CHAPTER 121 Tumors of the Small Intestine
EPIDEMIOLOGY
Small intestinal neoplasms are a diverse and rare group of tumors that affect the gastrointestinal tract from the duodenum to the ileum. Although the small intestine represents 75% of the length and 90% of the absorptive area of the gastrointestinal tract,1 less than 5% of all primary gastrointestinal tract tumors originate in the small intestine.
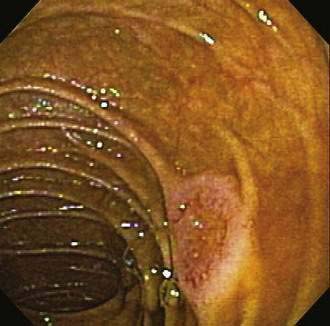
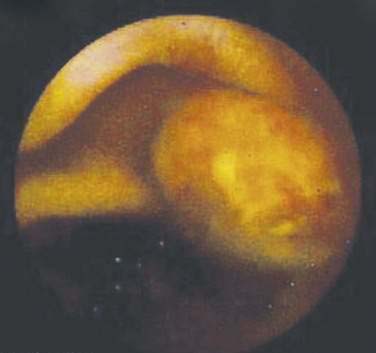
Many types of tumors arise from the small intestine, and about 65% of them are malignant.2 The four most common types of malignancies—adenocarcinomas, carcinoids, lymphomas, and sarcomas (including gastrointestinal stromal tumors or GISTs)—account for 95% of all small intestinal tumors. In most case series of Western patients, adenocarcinoma is the most common small intestinal neoplasm, followed closely by carcinoid tumor.3,4 Adenocarcinoma (Fig. 121-1) most commonly arises in the duodenum, and carcinoid tumor and lymphoma predominate in the jejunum and ileum. Sarcoma distributes equally among all three segments of the small intestine.5
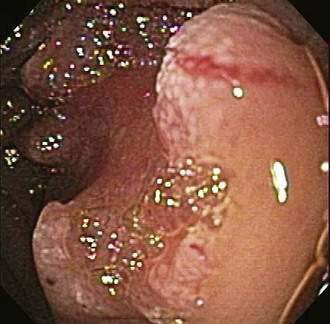
Figure 121-1. Endoscopic image of a mucosal neoplasm in the jejunum of a patient with metachronous small intestine adenocarcinomas.
(Courtesy of Mark A. Schattner, MD, New York.)
A review of 144 cases of malignant tumors of the small intestine showed that 64% of patients were men, with a median age of 55.7 years; 47% of the tumors were adenocarcinomas, 28% were carcinoid tumors, 13% were sarcomas, and 12% were lymphomas. Overall five-year survival was 57% and median survival was 53 months. Survival was highest for early-stage tumors and when curative resection was performed successfully.6 Survival was not related to location of tumor.
A recent study using the Connecticut Tumor Registry data reviewed the epidemiologic and clinical characteristics of 1260 cases of small intestinal tumors. Of these tumors, 49.8% were found in men; the mean age at presentation was 65.2 years. As for location, 25.4% occurred in the duodenum, 15.3% were in the jejunum, and 29.7% arose from the ileum; the remainder of tumors were either multifocal or their location was unspecified. By type, 33.1% were carcinoids, 30.1% were adenocarcinomas, 16.5% were lymphomas, and 7.1% were GISTs. Virtually all (99%) of the tumors were invasive. Surgery was the primary treatment in 87.7% of patients.7
Surveillance Epidemiology and End Results (SEER) data estimate that in the United States in 2008, small intestinal cancers were diagnosed in 3200 men and 2910 women, of whom 1110 people will die of these tumors. From data for 2001 to 2005, the median ages at diagnosis and death were 67 years and 71 years respectively. The overall five-year relative survival rate for 1996 to 2004 from 17 SEER geographic areas was 57.8%; 31% of patients had localized disease at diagnosis, 33% had locally advanced disease, and 29% had metastatic disease at presentation. The corresponding five-year relative survival rates were 77.0% with localized disease, 62.2% with locally advanced disease, 36.0% with metastatic disease, and 40.2% for patients whose stage of disease was not known.8
Worldwide, rates of small intestinal tumors vary; rates are higher in North America and Western Europe and lower in Asia. The Maori of New Zealand have an unusually high incidence of small intestinal tumors.9 Within the United States, incidence rates appear to be somewhat higher in African Americans and in men. Some have suggested an increasing incidence of small intestinal cancer over time.10
Carcinoid tumor (see Chapter 31) is the second most commonly diagnosed small intestinal malignancy, with an annual incidence of approximately three cases per million persons in the United States. The average age at diagnosis is 55 years. The most common sites of extranodal lymphomas (Fig. 121-2) are the stomach and small intestine (see Chapter 29). GISTs can be found throughout the small intestine (see Chapter 30).
