Ultrasonography for deep venous thrombosis
Background
Venous thromboembolism (VTE) is a spectrum of disease that encompasses both deep venous thrombosis (DVT) and pulmonary embolism (PE). It is a serious and often underrecognized condition in critically ill patients that can lead to significant morbidity and mortality. PE remains one of the leading causes of unexpected death in hospitalized patients.1,2
In the intensive care unit (ICU), the Virchow triad of “stasis, endothelial injury, and hypercoagulability” is a frequent occurrence, with most critically ill patients having one or more recognizable risk factors for VTE. Immobilization, mechanical ventilation, paralysis, sedation, and central venous catheters all increase the risk for DVT.3,4
The embolic risk for DVT is related to its location in the venous system. Ninety percent of cases of acute PE are due to emboli emanating from clots in the proximal veins of the lower extremities. The significance of upper extremity deep venous thrombosis (UEDVT) was underestimated for many years. Recently, this perception has changed, and it is now recognized that proximal UEDVT accounts for 5% to 10% of cases of VTE.3–6 The use of chronic venous access catheters such as peripherally inserted central venous and various tunneled catheters has greatly increased, and these catheters have been associated with an increased incidence of UEDVT. In its latest guidelines for antithrombotic therapy and prevention of thrombosis, the American College of Chest Physicians for the first time recommended treatment of UEDVT involving the axillary or more proximal veins.
Classic symptoms of DVT include swelling, pain, and erythema of the involved extremity. However, the signs and symptoms of DVT in critically ill patients are often unreliable, which makes diagnosis difficult. The true incidence and prevalence of acute DVT in critically ill patients are not known. Various studies have reported incidences ranging widely from 4% to 60% as a result of differences in the patient population, methods of detection, and use of surveillance programs.7–9
Because of rapid growth in the availability of portable ultrasound units, point-of-care ultrasonography performed at the bedside by critical care physicians is gaining increased popularity. A multicenter, retrospective review compared 128 bedside intensivist-performed compression ultrasound (IP-CUS) studies with a formal vascular study (FVS) performed by ultrasound technicians and interpreted by radiologists.10 All fellows and attending physicians performing IP-CUS had received formal training at a 3-day critical care ultrasonography course. When compared with the companion FVS study ordered immediately after completion of the IP-CUS study, IP-CUS studies had a sensitivity and specificity of 86% and 96%, respectively, for ruling in DVT. The median time between ordering the test and availability of the result of an FVS was 13.8 hours.
Anatomy
Upper extremity venous sonographic anatomy
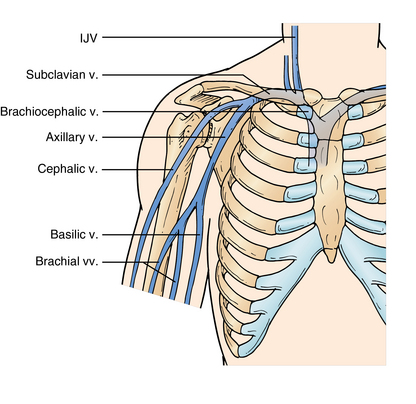
The axillary vein is a deep vein of the arm that branches distally into the paired brachial veins. It runs through the axilla to the outer border of the first rib and is joined by the cephalic vein medially close to its termination. The confluence of the axillary vein and the cephalic vein forms the subclavian vein (SCV). The SCV runs from the outer border of the first rib to the sternal head of the clavicle, where it is joined by the internal jugular (IJ) vein, and together they form the brachiocephalic vein. The brachiocephalic vein drains into the superior vena cava. The IJ extends from the jugular foramen in the base of the skull to its confluence with the SCV (Figure 9-1).11
Lower extremity venous sonographic anatomy
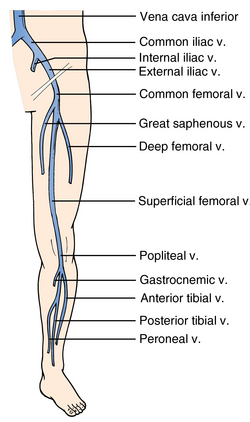
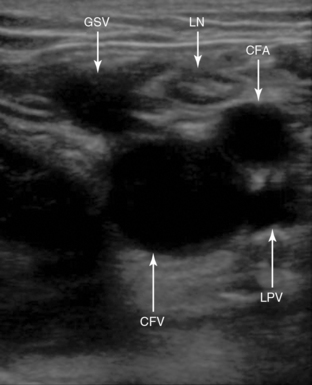
The two main veins of the superficial venous system are the great and small saphenous veins. The small saphenous vein runs along the lateral aspect of the calf and drains into the deep venous system at the level of the popliteal vein. The great saphenous vein (GSV) runs up from the ankle along the medial aspect of the leg and, in the proximal part of thigh, traverses the fossa ovalis and drains into the common femoral vein (CFV).
