46 Tumors of the Thoracic Spine
KEY POINTS
Introduction
Nearly 1.4 million new cases of cancer are diagnosed in the United States per year.1 In a majority of the approximately 724,000 cancer-related deaths per year, patients ultimately succumb to complications related to metastatic disease. Approximately 30% to 90% of cancer patients will have evidence of metastases to the spinal column at autopsy, and 5% to 10% of cancer patients develop symptomatic metastatic epidural spinal cord compression (MESCC), an oncologic emergency.1 There are approximately 25,000 cases of symptomatic MESCC in the united states each year.1 The thoracic spine is the most common site of metastatic disease involvement in the vertebral column and the most common site of primary vertebral column tumors. Because symptomatic degenerative disease is less common in the thoracic spine relative to the cervical or lumbar spine, thoracic region pain in a middle-aged or elderly patient should be considered a red flag symptom. Imaging should be considered expeditiously, particularly if the patient has a history of primary cancer. Nonetheless, delay in the diagnosis of thoracic region tumors is common. Surgical intervention for thoracic spinal cord and spinal column neoplasms continues to present significant challenges. Lesions in the thoracic spine can be subdivided into four major categories: metastatic disease, intradural extramedullary tumors, intramedullary spinal cord tumors, and primary neoplasms involving skeletal elements.
Metastatic Tumors
Approximately 70% of metastatic disease to the vertebral column involves the thoracic spine, making it the most common site of spinal metastases.2 As increased survival due to improved treatment strategies for primary neoplasms becomes a trend, the incidence of bone metastases to the spine will also increase. The unique venous drainage of visceral organs through the Batson plexus is one explanation for this anatomical phenomenon, along with the close proximity of the thoracic spine to thoracic and abdominal viscera.2 Eighty percent of metastases involve the vertebral body, while 20% involve the posterior elements.2 Of the four subtypes of tumors of the thoracic spine, metastatic tumors are by far the most common. Cancers that have a propensity to spread to the thoracic spine include breast, lung, leukemia/lymphoma, prostate, and renal cell.2 Surgical management requires consideration of the need for decompression of the neural elements, for restoration of spinal stability, and for pain relief. With tumors of breast, prostate, or renal origin, aggressive resection is indicated, as this has been shown to have a favorable impact on ambulatory function and may have a favorable impact on survival.3
Intradural Extramedullary Tumors
Intradural extramedullary (IDEM) tumors account for two thirds of intradural spinal tumors.4 The most common IDEM tumors of the thoracic spine are meningiomas, neurofibromas and schwannomas, which account for over 80% of tumors in this location.5 Meningiomas arise from arachnoid cap cells; 75% of spinal meningiomas are contained within the thoracic spine, and they show a strong predilection for women (80%).5 Meningiomas have a benign biological behavior in the spinal column and management should be directed toward gross total resection to achieve a cure. This may not be feasible with anteriorly situated, calcified tumors, and the risk of neurological injury must be weighed against the indolent growth rates usually observed after subtotal resection. Radiotherapy is thus reserved for recurrent tumors that cannot be completely resected.
Neurofibromas of spinal nerve root origin may be seen sporadically or in patients with neurofibromatosis. These tumors will have an extradural component in 30% of cases.5 The surgical goal in the treatment of thoracic schwannomas and neurofibromas is complete surgical resection. If the tumor cannot be separated from the nerve root of origin, then sacrifice of that root may be necessary. Fortunately, this is usually of little consequence in the thoracic spine.
Intramedullary Spinal Cord Tumors
Intramedullary spinal cord tumors (IMSCTs) make up 2% to 8.5% of all central nervous system(CNS) tumors and approximately one third of primary spinal column tumors.6 Approximately 90% are of glial origin.4 The major types observed in the thoracic spine are astrocytomas and ependymomas, which are seen with almost equal frequency in adults. Hemangioblastomas, which are less commonly observed, may be sporadic or a part of von Hippel-Lindau syndrome. Astrocytomas are most often seen at the cervicothoracic junction and in the lower thoracic spinal cord. In adults, ependymomas are the most common IMSCT. However, the majority of these occur in the cervical spine or arise from the filum terminale (myxopapillary ependymoma). Treatment of ependymomas is focused on gross total resection. Patients who have incompletely-removed tumors should be considered for re-resection or followed closely with serial imaging and considered for reoperation if growth is documented. Radiation therapy is often reserved for incomplete resection after reoperation. Astrocytomas in adults tend to be infiltrative and blend imperceptibly with the spinal cord at the margins of the tumor. For this reason, total removal may not be possible. Most commonly, infiltrative tumors treated with subtotal resection will undergo radiation therapy. For high-grade infiltrative lesions, biopsy or subtotal resection is also often followed by radiation therapy. For some low-grade lesions treated with subtotal resection, postoperative radiation therapy remains controversial.6
Pain is the most common presentation of IMSCT in the thoracic spine; it usually localizes to the level of the tumor and is either regional back pain or radicular.4 Up to one third of patients may also experience sensory or motor complaints and spasticity. The most common sign of an IMSCT within the thoracic spine is a mild scoliosis with spasticity and sensory disturbance.6 Until recently, it was believed that posterior decompression and radiation therapy were the limits of therapy, but aggressive gross total resection, with the aid of microsurgical tools and intraoperative monitoring, has been shown to be safe and to improve functional recovery and reduce tumor recurrence.