PATHOLOGY
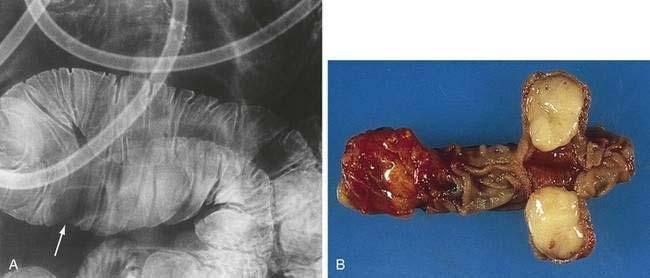
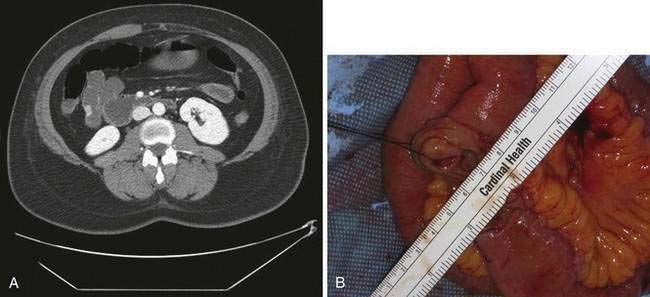
Neoplasms can arise from any of a wide range of epithelial and nonepithelial cells of the small intestine. Adenomas and adenocarcinomas arise from glandular mucosa; carcinoid tumors originate from argentaffin cells; lymphomas develop from clonal proliferation of lymphocytes; and GISTs have been identified as originating from the interstitial cells of Cajal, which help control the motility of the intestine and have elements of both smooth muscle and neural differentiation.11 Leiomyomas arise from smooth muscle cells in the muscularis propria or muscularis mucosa, can be intra- or extraluminal, and account for about 40% of benign intestinal neoplasms. Leiomyomas with more than two mitoses per high-power field are reclassified as leiomyosarcomas. Lipomas represent approximately 20% of benign small bowel neoplasms, are most commonly intraluminal, and are found in the ileum; they rarely bleed, but they can obstruct or intussuscept (Fig. 121-3).12 The remainder of benign neoplasms include desmoid tumors (see Chapter 122) and hemangiomas (see Chapter 36).
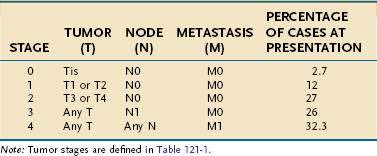
Small intestinal adenocarcinoma is staged using the TNM system as shown in Tables 121-1 and 121-2. Subtypes of this cancer include adenocarcinoma in situ, mucinous adenocarcinoma, signet cell carcinoma, squamous cell carcinoma, adenosquamous cell carcinoma, small cell carcinoma, medullary carcinoma, and undifferentiated carcinoma.
| CATEGORY | DESCRIPTION |
|---|---|
| Tumor (T) | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades lamina propria or submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through muscularis propria into subserosa |
| T4 | Tumor invades through visceral peritoneum or directly invades other organs or structures |
| Regional Lymph Nodes (N) | |
| Nx | Regional lymph nodes cannot be assessed |
| N1 | No regional lymph node metastases |
| N2 | Regional lymph node metastases |
| Distant Metastases (M) | |
| Mx | Distant metastases cannot be assessed |
| M1 | No distant metastases |
| M2 | Distant metastases |
Benign lesions of the small intestine can often be removed by endoscopic means, after being localized using some or all of the methods described later. Techniques for resection and destruction include snare polypectomy, endoscopic mucosal resection (EMR), and argon plasma coagulation. Early duodenal cancers also have been removed using EMR with good results. Because the duodenal wall is thin, the risk of perforation with EMR is high; careful submucosal injection of the duodenum is recommended before removing a duodenal lesion.13 Two studies have shown EMR to be safe and effective, with the potential of reducing the need for surgical intervention for these relatively rare conditions.14,15
Table 121-3 shows the variety of tumors that can arise in the small intestine.
Table 121-3 Some Benign and Malignant Tumors of the Small Intestine
| Epithelial Tumors |
* GIST may be a benign or malignant tumor, although all GISTs have malignant potential (see Chapter 29).
