44 Vessel-X
KEY POINTS
Introduction
Since vertebroplasty was introduced by Herve and Deramond in 1984, many methods of percutaneous osteoplasty (molding the bone) evolved to treat symptomatic vertebral compression fractures (VCFs), by injection of bone filler material (BFM): polymethylmethacrylate (PMMA), other kinds of bone cement, and bone grafts (autografts and allografts), or different kinds of osteoinductive or osteoconductive materials. The same risk in performing the previously mentioned techniques is the leakage of BFM, because the injected pressure will go to the fracture’s weakest area and lead to a leakage. Vesselplasty is an osteoplasty technique using the Vessel-X, which acts as an implant body expander to restore vertebral height in VCFs but prevents the potential risk of leakage.1,2
Indications and Contraindications
Description of the Device
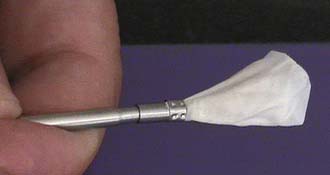
The idea behind the prototype originated in Taiwan, in February 2002 (Figure 44-1). The cadaveric study using the prototype was done by the author in Jakarta, Indonesia, in July 2003. The first generation used in the clinic was named threadplasty, because the connection used was thread (Figure 44-2).
A clinical trial was done in Jakarta, Indonesia, by the author from July 2004 until July 2005. As a preliminary report the first three cases were presented at the Asia Pacific Orthopaedic Association (APOA) Triennial Meeting in Kuala Lumpur on September 5-10, 2004. After the ten first-generation vesselplasty devices (Threadplasty) were made and during the clinical trial, major improvements and developments were made to formulate the last-generation instruments available for clinical use (Figure 44-3).1–6
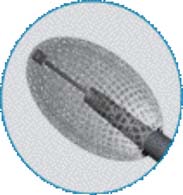
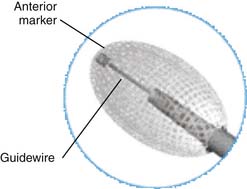
Vessel-X is a bone filler container made of polyethylene terephthalate (PET), a nonstretchable material. In deflated condition its shape is long, and when it is inflated the shape becomes short and bigger until a certain size is reached. When the pressure inside the container is equal to the surrounding resistance, the final size is achieved and the size will remain constant. This mechanical device is used to lift the vertebral endplate, acting like an implant body expander (Figure 44-4).
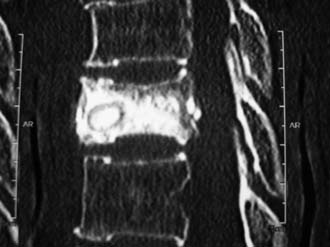
The Vessel-X container is a PET mesh and has 100-μm porosity. When the pressure inside the container is greater than the surrounding resistance, the BFM starts to interdigitate through the pores; some pressure is then relieved and the endplate is lifted further (Figures 44-5 and 44-6).