Primary Vertebral Column Tumors
Primary tumors of the vertebral column are rare, making up less than 10% of all tumors involving the spinal column.7 It should be emphasized that when patients present with a vertebral column mass in the absence of impending neurological compromise, minimally invasive, image-guided biopsy should be performed prior to definitive treatment planning. Many of the primary neoplasms of the thoracic spinal column are best treated via radical en bloc surgical resection. These types of surgical approaches require careful and detailed preoperative surgical planning by an experienced surgical team. Therefore, knowing the tumor histology up front is critical to the surgical plan.
Primary osseous tumors can be divided into three categories: benign, benign but locally aggressive, and malignant. Benign tumors include hemangiomas, osteoid osteomas/osteoblastomas, chondroma/osteochondromas, aneurysmal bone cysts, and eosinophilic granulomas. Hemangiomas are the most common primary neoplasm affecting the thoracic spine and have been seen in approximately 11% of all postmortem examinations.7 Infrequently, hemangiomas can behave in an atypical or locally aggressive fashion and can cause spinal cord compression. In this situation, aggressive surgical resection is advocated. Giant cell tumors have a variable biological behavior and may act in a locally aggressive fashion. Because of this, the authors often recommend radical en bloc excision of tumors exhibiting this pathology and will strongly consider adjuvant radiation therapy postoperatively. Chordomas occurring in the thoracic spine are almost uniformly locally aggressive and recurrences are common after intralesional resection, including gross total resection. Similarly, we recommend radical en bloc resection techniques for thoracic chordomas. Chemotherapy protocols for chordomas have not been promising to date, nor have conventional radiation therapy modalities. Proton beam irradiation therapy has been shown to be the most promising radiation modality and patients are evaluated postoperatively for this modality on an individual basis. However, it is our belief that the most effective treatment modality for achieving long term local disease control is aggressive en bloc resection with negative margins, including resection of the biopsy tract if possible. Proton beam irradiation should not be relied on for cases where gross total resection cannot be achieved. However, it may be employed even in cases of en bloc resection with negative margins.
Malignant types include plasmacytomas, chondrosarcomas, and osteosarcomas. Plasmacytomas make up nearly 30% of all primary tumors and have a propensity to occur in the thoracic spine.8 While these tumors are radiation-sensitive lesions, surgery may be necessary for failure of radiation therapy, acute neurological decline from spinal cord compression, and overt instability of the spinal column. Sarcomas present a specific treatment challenge. Biopsy is recommended to assess the specific histology and grade. Systemic workup for metastases is important prior to embarking on treatment. High-grade lesions that are large or those tumors associated with metastatic disease may be treated with neoadjuvant chemotherapy protocols to shrink the local disease and address metatstatic disease prior to surgical resection. En bloc resection is the technique that gives the patient the best opportunity for a longer-term survival with these tumor types, particularly when metastatic disease is not present.
Basic Science
Research related to the molecular biology and genetics of specific tumor types that affect the spinal column either as primary or metastatic lesions is beyond the scope of this text. However, several animal models of spinal column neoplasia and the translational research related to these animal models are worth discussion. Recently, animal models for metastatic disease to the spine and intramedullary spinal cord gliomas have been developed that will allow for more rigorous preclinical analysis of novel therapies. Mantha et al have established a reproducible model of metastatic breast adenocarcinoma to the vertebral column in rats.9 The establishment of this model represents the first of its kind and has allowed for the study of multiple treatment modalities focused on treating spinal metastatic disease, including surgery, radiation therapy, and the novel use of locally delivered chemotherapeutic agents.
Using the metastatic model developed by Mantha et al, Bagley et al have shown that radiation reliably delays paraparesis and death in this model, as does local delivery of paclitaxel.10,11 The combination of these two modalities has been shown to be more effective than either treatment alone. 11 Additionally, Gok et al. have shown that the combination of decompressive surgery, local chemotherapy, and radiation has the most profound results in terms of preservation of neurological function and prolongation of survival in this animal model with of metastatic epidural spinal cord compression.12 The hope is that this experimental data may translate into useful clinical trials designed to improve neurological morbidity and, potentially, survival in patients with MESCC due to metastatic breast cancer and other tumor histologies.
Pennant et al. have examined the efficacy of microsurgical excision in a rat model of intramedullary spinal cord tumor. The established model mimics the behavior of IMSCTS, both functionally and histopathologically.13 Following tumor implantation, animals were randomized into a treatment group (microsurgical resection) or to no treatment. The animals that underwent resection had a significant delay in the onset of functional paraplegia as compared to the controls and these results were highly reproducible. This new model allows for the study of new treatment options for high-grade intramedullary tumors.
