Ultrasound-guided central venous access: The basics
Overview
Central venous access is a core skill for anesthesiologists, intensivists, acute care physicians, and surgeons. It is indicated for fluid and drug administration, dialysis, and cardiovascular monitoring. More than 5 million central venous catheters (CVCs) are inserted annually in the United States.1 Because central venous access often remains poorly taught and left out of training curricula, inexperienced and less skilled practitioners frequently experience significant difficulties.
Ultrasound guidance (USG) for CVC placement was introduced in the 1980s. Since then, many reports have demonstrated increased success, enhanced safety, and effectiveness of USG in comparison to anatomic landmark or audio Doppler-based techniques.2–5
Several national and international recommendations6–10 have been developed that advocate the routine use of USG for placement of CVCs. Although the original practice guidelines11 focused mainly on USG for cannulation of the internal jugular vein (IJV), USG can be used at most sites.
Advantages of ultrasound-guided vascular access
To appreciate the importance of ultrasound-guided vascular access (USGVA) it is necessary to understand the potential complications of attempted vascular access, which can be grouped under immediate and delayed complications. The former include inadvertent arterial or venous puncture and cannulation, hematoma, posterior vessel wall (through-and-through) puncture, pneumothorax or hemothorax, and air embolism. Other complications include multiple skin punctures, patient anxiety and discomfort, and failure of the procedure. Thoracic duct injury and nerve injury may follow CVC placement. Late complications of vascular access are local and distant infection, including catheter-related bloodstream infection (CRBSI), arterial or venous thrombosis, vessel stenosis, occlusion of the lumen or device, arteriovenous fistula formation, and tip migration.1,6
USGVA can potentially reduce the incidence or completely prevent many of these complications and improve the safety of the procedure.2–5 This is due to a reduction or avoidance of primary damage caused by injury to collateral structures from the needle or secondary damage caused by misplaced guidewires, dilators, and catheters. USG enables direct visualization of the vessel or vessels of interest and surrounding structures. The depth and size of the target vessel can be measured and an optimal target vessel and site for venipuncture chosen. In trained hands, USGVA reduces the frequency of unsuccessful punctures and associated complications and facilitates first-pass cannulation.2–5 A preprocedural scan is performed to identify abnormal anatomy, thrombosis, or valves, and a postcannulation scan is used to confirm correct placement of the guidewire and catheter in the intended vessel. Ultrasound can be used reliably for the diagnosis of pneumothorax or pleural fluid collections. The same transducer used for cannulation can be used to examine the pleura.6 There is a potential reduction in CRBSI when USGVA is integrated within a multifaceted strategy.3 USGVA is cost-effective because of better clinical outcomes and reduced cost of care.2,6,11
The ultrasound transducer
Application of electric current to the piezoelectric element in the transducer creates ultrasound waves (see Chapter 1). The mechanical energy generated travels and penetrates through the tissues as pulsed longitudinal waves, some of which will be absorbed or lost in the tissues whereas the remainder will be reflected back at different tissue interfaces and received at the transducer. The phenomenon of attenuation describes the loss of ultrasound energy via absorption, reflection, refraction, and scattering and can cause distortion of the image and misinterpretation of anatomic relationship. The returned energy is processed into an anatomic representation of the underlying structures (see Chapter 1).12
Broad-frequency transducers (5 to 15 MHz) are typically used for vascular access. Higher frequencies provide better resolution, are best suited for superficial structures, and enable identification and avoidance of adjacent vulnerable structures (e.g., small arteries and nerves). High resolution is also required for neonatal and pediatric cannulation.9,12
Midrange frequencies (5 to 10 MHz) are used to visualize structures 3 to 6 cm under the surface of the skin. A low-frequency probe operates below 5 MHz and is usually reserved for deeper structures; it is not commonly used for vascular access.6,9