ETIOLOGY
Despite similarities in genetic mechanisms underlying adenocarcinoma of the small intestine and colon, the incidence of small intestinal adenocarcinoma is far lower than that of colonic adenocarcinoma. Given the identical genetic makeup and similar tissue anatomy of the small intestine and colon, the marked difference in cancer incidence between the two sites is difficult to explain. Both tumors in both locations likely develop via an adenoma-carcinoma sequence, and mutated K-ras, Ki-ras, TP53, DPC-4 (Smad-4), β-catenin, and mismatch repair genes are implicated in tumor progression (see Chapter 123).16 Factors that may reduce the incidence of small intestinal adenocarcinoma include higher intraluminal pH, liquidity of small intestinal content, rapid transit time of chyme, faster turnover rate of small intestinal epithelium, lower load of carcinogen-producing bacteria, and increased amounts of immunoglobulin (Ig)A-secreting lymphoid tissue surrounding the small intestinal lumen.7
RISK FACTORS
Despite the low incidence of malignant small intestinal tumors, several risk factors have been identified that contribute to their development (Table 121-4). A dietary study found that increased ingestion of refined carbohydrates, sugar, and red meat increased the risk of small intestinal adenocarcinoma, whereas a diet high in fish, fruits, and vegetables was protective. Alcohol consumption did not affect risk.17 A similar study also found a significantly increased risk with red meat consumption, as well as salt-cured or smoked foods, but did not show fruit and vegetable consumption to be protective.18 Tobacco use and alcohol consumption did not affect development of small intestinal cancer.18
Table 121-4 Risk Factors for Small Intestinal Tumors
Other etiologic factors for the development of small intestinal cancer include Crohn’s disease (see Chapter 111), in which the risk for both colon cancer and small intestinal adenocarcinoma is elevated.19–21 A recent meta-analysis pooling 34 studies of 60,122 patients with Crohn’s disease found a relative risk of 28.4 (95% confidence interval [CI]: 14.46 to 55.66) for the development of small intestinal adenocarcinoma.22 Celiac disease (see Chapter 104) also is thought to be a risk factor for developing non-Hodgkin’s lymphoma (NHL) and small intestinal adenocarcinoma.23 Another study showed an increase in esophageal cancer, melanoma, and NHL in patients with celiac disease; only the risk of NHL persisted despite patient adherence to a gluten-free diet.24
Familial adenomatous polyposis (FAP) predisposes to small intestinal adenomas with associated malignant potential (see Chapter 122). Most of these FAP-associated lesions are found in the periampullary duodenum, although they can occur throughout the length of the duodenum. More than 90% of patients with FAP have a varied number of duodenal adenomas, but only 5% to 10% develop duodenal cancer.25,26 Upper gastrointestinal polyps in patients with FAP might follow a different genetic pathway from that of colorectal cancer.27 FAP mice models show overexpression of the β-catenin gene and deregulation of its signaling pathway. In these animals, a diet of chenodeoxycholate results in more significant duodenal polyposis secondary to the overexpression of β-catenin.28 Bile has been proposed as a potential risk factor for small intestinal cancer,29,30 and there is some suggestion it may be synergistic with the genetic mutations seen in patients with FAP.31
Hereditary nonpolyposis colon cancer (HNPCC) patients also are at increased risk for developing small intestinal cancer (see Chapter 122). HNPCC patients can present with a small intestinal adenocarcinoma as their first and only tumor.32 Screening recommendations for patients with these inherited syndromes might change with the advent of newer imaging techniques for the small intestine.33,34
All adenomas of the small intestine, like adenomas of the colon, should be considered precancerous. Adenomas in both locations appear to progress through an adenoma-to-adenocarcinoma sequence. Malignant features are found in approximately one third of all small intestinal adenomas.35 For reasons that are not clear, adenomas at the ampulla of Vater are typically villous, large, and more likely to be malignant than adenomas found elsewhere in the small intestine.2
Patients with Peutz-Jeghers syndrome develop hamartomatous polyps of the small intestine and colon (see Chapters 22 and 122), and adenocarcinoma can arise within adenomatous foci in the hamartoma, although the overall risk of this is unknown.36 Other hamartomatous polyposis syndromes (see Chapter 122) include juvenile polyposis, which has an increased risk of gastrointestinal tract malignancy, also probably from adenocarcinoma arising from hamartomatous polyps. Other syndromes include Bannayan-Ruvalcaba-Riley syndrome, Cowden’s syndrome, Cronkhite-Canada syndrome, and Devon family syndrome. The gastrointestinal cancer risk in Cowden’s syndrome is well described, the others are less so.37
SMALL INTESTINAL ADENOCARCINOMA
CLINICAL FEATURES
Gastrointestinal bleeding, usually chronic, is the second most common symptom of neoplasms of the small intestine. Bleeding occurs in 20% to 50% of patients with benign lesions (e.g., leiomyomas) and, less commonly, in patients with malignant lesions. Massive hemorrhage is more common with sarcomas (e.g., GISTs) than it is with carcinomas, carcinoid tumors, or lymphomas. Weight loss is rare in patients with benign lesions, but, along with anorexia, it is noted in 50% of patients with malignancies. Intestinal perforation also is rare in patients with benign lesions but occurs in 10% of patients with sarcomas and lymphomas. Periampullary lesions can result in jaundice or pancreatitis. In patients with carcinoid tumors, symptoms of the carcinoid syndrome typically do not develop in the absence of hepatic metastases, and even with hepatic lesions, 28% to 50% of patients with such tumors remain free of the carcinoid syndrome (see Chapter 30).