Schuster et al have developed a novel model of spine metastasis using human osteoblasts implanted in immune incompetent (SCID) mice that has shown to be highly reproducible.14 This model allows for the study of bone-tumor interaction in metastatic disease, as well as the basic biology of bone metastases. Chordoma models have been difficult to establish, but through human cell culture, nude animal models have been conceptualized. Laboratory study of this extremely aggressive local tumor is much needed given the limited options for treatment outside of aggressive radical resection.
Clinical Practice Guidelines
Surgical intervention has a clear and proven role in the treatment of patients with metastatic disease involving the thoracic spine that results in spinal cord compression. Surgical treatment, followed by radiation therapy, not only extends ambulatory status but also results in a trend toward longer survival and reduces the need for steroids and pain medications.3
The indications for surgical intervention include the need to establish a diagnosis, the treatment of spinal instability, and the restoration or preservation of neurological function in the setting of epidural spinal cord compression. Additional indications include the treatment of radioresistant tumors, and the treatment of tumor recurrence following radiation therapy or the treatment of neurological decline during radiation therapy. A systemic approach for treatment of thoracic metastases has been developed and may aid in designing the optimal surgical strategy.15 The mnemonic “MAPS” stands for (1) method of resection; (2) anatomy of spinal disease; (3) patient’s level of fitness; and (4) stabilization.
Clinical Case Examples
Case 1: Posterolateral Approach for Metastatic Spine Disease
Patient Presentation
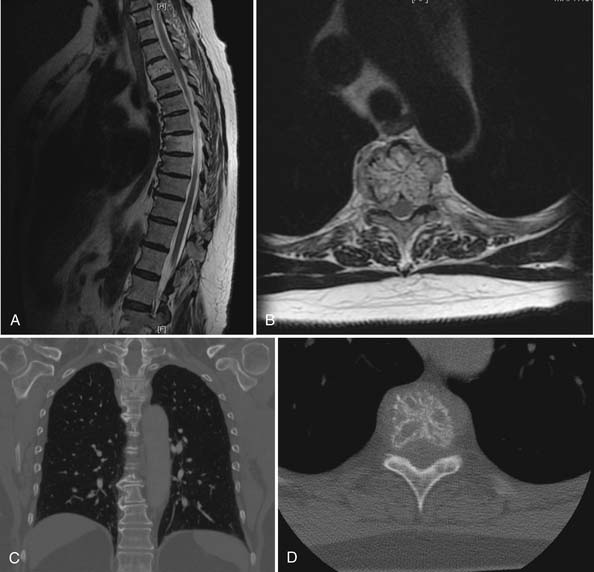
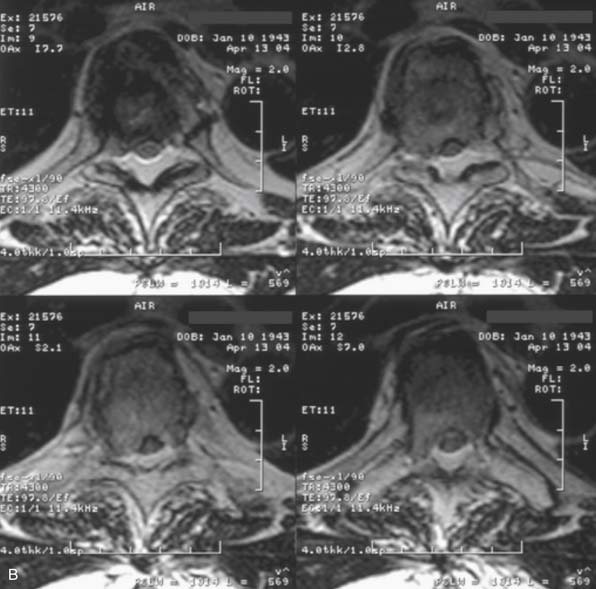
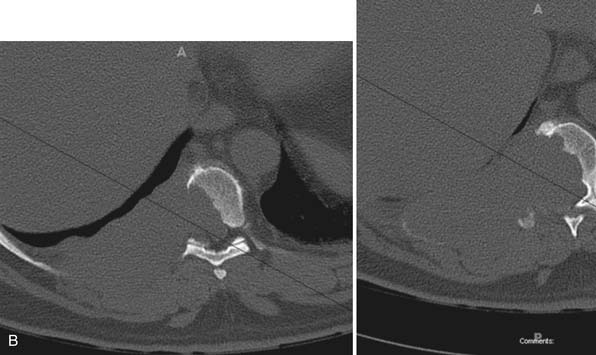
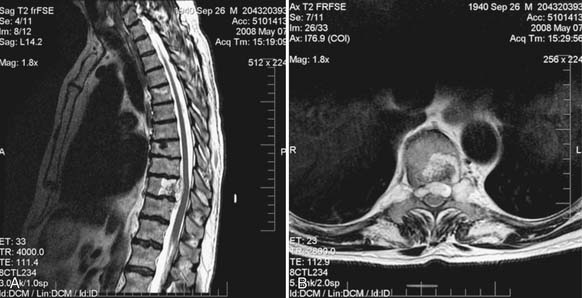
MRI of the thoracic spine and CT scan of the thoracic spine are shown in Figure 46-1. We felt that despite the negative biopsy, the imaging was most characteristic of an atypical vertebral hemangioma with epidural spinal cord compression. Imaging features that support this diagnosis include the appearance of vascular channels on the MRI and the “polka-dot” appearance with vascular channels also present on the CT scan. Also included in the differential diagnosis was plasmacytoma/multiple myeloma.
