Chapter 12 Ultrasound as a Complement to Electrodiagnostic Studies
As the technology continues to improve and the literature in this field develops, the role of ultrasound in the electrodiagnostic evaluation will continue to evolve. The goal of this chapter is to review the current indications for ultrasound in regards to localization of nerve and muscle, to highlight the additional information ultrasound can provide in neuromuscular disease diagnosed electrophysiologically, and to touch on future research and directions for this imaging modality with respect to electrodiagnosis.
The Role of Ultrasound in Nerve Conduction Studies
Ultrasound can be used during nerve conduction studies to better localize the nerve in question at the site of stimulation or recording.1,2 The accuracy of near-nerve needle stimulation or recording is increased when nerves and surrounding structures are imaged and needles are placed under ultrasound guidance. Ultrasound-guided needle placement is a relatively easy technical skill to develop. Formal training courses are available with hands-on demonstration sessions, and the technique can be refined using fresh frozen cadavers or gel phantoms with enclosed targets for simulation of image-guided needle placement (Box 12.1). Once this skill is acquired, it facilitates the use of ultrasound as a complement to electrodiagnosis by enhancing the accuracy and safety of the invasive aspects of nerve conduction studies and needle EMG.
Box 12.1 Examples of Diagnostic and Interventional Ultrasound Educational Resources
American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM): http://www.aanem.org/
American Institute of Ultrasound Medicine (AIUM): www.aium.org
European League Against Rheumatism (EULAR): www.eular.org
European Society of Skeletal Radiology (ESSR): www.essr.org
University of Michigan Ultrasound: www.med.umich.edu/rad/muscskel/mskus
Musculoskeletal Ultrasound Society (MUSOC): http://tdirad1.googlepages.com
Wake Forest University Center for Medical Ultrasound: http://www.wfubmc.edu/ultrasound/
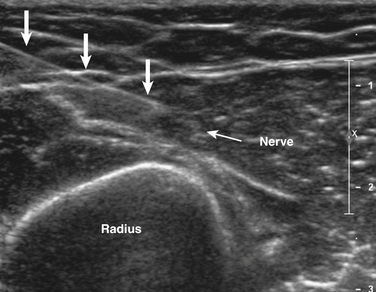
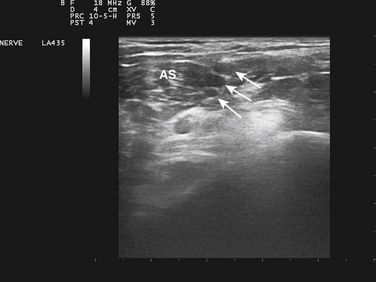
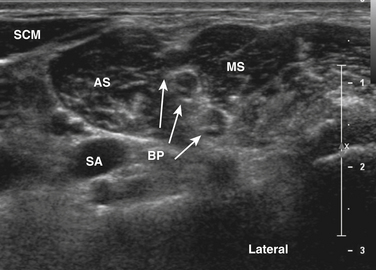
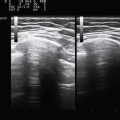
Ultrasound-guided needle placement can be very helpful in certain situations in the EMG laboratory. When apparent conduction block on a motor nerve conduction study is encountered but it is not clear whether technical factors such as submaximal stimulation may be contributing, a monopolar needle can be placed adjacent to the nerve, under ultrasound guidance, to allow for supramaximal stimulation (at much lower levels of current than required with surface stimulation). There are many potential applications for this, usually in nerves that are deeper and more difficult to stimulate with surface electrodes. Examples include the radial nerve at the spiral groove or elbow (Fig. 12.1), the sciatic nerve in the thigh, the tibial nerve at the knee, the femoral nerve in the proximal thigh, or even the brachial plexus or exiting cervical roots (Fig. 12.2).3 Femoral nerve stimulation may be indicated in cases of diabetic lumbosacral radiculoplexus neuropathy (diabetic amyotrophy) and select cases of Lambert-Eaton myasthenic syndrome, in which case repetitive stimulation before and after isometric exercise is necessary. In such cases, the neurovascular bundle is easily visualized with ultrasound and the needle can be placed directly adjacent to the nerve, allowing for less current, better patient comfort, and a technically superior response (Fig. 12.3). Such procedures can be also be more safely performed in anticoagulated patients, because the needle can be placed under direct ultrasound guidance, with visualization of nearby vascular structures facilitated by the use of color Doppler. The availability of ultrasound in this setting also allows for monitoring for hematoma formation after the needle is removed.