DIAGNOSIS
Plain Films
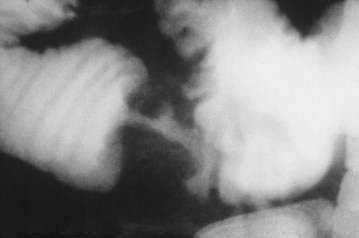
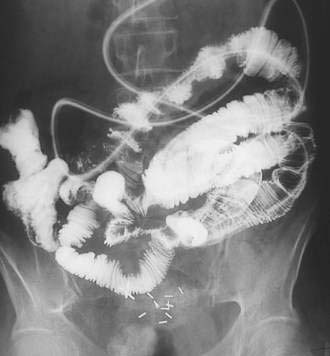
Plain abdominal films rarely are useful for localization of small intestinal tumors, but they may be helpful clinically in the setting of a suspected intestinal obstruction or perforation. In 50% to 80% of patients with small intestinal neoplasms, an upper gastrointestinal series with a small intestinal follow-through (UGIS-SBFT) reveals an abnormality38 and specifically demonstrates a tumor in 30% to 44% of cases (Fig. 121-4); this figure increases to 90% if an enteroclysis study (small bowel enema) is performed (see Fig. 121-3).39 A barium enema can reveal lesions in the terminal ileum if there is sufficient reflux of barium through the ileocecal valve.
Video Capsule Endoscopy
Video capsule endoscopy (VCE) uses a capsule that contains a radiotransmitter to generate photographic images of the small intestine. The patient swallows the capsule, which is propelled by peristalsis through the small intestine. The capsule generates two images per second and has a battery life of eight hours. Images are captured by a digital recorder worn by the patient, and the images are downloaded in a computer to be reviewed later by the physician.40,41 This technology is useful for detecting tumors of the small intestine, especially when they are beyond the reach of push enteroscopy (Fig. 121-5). In 2002, a prospective trial compared traditional barium UGIS-SBFT with VCE in 22 patients with suspected small intestinal abnormalities. SBFT was found to be diagnostic in four (20%) patients; VCE was diagnostic in nine (45%) patients and suspicious in eight (45%).42 VCE was well tolerated. A much larger study published in 2006 looked at 562 patients who underwent VCE from August 2001 to November 2003 for a variety of indications; 79% (443/562) had evidence of occult gastrointestinal bleeding. A diagnosis was made by VCE in 277 patients (49.3%). Of the 562 patients studied, 50 (8.9%) were found to have a small intestinal tumor, 48% of which were malignant.43
VCE also has been used to locate the primary small intestinal lesion in patients with metastatic neuroendocrine tumors. In a study performed in 2006, 20 such patients were evaluated by CT scan, enteroclysis, nuclear imaging (111In pentetreotide scintigraphy) and VCE.44 Nuclear imaging revealed an abnormality in the abdominal region in 13 patients but could not localize the abnormality further. In contrast, VCE found nine small intestinal tumors, of which five were carcinoid tumors, for a diagnostic yield similar to that of nuclear imaging; VCE findings were felt to be more clinically helpful and relevant.
Other studies have shown the superiority of VCE in detecting small intestinal cancer over enteroclysis, push enteroscopy, colonoscopy, and CT scans. VCE was instrumental in diagnosis or management in 55% to 77% of patients evaluated.45–48 A study reported in 2006 by Bailey and colleagues looked at the diagnosis and outcome of small intestinal tumors found by VCE.49 In their study, 416 VCEs were performed in Australia between May 2001 and May 2004, identifying 27 tumors in 26 patients. Twenty-one of the 26 evaluations were for occult gastrointestinal bleeding. Twenty-three patients had both colonoscopy and upper endoscopy, which were unrevealing,23 and had prior radiologic or nuclear evaluation (SBFT, enteroclysis, abdominal CT, tagged RBC study); eight (34.7%) of these patients had only a possible finding. VCE revealed nine benign lesions, eight of which were resected. Four were hamartomatous polyps.17 Thirteen patients were found to have small intestinal malignancies. Adenocarcinoma was identified in five, carcinoid tumor in six, and GIST in two. Most importantly, 23 of 26 patients underwent surgery, and in 12 of these 23 patients, surgery was regarded as curative, including six patients with malignant small intestinal tumors. None of the patients who underwent a curative resection developed evidence of recurrent disease at a follow-up interval that ranged from 26 to 51 months.
Although VCE is a useful technology for detecting small intestinal tumors, it is not without its limitations. As yet, no tissue for definitive diagnosis can be obtained by VCE. Tumors that can be seen on endoscopic push enteroscopy can be missed by VCE because views are often rapidly fleeting.50 Push enteroscopy, however, is limited by its ability to proceed only to about 50 cm beyond the ligament of Treitz.51
Double-Balloon Endoscopy
The development of double-balloon endoscopy (DBE) has allowed evaluation of the entire small intestine, much like VCE, but with the important added ability to biopsy tissue and potentially to perform interventional maneuvers, such as coagulation of bleeding lesions and polypectomy. Yamamoto and colleagues first described DBE in 200152 and elaborated on the clinical experience and outcome using it in 2004.53 In this report, 66 of 123 patients were evaluated for occult gastrointestinal bleeding; 10 patients who presented with occult bleeding had polyps or tumors identified by the procedure.51 Another study of DBE demonstrated similar findings in investigating 64 patients with occult gastrointestinal bleeding: 13% had evidence of tumors or polyps.54 Other studies have shown the efficacy and utility of DBE for the investigation of the small intestine.55,56 A case series showed that some small intestinal lesions missed on VCE are seen on DBE, suggesting that the two technologies are complementary in the evaluation and management of patients with small intestinal diseases.57
Computed Tomography
Computed tomography (CT) in conjunction with newer modalities has been used with success to better image the small intestine. A study by Pfannenburg and colleagues showed that anatomic functional image fusion using combined transmission and emission tomography (SPET/CT) had a diagnostic accuracy of 99% in classifying neuroendocrine lesions and improved tumor localization and characterization in patients with neuroendocrine tumors.58
The advent of multidetector CT enteroclysis (MDCT-E) has enabled demonstration of various findings including wall thickening, hypervascularity, and mural and extramural abnormalities.59 MDCT-E requires nasojejunal intubation to enable rapid delivery of an enteral contrast medium to distend the small intestinal loops homogenously; water has been found to be the most useful neutral contrast medium.60 Intravenous contrast also is necessary. MDCT-E has been shown to be a useful technology for detecting small intestinal abnormalities (Fig. 121-6).
