Ultrasonography in circulatory failure
Overview
Shock is an emergency medical condition caused by inadequate tissue oxygenation. Treatment of shock depends on the underlying cause of the circulatory failure. Ultrasound facilitates rapid evaluation of hemodynamically unstable patients by providing valuable information about not only myocardial function but also the peripheral vasculature.1 When performed in real time, intensivist-guided bedside ultrasonography can be used to differentiate between shock states, facilitate efficient early goal-directed therapy, and monitor the response to therapy.2,3 Proficiency in basic critical care ultrasonography allows the intensivist to distinguish among shock secondary to obstruction (pulmonary embolism or cardiac tamponade), hypovolemia, and distributive causes (septic shock). Ultrasound is a bedside, reproducible, and noninvasive imaging modality. Furthermore, it obviates the need to transport unstable patients to the radiology department. This chapter discusses the role of ultrasound in the diagnosis and management of circulatory failure in the intensive care unit (ICU).
Ultrasound-based evaluation of circulatory failure in the intensive care unit
We start by first noting that ultrasound should not in any way substitute for a thorough history and physical examination. The presence of heart murmurs, pericardial rubs, elevated neck veins, previous history of gastrointestinal bleeding, known malignancy, or unilateral absence of air entry on examination will suggest the cause of the shock. Ultrasound can be used to confirm clinical suspicion. Specific emergency department protocols exist for patients in shock with and without a history of trauma4–7; however, these protocols are not reviewed in this chapter. Moreover, ultrasound techniques have been described in detail elsewhere in this book and are not restated here. Implicit in the discussion of the use of ultrasound in shock patients is the dynamic nature of the symptoms and signs. The state of patients in circulatory failure is constantly changing secondary to both the initial insult and its subsequent treatment. Accordingly, ultrasound can be used to aid in both diagnosis of the cause of the shock and the response to interventions.8
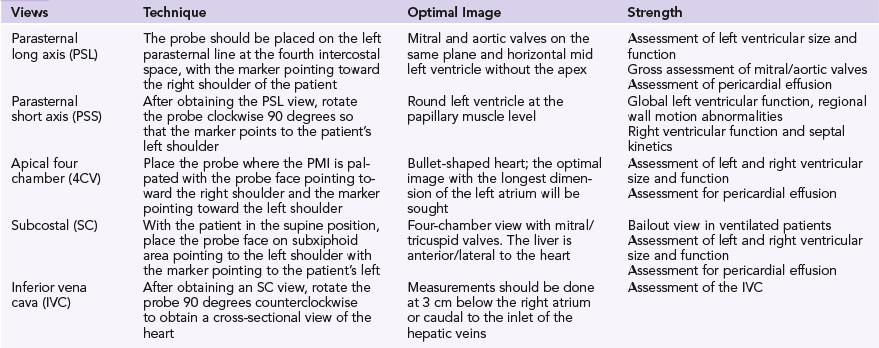
Ultrasound examination of patients usually starts by assessing the heart with a low-frequency transducer. The following views are obtained1: parasternal long-axis (PSL), parasternal short-axis (PSS), apical four-chamber (4CV), subcostal (SC), and inferior vena cava (IVC) views (Table 39-1). The purpose is to evaluate myocardial function. The basic algorithm is shown in Figure 39-1. We assess left ventricular (LV) size and function, right ventricular (RV) size and function, gross valvular abnormalities, the pericardial space, and the size and variation of the IVC with respiration. Occasionally, the cause of the shock can be unambiguously diagnosed with ultrasonography. For example, visualization of a thrombus in one of the main pulmonary arteries necessitates rapid intervention. However, the cause of the shock is more likely to be found by ultrasound examination of several organs in a coordinated fashion, once again underlining the benefits of the holistic approach (HOLA) critical care ultrasound concept (see Chapters 1 and 57). A normal finding on ultrasound examination is very useful because it rules out components of the differential diagnosis. For example, a hypotensive patient with a known underlying malignancy and a normally functioning right ventricle on ultrasound assessment virtually rules out the presence of a massive pulmonary embolus.