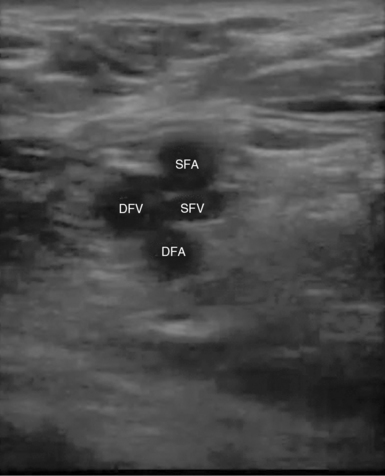
The external iliac vein originates from the inferior vena cava and exits the pelvis deep to the inguinal ligament to become the CFV. The CFV is then joined on its medial aspect by the GSV. Approximately 2 cm below this point the CFV divides into the deep femoral vein (DFV) and the superficial femoral vein (SFV). The DFV can be tracked for only a short distance before it disappears into the deeper tissue away from the acoustic window. It is important to note that the SFV is part of the deep venous system and is also called the “femoral vein” to avoid confusion. The SFV travels down the anteromedial aspect of the leg to the adductor canal and then courses posteriorly into the popliteal fossa to become the popliteal vein. The popliteal vein then trifurcates into the anterior tibial, peroneal, and posterior tibial veins in the proximal part of the calf (Figure 9-2).
Basic ultrasound techniques
Several ultrasound techniques can be used when assessing a vessel for the presence of a clot.
Duplex ultrasound
This method adds color Doppler to the 2D analysis. The energy of the returning sound waves is assigned a color. By convention, flow toward the transducer probe appears as red and flow away from the probe is visualized as blue. This is superimposed on the 2D image to allow visualization of anatomy and flow simultaneously (see Chapters 1 and 8).
Triplex ultrasound
This method adds spectral analysis to the 2D and color Doppler analysis (see Chapter 1). In this mode, flow velocities are displayed graphically, with time on the x-axis and velocity on the y-axis.
Traditionally, an FVS included all of the aforementioned techniques. However, studies have shown that CUS with color and pulsed wave Doppler does not increase the accuracy of the examination over CUS alone.12–15 Therefore, in the majority of critical care studies, CUS without Doppler interrogation is used for evaluating the venous system.
Transducer selection
A high-frequency linear transducer (7.5 to 10 MHz) is often ideal. For larger patients and deeper venous circuits, lower-frequency transducers can be used alternatively (see Chapter 1). Color Doppler analysis is not part of a routine CUS study but is usually helpful in identifying vascular structures, especially when scanning obese or edematous patients.
Transducer orientation and image acquisition
To begin scanning, by convention the transducer is held in a transverse plane with the marker pointing toward the patient’s right. Gain should be adjusted so that the resolution and brightness of the image are uniform in both the near and far fields (Chapter 1). The depth should also be adjusted so that the structures of interest take up almost three fourths of the screen. Once the vessels are identified, the vein is compressed on its transverse plane.
Diagnostic criteria
Acute versus chronic deep venous thrombosis
Detecting the relative age of a thrombus by ultrasound requires expertise. In brief, the sonographic features of chronic thrombosis include a contracted venous segment, clot adherence, detection of hyperechoic and rather heterogeneous thrombi and partial recanalization, and the presence of venous collaterals. Ultrasound characteristics that usually favor acute thrombosis include venous distention; venous lumen partially compressible or noncompressible; and detection of hypoechoic, free-floating, and rather homogeneous thrombi. Recently, another sonographic feature has been observed in cases of acute thrombosis: a double hyperechoic line along the thrombus–venous wall interface. It was suggested that this double hyperechoic line might represent fibrin fibers coating acute clots (Figure 9-3).16
Ultrasound examination strategies in the intensive care unit
Patient positioning
For examination of a lower extremity, the patient should be placed in a supine and, if tolerated, a reverse Trendelenburg position. The knee should be bent slightly and the hip externally rotated. To image the popliteal vein and artery the patient should ideally be in a prone position. However, this is not possible in critically ill patients, and imaging is usually performed with the knee flexed at a 45-degree angle or with the patient in a lateral decubitus position.