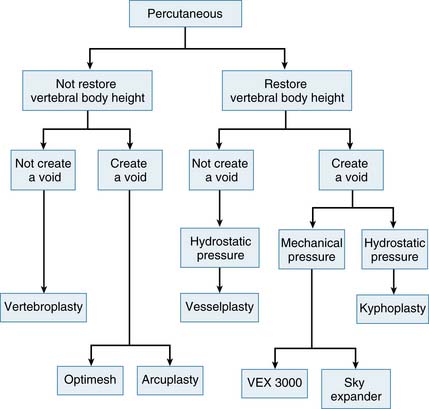
The Variations in technical concepts in percutaneous osteoplasty have led to the differences in techniques and results for the numerous methods: vesselplasty (KYPHON INC. CA), vertebroplasty, kyphoplasty, VEX-3000 (Taeyeon Medical CO., LTD, South Korea), Sky Expander (DISC-O-TECH MEDICAL TECHNOLOGIES, LTD., IS), arcuplasty (Warsaw Orthopedic, Inc, IN), and the Optimesh system (Spineology, Inc., MN) (Figure 44-7). Vertebroplasty is not used to restore the vertebral body height (VBH), whereas the other techniques are accomplishing the same goal by first creating a void. The other concepts do restore VBH and create a void by mechanical or hydrostatic pressure. The difference between the restoring VBH group is based on the technical methods and instruments to lift the vertebral endplate. To restore VBH, all techniques except vesselplasty need to first create a void by mechanical or hydrostatic pressure, followed by filling the void with PMMA or other BFM. All the previously mentioned techniques carry different risks of leakage, because if the BFM is injected directly into the bone or void, it will go to the weakest fracture area. The vesselplasty technique requires only that a hole be drilled into the vertebral body as a place to be occupied by the deflated PET container (almost like screw insertion into the bone). Then the container is inflated by injecting viscous PMMA or other BFM, and the hydrostatic pressure lifts the vertebral endplate, acting as an implanted vertebral body expander.1,6–16
Sequential injection of BFM into a nonstretchable container will prevent leakage, because inside the container the pressure will be distributed equally to all direction. Under a continuous sequential injection the pressure is released outside the Vessel-X through the pores sequentially, because the container size is constant, and starting the interdigitation works like vertebroplasty. The sequential pressure release and the interdigitation