A prospective study reported by Boudiaf and colleagues in 2004, looked at 107 consecutive patients with suspected small intestinal pathology.61 Results were compared with the results of endoscopy, enteroscopy, VCE, pathology findings, and clinical follow-up. MDCT-E revealed 21 patients with small intestinal mass lesions, nine patients with active Crohn’s disease, two patients with small intestinal tuberculosis, four patients with small intestinal lymphoma complicating celiac disease, and 12 patients with low-grade small intestinal obstruction.58 Sensitivity and specificity of MDCT-E were 100% and 95%, respectively, compared with the appropriate gold standard (endoscopy, enteroscopy, VCE, or pathology).61
Another study looked MDCT-E in symptomatic patients with suspected small intestinal neoplasm; researchers used endoscopic, pathology, and clinical follow-up findings as reference standards.62 After having normal endoscopy and colonoscopy, 219 patients underwent MDCT-E. Fifty-five patients had positive findings on MDCT-E, for an overall sensitivity and specificity of 84.7% and 96.9%, respectively. Pathologic evaluation revealed 50 patients with small intestinal tumors, including 19 with carcinoid, seven with adenocarcinoma, five with lymphoma, nine with jejunal adenoma, five with GIST, two with ectopic pancreas, two with angiomatous masses, and one with a metastatic lesion. Five patients had false-positive evaluations.62
Endoscopic Ultrasonography
Endoscopic ultrasonography (EUS) is potentially helpful in determining the origin of intestinal tumors and whether they are benign or malignant. To date, however, the role of EUS in the small intestine has mainly been in the evaluation of pancreatic and ampullary tumors,63 although its role is sure to evolve with more experience (Fig. 121-7).
TREATMENT
Malignant Neoplasms
Surgery remains the only known curative treatment for small intestinal adenocarcinoma. Curative surgical resection is successful in 40% to 65% of patients.6,64 Surgical treatment for adenocarcinoma of the duodenum, because of anatomic location, is a pancreaticoduodenectomy (Whipple’s procedure). In patients with resectable adenocarcinoma of the jejunum, removal of the tumor with wide margins and adjacent lymph nodes is performed. Terminal ileal adenocarcinoma is managed similar to cecal cancer, with a terminal ileal resection, right hemicolectomy, and lymph node resection.
Dabaja and colleagues presented a large retrospective review of 217 patients with small intestinal adenocarcinoma in 2004.65 Surgery with curative intent was performed in 146 patients (67%). Adjuvant chemotherapy was administered in 59 patients (27%) and palliative chemotherapy in 48 patients (22%). The median overall survival time was 20 months. The five-year overall survival rate was 26%. Cancer-directed surgery, early-stage disease, and lymph node involvement ratio were significantly associated with overall survival by univariate analysis. Cancer-directed surgery and lymph node involvement ratio were independent predictors of overall survival in a multivariate analysis (adjusted rate ratio, 0.14; 95% CI: 0.04 to 0.46; P = 0.001). In a study reported from British Columbia, 47 patients with small bowel adenocarcinoma were identified between 1990 and 2000; 21 of the 47 were given chemotherapy, with the majority receiving 5-fluorouracil (5-FU)-based therapy. Of 19 patients treated with curative intent, the median overall survival was 38.6 months. Sixteen of 37 patients with advanced disease received chemotherapy. The median overall survival for those who received palliative chemotherapy was 15.6 months, compared with 7.7 months for those who did not.66
There have been no large prospective studies of chemotherapy for adenocarcinoma of the small intestine because this tumor is uncommon. Most of the chemotherapy data, therefore, come from retrospective analyses, case reports, and case series. One such large retrospective review of continuous infusion 5-FU in advanced small intestinal adenocarcinoma showed an overall response rate of 37.5% and a median survival of 13 months.67 In another retrospective study, combination chemotherapy with 5-FU plus cisplatin, carboplatin, or oxaliplatin in advanced small intestinal adenocarcinoma had an overall response rate of 21% and an overall survival of 14 months. Second-line chemotherapy using 5-FU plus irinotecan has shown promise, but use of continuous infusion 5-FU or 5-FU with cisplatin is not effective.68