Upper extremity
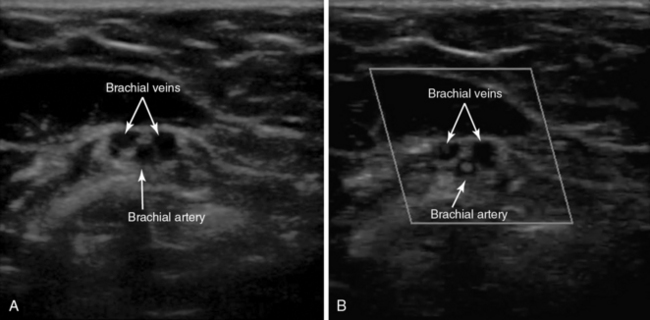
For examination of an upper extremity, transverse compression of the IJ, axillary, and brachial veins should be demonstrated. To identify the paired brachial veins on either side of the brachial artery, the transducer should be placed on the medial aspect of the upper part of the arm approximately midway between the shoulder and the elbow. Compression should be applied every few centimeters along the brachial veins centrally up to the axillary vein. The basilic vein is a large superficial vein that is not paired with an artery, which allows differentiation from the deeper brachial veins. However, since the basilic vein joins with the brachial veins to form the axillary vein, a large clot burden in this vein may be clinically significant (Figure 9-4).17
Lower extremity
A bedside CUS study may be limited or complete. Most of the data for CUS studies come from venous examination of the lower extremity. A complete examination is defined as sequential compressions every 2 cm from the groin crease to the distal popliteal fossa. However, previous work has shown that strategies with a limited number of compression sites have a sensitivity and specificity similar to that of a complete examination in detecting lower extremity DVT.13,14,18
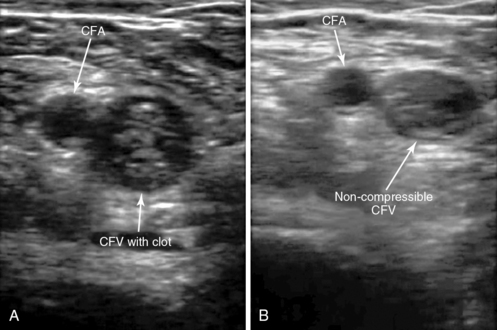
Compressive interrogation of the vessel commences at the level of the inguinal ligament. The common femoral artery and vein should be identified and a compression maneuver performed at this level (Figure 9-5).
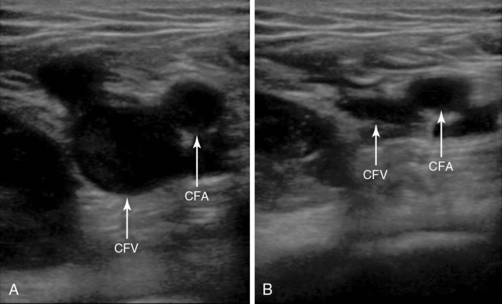
Figure 9-5 The common femoral vein with and without compression. The image on the left is without compression and the image on the right is with compression and shows complete apposition of the anterior and posterior walls of the common femoral vein. No deep venous thrombosis is present.
By sweeping the transducer below the inguinal ligament, the GSV should be seen joining the CFV medially. The CFV-GSV junction should be compressed because a thrombus at this level is associated with high risk for extension into the CFV (Figure 9-6).
Sequential compressions are then performed every 2 cm along the CFV to its bifurcation into the DFV and SFV and continued along the SFV to the adductor canal (Figure 9-7).
Limitations of bedside compression ultrasound
• Performing a bedside CUS study can be challenging in obese patients. To improve image acquisition, the patient is placed in a reverse Trendelenburg position to distend the veins. The depth is increased to appropriate levels and firm pressure applied to clearly visualize the deeper vessels. Identification of the companion artery will prevent misidentification of large superficial veins as deep veins in obese patients.
• The presence of edema, obscuring dressings, local areas of tenderness, burns, or recent surgical sites may obstruct ultrasound scanning.
• An inexperienced sonographer may sometimes misinterpret other semisolid structures such as inguinal lymph nodes or bands of muscle for a thrombosed vein. Similarly, fluid-filled structures such as an abscess or soft tissue cysts may resemble blood vessels. In these scenarios it is important to identify the paired artery and scan the structure of interest distally in both the transverse and longitudinal planes. Lymph nodes, muscle bands, and cystic structures will disappear and not be accompanied by an artery.