will further lift the endplate yet still preventing leakage, because the pressure is equally distributed in all directions. The most important point is the experienced surgeon’s judgment of when to end the procedure, which is related to the individual patient’s condition (Figures 44-8 and 44-9).
Background of Scientific Testing and Clinical Outcomes
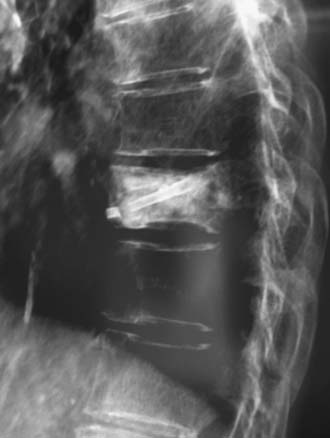
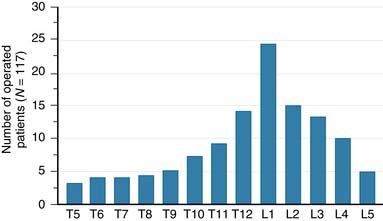
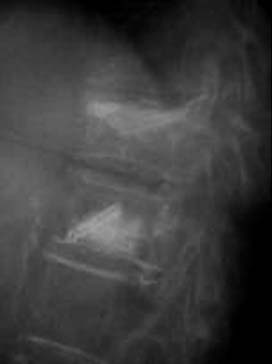
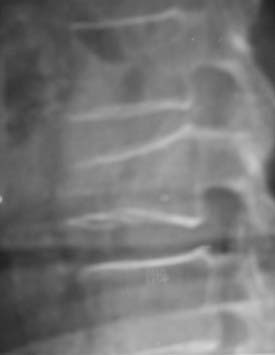
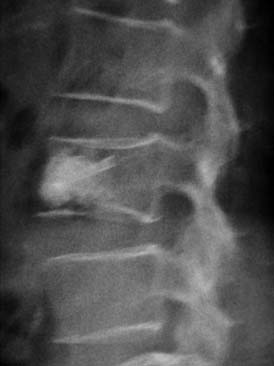
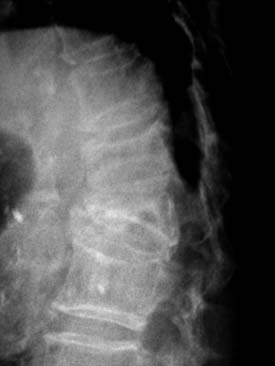
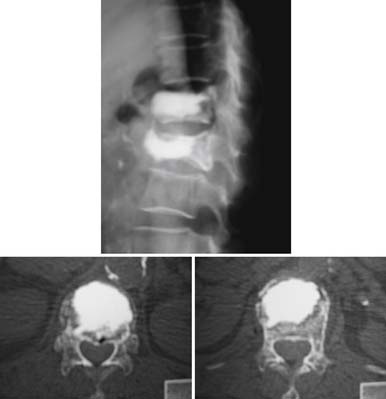
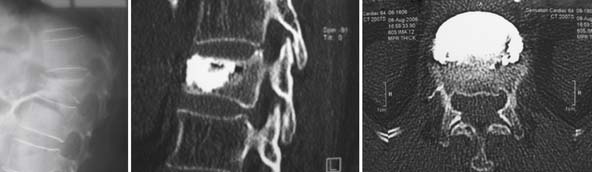
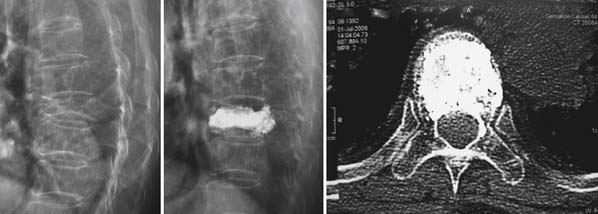
A nonrandomized 3-year prospective follow-up study on 103 patients who had single- or multiple-level stable VCFs from T5 to L5, involving a total of 117 vertebrae (Figure 44-10). In 86 cases, fractures were in osteoporotic vertebrae, compared to 17 cases where the fractures were due to high energy trauma. The number of females, 69 cases, was twice the number of males, at 34 cases. The average patient age was 70.3 years, with the youngest 34 years old and the oldest 98 years old. Fracture age ranged from 1 day to 70 days after the trauma.
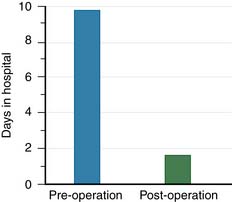
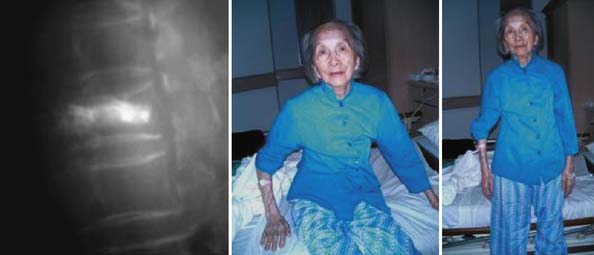
One day after treatment, all patients gained significant pain relief, as determined from the VAS, which dropped from 9.9 to 1.7 (p < .001), and the average hospital stay was 2.2 days (Figure 44-11). The average vertebral height restoration was 96.4% (range, 100% to 50%) related to the variable bone density from old to young patients, fracture type, and fracture age. A variable amount of BFM was injected into the small 20-mm Vessel-X, from 2.5 ml to 10.25 ml, without any leakage, bleeding, or neurologic deficits, and only two adjacent level fractures were detected 1 year after treatment on the eldest patients, who were older than 90 years.
