJR Am J Roentgenol. 2002; 179:699–702.
11. Neal, JM, Brull, R, Chan, VWS, et al. The ASRA evidence-based medicine assessment of ultrasound-guided regional anesthesia and pain medicine. Reg Anesth Pain Med. 2010; 35(suppl 1):S1–S9.
12. Marhofer, P. Ultrasound guidance in regional anesthesia. Principles and practical implementation. Oxford: Oxford University Press; 2010.
13. Schoenmakers, K. Effect of local anesthetic volume (15 vs 40 mL) on the duration of ultrasound-guided single shot axillary brachial plexus block. Reg Anesth Pain Med. 2012; 37(3):242–247.
14. Smith, HM, Clinical utility of low-volume ultrasound-guided interscalene blockade: contraindications reconsidered. J Ultrasound Med. 2009;28(9):1251–1258.
15. Nishimori, M, Low, JHS, Zheng, H, Ballantyne, JC. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. 7, 2012. [CD005059].
16. Stundner, O, Memtsoudis, SG, Regional anesthesia and analgesia in critically ill patients. Reg Anesth Pain Med. 2012;37(5):537–544.
17. Andreae, MH, Andreae, DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev. 10, 2012. [CD007105].
18. Salengros, JC, Huybrechts, I, Ducart, A, et al, Different anesthetic techniques associated with different incidences of chronic post-thoracotomy pain: low-dose remifentanil plus presurgical epidural analgesia is preferable to high-dose remifentanil with postsurgical epidural analgesia. J Cardiothorac Vasc Anesth. 2010;24(4):608–616.
19. Wiebalck, A, Grau, T. Ultrasound imaging techniques for regional blocks in intensive care patients. Crit Care Med. 2007; 35(5 suppl):S268–S274.
20. Arain, MR, Buggy, DJ. Anaesthesia for cancer patients. Curr Opin Anaesthesiol. 2007; 20:247–253.
21. Kurosawa, S. Anesthesia in patients with cancer disorders. Curr Opin Anaesthesiol. 2012; 25(3):376–384.
22. Horlocker, TT, Wedel, DJ, Rowlingson, JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anesth Pain Med. 2010; 35(1):64–101.
23. Bernards, C, Hadzic, A, Suresh, S, Neal, J. Regional anesthesia in anesthetized or heavily sedated patients. Reg Anesth Pain Med. 2008; 33(5):449–460.
24. Davis, ET, Harris, A, Keene, D, et al. The use of regional anaesthesia in patients at risk of acute compartment syndrome. Injury. 2006; 37(2):128–133.
25. Schulz-Stübner, S. The critically ill patient and regional anesthesia. Curr Opin Anaesthesiol. 2006; 19:538–544.
26. Chin, KJ, Tse, C, Chan, V. Ultrasonographic identification of an anomalous femoral nerve. Anesthesiology. 2011; 115(5):1104.
27. Bhat-Bhatia, A, Lai, J, Chan, VW, Brull, R. Pneumothorax as a complication of the ultrasound guided supraclavicular approach for brachial plexus block. Anesth Analg. 2010; 111(3):817–819.
28. Christophe, JL, Berthier, F, Boillot, A, et al. Assessment of topographic brachial plexus nerves variations at the axilla using ultrasonography. Br J Anaesth. 2009; 103(4):606–612.
29. Liu, SS, YaDeau, JT, Shaw, PW, et al. Incidence of unintentional intraneural injection and postoperative neurological complications with ultrasound-guided interscalene and supraclavicular nerve blocks. Anaesthesia. 2011; 66(3):168–174.
30. Reiss, W, Kurapati, S, Shariat, A, Hadzic, A. Nerve injury complicating ultrasound/electrostimulation-guided supraclavicular brachial plexus. Reg Anesth Pain Med. 2010; 35(4):400–401.
31. Fredrickson, MJ, Wolstencroft, P, Kejriwal, R, et al. Single versus triple injection ultrasound-guided infraclavicular block. Anesth Analg. 2010; 111(5):1325–1327.
32. Chin, KJ, Karmakar, MK, Peng, P. Ultrasonography of the adult thoracic and lumbar spine for central neuraxial blockade. Anesthesiology. 2011; 114(6):1459–1485.
33. Dulaney-Cripe, E, Hadaway, S, Bauman, R, et al, Continuous infusion fascia iliaca compartment block in hip fracture patients: a pilot study. J Clin Med Res. 2011;4(1):45–48.
34. Chan, VWS, Nova, H, Abbas, S, et al. Ultrasound examination and localization of the sciatic nerve. Anesthesiology. 2006; 104(2):303–309.
35. Niraj, G, Kelkar, A, Fox, AJ. Application of the transversus abdominis plane block in the intensive care unit. Anaesth Intensive Care. 2009; 37(4):650–652.
36. Piraccine, E, Pretto, EA, Corso, RM, Gambale, G, Analgesia for thoracic surgery: the role of the paravertebral block. HSR Proc Intensive Care Cardiovasc Anesth. 2011;3(3):57–60.
37. Boezaart, AP, Lucas, SD, Elliott, CE, Paravertebral block: cervical, thoracic, lumbar, and sacral. Curr Opin Anaesthesiol. 2009;22(5):637–643.
38. Mai, CL, Young, MJ, Quraishi, SA. Clinical implications of the transversus abdominis plane block in pediatric anesthesia. Pediatr Anesth. 2012; 22(9):831–840.
39. Anahi, P. Evidence for the use of ultrasound in neuraxial blocks. Reg Anesth Pain Med. 2010; 35(suppl 1):S43–S46.