Other regimens with activity in gastric and colon cancers have been tried with varying benefit, including 5-FU alone or in combination with doxorubicin, mitomycin-C, cisplatin, and carmustine. A phase II study of 5-FU, doxorubicin, and mitomycin-C in 38 patients with unresectable, biopsy-proven adenocarcinoma of the small intestine showed a response rate of 18.4%, which was no better than that of previous studies of 5-FU-containing regimens.69 In a small case series of three patients who had not responded to 5-FU, irinotecan was used as salvage therapy and appeared to have some efficacy, with marked improvement in symptoms, in two of the three patients.70 Another study reported on six patients with carcinomatosis from small intestinal adenocarcinoma. The authors demonstrated that patients treated with cytoreductive surgery followed by intraperitoneal hyperthermic chemotherapy with mitomycin C given intraoperatively had a median survival of 45.1 months compared with various historical controls of 3.1 months and 12 months.71 A retrospective review of chemotherapy in 113 patients with advanced small bowel adenocarcinoma looked at newer agents compared with 5-FU-based regimens. Forty-four patients received palliative chemotherapy, with an overall response rate of 36% during a first- or second-line regimen (9% complete responses and 27% partial responses). Palliative chemotherapy predicted for overall survival in a multivariate analysis (hazard ratio, 0.47; P = 0.035).72
PROGNOSIS
Long-term survival generally is poor for patients with adenocarcinoma of the small intestine. Curative surgical resection is the mainstay of treatment and improves the five-year survival from 0% after palliative treatment to 54%. The absence of involved lymph nodes at the time of surgical resection improves the five-year survival further up to about 63%. Anemia at the time of initial presentation dramatically improves survival, likely because it leads to evaluation at an earlier stage.73
Several studies have looked at prognostic indicators for survival with small intestinal adenocarcinoma. Brucher and colleagues looked a series of 94 patients with primary tumors of the small intestine, 62 of which were malignant; 22 of these were adenocarcinomas. They found a five-year overall survival of 45%.74 Univariate analysis showed degree of resection (R status) and advancing tumor stage; the presence of lymph node metastases, distant metastases, carcinomatosis, and vascular invasion were poor prognostic indicators.
Dabaja and colleagues also looked at survival in 217 cases of small intestinal adenocarcinoma.65 The median overall survival of these patients was 20 months (95% CI: 16 to 24 months). The five-year overall survival of all patients was 26%. Outcome, as expected, was closely related to stage of disease, with stage IV patients having the worst five-year overall survival (5%) and median survival period of 11 months. The five-year overall survival was significantly shorter for patients with positive lymph nodes compared with patients with negative lymph nodes (32% vs. 52%; median survival, 22 months vs. 78 months; P = 0.0039). The five-year overall survival rate was significantly higher for patients who underwent cancer-directed surgery than for patients who did not, and it was particularly high for those who underwent pancreaticoduodenectomy compared with other types of surgical resection (82 months vs. 29 months; P < 0.0001). Adjuvant chemotherapy did not appear to significantly affect overall survival when given after resection, but chemotherapy for patients with unresectable (stage IV) disease did appear to afford some benefit (12 months vs. 2 months, P < 0.02).
Wu and colleagues retrospectively reviewed prognostic factors in 80 patients with primary small intestinal adenocarcinoma; 60 of 80 underwent surgical management, and 45 of 60 had a curative resection. The five-year survival for patients undergoing resection was 27%; overall survival was 17.5%. In multivariate analysis, earlier tumor stage (I and II) and curative resection were two independent factors with a positive impact on survival. Conversely, the presence of lymph node metastases was an independent variable for poor disease-free survival in patients who underwent a curative resection.74
OTHER MALIGNANT SMALL INTESTINAL NEOPLASMS
For diagnosis, management, and prognosis of lymphomas, GISTs, and carcinoids please refer to Chapters 29, 30, and 31, respectively.
Metastatic tumors are more commonly found in the small intestine than are primary tumors. Melanoma is the most common cancer to spread to the small intestine (Fig. 121-8). Patients with metastatic melanoma can present with occult or overt gastrointestinal bleeding. Surgical intervention may be performed as a useful palliation. Other cancers such as breast, lung, colon, stomach, and ovary can directly invade or metastasize to the small intestine (see Fig. 121-9). In these situations, surgery can be useful for palliation, and systemic chemotherapy can help control disease progression. Prognosis remains poor, however.
Bailey AA, Debinski HS, Appleyard MN, et al. Diagnosis and outcome of small intestinal tumors found by capsule endoscopy: A three-center Australian experience. Am J Gastroenterol. 2006;101:2237-43. (Ref 49.)
Boudiaf M, Jaff A, Soyer P, et al. Small-intestinal disease: Prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338-44. (Ref 61.)
Brucher BL, Stein HJ, Roder JD, et al. New aspects of prognostic factors in adenocarcinomas of the small bowel. Hepatogastroenterology. 2001;48:727-32. (Ref 74.)
Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small intestinal tumors with capsule endoscopy. Cancer. 2006;107:22-7. (Ref 43.)
Czaykowski P, Hui D. Chemotherapy in small intestinal adenocarcinoma: 10-year experience of the British Columbia cancer agency. Clinical Oncology. 2007;19:143-9. (Ref 66.)
Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-26. (Ref 65.)
Egberts J-H, Scharrer M-L, Hinz S, et al. Small intestinal cancer: single-centre results over a period of 12 years. Hepatogastroenterology. 2007;54:129-34. (Ref 3.)
Filippone A, Cianci R, Milano A, et al. Obscure gastrointestinal bleeding and small intestinal pathology: comparison between wireless capsule endoscopy and multidetector-row CT enteroclysis. Abdom Imaging. 2008;33:398-406. (Ref 59.)
Ginsberg GG, Barkun AN, Bosco JJ, et al. Wireless capsule endoscopy. Gastrointest Endosc. 2002;56:621-4. (Ref 40.)
Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small intestinal cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781-7. (Ref 9.)
Hatzaras I, Palesty A, Abir F, et al. Small bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229-35. (Ref 7.)
Locher C, Malka D, Boige V, et al. Combination chemotherapy in advanced small intestinal adenocarcinoma. Oncology. 2005;69:290-4. (Ref 68.)
Schwartz GD, Barkin JS. Small-intestinal tumors detected by wireless capsule endoscopy. Dig Dis Sci. 2007;52:1026-30. (Ref 45.)
Wheeler JM, Warren BF, Mortensen NJ, et al. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut. 2002;50:218-23. (Ref 26.)
Yamamoto H, Kita H, Sunada K, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol.. 2004;2:1010-16. (Ref 53.)
1. Hirsch JE, Ahrens EHJr, Blankenhorn DH. Measurement of the human intestinal length in vivo and some causes of variation. Gastroenterology. 1956;31:274-84.
2. Wilson JM, Melvin DB, Gray GF, Thorbjarnarson B. Primary malignancies of the small bowel: a report of 96 cases and review of the literature. Ann Surg. 1974;180:175-9.
3. Egberts J-H, Scharrer M-L, Hinz S, et al. Small intestinal cancer: single-centre results over a period of 12 years. Hepatogastroenterology. 2007;54:129-34.
4. Mittal VK, Bodzin JH. Primary malignant tumors of the small bowel. Am J Surg. 1980;140:396-9.
5. Neugut AI, Jacobson JS, Suh S, et al. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomarkers Prev. 1998;7:243-51.
6. North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg. 2000;66(1):46-51.
7. Hatzaras I, Palesty A, Abir F, et al. Small bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142:229-35.
8. SEER.cancer.gov [Internet]. Bethesda, Md: National Cancer Institute [cited 2009 Jun 12]. Available from http://seer.cancer.gov/csr/1975_2005/
9. Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small intestinal cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781-7.
10. Severson RK, Schenk M, Gurney JG, et al. Increasing incidence of adenocarcinomas and carcinoid tumors of the small intestine in adults. Cancer Epidemiol Biomarkers Prev. 1996;5:81-4.
11. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-69.
12. Wright NH, Howe JR, Rossini FP, et al. Tumours of the small intestine. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics: tumours of the Digestive System. Lyon: IARC Press; 2000:70-92.
13. Friedrich-Rust M, Ell C. Early-stage small-intestinal adenocarcinoma: a review of local endoscopic therapy. Endoscopy. 2005;37(8):755-9.
14. Hirasawa R, Iishi H, Tatsata M, Ishiguro S. Clinicopathological features and endoscopic resection of duodenal adenocarcinomas and adenocarcinomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507-13.
15. Oka S, Tanaka S, Nagata S, et al. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381-6.
16. Delaunoit T, Neczyporenko F, Limburg PJ, Erlichman C. Small bowel adenocarcinoma: a rare but aggressive disease. Clin Colorectal Cancer. 2004;4:241-48.
17. Negri E, Bosetti C, La Vecchia C, et al. Risk factors for adenocarcinomas of the small intestine. Int J Cancer. 1999;82:171-4.
18. Chow W-H, Linet MS, McLaughlin JK, et al. Risk factors for small intestinal cancer. Cancer Causes Control. 1993;4:163-9.
19. Sigel JE, Petras RE, Lashner BA, et al. Intestinal adenocarcinoma in Crohn’s disease: a report of 30 cases with a focus on coexisting dysplasia. Am J Surg Path. 1999;23:851-5.
20. Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small intestinal cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2005;23:1097-104.
21. Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-29.
22. vonRoon AC, Reese G, Teare J, et al. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839-55.
23. Card TR, West J, Holmes GKT. Risk of malignancy in diagnosed celiac disease: a 24-year prospective, population-based cohort study. Aliment Pharmacol Ther. 2004;20:769-75.
24. Green PHR, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191-5.
25. Heiskanen I, Kellokumpu I, Jarinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412-16.
26. Wheeler JM, Warren BF, Mortensen NJ, et al. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut. 2002;50:218-23.
27. Norheim Anderson S, Lovig T, Fausa O, Rognum TO. Germline and somatic mutations in exon 15 of the APC gene and K-ras mutations in duodenal adenomas in patients with familial adenomatous polyposis. Scand J Gastroenterol. 1999;34:611-17.