The scanning surface (footprint) may be linear or curvilinear. Typical footprints vary from 20 to 50 mm in length. Smaller footprints enable access to confined anatomic locations (e.g., infraclavicular imaging or children), whereas large footprints allow the acquisition of a wider image.6,9
The display
Safe, successful vascular access requires attention to the surrounding environment: ultrasound display position and transducer orientation. Operator experience and patient and equipment factors contribute to the final outcome. Adequate knowledge of the physics of USG and the mechanism of image acquisition and interpretation is essential before attempting USGVA. The operator must have the ability to interpret the displayed two-dimensional (2D) image of the vessels of interest and associated tissues, use this 2D image to perform a three-dimensional (3D) task, and possess the necessary hand-eye coordination and manual dexterity skills to manipulate the needle and transducer according to the displayed image.9
The display should be positioned at an appropriate height for the procedure and on the contralateral side of the patient.6,9 The displayed image should be in the same anatomic orientation as seen from the position of the practitioner. A palpable marker on the side of the transducer corresponds to an orientation cue on the display. Touching one end of the probe and observing the associated display artifact will eliminate confusion.12
Typically, the operator holds the ultrasound probe with the nondominant hand in such a way that the structures beneath the left end of the probe appears on the left side of the screen and vice versa, and subsequently each side of the screen will display the ipsilateral structures.9,12
Image optimization
Depth and gain control is used to improve the quality of the displayed image.12 This typically follows a preliminary scan and identification of the structures of interest. Initially, the depth must be adequate to allow visualization of a wider anatomic field that includes the structure or structures of interest. This will identify vulnerable structures and those at risk with needle advancement, for example, the pleura and other vessels. After a preliminary scan, the depth can be reduced to focus on the target vessel and associated structures.
Sometimes the operator may want to increase or decrease the gray scale for optimization of the image. The gain control is used to reduce or amplify the ultrasound signals received and subsequently image brightness.12 Different structures have different echogenic characteristics, and this will influence the amount of gain control required.
Relative echogenicity refers to the ability of some structures to reflect back more of the emitted ultrasound energy. Hyperechoic or echogenic structures (e.g., bones and pleura) reflect more ultrasound signal than do the surrounding tissues and appear brighter. In contrast, blood and fluid are hypoechoic.12
Vascular scanning
Ultrasound-assisted vascular access refers to the use of ultrasound to verify the presence and patency of vessels whose approximate position can then be marked on the skin. This is followed by an attempted “blind” vascular puncture.6 Ultrasound-guided cannulation refers to ultrasound scanning to visualize and verify a vessel and subsequently guide the tip of the needle in real time throughout the insertion process.6
Vascular structures have different morphologic and anatomic features. Ultrasound examination is used to distinguish veins and arteries and avoid unintentional puncture or cannulation. Veins are typically elliptic or ovoid, thin walled, and readily collapsible, whereas arteries are circular, thick walled, and less compressible.9
Though not routinely required for vascular access, Doppler examination can demonstrate vessel patency and the direction and nature of blood flow and enable differentiation of deeper veins from arteries. Color Doppler and spectral Doppler waveform examination require further training and can establish vessel patency, as well as differentiate between a vascular and a nonvascular hypoechoic structure.6,9
Various terms have been used to describe the spatial relationship between the advancing needle and probe and the target vessel. Safe and successful cannulation requires understanding of such relationships. The first step is to confirm correct orientation of the scanning probe. This is followed by visualization and verification of the target vessels and surrounding structures.9 Thorough preliminary scanning will allow the operator to identify the most appropriate site for needle puncture and avoid anatomic misinterpretation.6