Approaches to this lesion were discussed, with several considerations in mind. First, the epidural compression was noted to occur from an anterolateral direction at one segment, making an anterior approach appealing. However, at the T3 level, anterior approaches are more complicated and involve splitting the sternum to gain access to this location and working around the great vessels. We recommended a posterolateral approach to allow for decompression, resection of tumor, and both anterior column and posterior reconstruction.
Surgical Technique
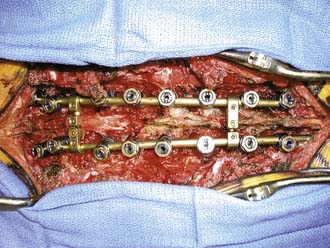
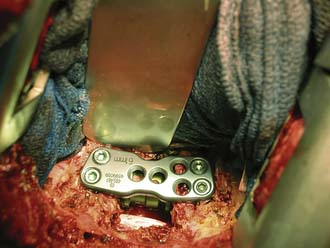
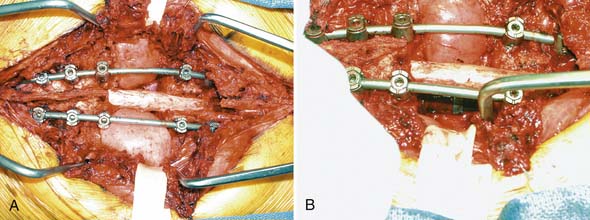
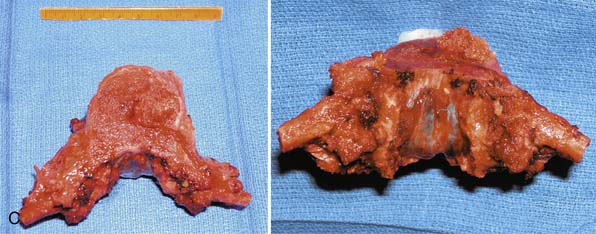
With the patient in a prone position on the Jackson table with neurological monitoring, T2-T4 laminectomies, T3 costotransversectomy, transpedicular decompression of the spinal canal, and T3 corpectomy with intralesional resection of the tumor were performed. Reconstruction was achieved with an expandable cage placed from T2 to T4 and supplemented with pedicle screw instrumentation extending from T1 to T9. An intraoperative photo is shown in Figure 46-2. We elected to extend the construct multiple levels below the site of tumor resection because we did not want to stop our instrumentation just above or at the site of the apex of the thoracic kyphosis for fear of inferior construct failure. Bone grafting was performed with allograft and demineralized bone matrix. In the setting of potential malignant disease affecting the bone marrow and with spinal tumor surgery in general, we tend to avoid the use of autograft bone or BMP.
Postoperative Course
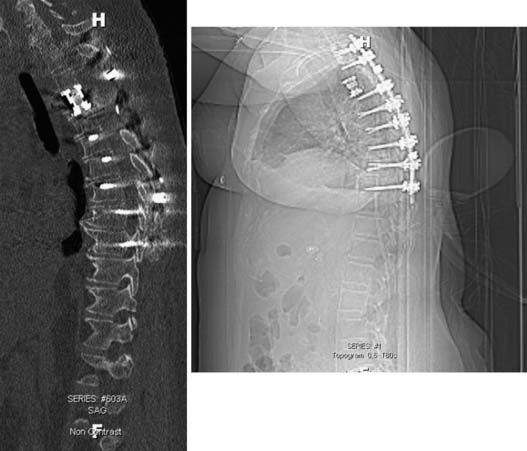
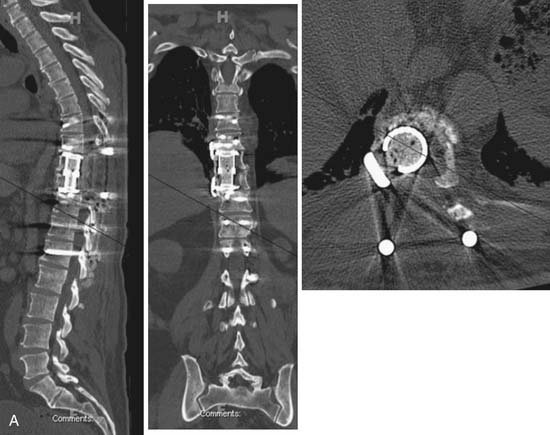
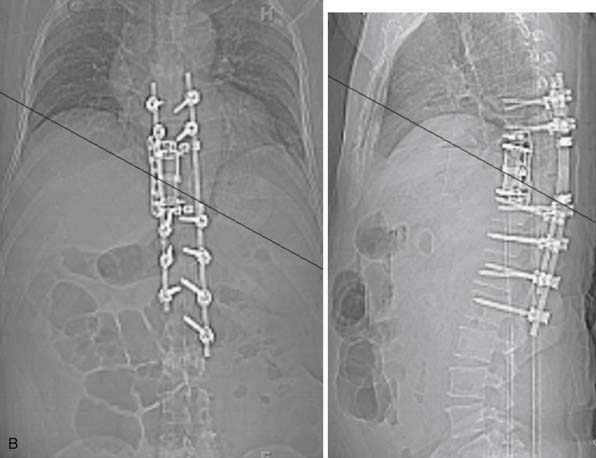
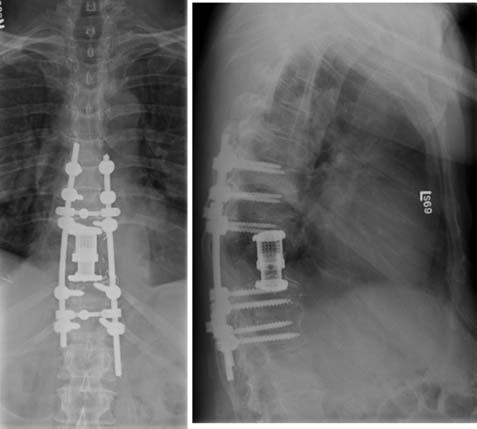
The patient tolerated the procedure well and spent one night in the neuro intensive care unit. The patient had no new neurological deficits. Postoperative CT scan demonstrating the cage reconstruction and pedicle screw instrumentation is shown in Figure 46-3. Pathological analysis confirmed the tumor to be a hemangioma. The patient was discharged to rehab and is being followed with serial imaging and clinical evaluation to assess for tumor recurrence. However, given the benign nature of the histopathology despite aggressive local behavior, it is suspected that the patient is cured of her disease, and adjuvant therapy has not been recommended.