28. Romagnolo B, Berrebi D, Saadi-Keddoucci S, et al. Intestinal dysplasia and adenoma in transgenic mice after over-expression of an activated β-catenin. Cancer Res. 1999;59:3875-9.
29. Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001;121:542-7.
30. Ross RK, Hartnett NM, Bernstein L, et al. Epidemiology of adenocarcinoma of the small intestine: is bile a small intestinal carcinogen? Br J Cancer. 1991;63:143-5.
31. Spigelman AD, Owen RW, Hill MJ, et al. Biliary bile acid profiles in familial adenomatous polyposis. Br J Surg. 1991;78:321-5.
32. Park J-G, Kim D-W, Hong CW, et al. Germ line mutations of mismatch repair genes in hereditary nonpolyposis colorectal cancer patients with small intestinal cancer: International Society for Gastrointestinal Hereditary Tumours collaborative study. Clin Cancer Res. 2006;12(11):3389-93.
33. ten Kate GL, Kleibeuker JH, Nagengast FM, et al. Is surveillance of the small intestine indicated for Lynch syndrome families? Gut. 2007;56:1198-201.
34. Schulmann K, Brasch FE, Kunstmann E, et al. HNPCC-associated small intestinal cancer: clinical and molecular characteristics. Gastroenterol. 2005;128:590-9.
35. Rose DM, Hochwald SN, Klimstra DS, Brennan MF. Primary duodenal adenocarcinoma: A ten-year experience with 79 patients. J Am Coll Surg. 1996;182:89-96.
36. Hizawa K, Iida M, Matsumoto T, et al. Neoplastic transformation arising in Peutz-Jeghers polyposis. Dis Colon Rectum. 1993;36:953-7.
37. Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4:492-502.
38. Ekberg O, Ekholm S. Radiography in primary tumors of the small intestinal. Acta Radiol. 1980;21:79-84.
39. Maglinte DD, Hall R, Miller RE, et al. Detection of surgical lesions of the small intestinal by enteroclysis. Am J Surg. 1984;147:225-9.
40. Ginsberg GG, Barkun AN, Bosco JJ, et al. Wireless capsule endoscopy. Gastrointest Endosc. 2002;56:621-4.
41. Swain P. Wireless capsule endoscopy. Gut. 2003;52(Suppl 4):iv48-iv50.
42. Costamagna G, Shah SK, Riccioni ME, et al. A prospective trial comparing small intestinal radiographs and video capsule endoscopy for suspected small intestinal disease. Gastroenterology. 2002;123:999-1005.
43. Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small intestinal tumors with capsule endoscopy. Cancer. 2006;107:22-7.
44. van Tuyl SAC, van Noorden JT, Timmer R, et al. Detection of small intestinal neuroendocrine tumors by video capsule endoscopy. Gastrointest Endosc. 2006;64:66-72.
45. Schwartz GD, Barkin JS. Small-intestinal tumors detected by wireless capsule endoscopy. Dig Dis Sci. 2007;52:1026-30.
46. Urbain D, De Looze D, Demedts I, et al. Video capsule endoscopy in small-intestinal malignancy: a multicenter Belgian study. Endoscopy. 2006;38(4):408-11.
47. Spada C, Riccioni ME, Familiari P, et al. Video capsule endoscopy in small-intestinal tumours: a single center experience. Scand J Gastro. 2008;43:497-505.
48. Baichi MM, Arifuddin RM, Mantry PS. Small-intestinal masses found and missed on capsule endoscopy for obscure bleeding. Scand J Gastro. 2007;42:1127-32.
49. Bailey AA, Debinski HS, Appleyard MN, et al. Diagnosis and outcome of small intestinal tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol. 2006;101:2237-43.
50. Madisch A, Schimming W, Kinzel F, et al. Locally advanced small-intestinal adenocarcinoma missed primarily by capsule endoscopy but diagnosed by push enteroscopy. Endoscopy. 2003;35(10):861-4.
51. Pennazio M, Arrigoni A, Risio M, et al. Clinical evaluation of push-type enteroscopy. Endoscopy. 1995;27:164-70.
52. Yamamoto H, Sekine Y, Sato Y, et al. Total enteroscopy with a non-surgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-20.
53. Yamamoto H, Kita H, Sunada K, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2(11):1010-16.
54. Ell C, May A, Nachbar L, et al. Push-and-pull enteroscopy in the small intestinal using the double balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613-16.
55. Heine GD, Al-Toma A, Mulder CJ, Jacobs MA. Milestone in gastrointestinal endoscopy: double-balloon enteroscopy of the small bowel. Scand J Gastroenterol Suppl. 2006;243:32-8.
56. Schafer C, Rothfuss K, Kreichgauer H-P, Stange EF. Efficacy of double-balloon enteroscopy in the evaluation and treatment of bleeding and non-bleeding small intestinal disease. Z Gastroenterol. 2007;45:237-43.
57. Chong AKH, Chin BWK, Meredith CG. Clinically significant small-intestinal pathology identified by double balloon enteroscopy but missed by capsule endoscopy. Gastroenterol Endo. 2006;64:445-9.
58. Pfannenburg AC, