Ultrasound-guided placement of inferior vena cava filters
(CONSULTANT-LEVEL EXAMINATION)
Venous thromboembolism (VTE) is a major problem in patients in the intensive care unit (ICU), as previously analyzed (Chapter 9). VTE will develop in approximately 12% of ICU patients despite appropriate prophylaxis.1 Furthermore, pulmonary embolism (PE) is common and underrecognized in critically ill patients.2
Inferior vena cava (IVC) filter placement is indicated in a small group of patients with VTE who have a contraindication to anticoagulation (in whom it results in complications, fails, or is insufficient) or following massive PE with residual deep vein thrombosis (DVT). The only prospective randomized trial of the use of IVC filters demonstrated that even though patients with IVC filters have a lower rate of PE than do those who undergo anticoagulation, they also have an increased rate of subsequent DVT without a difference in mortality or postphlebitic syndrome.3,4
IVC filters are most commonly placed in an interventional radiology (IR) suite equipped with fluoroscopic guidance. Fluoroscopically guided filter placement has the advantage that IVC venography can be performed, which can ensure that the IVC is patent (Figure 18-1) without any duplication (Figure 18-2) or anatomic variant and is of appropriate size for placement of the filter. It is recommended that anticoagulation be initiated once the contraindication resolves.5
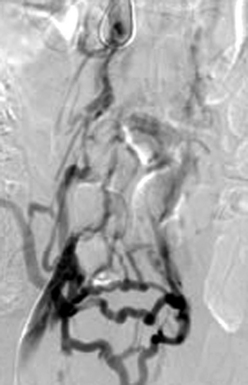
Figure 18-1 Occluded inferior vena cava (injection of contrast material into the right common femoral vein) with visualization of multiple pelvic collateral veins.
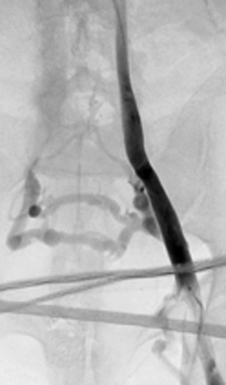
Figure 18-2 Duplicated inferior vena cava (injection of contrast material into the left external iliac vein).
Many ICU patients require IVC filters to prevent PE and death since they usually have contraindications to anticoagulation. Concerns over transportation-related risks have led some investigators to evaluate ultrasound-guided IVC filter placement at the bedside in an attempt to limit transport-associated complications in critical care patients. Papson et al reported 230 unexpected events occurring during the transport of ICU patients, 30 (8.9%) of which were considered serious.6
Abdominal ultrasound has been used to guide IVC filter placement. However, failure to visualize the IVC adequately limits the use of abdominal ultrasound guidance.7 Intravascular ultrasound (IVUS) offers the ability to accurately measure IVC diameter and visualize tributaries in great clarity. A review of the current literature regarding IVUS guidance for IVC filter placement revealed 1 animal study and 13 retrospective human case series. Only three of these studies included more than 50 patients. The largest case series, by Rosenthal et al, reported a 93.1% technical success rate of placing retrievable IVC filters (97 Gunther Tulip and 90 Celect) under IVUS guidance at the bedside, with 6 Tulip filters being misplaced in the iliac veins and 12% tilted.8 When compared with the 3.4% tilt rate described in 100 patients in the prospective Celect filter registry, it is clear that fluoroscopic guidance allows more accurate placement of retrievable IVC filters.9
Although a bedside technique has its advantages, observational studies have reported an increased incidence of filter malposition.8 It is well known that malpositioned filters can result in poorer cross-sectional protection of the IVC (Figure 18-3), and lead to PE despite the filter’s presence and can increase the challenge at the time of retrieval. Another disadvantage of IVUS-guided placement is that no venogram is performed, thereby missing any anatomic variants, in which case the filter may not be protective (e.g., circumaortic left renal vein, duplicated IVC). If managed carefully, the cost related to transport, monitoring equipment, and nursing staff will be negligible in comparison to bedside placement of the filters. Adherence to guidelines for the transportation of critically ill patients, when implemented, can dramatically reduce the morbidity associated with the transportation of such patients.10 Further evidence based on a randomized trial of bedside IVUS-guided placement versus the commonly used fluoroscopically guided placement in an IR suite is warranted.
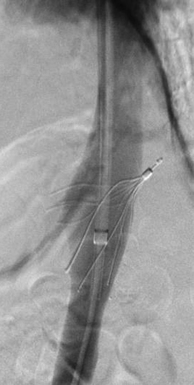
Figure 18-3 Vena cavagram performed during removal of a Gunther Tulip IVC filter. Marked tilting and projection of the retrieval hook outside the lumen of the inferior vena cava are seen.
Technique of bedside intravascular ultrasound–guided inferior vena cava filter placement
Technique
• Perform ultrasound of the femoral veins to ensure their patency.
• Access a patent femoral vein (the right femoral vein is preferred because of less tortuosity).
• Place a 7-Fr sheath in the femoral vein.
• Advance the IVUS catheter into the IVC and image the renal veins (Figure 18-4).
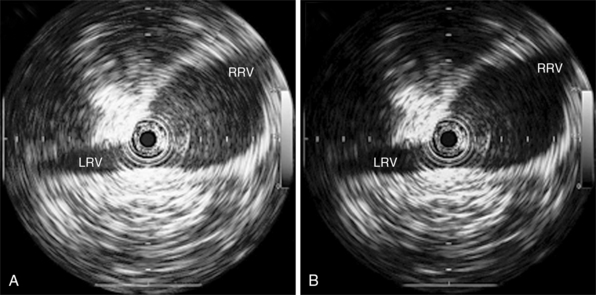
Figure 18-4 Visualization of the right and left renal veins opening into the inferior vena cava. The most central ring represents the intravascular ultrasound catheter. (Reproduced with permission from the Journal of Endovascular Therapy8. ©Copyright (2009). International Society of Endovascular Specialists. Tulip G: Select IVC filters in multiple-trauma patients, J Endovasc Ther 16:494-499, 2009.)