Ultrasound scanning of a blood vessel in its short axis generates a transverse image. Turning the position of the probe 90 degrees from the short axis generates a long-axis or longitudinal view of the same vessel. A hybrid oblique axial view has also been described and is useful with more tortuous vessels.6
Needle orientation
With respect to the ultrasound beam, the needle can be advanced either in plane or out of plane. The former allows continuous visualization of the entire needle and tip throughout their complete trajectory from the surface of the skin to the target vessel, thereby enabling precise real-time control. This should improve the safety of the procedure and minimize inadvertent injury; nevertheless, it is more challenging with deeper structures (e.g., the axillary vein in the morbidly obese) and requires further training. An in-plane approach does not provide simultaneous imaging of surrounding structures, nor does it necessarily avoid transfixion of the vessel when it is at low pressure and collapsible.6
The more widely used out-of-plane puncture allows concomitant visualization of associated at-risk structures and simultaneous anterior-posterior and medial-lateral views of the vessel or vessels; however, the position of the needle tip is more difficult to ascertain and requires considerable experience and practice. It is easy to advance the tip of the needle across the very thin (<1 mm) ultrasound beam and misinterpret the shaft for the tip. Failure to visualize the needle tip as it penetrates through the tissues may expose the patient to avoidable harm and reduce the expected benefits of USGVA. Many operators are now combining both approaches such that the vessel is viewed in cross section (wholly or in part) and the needle introduced from the side in plane with the probe beam.6 Irrespective of the approach taken, there are still difficulties with very collapsible veins because it is difficult to know when the tip of the needle has penetrated the anterior or posterior vein walls.6
Needle guides can be used to align the position of the needle in either a transverse or longitudinal view. They could be useful for less experienced operators, for whom a mechanical device to hold the needle in an appropriate orientation can be valuable.6,9 A recent study reported better visibility, faster access, and fewer mechanical complications with an echogenic needle.13
Selection of the site of cannulation
Various factors are important when deciding on the cannulation site, including patient and operator factors, as well as the intended indication for and duration of central venous catheterization and the clinical scenario (e.g., rapid access in an emergency situation).6,10 A preprocedural scan will help identify difficult or impossible access sites inasmuch as 10% of patients may have abnormal vascular anatomy.6 Assessment of vessel size, position, and patency helps in identifying the most suitable site and choosing the correct size of catheter.6 USG can influence the choice of cannulation site and the long-term outcome of the procedure:
• Vein size: Smaller veins are difficult to cannulate and more predisposed to the development of thrombosis and stenosis. As a general role, the outer diameter of the CVC should not be more than a third of the vein’s caliber. A high catheter-to-vein ratio impedes blood flow and should be avoided.6
• Vein depth: In most individuals the IJV is less than 2 cm underneath the skin. The brachiocephalic vein and subclavian vein (SCV) are relatively deeper structures, especially in obese patients.
• Surrounding structures: The SCV lies immediately above the pleura. A more lateral infraclavicular approach allows venipuncture away from the pleura. At-risk vessels may be located in front of or behind the target vein (Figure 10-1A).
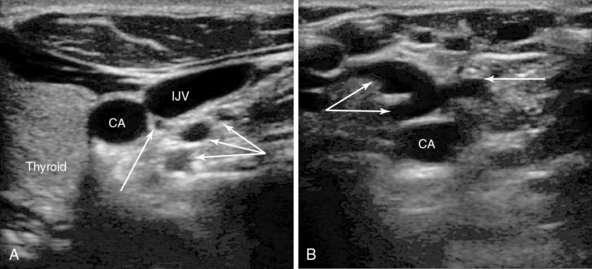
Figure 10-1 A, Right carotid artery, internal jugular vein (IJV), and thyroid. Note the vessels in close proximity behind the IJV (arrows). These three vessels are all from the thyrocervical trunk, which arises from first part of the subclavian artery and ascends for a short distance behind the IJV before branching. Such vessels are at risk with needle transfixion of the vein. A vein is also present just below these arterial branches. B, Left carotid artery with a plexus of tortuous collateral veins anteriorly (arrows). No obvious larger IJV is present, which has been blocked after previous long-term catheterization. The operator should move to another site of access.