Case 2: Anterior Approach
Patient Presentation
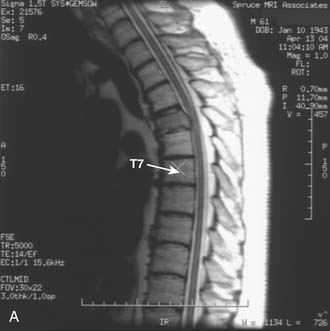
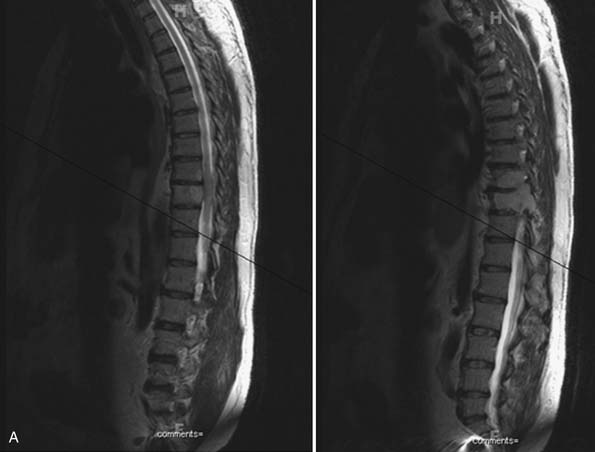
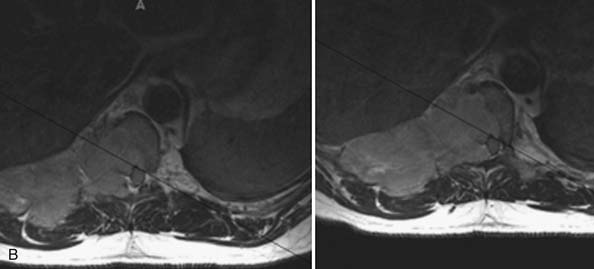
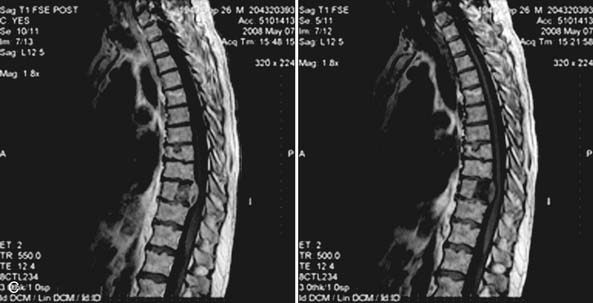
A 61-year-old male with a history of pheochromocytoma presented with progressive midthoracic pain. Nineteen years prior to presentation the patient was diagnosed with a pheochromocytoma and was treated successfully with left adrenalectomy. Six years prior to presentation the patient developed midthoracic region pain and was treated conservatively with physical therapy and NSAIDs. The patient developed myelopathic signs and symptoms to include lower extremity weakness and gait dysfunction. And an MRI of the thoracic spine disclosed a lesion at T5 with significant epidural spinal cord compression. He was then treated with radiation therapy and his pain and neurological dysfunction improved. Several years following the radiation therapy, the patient developed worsening and severe midthoracic pain that had a mechanical component to it. Associated symptoms included bilateral thoracic radicular pain and subjective lower extremity weakness. He described dragging his feet, but denied sphincter dysfunction. On exam he had 4/5 strength of the iliopsoas and dorsiflexors bilaterally. He was also noted to be hyperreflexic in his lower extremities. MRI of the thoracic spine (Figure 46-4) showed partial collapse of the T5 vertebral body with kyphosis and epidural spinal cord compression. T2 signal change was noted in the spinal cord at this level, consistent with myelomalacia. Surgery was recommended, given evidence of spinal cord compression, clinical myelopathy, anterior vertebral column compromise and kyphosis. An all-posterior approach with transpedicular corpectomy and reconstruction of the anterior column was considered. However, it was felt that an anterior approach would be the best approach to resect the anterior compressive tumor, correct the kyphosis, and reconstruct the anterior column without having to perform a multilevel instrumented fusion that would be required with an all-posterior approach.