Ultrasound can also facilitate nerve conduction studies in challenging cases in which anatomy is altered or usual landmarks are not palpable as a result of body habitus or dysmorphism. In such cases, the nerve of interest can be localized ultrasonographically prior to proceeding with standard surface stimulation, for example, after ulnar nerve transposition surgery in which the altered position of the nerve can be mapped out in the antecubital fossa and inching studies performed along the actual course of the nerve.
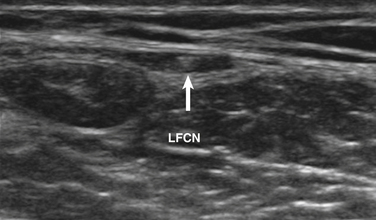
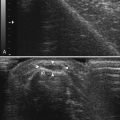
Ultrasound-guided nerve conduction techniques have been described to allow more accurate and reliable evaluation of nerves that previously had been difficult to study for technical or anatomic reasons. Examples include the lateral femoral cutaneous nerve in the proximal thigh and the saphenous nerve in the calf. Bailey and associates reported on a cohort of 50 normal subjects, including many with body mass indices in the obese range, in which the lateral femoral cutaneous nerve was localized with ultrasound, approximately 11 cm distal to the stimulation site at the inguinal crease (Fig. 12.4). The G1 electrode was placed directly over the nerve, and responses were elicited in 49 of 50 patients, with an average amplitude of 9.3 μV and side-to-side variability of 34%.4 Similarly, the saphenous nerve can be easily identified with ultrasound in the proximal calf and followed distally, to allow for placement of recording or stimulating electrodes directly over the nerve.5 If no response is obtained on standard conduction studies and there is a question as to whether technical factors may be contributing, it is simple to examine the limb with ultrasound and identify the nerve of interest, to ensure setup is optimal. In critical cases placing a needle next to the nerve for either stimulating or recording can be performed when necessary.
Imaging of Nerve
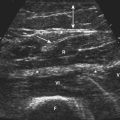
Ultrasound imaging of nerve is rapidly emerging as a useful diagnostic technique. In cases of entrapment neuropathy, electrodiagnosis helps to determine the presence and severity of entrapment, and to distinguish demyelination from axonal loss, but no information regarding the underlying cause can be elicited. Ultrasound can identify underlying space-occupying or compressive lesions such as lipoma, fibroma, hemangioma, hematoma, amyloid, synovitis, tenosynovitis, ganglion cyst, intraneural ganglion, pseudoaneurysm, anomalous muscle, and primary nerve tumors including neurofibromas, schwannomas, malignant nerve sheath tumors, and pericytomas.6–8 Typical sites of entrapment that can be evaluated with ultrasound are listed in Table 12.1.
Table 12.1 Common Sites of Entrapment that Can Be Evaluated with Ultrasound
| Nerve | Site of Entrapment |
|---|---|
| Suprascapular | Spinoglenoid notch, supraspinous notch |
| Axillary | Quadrilateral space |
| Radial | Spiral groove |
| Posterior interosseous | Supinator |
| Superficial radial | Wrist |
| Ulnar | Groove, cubital tunnel, wrist |
| Anterior interosseous | Mid-forearm |
| Median | Wrist |
| Sciatic | Thigh, posterior hip∗ |
| Peroneal | Fibular head |
| Superficial peroneal | Ankle, distal leg |
| Tibial | Ankle |
∗ The sciatic nerve can be difficult to visualize in the buttock/posterior hip region without the use of a curvilinear probe, particularly in obese patients.
Padua and colleagues used ultrasound imaging in a series of 77 cases presenting to the EMG laboratory with various mononeuropathies, including peroneal neuropathy at the fibular head, ulnar neuropathy at the elbow, and atypical median neuropathy at the wrist. In 26% of cases, an underlying cause for the neuropathy was identified on ultrasound, which led to an alteration in the management of those cases.8 Visser found intraneural ganglion cysts on ultrasound in 18% of patients with electrophysiologic evidence of common peroneal neuropathy at the fibular head.9 Similarly, in cases of suprascapular neuropathy, ultrasound of the posterior shoulder may identify a spinoglenoid cyst.10 In a series of 77 patients (96 wrists) with clinical and electrophysiologic evidence of carpal tunnel syndrome, without typical risk factors such as rheumatoid arthritis, diabetes, or pregnancy, 17% had evidence of underlying flexor tenosynovitis on ultrasound, which has implications with regard to management in comparison with cases of idiopathic carpal tunnel syndrome.7 Nakamichi and Tachibana showed that in patients presenting with unilateral carpal tunnel syndrome, 35% had an underlying structural abnormality, which altered the management in those cases.11 Anatomic variants, such as a bifid median nerve or persistent median artery, are easily identified on ultrasound and can have surgical implications, particularly with use of an endoscopic surgical technique, arguing for routine preoperative ultrasonography.