• The skin exit site should be chosen carefully because it will affect both the short- and longer-term outcome of central venous catheterization. A conveniently located exit site enables better maintenance of the CVC, is easier to dress and secure, and enhances patient compliance and nursing care. Some exit sites are associated with a higher rate of infection (e.g., femoral veins).9
• Anatomic and morphologic abnormalities: Mediastinal tumors and masses may cause external compression or stenosis of a great vein. An occluding thrombus or prolonged cannulation may cause blockade of the vein. Ultrasound features include demonstration of engorged collaterals and difficult-to-compress veins because of high pressure and reversed flow on Doppler examination. These sites need to be avoided since successful central guidewire and catheter insertion is unlikely. This situation is more likely to be encountered in patients requiring long-term or repeated central venous catheterization (Figure 10-1B).6
Ultrasound-guided vascular access techniques and current recommendations
Internal jugular vein cannulation
A substantial body of scientific evidence recommends USG over landmark techniques for cannulation of the IJV in adult and pediatric patients in both elective and acute settings.2–6,11 USG for IJV access offers a higher success rate, typically on the first needle pass, reduced complications from collateral damage, decreased procedural time, and potential overall savings.2–6 A procedural failure rate of 7% to 19% has been demonstrated with traditional anatomic landmark approaches. Furthermore, a success rate of less than 25% per attempt has been reported when initial blind punctures have failed.4
Routine USG is invaluable inasmuch as anatomic variability was observed in 36% of the population in one study.14 Head rotation from a neutral to a lateral position can increase vein-to-artery overlap nearly threefold, and this occurs more frequently in obese patients. Precise needle control with USG reduces the risk for this complication. An ultrasound-measured IJV diameter smaller than 7 mm suggests a poor success rate, and 3% of patients had small fixed IJVs in one study. The right IJV is usually larger than the left, and USG can verify size and patency and help in determining best side for cannulation, thus improving success and minimizing complications.11
Patients with carotid artery disease, such as stenosis or a patch, are at particular risk, and puncture of the carotid artery with even a 21-gauge seeker needle can lead to catastrophic sequelae. High-resolution ultrasound can identify nearby at-risk structures, such as the carotid, vertebral, and subclavian arteries; thyroid gland and arteries; and lymph nodes. The subclavian, vertebral, and inferior thyroid arteries and the thyrocervical trunk cross behind the IJV low in the neck and can pose significant risk (Figure 10-2A and B).
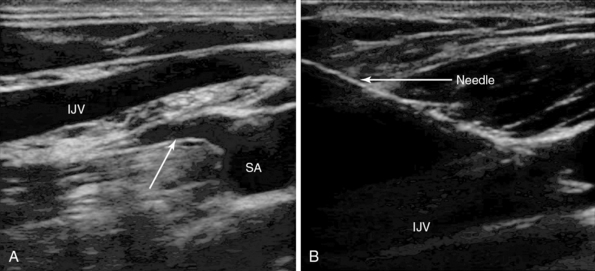
Figure 10-2 A, Longitudinal view of the lateral edge of the lower section of the right internal jugular vein (IJV) crossing the right subclavian artery (SA). A major branch of the artery is arising from the superior aspect of artery, the thyrocervical trunk (arrow), which is about 4 mm behind the vein. The artery and branches are at risk with vein transection. B, In-plane needle visualization and puncture of the IJV.
Axial and lateral approaches refer to out-of-plane and in-plane transverse views of the needle during ultrasound-guided cannulation of the IJV. Precise control of the needle during the in-plane lateral approach should lessen the likelihood of arterial or pleural puncture and provide a more conveniently located exit site in the lower part of the neck. The more commonly used out-of-plane axial puncture usually results in a midneck exit site with potential difficulty in securement and dressings. The more technically challenging in-plane needle insertion via a longitudinal view should be associated with fewer complications in experienced hands.6 In conclusion, level 1 evidence mandates the routine use of real-time USG for cannulation of the IJV by adequately trained operators.
Subclavian vein cannulation
The SCV is the continuation of the axillary vein and runs from the apex of the axilla across the first rib in the subclavian groove and is separated from the axillary artery by the insertion of the anterior scalene muscle. It joins the IJV behind the sternoclavicular junction to form the brachiocephalic vein. Throughout most of its course the SCV lies behind the clavicle, which impedes ultrasound visualization; however, a supraclavicular and infraclavicular lateral approach can be used for USG. The SCV is close to the pleura, subclavian artery, and brachial plexus (Figure 10-3). Because of currently insufficient evidence, routine use of USG for SCV cannulation is not mandated despite its perceived advantages.9,10
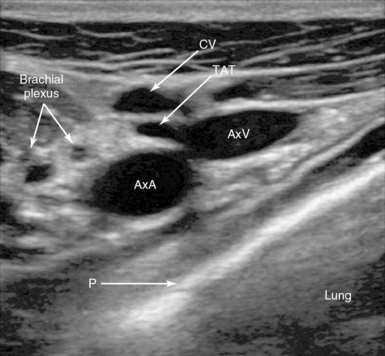
Figure 10-3 Lateral infraclavicular approach to the right axillary vein. Note the right axillary artery (AxA) and vein (AxV) with the thoracoacromial trunk (TAT) branch in front, the cephalic vein (CV), and the pleura (P).
Landmark-based techniques for SCV cannulation are associated with up to 12% complication rates.9 There is increasing experience and interest in ultrasound scanning for SCV cannulation. Fragou et al15 used a parasternal approach to SCV cannulation and reported a higher success rate, faster access, and fewer needle passes and complications. USG via a suprasternal approach facilitated success on the first needle pass in more than 98% of attempts with no reported major complications.16
Many clinicians are probably actually accessing the axillary vein rather than the SCV, which starts at the level of the first rib. The axillary vein lies entirely outside the rib cage, and a slightly more lateral infraclavicular approach allows easy visualization with ultrasound and a greater distance between the pleura and vein and can largely avoid pleural or lung damage.17
A recent analysis confirmed the safety and effectiveness of this approach.18 Real-time needle visualization and depiction of the axillary artery and brachial plexus can avoid injury to these structures. The cephalic vein and thoracoacromial branch of the axillary artery (see Figure 10-3) may lie anterior to the axillary vein and can be avoided.18
Femoral vein cannulation
The common femoral vessels lie within the femoral sheath in the femoral triangle formed by the inguinal ligament, adductor longus, and sartorius muscle. Many patients will not have the classic textbook description of the femoral artery and vein running side by side, and significant overlap has been observed in adults and children.9 Ultrasound imaging identifies the common femoral vein (FV) with the great saphenous vein joining it and the superficial and deep branches of the common femoral artery. With USG, vessel patency, depth, and overlap can be verified (see Figure 10-4A,B).
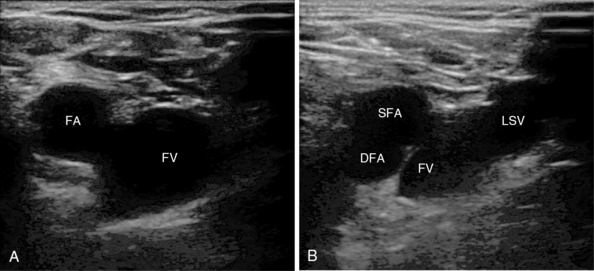
Figure 10-4 A, Femoral vessels at the level of the inguinal ligament with side-by-side orientation. B, Vessels just below the inguinal ligament with overlap: superficial (SFA) and deep (DFA) femoral arteries. Note the long saphenous vein (LSV) as it joins the femoral vein (FV).