Surgical Technique
Given the history of pheochromocytoma, the patient was seen by endocrinology and anesthesia preoperatively and was premedicated with phenoxybenzaprine for alpha blockade. Pheochromocytomas are highly vascular tumors and preoperative embolization was also recommended. The right and left T5 intercostal vessels were entered and the tumor was successfully embolized with embospheres. A right-sided, high posterolateral thoracotomy approach was selected to allow for access to the anterolateral vertebral column, because of the prominence of the aortic arch on the left side. The high posterolateral thoracotomy requires mobilization of the scapula, and in this case, partial resection of the right 5th rib. A T5 corpectomy was performed with opening of the posterior longitudinal ligament for complete decompression of the spinal cord (Figure 46-5). Reconstruction of the anterior column was achieved with a distractible cage and plate. Bone grafting was performed using allograft bone. Autograft bone was avoided to limit the rest of metastatic spread of tumor potentially present in the bone marrow of the adjacent rib.
Postoperative Course
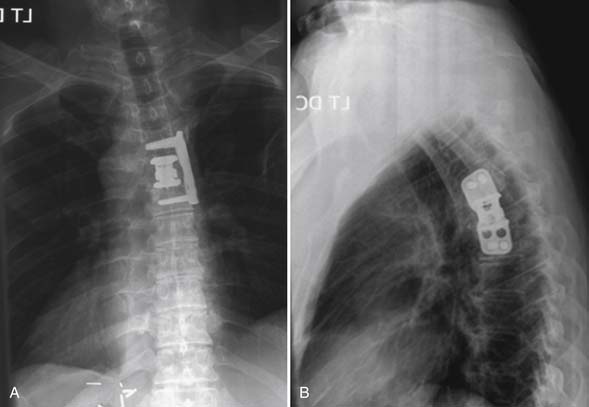
The patient was observed in the neuro intensive care unit overnight. A chest tube was placed at the time of surgery and removed 72 hours postoperatively. He remained at his baseline neurological exam. He was discharged to home, fully ambulatory, on postoperative day number 8. Postoperative x-rays demonstrated excellent position of the cage and plate (Figure 46-6). At 1-year follow-up the patient had excellent pain control and stable postoperative imaging.
Case 3: Anterior/-Posterior Combined Approach
Patient Presentation
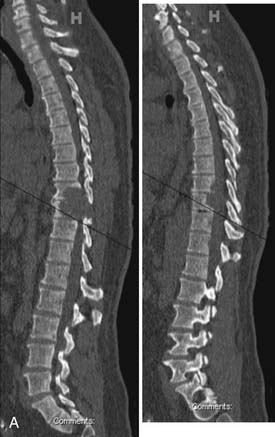
A 52-year-old male with a history of hypertension presented with a 1-year history of progressive right-sided abdominal and flank region pain. His pain subsequently progressed to include axial back pain that was most severe at night when recumbent in bed. A CT scan (Figure 46-7) of the thoracic spine revealed a lytic/destructive lesion at T9-T10 with associated large paraspinal posterior pleural-based mass on the right side. MRI (Figure 46-8) disclosed significant epidural extension and spinal cord compression. The patient denied lower extremity sensory changes, weakness, or bowel or bladder dysfunction. He had no history of systemic cancer. Neurological exam was significant for 5/5 strength in his lower extremities, and a normal sensory exam except for dermatomal sensory loss in the right T9-T10 location. He was also noted to have hyperreflexia in his lower extremities and two to three beats of bilateral ankle clonus. Given the prolonged history, it was felt that this lesion might represent a relatively slow-growing histopathological entity. Considerations were sarcoma, plasmacytoma/multiple myeloma, and possibly metastatic carcinoma. Given his preserved motor function, an expedited CT-guided biopsy of the lesion was recommended. The pathology proved the lesion to be a plasmacytoma/multiple myeloma. Further staging disclosed this to be stage 1A IgG kappa multiple myeloma. Despite the lytic appearance of this tumor, the patient initially did not have mechanical pain and the anterior column height was preserved without pathological fracture or deformity. Given the high radiation sensitivity of multiple myeloma, radiation therapy was recommended with close follow-up. The patient failed to respond to radiation therapy and developed mild gait dysfunction. Surgical resection was recommended. The lack of response to radiation called into question the diagnosis of multiple myeloma. Concomitant thoracotomy and posterior approach was planned. In any situation when an anterior approach is planned and a greater than 1 level corpectomy is performed, we recommend supplemental posterior stabilization. In this case, we felt as well that a combined posterior approach would also assist with the tumor resection and decompression.