Operative Technique
Anesthesia
During the procedure both local anesthesia and conscious sedation to make the patient comfortable and relaxed are recommended rather than general anesthesia. The combination of conscious sedation and local anesthesia could reduce the risk of injuring the nerve root because the patient can feel the radiating pain caused by the procedure, which is not possible with general anesthesia. The local anesthetic preparation should involve the skin and subcutaneous tissues along the expected needle tract, and the periosteum of the bone at the bone entry site must be thoroughly infiltrated. Once this is accomplished, the patient will experience only mild discomfort while the bone needle is being placed, and the patient will become more relaxed if conscious sedation is used. The local anesthesia being used is a mixture of 0.5% lidocaine and 1:200,000 epinephrine, because it allows the use of a more generous volume locally with less risk of toxicity.4
Position
Patient positioning should be prone on a beanbag or just supported by pillows located under the chest and the hip. If hyperextension is needed to promote some reduction of the fractured vertebra, additional pillows can be added under the hip and the legs of the patient. This position allows a clear visualization during fluoroscopy using the C-arm in both the anteroposterior and lateral views because there is no metal in between.2
Procedure
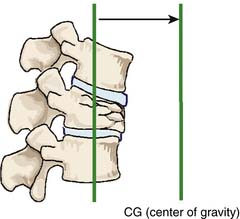
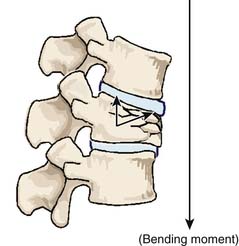
Related to the biomechanical theory of VCFs, the restoration of VBH could be achieved by delivering enough pressure inside the vertebral body to counteract the resistance of the surrounding bone density and the large bending moment due to the shift of the center of body gravity toward the anterior vertebral body side (Figures 44-22 and 44-23).1–8,9,17–19 The restoration is more related to the amount of pressure that can be created, rather than the amount of BFM to be injected. The BFM being delivered with pressure into a vertebral body tends to fill the cavity or void, going toward the weakest area of the fracture, which is its side, and leads to a leakage risk. A nonstretchable container can be used to control the leakage risk because the delivered BFM will be distributed equally in all directions inside the container, and the created pressure inside the container can be used to lift the vertebral endplate toward its normal position accordingly.1–4
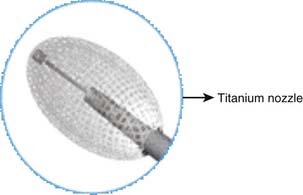
The Vessel-X container (A-Spine Holding Taipei, Taiwan) was designed to meet this purpose. It is made of polyethylene terephthalate (PET), a biocompatible material that is ordinarily used for blood vessel grafts and mesh grafts in herniorrhaphy. The PET mesh container has multipores of 100-μm diameter and is available in one or two layer containers. The number of layers, the pore diameter, and size of a nonstretchable PET container are used to control the amount of the pressure created and the volume of BFM. The relatively weakest area of the container is the posterior part, where the pressure is applied. A titanium nozzle (also a biocompatible material) is used to facilitate the pressure delivery and also to counteract the rebound pressure (Figure 44-24).1,2,6
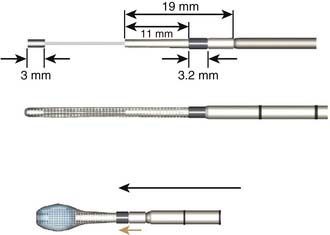
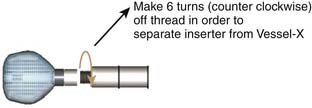
Because the Vessel-X is strongly connected to the inserter by a six-turn clockwise-threaded surface, the inserter should be turned six times counter- clockwise to release it (Figure 44-25).
An anterior titanium marker is available for intraoperative confirmation after inserting the Vessel-X container. A preloaded 1.2-mm guidewire is positioned within the inserter, engaged together with the anterior marker in maintaining the overall length of the Vessel-X during insertion (Figure 44-26).