The FV is the preferred route of access for many cardiac procedures in both children and emergency scenarios.9 Attempted FV cannulation does not interfere with cardiopulmonary resuscitation, breathing tubes, and monitoring cables and avoids the risk for pneumothorax and hemothorax.9 However, vascular complications may follow FV access and include bleeding, arterial puncture, hematoma, thrombosis, and formation of an arteriovenous fistula and pseudoaneurysm. Rare but serious complications include bowel and bladder perforation and peritoneal or retroperitoneal bleeding following high puncture sites.9
These complications are less likely when USG is used. FV catheters are predisposed to contamination because of their close proximity to the perineal area. Both prolonged cannulation and repeated needle passes increase this risk; the latter can be minimized by USG, and it should be used routinely for FV catheterization.6,10
Pearls and highlights
• Competency in ultrasound image acquisition, interpretation, and management is a prerequisite before undertaking practical procedures. The operator must be able to appreciate the difference between the virtual 3D patient anatomy and the 2D displayed ultrasound image. Skill in needle visualization, guidance, and navigation in real time requires considerable training.
• Doppler shift differentiates vascular from nonvascular structures. Color Doppler and spectral Doppler waveform examination are used to differentiate arterial from venous flow.
• Accurate interpretation of the ultrasound image, identification of the target vessel, and continual visualization of the tip of the needle are fundamental to the safety and success of the procedure.
• USGVA improves patient safety by reducing mechanical and possibly infectious complications, efficacy of the procedure by reducing the number of attempts and duration, and maximizes patient satisfaction.
References
1. McGee, DC, Gould, MK, Preventing complications of central venous catheterization. N Engl J Med 2003;348(12):1123–1133.
2. Hind, D, Calvert, N, McWilliams, R, et al, Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361–368.
3. Karakitsos, D, Labropoulos, N, De Groot, E, et al, Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162.
4. Troianos, CA, Jobes, DR, Ellison, N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991; 72(6):823–826.
5. Augoustides, J, Horak, J, Ochroch, A, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005; 19(3):310–315.
6. Lamperti, M, Bodenham, AR, Pittiruti, M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012; 38(7):1105–1117.
7. Rothschild, JM, Ultrasound guidance of central vein catheterization. In Making healthcare safer: a critical analysis of patient safety practices, AHRQ Publication No. 01-E058. Agency for Healthcare Research and Quality, Rockville, MD, 2001:245–253.
8. Bishop, L, Dougherty, L, Bodenham, A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol. 2007; 29(4):261–278.
9. Troianos, CA, Hartman, GS, Glas, KE, et al, Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291–1318.
10. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp, SM, Apfelbaum, JL, Blitt, C, et al, Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
11. National Institute for Health and Clinical Excellence, NICE Technology Appraisal No. 49: guidance on the use of ultrasound locating devices for placing central venous catheters Available at http://publications.nice.org.uk/guidance-on-the-use-of-ultrasound-locating-devices-for-placing-central-venous-catheters-ta49, September 3, 2012 [Accessed].
12. Chapman, GA, Johnson, D, Bodenham, AR. Visualisation of needle position using ultrasonography. Anaesthesia. 2006; 61(2):148–158.
13. Stefanidis, K, Pentilas, N, Dimopoulos, S, et al. Echogenic technology improves cannula visibility during ultrasound-guided internal jugular vein catheterization via a transverse approach. Crit Care Res Pract. 2012; 2012:306182.
14. Benter, T, Teichgräber, UK, Klühs, L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001; 22(1):23–26.
15. Fragou, M, Gravvanis, A, Dimitriou, V, et al, Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607–1612.
16. Cavanna, L, Civardi, G, Vallisa, D, et al, Ultrasound-guided central venous catheterization in cancer patients improves the success rate of cannulation and reduces mechanical complications: a prospective observational study of 1978 consecutive catheterizations. World J Surg Onco. 2010; 8:91.
17. Sharma, A, Bodenham, AR, Mallick, A. Ultrasound-guided infraclavicular axillary vein cannulation for central venous access. Br J Anaesth. 2004; 93(2):188–192.
18. O’Leary, R, Ahmed, SM, McLure, H, et al, Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anesth. 2012;109(5):762–768.