• Place the IVUS catheter at the level of the renal veins.
• Mark the outside of the catheter at the hub of the sheath.
• Pull the catheter back 1 cm plus the length from the hub to the skin entry site and remark the outside of the catheter.
• Remove the IVUS catheter and measure the distance from the IVUS transducer to the final catheter marking. This is the distance that the tip of the filter should be inserted from the skin entry site.
• Mark the outside of the delivery sheath at the same distance just measured.
• Exchange the existing sheath over a wire for the delivery sheath.
• Place the filter into the delivery sheath according to the manufacturer’s instructions. With the filter deployment system connected to the delivery sheath, the tip of the filter should be at the desired location.
• Deploy the filter according to the manufacturer’s instructions.
• Reinsert the IVUS catheter to ensure that the apex of the filter is in the midportion of the IVC and the tip is below the renal veins (Figure 18-5).
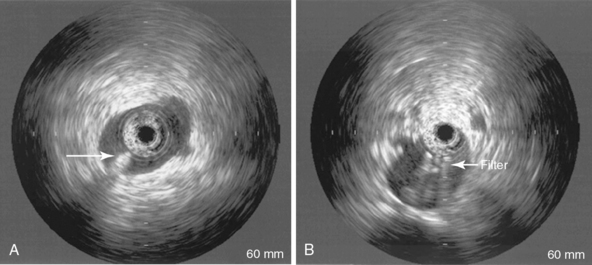
Figure 18-5 Intravascular ultrasound after deployment of the filter for confirmation of the position of the tip of the filter and adequate deployment of the filter legs. (From Bedside intravascular ultrasound-guided vena cava filter placement. Wellons ED, Matsuura JH, Shuler FW, Franklin JS, Rosenthal D, from the Department of Vascular Surgery, Atlanta Medical Center. Presented at the Twenty-seventh Annual Meeting of the Southern Association for Vascular Surgery, Tucson, Ariz, Jan 15-18, 2003, J Vasc Surg 38:455-458, 2003. Copyright © 2003 by The Society for Vascular Surgery.)
Modification with two venous accesses:
• In addition to the steps just outlined, a second venous access can be obtained for placement of an IVUS catheter at the level of the renal veins.
• With the IVUS in place, the sheath and filter can be advanced, the tip of the filter placed 1 cm below the renal veins under direct visualization, and the filter deployed.
• The monitoring IVUS catheter should be withdrawn below the filter before deployment and then readvanced to image the filter after deployment.
The sheaths are then removed and hemostasis achieved with manual compression for 5 to 10 minutes.
Pearls and highlights
• VTE and its management are a major problem in critically ill patients.
• Based on strict guidelines, IVC filter placement is indicated in only a small group of patients with VTE.
• It is recommended that anticoagulation be initiated as soon as the contraindication resolves.
• IVC filters are most commonly placed in an IR suite equipped with fluoroscopic guidance, which has the advantage of real-time assessment of the procedure and associated difficulties.
• A bedside technique under IVUS guidance has advantages but also more procedure-related complications such as filter malposition, which can lead to PE and increase the challenge at the time of retrieval.
• With appropriate checklists and adherence to transportation guidelines, significant complications associated with moving patients to the IR suite can be minimized and allow more accurate IVC filter placement, which should minimize any long-term sequelae of filter malposition.
• Further evidence based on a randomized trial of bedside IVUS-guided placement versus the commonly used fluoroscopically guided placement in an IR suite is warranted.
References
1. Marik, PE, Andrews, L, Maini, B. The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111:661–664.
2. Twigg, SJ, McCrirrick, A, Sanderson, PM. A comparison of post mortem findings with post hoc estimated clinical diagnoses of patients who die in a United Kingdom intensive care unit. Intensive Care Med. 2001; 27:706–710.
3. Decousus, H, Leizorovicz, A, Parent, F, et al, for the PREPIC Study Group: A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Me. 1998; 338:40–415.
4. Decousus, H, Eight-year follow-up of a randomized trial investigating vena cava filters in patients presenting a proximal DVT: the PREPIC trial. J Thromb Haemost. 2003;1(suppl) [OC440].
5. Geerts, WH, Bergqvist, D, Pineo, GF, et al, Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Ches. 2008; 133:381S–453S.
6. Papson, JPN, Russel, KL, Taylor, DM. Unexpected events during the intrahospital transport of critically ill patients. J Acad Emerg Med. 2007; 14:574–578.
7. Benjamin, ME, Sandager, GP, Cohn, EJ, Jr., et al. Duplex ultrasound insertion of inferior vena cava filters in multi-trauma patients. Am J Surg. 1999; 178:92–97.
8. Rosenthal, D, Kochupura, PV, Wellons, ED, et al. Gunther Tulip and Celect IVC filters in multiple-trauma patients. J Endovasc Ther. 2009; 16:494–499.
9. Lyon, SM, Riojas, GE, Uberoi, R, et al, Short- and long-term retrievability of the Celect vena cava filter: results from a multi-institutional registry. J Vasc Interv Radio. 2009; 20:1441–1448.
10. Warren, J, Fromm, RE, Jr., Orr, RA, et al. Guidelines for the inter- and intrahospital transport of critically ill patients. Crit Care Med. 2004; 32:256–262.