Surgical Technique
The patient was placed in the left lateral decubitus position to allow access to the right thoracoabdominal region and the posterior thoracolumbar spine. The posterior thoracolumbar spine was exposed from T7 to L2. Pedicle screws were placed at T7 to T8, and T11-L1. Only one screw could be placed at L2, and in the lateral position, screws could not be successfully placed at T6. A temporary rod was placed on the left side of the spine to stabilize the spine during the decompression. T9 and T10 laminectomies were fashioned and tumor in the epidural space was resected. A right thoracotomy incision was fashioned over the 9th and 10th ribs and connected to the thoracolumbar incision dorsally. A portion of the 9th and 10th ribs distal to the tumor was resected. This allowed access to the pleural cavity. The 9th and 10th ribs were disarticulated from the spine to allow the paraspinal component of the tumor to be resected with the chest wall. Corpectomies of T9 and T10 were performed to resect the remaining tumor. The segment was reconstructed with an expandable cage encompassing T8 to T11. A lateral plate was also placed from T8 to T11. Final rods were placed posteriorly and bone grafting was performed with allograft. Plastic surgery assisted with the closure and reconstruction of the chest wall.
Postoperative Course
The patient remained at his neurological baseline postoperatively. His pathology again confirmed the diagnosis of multiple myeloma. His postoperative course was complicated by a prolonged air leak requiring a chest tube. He was discharged on postoperative day 17. Postoperative imaging showed good position of the instrumentation (Figure 46-9). At 2.5 years post surgery he remains neurologically intact with an intact construct. He has had skeletal progression of the myeloma to stage III.
Discussion
Metasatic tumors are the most common neoplasms affecting the thoracic spine. Based on the work of Patchell et al surgery followed by radiation therapy has replaced radiation therapy alone as the gold standard for treatment of MESCC. Extremely radiation-sensitive tumors include small cell lung carcinoma, lymphoma, and multiple myeloma. For these lesions, radiation therapy may still be employed as a first-line treatment modality for MESCC. The surgical approach may be anterior, posterior, or combined anterior/posterior. En bloc spondylectomy may be employed specifically for certain locally aggressive primary spinal column neoplasms. Prior to embarking on treatment, it is important to know the tumor histology. Therefore, CT-guided biopsy is recommended in patients who have stable or preserved neurological function. Tumor history, tumor location, anatomy of spinal disease, extent of systemic disease/medical comorbidities, and vertebral column compromise are factors that dictate the surgical approach. Thoracic spinal column neoplasms require reconstruction with instrumentation, with the exception of IMSCTs and some IDEM tumors. The clinical goals of surgery for thoracic spinal column neoplasms include local disease control, preservation or restoration of neurological function, stabilization of the spinal column, and pain control.
Case 4: En Bloc
Patient Presentation
A 67-year-old male with a history of prostate cancer treated with resection and thought to be in remission presented with upper lumbar/lower thoracic and right paraspinal pain. Associated symptoms included local numbness and paresthesias and more recent numbness and paresthesias in his feet. An MRI of the thoracic and lumbar spine (Figure 46-10) was performed with and without contrast as well as plain films. The plain films did not demonstrate evidence of bony changes; however, MRI showed a T2-hyperintense and contrast-enhancing lesion at T10 with epidural extension and spinal cord compression. Although the patient had some mild myelopathic signs on exam (hypereflexia), his motor exam was normal and his gait and sphincter function were both normal. The differential diagnosis included chordoma, given the T2 hyperintensity that is typical of this lesion. Also included on the list was metastatic disease, with prostate being the highest possibility on the list given his previous history. Because chordoma was on the list, representing a locally aggressive tumor that is rarely cured with intralesional resection alone, a CT-guided biopsy was recommended through a posterior approach to confirm the pathology prior to recommending further treatment. The report confirmed a chordoma. An en bloc surgical resection was recommended. An all-posterior approach was recommended with the caveat that if en bloc resection could not be achieved in this fashion, the posterior resection of the posterior elements at T10 would be followed by a thoracotomy with anterior en bloc vertebral body resection.