Ultrasound imaging is also useful in detecting complete nerve transection, which can be particularly helpful acutely, when electrodiagnostic studies cannot differentiate between conduction block and axonal injury.12 Neuromas can be easily identified on ultrasound. In addition, sonopalpation (direct pressure from the transducer applied over the area of interest) can be used to determine if a neuroma is symptomatic.13 Dynamic imaging is also useful in the diagnosis of various nerve disorders. For example, one can confirm the presence of a subluxing ulnar nerve, but more importantly, evaluate for a snapping head of the medial triceps, an entity that commonly accompanies a symptomatic subluxing ulnar nerve, and if unrecognized, can lead to a poor surgical outcome.14–15
When diagnosing nerve entrapment per se, as opposed to the underlying cause of the entrapment, there is a need for more studies evaluating both normal controls, diseased controls, and those with the entrapment of interest to delineate more accurately normal cross-sectional areas, the most appropriate site for measurement, the utility of patient-derived control measurements,16,17 and the effects of various body metrics on the normal values. Normal values for a number of nerves have been published.18 In particular, a number of studies have focused on median neuropathy at the wrist and ulnar neuropathy at the elbow with considerable overlap demonstrated between normal controls and those with the disease.7,14,18–22
Clinical Application—Case 1
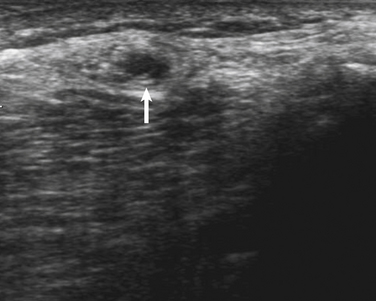
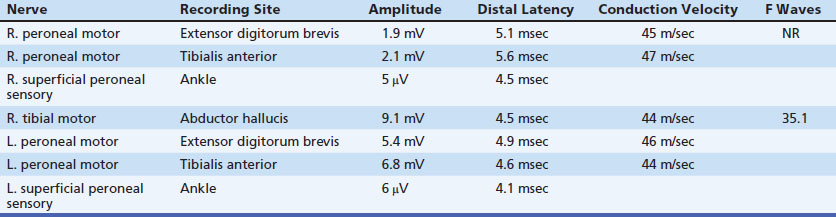
A 53-year-old man presented with a 6-month history of progressive pain during and after soccer games, progressing to pain and mild ankle weakness when hiking, that was unresponsive to antiinflammatory medication. On examination, the patient was noted to have mild to moderate weakness of ankle dorsiflexors and toe extensors on the affected side. Nerve conduction studies and needle examination demonstrated a common peroneal neuropathy, without focal conduction block or slowing (Tables 12.2 and 12.3). Ultrasound of the peroneal nerve showed a large intraneural ganglion cyst that was surgically decompressed (Fig. 12.5).
Intraneural ganglion cysts involving the peroneal nerve arise from the proximal tibiofibular joint, caused by communication via the intraarticular branch of the peroneal nerve. Typically patients have pain and fluctuating weakness (worse with prolonged weightbearing), as opposed to more typical peroneal neuropathies associated with weight loss, habitual leg crossing, and frequent squatting, which tend to be painless and are more frequently associated with conduction block on nerve conduction studies.23 It is important to differentiate these etiologies because surgical intervention is indicated in intraneural ganglion cysts as opposed to the natural history of spontaneous improvement with other causes of peroneal neuropathy.
The Role of Ultrasound in Needle EMG
There are many potential applications of ultrasound with respect to accurate identification of muscle during needle EMG. In select circumstances ultrasound enhances the accuracy and safety of needle EMG. Several cadaveric studies evaluated the accuracy of non–image-guided needle placement in the hands of experienced electromyographers, with accuracy ranging from 0% to 83% depending on the muscle examined.24–26 The authors evaluated the accuracy of nonguided versus ultrasound-guided EMG needle placement in 14 different muscles in the lower limb in fresh frozen cadavers and found overall nonguided accuracy rates of 50% for a fully trained resident electromyographer compared with 83% for an experienced staff electromyographer at a large academic EMG laboratory. With the use of ultrasound guidance, accuracy rates were markedly enhanced, with improvement to 96%.