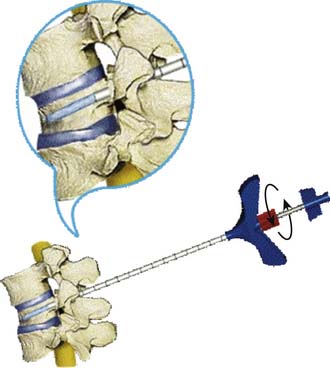
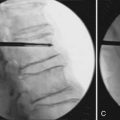
A bone access needle and precision drill are used to facilitate the delivery of the Vessel-X into the vertebral body through a transpedicular or extrapedicular approach (similar to screw delivery into the bone) (Figure 44-27.) Once the Vessel-X is in its proper position inside the vertebral body, the guidewire is removed, and the inserter is pushed a few mm anterior to facilitate the inflation of the nonstretchable container (Figure 44-28).1,3
To prevent inserter migration, the position of the inserter is secured by tightening the Luer connector of the lock knob of the inserter to the working cannula tube before removing the guidewire (Figure 44-29). The final position of the Vessel-X and the inserter before the delivery of BFM is shown in Figure 44-30.1,3
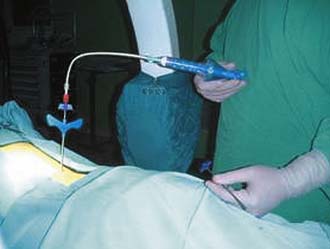
A proper viscosity of the BFM is important to create the hydrostatic pressure to lift the vertebral endplate. (Note: Powder has no hydrostatic pressure, whereas paste has some.) When the proper viscosity is reached, the BFM is delivered through the controllable cement delivery (CCD) system and extension tube (Figure 44-31). The extension tube is connected to the CCD, and the BFM is slowly injected until it comes out from the distal tip of the extension tube. Then by turning the handle of the CCD 180 degrees, amount of 0.25 ml of BFM will be ejected.1,3,5,6
The extension tube is connected to the Vessel-X inserter by tightening the Luer-lock connector to prevent the disengagement of the extension tube. The final setting is achieved and it is now ready for the injection of the BFM (Figure 44-32).1,3
The maximum volume of BFM to be added to the respective Vessel-X container outside the bone is:
The injected volume of BFM inflates the Vessel-X into its final shape, and the pressure inside the container will be equal to the air resistance: 1 atm. As more BFM is injected inside, the pressure will increase above 1 atm, the BFM starts to penetrate the pores, and the released pressure will lift the vertebral endplate (Figure 44-33).1,3,6
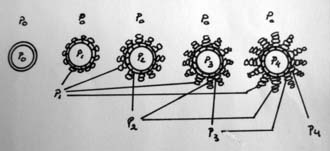
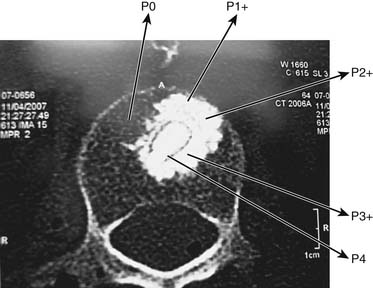
Inside the bone, the resistance is above 1 atm depending on the variable bone density (fracture’s age, osteoporosis, bone age) and the large bending moment due to kyphotic deformity. Restoring the VBH requires a different pressure to counteract the different bone resistance and the kyphotic bending moment. For example, if the bone resistance is P0 (P0 > 1 atm), the volume of BFM to be injected into a 20-mm container will be over 2 ml until the pressure inside the container is equal to P0, then the final shape of the container is achieved and constant. The final shape of the container, which is bigger than before, allows some restoration of the vertebral body height. As more BFMs are injected inside the container, the pressure will increase until P1 (P1 > P0) and the BFM starts to penetrate the pores to the surrounding bone.1,3
By doing the procedure step by step, gradual pressure lifts the endplate until the desired restoration of VBH is achieved. The final outcome is a creation of gradual resistance or stiffness of the bone plus BFM; the central core of the container has the highest pressure, and this might prevent fractures at the adjacent or same level (Figures 44-34 and 44-35).1,4–6
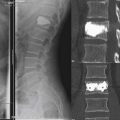
The first 1.25 ml of BFM to be injected fills the inserter, and the following gradual injections will fill the container. After each 0.25 ml injection of BFM, the procedure should be stopped to perform a fluoroscopy check and to achieve some hardening of the penetrated BFM. Then injection is repeated until the properly desired volume is injected. Once the desired restoration of VBH is properly achieved, based on surgeon’s judgment under fluoroscopic control, the injection is stopped (Figures 44-36 and 44-37).1,3
The next step is to detach the extension tube and use a pusher to push the 1.25 ml BFM inside the inserter into Vessel-X to achieve the final interdigitation through the 100-μm pores. The gradual interdigitation and stiffness of BFM could stabilize the Vessel-X in the surrounding bone and might prevent later fractures of the adjacent or same level.7 When the BFM starts to change from viscous to paste condition, the Vessel-X container should be detached from the inserter by loosening the Luer connector, turning the handle counterclockwise for six full turns, and pulling the inserter out (the working cannula should always stay in position without moving). The needle is inserted into the cannula and they are removed together, leaving the Vessel-X as an implant (Figure 44-38).1,3