• Because the femoral vein within the adductor canal is inadequately imaged, a thrombus in this area may be missed.
• In the presence of low-flow states, internal echoes or “smoke” may be visualized in patent veins. This is more easily seen in large veins such as the IJ, subclavian vein, and CFV. Vessel compressibility should be adequately assessed and “smoke” should not be confused with a thrombus.
• The iliac veins and inferior vena cava are not directly visualized during a lower extremity CUS study. However, excluding thrombosis in these venous segments can be facilitated by the use of color Doppler and other imaging techniques.
Pearls and highlights
• The transducer is held in a transverse plane with the marker pointing toward the patient’s right.
• Identification of the companion artery prevents misidentification of a superficial vein as a deep vein.
• Compression should be performed perpendicular to the vessel of interest.
• Lack of compressibility of the vein is diagnostic of a thrombus.
• If complete venous compression is not attained with pressure sufficient to deform the artery, an obstructing venous thrombus is likely to be present.
• The SFV is part of the deep venous system.
• If a thrombus is identified, further compression should be limited because of the possibility of dislodging the clot.
• A negative scan for lower extremity DVT does not rule out the presence of PE.
• PE remains one of the leading causes of unexpected death in hospitalized patients.
• The true incidence and prevalence of acute DVT in critically ill patients are not known.
References
1. Rubinstein, I, Murray, D, Hoffstein, V, Fatal pulmonary emboli in hospitalized patients: an autopsy study. Arch Intern Me. 1988; 148:1425–14266.
2. Sandler, DA, Martin, JF, Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis. J R Soc Me. 1989; 82:203–205.
3. Lineman, LE, Van Deer Meer, FJ, Roosendaal, FR, Dogged, CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost. 2008; 6:1262–1266.
4. Spencer, FA, Emery, C, Lessard, D, Goldberg, RJ, Worcester Venous Thromboembolism Study: Upper extremity deep vein thrombosisa community-based perspective. Am J Me. 2007; 120:678–684.
5. Owens, CA, Bui, JT, Knuttinen, MG, et al, Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radio. 2010; 21:779–787.
6. Mai, C, Hunt, D, Upper-extremity deep venous thrombosis: a review. Am J Me. 2011; 124:402–407.
7. Burns, GA, Cohn, SM, Frumento, BJ, et al. Prospective ultrasound evaluation of venous thrombosis in high-risk trauma patients. J Trauma. 1993; 35:405–408.
8. Harris, LM, Curl, GR, Booth, FV, et al. Screening for asymptomatic deep vein thrombosis in surgical intensive care patients. J Vasc Surg. 1997; 26:764–769.
9. Marik, PE, Andrews, L, Maini, B. The incidence of deep venous thrombosis in ICU patients. Chest. 1997; 111:661–664.
10. Kory, PD, Pellecchia, CM, Shiloh, AL, et al. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011; 139:538.
11. Rumack, C, Wilson, SR, Charboneau, JW, Levine, D. Diagnostic ultrasound, ed 4. St Louis: Elsevier Health; 2011.
12. Birdwell, BG, Raob, GE, Whitsett, TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998; 128:1–7.
13. Bernardi, E, Camporese, G, Buller, HR, et al, Serial 2-point ultrasonography plus D-dimer vs. whole-leg color-code Doppler ultrasonography for diagnosing suspected symptomatic deep venous thrombosis: a randomized controlled trial. JAM. 2008; 300:1653–1659.
14. Jacoby, J, Cesta, M, Axelband, J, et al. Can emergency medicine residents detect acute deep venous thrombosis with a limited, two site ultrasound exam. J Emerg Med. 2006; 32:197–200.
15. Lensing, AW, Prandoni, P, Brandjes, D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989; 320:342–345.
16. Blaivas, M, Stefanidis, K, Nanas, S, et al. Sonographic and clinical features of upper extremity deep venous thrombosis in critical care patients. Crit Care Res Pract. 2012; 2012:489135.
17. Chin, EE, Zimmerman, PT, Grant, EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med. 2005; 24:829–838. [quiz 839-840].
18. Blaivas, M, Lambert, MJ, Harwood, RA, et al. Lower-extremity Doppler for deep venous thrombosis—can emergency physicians be accurate and fast. Acad Emerg Med. 2000; 7:121–126.