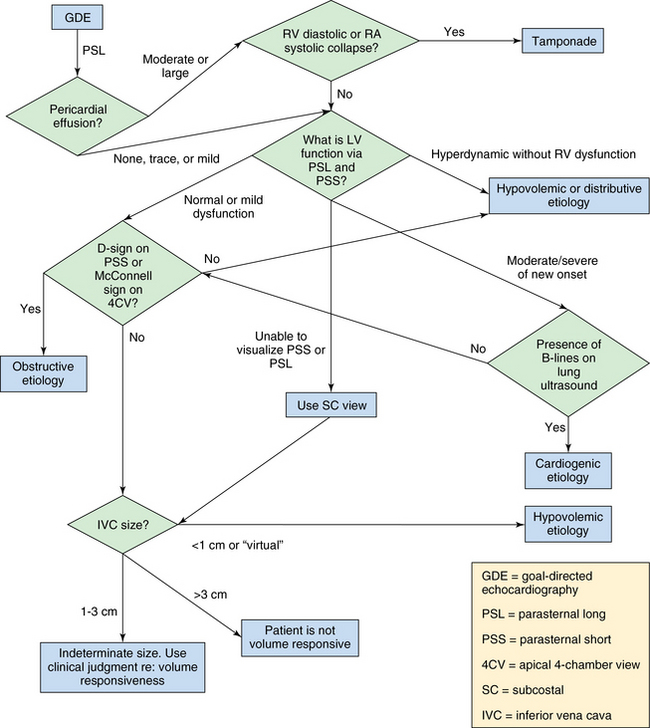
The suggested algorithm begins with evaluation of the pericardial space. Here it is important to clearly differentiate pleural from pericardial fluid. On the PSL view, it is easy to differentiate a pericardial from a pleural effusion by noting whether the fluid travels anteriorly in front of the aorta. A significant pericardial effusion should prompt the intensivist to assess for RV diastolic or right atrial systolic collapse. If either is present, tamponade physiology is probably present and drainage of the pericardial fluid is recommended. Ultrasound-guided pericardiocentesis can be then performed.1,9
Assessment of the right ventricle follows. RV dilatation (as seen on the PSS and 4CV views) with bowing of the interventricular septum into the left ventricle suggests obstructive shock (Figure 39-2). Furthermore, akinesia of the midportion of the RV free wall with sparing of the RV apex (McConnell sign) is also suggestive of pulmonary embolism causing obstructive shock.10
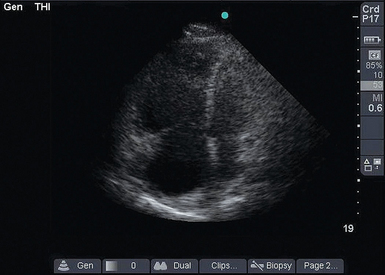
Figure 39-2 Dilated right ventricle. In this apical four-chamber view, the right atrium and right ventricle are much larger than the left side of the heart.
Next, the IVC is evaluated 3 cm below the right atrium or caudal to the inlet of the hepatic veins. One of the central questions in a hypotensive patient is whether a volume challenge will increase preload and therefore cardiac output. If the patient is passive and mechanically ventilated, dynamic changes in the IVC are assessed with M-mode in the SC or IVC view. The maximum and minimum diameters are recorded. A 12% or greater difference between minimum and maximum diameters suggests that the patient’s blood pressure will increase following an infusion of fluids.11 Of note, patients need to be fully sedated and on volume control mode with a tidal volume of 8 to 10 mL/kg, as has previously been reported.11 Alternatively, if the patient is breathing spontaneously or triggering the ventilator, a small (<1 cm) IVC (along with end-systolic effacement of the papillary muscles on a PSS view and a hyperdynamic left ventricle) argues in favor of a volume challenge (Figure 39-3). A large (>3 cm) IVC argues against a fluid challenge (Figure 39-4).12 Between these two extremes, the result is indeterminate and intensivists should use their clinical judgment. Another validated method for assessing fluid responsiveness in spontaneously breathing patients without arrhythmias is passive leg raising with evaluation of the variation in stroke volume (threshold of 12.5%).13 This requires knowledge of advanced critical care echocardiography and is analyzed elsewhere in this section of the book.