Surgical Technique
The patient was positioned prone on the Jackson table. The posterior spine was exposed from T7 to T12. The surgical plan was to stop the construct at T12/the thoracolumbar (TL) junction. Although stopping a construct at a junctional level may not be ideal, the authors feel that when this is done with a construct ending on the superior side of the TL junction, it is better tolerated than ending on the inferior side of the TL junction. However, there is no solid data to support this clinical belief. We also felt that ending at T7 was high enough above the apex of the patient’s thoracic kyphosis, which we estimated to be at T8-9 or T9-10 based on the imaging. Pedicle screws were therefore placed at T7-T9 and T11-T12 bilaterally. Laminectomies at T9 and T11 were subsequently fashioned. Two Tomita saws were then placed between the lamina of T10 and the dura at the junction with the facet joint. Using the Tomita saws, the spinous process and bilateral lamina of T10 were removed in en bloc fashion. Using silk ligatures to prevent a CSF leak, the bilateral T9 and T10 nerve roots were ligated proximal to the dorsal root ganglion. The nerve roots typically have to be sectioned at one or two levels to deliver the vertebral body posteriorly in an en bloc spondylectomy. It is felt that sectioning the roots proximal to the dorsal root ganglion decreases the possibility of postoperative neuropathic radicular pain. The thecal sac was carefully dissected from the anterolaterally situated epidural tumor. The paraspinal muscles were dissected off of the rib cage and retracted medially with Penrose drains. The bilateral T10 ribs were then resected from 1 cm distal to the costotransverse junction to 5 cm distal to that point. The pleura was opened and a chest spreader was placed, first on the left and then on the right. The lung was retracted away from the field and the vessels (aorta and vena cava) were dissected off the pleura and T9 and T10 vertebral bodies in a circumferential fashion. Both T9 and T10 segmental vessels were ligated bilaterally. Rods were then attached to the screw heads from T7 through T12. Using Tomita saws, the midvertebral body of T9 was transected, as was the T10-T11 disc space. This completely mobilized the specimen. The specimen was then removed posterolaterally in en bloc fashion (Figure 46-11). A distractible cage was placed from T9 to T11. Bone grafting was done with allograft. Neurological monitoring remained stable throughout the case.
Postoperative Course
The patient was taken to the intensive care unit with preserved lower extremity neurological function. He remained intubated until postoperative day 1 when he was successfully extubated. Postoperative imaging disclosed good position of his cage and posterior screws (Figure 46-12). His postoperative course was complicated by new-onset atrial fibrillation. His chest tubes were discontinued on postoperative days 5 and 6 respectively. He was discharged on postoperative day 8. At 3 months after surgery, he was off narcotics and returned to work. Proton beam irradiation was scheduled.
1. Witham T.F., et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat. Clin. Pract. Neurol.. 2006;2(2):87-94.
2. Bilsky M. Metastatic tumors of the spine and spinal cord. In: Dickman C.A., Fehlings M.G., Gokaslan Z.L., editors. Spinal Cord and Spinal Column Tumors. New York: Thieme, 2005.
3. Patchell R.A., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
4. McCormick P.C. BB: Spinal tumors. In: L.C., Grossman R.G. Principles of neurosurgery. Philadelphia: Lippincott-Raven, 1999.
5. Hentschel S.J., M.I. Intradural extramedullary spinal tumors. In: Fehlings M.G., Dickman C.A., Gokaslan Z.L., editors. Spinal cord and spinal column tumors. New York: Thieme, 2005.
6. Cooper P.R., H.K. Intramedullary spinal cord tumors. In: Fehlings M.G., Dickman C.A., Gokaslan Z.L., editors. Spinal cord and spinal column tumors. New York: Thieme, 2005.
7. Jacobs W.B., F.M. Primary vertebral column tumors. In: Fehlings M.G., Dickman C.A., Gokaslan Z.L., editors. Spinal cord and spinal column tumors. New York: Thieme, 2005.
8. Chi J.H., et al. Epidemiology and demographics for primary vertebral tumors. Neurosurg. Clin. N. Am.. 2008;19(1):1-4.
9. Mantha A., et al. A novel rat model for the study of intraosseous metastatic spine cancer. J Neurosurg. Spine. 2005;2(3):303-307.
10. Bagley C.A., et al. Fractionated, single-port radiotherapy delays paresis in a metastatic spinal tumor model in rats. J. Neurosurg. Spine. 2007;7(3):323-327.
11. Bagley C.A., et al. Local delivery of oncogel delays paresis in rat metastatic spinal tumor model. J. Neurosurg. Spine. 2007;7(2):194-198.
12. Gok B., et al. Surgical resection plus adjuvant radiotherapy is superior to surgery or radiotherapy alone in the prevention of neurological decline in a rat metastatic spinal tumor model. Neurosurgery. 2008;63(2):346-351.
13. Pennant W.A., et al. Microsurgical removal of intramedullary spinal cord gliomas in a rat spinal cord decreases onset to paresis, an animal model for intramedullary tumor treatment. Childs Nerv. Syst.. 2008;24(8):901-907.
14. Schuster J., Zhang J., Longo M. A novel human osteoblast-derived severe combined immunodeficiency mouse model of bone metastasis. J Neurosurg. Spine. 2006;4(5):388-391.
15. Fourney D.R., Gokaslan Z.L. Use of “MAPs” for determining the optimal surgical approach to metastatic disease of the thoracolumbar spine: anterior, posterior, or combined: invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J. Neurosurg. Spine. 2005;2(1):40-49.