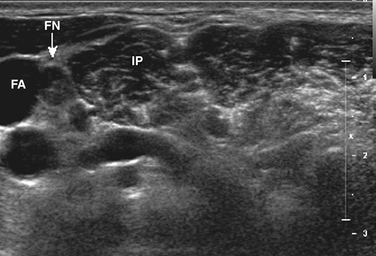
EMG guidance has been used to allow accurate injection of chemodenervating agents for many years, but the addition of ultrasound may be complementary or in some cases can be used in place of EMG guidance. When targeting small muscles in a very spastic or dystonic limb, the patient will be unable to selectively activate and ultrasound can provide excellent anatomic localization. Also, when targeting muscles in proximity to vital structures, such as the scalenes, subclavius, and pectoralis minor muscles when treating myofascial pain with associated thoracic outlet symptoms, the use of power Doppler and visualization of the brachial plexus significantly enhances safety (Fig. 12.6)27 (further discussion of ultrasound-guided injections can be found in Chapter 11).
Clinical Application—Case 2
A 51-year-old woman presented with a 12-month history of progressive difficulty with shoulder range of motion, with associated pain, after undergoing modified radical mastectomy for breast cancer, followed by breast reconstruction surgery with saline implants. On examination she had markedly limited shoulder abduction and forward flexion, with marked contraction of the pectoralis muscle. Her treating physician requested botulinum toxin injection of the pectoralis major muscle to assist with regaining shoulder range of motion and to decrease pain. Her surgeon was reluctant to consider EMG-guided chemodenervation because of the risk of saline implant rupture; however, with the complementary use of ultrasound, the extent of the saline implant was easily identified and marked on the skin and the botulinum toxin was then injected under EMG guidance above and lateral to the implant.
Clinical Application—Case 3
Ultrasound added several facets to the electrodiagnostic examination in this case and allowed the clinician to rule out any role for phrenic nerve pacing. The phrenic compound muscle action potentials recorded on prior studies were likely volume conducted from nearby chest wall muscles, a technical issue that is not uncommon secondary to proximity of the brachial plexus to the phrenic nerve with supraclavicular stimulation.28 Needle examination was able to be performed in a controlled manner in the setting of elevated bleeding risk, because direct visualization of the needle enabled avoidance of vital structures such as intercostal vessels, lung, and liver, and enabled monitoring of the area after completion of the needle examination to exclude hematoma formation and facilitate immediate intervention if observed.
Needle Examination of the Diaphragm with Ultrasound
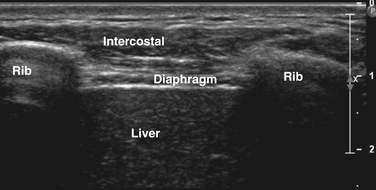
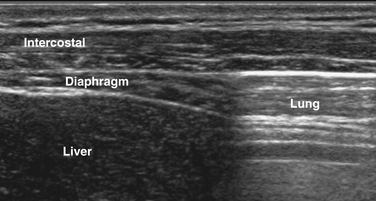
The diaphragm can be easily visualized in most patients using a high-frequency (13-8 MHz) linear array transducer, although in larger patients the authors have found a cardiac curvilinear probe useful because it provides better visualization of the diaphragm at greater depths. The linear probe is placed perpendicular to the lower ribs, at approximately the anterior axillary line, spanning two ribs. The ribs are easily identified by the bright signal generated by the bony cortex, and the intercostal muscles are seen between the ribs, with subcutaneous tissue superficial and diaphragm muscle layer deep to the intercostal muscles (Fig. 12.7). Deep to the diaphragm the liver can be identified, because of a more homogeneous, nonfibrillar appearance, punctuated by small blood vessels. The diaphragm is identified by its location, activation pattern (contraction/thickening with inspiration), curved appearance in some cases, and displacement by pleura and/or lung tissue coming into the field of view with end inspiration (Fig. 12.8).