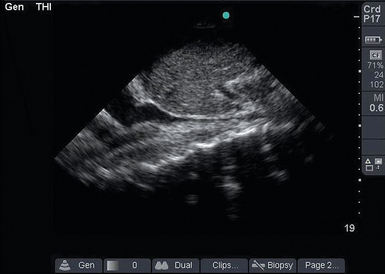
Figure 39-3 Collapsible inferior vena cava (IVC). In this longitudinal IVC view, the IVC is seen to be small and collapsible. This patient’s blood pressure is likely to improve with fluid administration.
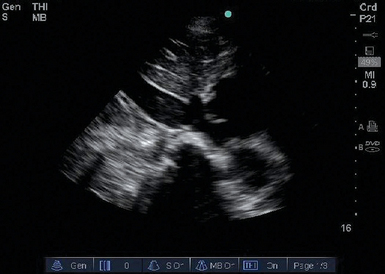
Figure 39-4 Distended inferior vena cava (IVC). The IVC is dilated and does not collapse with inspiration, thus indicating that the IVC is “filled.” The patient’s blood pressure is unlikely to improve following fluid administration.
Acute valvular dysfunction as a cause of circulatory failure is most commonly observed in the coronary care unit. However, a patient in the medical or surgical ICU will occasionally have an undiagnosed or previously unknown valvular pathology. Examples include a flail mitral valve leaflet or a severely stenotic aortic valve with decreasing excursion.14 The PSL view is ideal for this evaluation. Color Doppler is crucial for a complete diagnosis of valvular disease but is beyond basic critical care echocardiography. The intensivist should be able to recognize that a major valvular abnormality exists and then seek consultation from a cardiologist (Chapter 57).1
Lung ultrasound completes the evaluation. Even though lung ultrasound is covered in detail elsewhere, it is quite useful in the assessment of shock for evaluating pulmonary edema. Lung auscultation remains an important part of the physical examination; however, its sensitivity is poor, especially in a supine, mechanically ventilated, critically ill patient who is unable to cooperate. In contrast to chest auscultation and plain chest radiography, lung ultrasonography has greater than 90% sensitivity with regard to pulmonary edema and provides objective data.15 Additionally, the presence of decreased or absent breath sounds on physical examination can point to the presence of massive pleural effusion, exacerbation of chronic obstructive pulmonary disease, or pneumonia. These conditions can easily be distinguished with bedside ultrasonography since they demonstrate different patterns (see the chapters on pleural and lung diseases), thereby reducing the need for portable radiography.
Ultrasound findings such as predominance of an A- or B-line pattern can further guide fluid resuscitation as described in the FALLS protocol (fluid administration limited by lung sonography).3 For example, an initial A-line predominance (a surrogate for pulmonary artery occlusion pressure ≤18 mm Hg) that changes to a B-line pattern following aggressive fluid resuscitation argues against further fluid administration. However, the opposite does not hold true. The initial presence of diffuse B-lines on lung ultrasound cannot be assumed to be of cardiogenic etiology since they can found in multiple interstitial pathologies such as pulmonary edema (cardiogenic or noncardiogenic), chronic lung diseases, and certain infections such as Pneumocystis pneumoniae. We again stress two previously noted issues: (1) the importance of the history and physical examination and (2) the importance of continuous assessment by both clinical examination and ultrasound.
Ultrasound may also be used to determine the cause of the decreased urine output that often accompanies shock states. For example, bladder ultrasound, in the setting of decreased urine output, can be used to confirm that the Foley catheter is in the proper position (Figure 39-5). The presence of hydronephrosis on kidney ultrasound can also be ascertained.
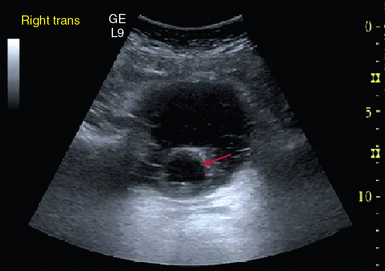
Figure 39-5 Foley catheter in the bladder. The clinician is reassured that misplacement of the catheter is not the cause of the decreased urine output.