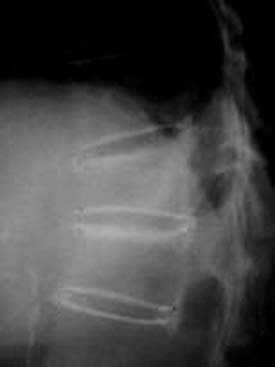
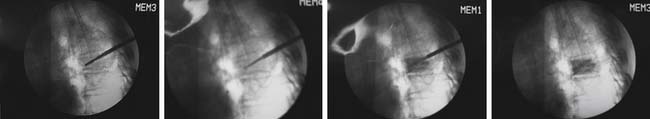
It is critically important that the vesselplasty procedure be performed under fluoroscopic imaging control (Figure 44-39).1,3,7, 8
Postoperative Care
When the conscious sedation anesthesia has worn off, the patient is allowed to sit and walk. Patient activity should be adjusted to the healing process of the bone, which will take around 3 months. Two activities in particular should be restricted: bending forward and lifting. The patient is discharged from the hospital the same day or one day after vesselplasty, and assessments by x-ray are done every month until the bone heals.2,3
Complications and Cautions
The complications are related to errors in patient selection and improper handling of the procedure. The indications should be restricted only for stable fractures and the symptomatic levels only, because the nonfusion technique will not stabilize the instability. Injection of the viscous BFMs should be done slowly, because the delivery of this material needs time to reach the Vessel-X inside the bone, because the viscosity is greater than water. If the injection is done too quickly, it will suddenly elevate the pressure inside the inserter very high and cause the system to fail, which will break the delivery system. Selection of the proper viscosity of BFM with a setting time of at least 10 minutes is very important. A fast-setting cement could force the procedure to end too soon. The container should be delivered gently, because a rough insertion could break the predeployment mesh container; an improper positioning of the Vessel-X inside the vertebral body, such as too close to the vertebral body wall, spinal canal, or partially outside the bone, could cause the BFM to leak outside the vertebral body.2,3
Conclusion
In comparison to the other osteoplasty techniques, the advantage of Vesselplasty is its ability to control the leakage of BFM, by injecting the BFM into a nonstretchable PET container previously inserted inside the vertebral body. The hydrostatic pressure is created by the resistance of the PET container related to the pore diameter of 100 μm, PET layers, and the container size (20, 25, or 30 mm). The viscosity of BFM also plays an important role in achieving the optimum hydrostatic pressure, because the paste condition of BFM provides a lower hydrostatic pressure.1,3
The maximum pressure can be created inside the container, and it is related to the relative resistance of the surrounding individual bone density. The density of the bone is totally different between fresh and old fractures, or between young and osteoporotic bone. Once the created pressure exceeds the resistance of the surrounding bone density, the BFM starts to penetrate the 100-μm pore, interdigitating and stabilizing the container, and the increased pressure can lift the vertebral endplate. Injecting more BFM increases interdigitation and pressure. Once the penetrated BFM contacts body fluids and their higher temperature, it becomes harder than the BFM inside the container, and it increases the surrounding bone density. When this procedure is done step by step, injecting BFM and releasing pressure, the end result is a restoration of vertebral body height and a gradual stiffness of the bone plus BFM from periphery to the central container. This gradual stiffness theoretically might prevent fractures in the same and adjacent levels. In vivo studies showed that up to 9.5 ml BFM can be injected into a 20-mm Vessel-X container without leakage, and restore vertebral height 100%.1,2,4–6
ADVANTAGES AND DISADVANTAGES
The Vessel-X system is designed to prevent the leakage of BFM, but it should be done properly. The amount of BFM to be injected is related to the pressure created, and the end restoration of vertebral body height is different for each case. Every patient has different bone density, different fracture type, and different fracture age and stage of healing. A wise surgeon’s judgment of when to end the procedure is very important; it plays the key role in achieving the best results for the patient and prevents leakage of the BFM outside the bone.1–3
1. Darwono B. Vesselplasty: a novel concept of percutaneous treatment for stabilization and height restoration of vertebral compression fractures. J. Musculoskelet. Res.. 2008;11:71-79.