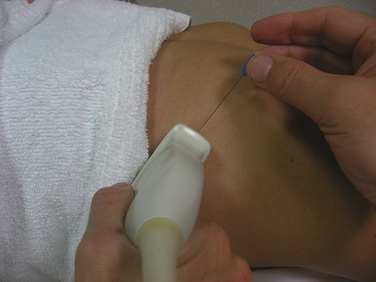
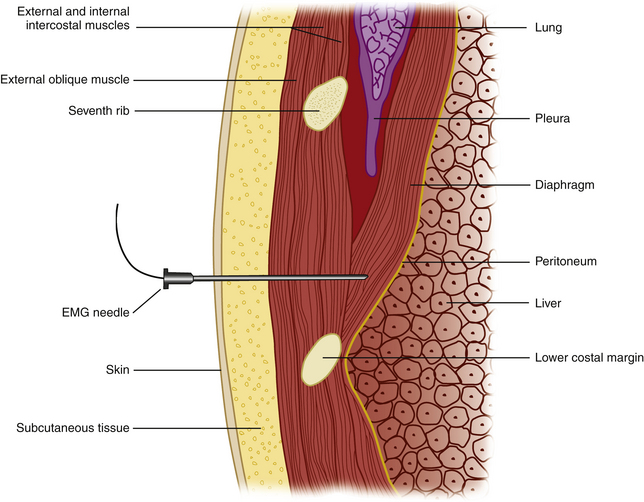
Once the diaphragm is identified, the transducer is then turned 90 degrees, parallel to the ribs, overlying one of the lowest intercostal spaces (usually the seventh or eighth space is targeted). The needle can then be inserted using a long axis approach (i.e., the needle is inserted parallel and deep to the transducer; Fig. 12.9), with the entry point medial to the transducer, thereby positioning the lung farthest from the needle. These authors have found that when the diaphragm is deeper, in larger patients, an oblique standoff approach allows one to visualize the needle easily. This technique uses heaped up gel beneath one end of the transducer (at the site of needle entry), so that the transducer is parallel with the needle as it is inserted obliquely through the heaped up gel and into the chest wall. Motor units in the diaphragm fire during inspiration and tend to be shorter duration and more rapidly recruited than motor units in the intercostal or limb muscles. Sharp pain during needle examination usually occurs with penetration of rib periosteum, pleura, or peritoneum, with dull aching discomfort sometimes experienced when the needle is in the diaphragm itself.
Ultrasound guidance can enhance the safety and accuracy of the needle examination of the diaphragm. Even in relatively straightforward cases, the optimal intercostal space for needle entry (where the diaphragm is thickest and there is little or no encroachment of the lung during inspiration) can be identified, and the depth of the diaphragm is noted. Thus if one is using a standard nonguided technique as described by Bolton and colleagues,29 with needle insertion perpendicular to the skin/chest wall (Fig. 12.10), knowing the anticipated depth of the diaphragm is helpful. In more difficult patients, direct visualization of the needle with ultrasound throughout insertion can minimize risk and maximize the chance of entering the diaphragm. An additional advantage of ultrasonographic evaluation of the diaphragm is evaluation of function. Is the muscle contracting with inspiration? Is it contracting with phrenic nerve stimulation? What is the quality of motion? Normal values for diaphragm muscle thickness at end inspiration and expiration are not available but with the development of such data, ultrasound may be able to provide additional diagnostic information noninvasively in the future.
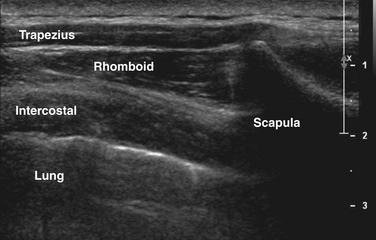
The greatest concern when performing needle EMG of the diaphragm is pneumothorax. However, in practice, relative risk of this is very low with examination of the diaphragm, and in fact pneumothorax is more likely to occur as a complication of needle examination of chest wall muscles such as serratus anterior, rhomboid, and thoracic paraspinal muscles. Ultrasound can similarly be used to localize these muscles particularly in obese subjects in whom ribs and other anatomic landmarks cannot be palpated, ensure accuracy (because most chest wall muscles cannot be activated in isolation), and evaluate qualitatively for atrophy or signs of denervation in cases in which needle examination is technically difficult or contraindicated (Fig. 12.11). In cases of unilateral disease, the patient can serve as his or her own control with comparing with the contralateral side.
Clinical Pearl
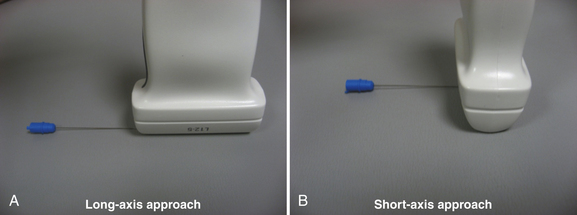
When performing ultrasound-guided needle examination, if a short axis approach is used (Fig. 12.12), the tip of the needle will appear almost identical to the midshaft of the needle (i.e. a bright, hyperechoic spot on the screen). Therefore, as soon as the needle tip is identified, advancement of the needle must stop while the ultrasound probe is repositioned to allow re-identification of the needle tip as it is advanced deeper.
Risk of Needle EMG in Anticoagulated Patients
The availability of portable, high-resolution ultrasonography in the authors’ laboratory has allowed for modifications in practice related to anticoagulation. To date, the literature has been sparse in relation to this issue, with scattered case reports of compartment syndromes and hematomas, the majority in nonanticoagulated patients.30–35 One study by Caress and associates in which MRIs were reviewed from 17 patients who underwent EMG of the paraspinal muscles followed by MRI, found small subclinical hematomas in 5 out of 45 paraspinal muscles on retrospective review.36 This study has led to widespread teaching that paraspinal muscles should not be examined in anticoagulated patients. There was also a survey of 47 electrodiagnostic laboratories in which four laboratories reported via retrospective recall at least one case of bleeding in anticoagulated patients requiring medical or surgical intervention. Based on the available literature, many EMG laboratories have required patients to discontinue warfarin before undergoing EMG, a practice that is controversial given the relative risk of discontinuing anticoagulation as it relates to stroke.37
These authors evaluated more than 200 patients, of whom one half were anticoagulated with warfarin, who underwent EMG of the tibialis anterior muscle.38 Ultrasound examination of the tibialis anterior muscle was performed shortly after the needle EMG to examine for hematoma development. Three very small hematomas were identified, two in patients taking warfarin and one in a patient on aspirin and nonsteroidal antiinflammatory (NSAID) medication. None of the hematomas was clinically evident, and the authors concluded that there was no increased risk of significant bleeding after needle EMG of the tibialis anterior in anticoagulated patients. Based on the results of this study, with larger numbers of data currently being collected, these authors changed their practice such that patients are no longer required to discontinue anticoagulation prior to needle EMG, although the upper limit of INR and the specific muscles examined are determined by the individual electromyographer performing the study. Other laboratories across the United States have since changed their laboratories practices to avoid temporary cessation of anticoagulation prior to needle EMG based on the results of this study. This is one example of how the availability of high-resolution ultrasonography in the EMG laboratories setting can lead to improvements in quality and safety.
Imaging of Muscle
Ultrasound has high sensitivity in screening for neuromuscular disease,39–41 with 87% to 92% sensitivity demonstrated by some investigators, and as such this can be a very useful tool in the pediatric population in whom standard electrodiagnostic evaluation can be challenging. Visual evaluation of gray scale signal on ultrasound has significant inter- and intrarater variability; as a result, gray scale imaging via quantification of muscle echogenicity has been developed as a more reliable means of identifying changes in homogeneity as well as overall echo-texture.42 Muscle ultrasound was able to differentiate between neurogenic and myopathic disorders in 92% of hypotonic infants in one study.43
Quantitative gray scale imaging is discussed in more detail elsewhere in this text (see Chapters 3 and 10), including current limitations that need to be taken into consideration, including the need for normal values for different ultrasound machines (using the same anatomic landmarks and standardized muscle position), and the necessity of maintaining consistent machine settings for serial examinations. System settings adjustments for each machine as well as correction models may allow for transposition of normal values to different machines in the future but such software is not yet commercially available. There certainly appears to be a role in targeting muscle biopsy if heterogeneity or atrophy can be recognized. This could address the issue of inadvertently selecting very severely involved muscles, which may give nonspecific findings on biopsy, and avoid biopsy of an uninvolved muscle.
Clinical Application—Case 4
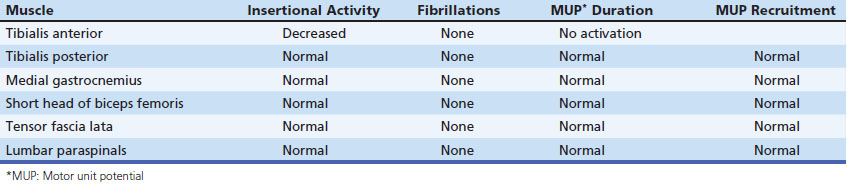
Nerve conduction studies and needle examination are presented in Tables 12.4 and 12.5. The resident performing the study had a working diagnosis of peroneal neuropathy based on the clinical presentation and findings on nerve conduction studies. However, he was surprised by the needle EMG findings of decreased insertional activity and no motor unit activation in the tibialis anterior, particularly given the presence of compound muscle action potentials, albeit small, in the peroneal distribution. The attending physician repeated the needle examination of select muscles and noted similar findings in the tibialis anterior but did find fibrillation potentials and small motor unit potentials more laterally in that muscle, as well as in the peroneus longus and extensor hallucis longus muscles.
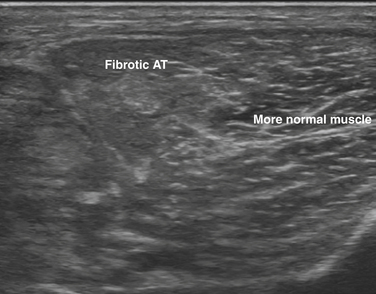
With palpation, the tibialis anterior muscle was noted to feel fibrotic. It was decided to evaluate the muscle with ultrasound, and there was clear change in muscle architecture, with loss of the normal fibrillar pattern and increase in echogenicity. Findings were patchy and not consistent throughout the entire muscle (Fig. 12.13). Based on the combination of ultrasound and electrodiagnostic findings, in the clinical context of onset of painful red swollen leg after an 11-hour brachial plexus surgery, unresponsive to antibiotics, the patient was diagnosed with direct muscle crush injury, without evidence of peroneal neuropathy.
Dynamic Muscle Ultrasound
A significant advantage of ultrasonography compared with other imaging modalities in the evaluation of neuromuscular disease is the ability to image both statically and dynamically. Abnormal spontaneous activity including fasciculation and fibrillation potentials can be identified and a larger area can be sampled compared with needle EMG39 (see further discussion in Chapter 3). Unusual patterns of spontaneous activity such as that seen in rippling muscle disease can be visualized. Real-time imaging allows the electromyographer to identify muscle contraction in response to electrical stimulation, if there is a question as to whether a response is a true compound muscle action potential or merely a volume-conducted response from nearby muscles. For example, with supraclavicular stimulation of the phrenic nerve, the diaphragm can be seen to contract, and with stimulation of the suprascapular nerve in suprascapular neuropathy, contraction of the supraspinatus can be differentiated from the overlying trapezius. Muscles shorten and thicken with contraction, which is easily appreciated under ultrasound visualization. A muscle can also be passively flexed and extended while visualizing with ultrasound to allow accurate identification of a targeted muscle, which can be particularly useful in patients with altered anatomy or a severely weak muscle.
Dynamic entrapment can be identified using ultrasound; for example, there have been case reports of carpal tunnel syndrome secondary to an anomalous muscle belly entering the carpal tunnel when the fingers are flexed or extended, or of ulnar nerve entrapment secondary to a hypertrophied medial head of the triceps snapping over the medial epicondyle during elbow flexion and impinging the subluxing ulnar nerve (in the latter case, both the snapping muscle and subluxing ulnar nerve can be identified as the patient performs the provocative activity).13,18
1. Oh S.J. Clinical electromyography and nerve conduction studies, ed 3. New York: Lippincott Williams and Wilkins; 2003. 365–374
2. Kamm C.P., Scheidegger O., Rösler K.M. Ultrasound-guided needle positioning in sensory nerve conduction study of the sural nerve. Clin Neurophysiol. 2009;120:1342-1345.
3. Demondion X., Herbinet P., Boutry N., et al. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24:1303-1309.
4. Bailey P.W., Boon A.J., Smith J., et al. Ultrasound assisted electrophysiologic technique for evaluation of the lateral femoral cutaneous nerve. Muscle Nerve (Suppl). 2009.
5. Watson J.C., Pingree M.J., Boon A.J., et al. A novel ultrasound-guided proximal saphenous nerve conduction study,. Muscle Nerve. (Suppl):2009.
6. Cartwright M.S., Donofrio P.D., Ybema K.D., Walker F.O. Detection of a brachial artery pseudoaneurysm using ultrasonography and EMG. Neurology. 2005;65:649.
7. El Miedany Y.M., Aty S.A., Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology. 2004;43:887-895.
8. Padua L., Aprile I., Pazzaglia C., et al. Contribution of ultrasound in a neurophysiological lab in diagnosing nerve impairment: a one-year systematic assessment. Clin Neurophysiol. 2007;118:1410-1416.
9. Visser L.H. High-resolution sonography of the common peroneal nerve: detection of intraneural ganglia. Neurology. 2006;67:1473-1475.
10. Weiss C., Imhoff A.B. Sonographic imaging of a spinoglenoid cyst. Ultraschall in der Medizin. 2000;21:287-289.
11. Nakamichi K., Tachibana S. Unilateral carpal tunnel syndrome and space-occupying lesions. J Hand Surg Br. 1993;18:748-749.
12. Cartwright M.S., Chloros G.D., Walker F.O., et al. Diagnostic ultrasound for nerve transection. Muscle Nerve. 2007;35:796-799.
13. Chipman J.N., Mott R.T., Stanton C.A., Cartwright M.S. Ultrasonographic tinel sign. Muscle Nerve. 2009;40:1033-1035.
14. Spinner R.J., Goldner R.D. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve: anatomical and dynamic factors. J Bone Joint Surg. 1998;80:239-247.
15. Spinner R.J., Hayden F.R.Jr., Hipps C.T., Goldner R.D. Imaging the snapping triceps. Am J Roentgenol. 1996;167:1550-1551.
16. Hobson-Webb L.D., Massey J.M., Juel V.C., Sanders D.B. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:1353-1357.
17. Visser L.H., Smidt M.H., Lee M.L. Diagnostic value of wrist median nerve cross sectional area versus wrist-to-forearm ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:2898-2899.
18. Cartwright M.S., Passmore L.V., Yoon J.S., et al. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve. 2008;37:566-571.
19. Beekman R., Schoemaker M.C., Van Der Plas J.P., et al. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62:767-773.
20. Kamolz L.P., Schrögendorfer K.F., Rab M., et al. The precision of ultrasound imaging and its relevance for carpal tunnel syndrome. Surg Radiol Anat. 2001;23:117-121.
21. Mondelli M., Filippou G., Frediani B., Aretini A. Ultrasonography in ulnar neuropathy at the elbow: relationships to clinical and electrophysiological findings. Neurophysiol Clin. 2008;38:217-226.
22. Visser L.H., Smidt M.H., Lee M.L. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 2008;79:63-67.
23. Young N.P., Sorenson E.J., Spinner R.J., Daube J.R. Clinical and electrodiagnostic correlates of peroneal intraneural ganglia. Neurology. 2009;72:447-452.
24. Chiodo A., Goodmurphy C., Haig A. Cadaveric study of methods for subscapularis muscle needle insertion. Am J Phys Med Rehabil. 2005;84:662-665.
25. Chiodo A., Goodmurphy C., Haig A. Cadaver evaluation of EMG needle insertion techniques used to target muscles of the thorax. Spine. 2006;31:E241-E243.
26. Haig A.J., Goodmurphy C.W., Harris A.R., et al. The accuracy of needle placement in lower-limb muscles: a blinded study. Arch Phys Med Rehabil. 2003;84:877-882.
27. Jordan S.E., Ahn S.S., Gelabert H.A. Combining ultrasonography and electromyography for botulinum chemodenervation treatment of thoracic outlet syndrome: comparison with fluoroscopy and electromyography guidance. Pain Physician. 2007;10:541-546.
28. Chen R., Collins S., Remtulla H., et al. Phrenic nerve conduction study in normal subjects. Muscle Nerve. 1995;18:330-335.
29. Bolton C.F., Grand’Maison F., Parkes A., Shkrum M. Needle electromyography of the diaphragm. Muscle Nerve. 1992;15:678-681.
30. Al-Shekhlee A., Shapiro B.E., Preston D.C. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve. 2003;27:517-526.
31. Baba Y., Hentschel K., Freeman W.D., et al. Large paraspinal and iliopsoas muscle hematomas. Arch Neurol. 2005;62:1306.
32. Butler M.L., Dewan R.W. Subcutaneous hemorrhage in a patient receiving anticoagulant therapy: an unusual EMG complication. Arch Phys Med Rehabil. 1984;65:733-734.
33. Hough D.M., Wittenberg K.H., Pawlina W., et al. Chronic perineal pain caused by pudendal nerve entrapment: anatomy and CT-guided perineural injection technique. AJR Am J Roentgenol. 2003;181:561-567.
34. Rosioreanu A., Dickson A., Lypen S., Katz D.S. Pseudoaneurysm of the calf after electromyography: sonographic and CT angiographic diagnosis. AJR Am J Roentgenol. 2005;185:282-283.
35. Vaienti L., Vourtsis S., Urzola V. Compartment syndrome of the forearm following an electromyographic assessment. J Hand Surg Br. 2005;30:656-657.
36. Caress J.B., Rutkove S.B., Carlin M., et al. Paraspinal muscle hematoma after electromyography. Neurology. 1996;47:269-272.
37. Gruis K.L., Little A.A., Zebarah V.A., Albers J.W. Survey of electrodiagnostic laboratories regarding hemorrhagic complications from needle electromyography. Muscle Nerve. 2006;34:356-358.
38. Lynch S., Boon A.J., Smith J., et al. Complications of needle electromyography: hematoma risk and correlation with anticoagulation and antiplatelet therapy. Muscle Nerve. 2008;38:1225-1230.
39. Pillen S., Arts I.M., Zwarts M.J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37:679-693.
40. Pillen S., Scholten R.R., Zwarts M.J., Verrips A. Quantitative skeletal muscle ultrasonography in children with suspected neuromuscular disease. Muscle Nerve. 2003;27:699-705.
41. Pillen S., Verrips A., van Alfen N., et al. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007;17:509-516.
42. Pillen S., van Keimpema M., Nievelstein R.A., et al. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32:1315-1321.
43. Aydinli N., Baslo B., Calişkan M., et al. Muscle ultrasonography and electromyography correlation for evaluation of floppy infants. Brain Dev. 2003;25:22-24.