Finally, ultrasound can assist in resuscitation. Rapid insertion of central venous lines is easier and safer with ultrasound. The complication rate associated with venous central line placement can be greater than 15%.16,17 In a randomized study, 450 critical care patients who underwent real-time ultrasound-guided cannulation of the internal jugular vein were compared with 450 patients in whom the landmark technique was used. Access time (skin to vein) and number of attempts were significantly reduced in the ultrasound group. In the landmark group, puncture of the carotid artery occurred in 10.6% of patients, hematoma in 8.4%, hemothorax in 1.7%, pneumothorax in 2.4%, and central venous catheter–associated bloodstream infection in 16%. All these complications were significantly reduced in the ultrasound group. Indeed, the Agency for Healthcare Research and Quality presently recommends the use of ultrasound for central venous placement as one of their 11 practices to improve patient care.18
Pearls and highlights
• Circulatory collapse is a common admitting diagnosis in the ICU; rapid determination of its cause and subsequent treatment are necessary for improved outcomes.
• Bedside critical care ultrasound aids the intensivist in assessing the cause of shock, change in the patient’s status, and response to interventions.
• Real-time ultrasound is portable and reproducible, does not require transport of patients, and is performed by the treating intensivist.
• Critical care ultrasound has a steep learning curve, but its basic techniques can be mastered at the bedside in a relatively short time.
References
1. Kaplan, A, Mayo, PH. Echocardiography performed by the pulmonary/critical care medicine physician. Chest. 2009; 135:529–535.
2. Dark, PM, Delooz, HH, Hillier, V, et al. Monitoring the circulatory responses of shocked patients during fluid resuscitation in the emergency department. Intensive Care Med. 2000; 26:173–179.
3. Lichtenstein, D, Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Me. 2012; 6:155–162.
4. Atkinson, PR, McAuley, DJ, Kendall, RJ, et al, Abdominal and cardiac evaluation with sonography in shock (ACES): an approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg Med . 2009; 26:87–91.
5. Perera, P, Mailhot, T, Riley, D, Mandavia, D, The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically ill. Emerg Med Clin North A. 2010; 28:29–56. [vii].
6. Jones, AE, Craddock, PA, Tayal, VS, et al. Diagnostic accuracy of left ventricular function for identifying sepsis among emergency department patients with nontraumatic symptomatic undifferentiated hypotension. Shock. 2005; 24:513–517.
7. Rose, JS, Bair, AE, Mandavia, D, et al, The UHP ultrasound protocol: a novel ultrasound approach to the empiric evaluation of the undifferentiated hypotensive patient. Am J Emerg Me. 2001; 19:299–302.
8. Lichtenstein, DA. Analytic study of frequent and/or severe situations. In: Heilmann U, ed. General ultrasound in the critically ill. Berlin: Springer-Verlag; 2007:177–183.
9. Tsang, TS, Enriquez-Sarano, M, Freeman, WK, et al, Consecutive 1127. therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Pro. 2002; 77:429–436.
10. Koenig, S, Cohen, R. The use of ultrasonography in circulatory failure. Open Crit Care Med J. 2009; 2:66–68.
11. Feissel, M, Michard, F, Faller, J-P, Teboul, JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004; 30:1834–1837.
12. Schmidt, GA, Koenig, S, Mayo, PH, Shock: ultrasound to guide diagnosis and therapy. Ches. 2012; 142:1042–1048.
13. Preau, S, Saulnier, F, Dewavrin, F, et al. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010; 38:819–825.
14. Stone, MB. Emergency ultrasound diagnosis of cardiogenic shock due to acute mitral regurgitation. Acad Emerg Med. 2010; 17:E1–E2.
15. Lichtenstein, DA, Meziere, GA, Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Ches. 2008; 134:117–125.
16. Olivier, AF, Real-time sonography with central venous access: the role of self-training. Ches. 2007; 132:20–61.
17. Feller-Kopman D, Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Ches. 2007; 132:302–309.
18. Karakitsos, D, Labropoulos, N, De Groot, E, et al, Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Car. 2006; 10:R162.