2. Darwono A.B. Vesselplasty as an alternative to kyphoplasty: a preliminary report. Kuala Lumpur, Malaysia: Triennial APOA meeting; 2004; September 5-10. Abstract not published
3. Darwono A.B. Surgical technique of vertebroplasty and vesselplasty. Coimbatore, India: 13th APOA Spine Surgery Course; 2007; March 8–11. Abstract not published
4. Darwono A.B. Vesselplasty as an alternative to Kyphoplasty: a new concept. Changsha, Hunan, PRChina: 2nd CAMISS congress; 2007; June 17. Abstract not published
5. Darwono A.B. Vesselplasty as an alternative to kyphoplasty: 2 years follow-up study. Qeongju, SouthKorea: 7th PASMISS Congress; 2007; August 17. Abstract not published
6. DarwonoVesselplasty A.B. A new concept to treat vertebral compression fractures: 3 years follow-up study. Athens, Greece: 1st Panhellenic Congress; 2007; September 21. Abstract not published
7. Galibert P., Deramond H., Rosat P., et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166-168.
8. Gangi A., Guth S., Imbert J.P., et al. Percutaneous vertebroplasty: indications, technique, and results. Radiographics. 2003;23:10.
9. Johnell O., Kanis J., Oden A., et al. Mortality after osteoporotic fractures. Osteoporos. Int.. 2001;15:35-42.
10. Kado D.M., Huang M.H., Karlamangla A.S., et al. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J. Am. Geriatr. Soc.. 2004;52:1662-1667.
11. Kasperk C., Hillmeier J., Noldge G., et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J. Bone Miner. Res.. 2005;20:604-612.
12. Ledlie J.T., Renfro M.B. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine. 2006;31:57-64.
13. Lieberman I.H., Dudeney S., Reinhardt M.K., et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631-1638.
14. Majd M.E., Farley S., Holt R.T. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J.. 2005;5:244-255.
15. Moreland D.B., Landi M.K., Grand W. Vertebroplasty: techniques to avoid complications. Spine J.. 2001;1:66-71.
16. Nussbaum D.A., Gailloud P., Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related website. J. Vasc. Interv. Radiol.. 2004;15:1185-1192.
17. Rao R.D., Singrakhia M.D. Painful osteoporotic vertebral fracture. Pathogenesis, evaluation, and roles of vertebroplasty and kyphoplasty in its management. J. Bone Joint Surg. Am.. 2003;85-A:2010-2022.
18. Cauley J., Thompson D., Ensrud K., et al. Risk of mortality following clinical fractures. Osteoporos. Int. 2000;11:556-561.
19. Cockerill W., Lunt M., Silman A., et al. Health-related quality of life and radiographic vertebral fracture. Osteoporos. Int. 2004;15:113-119.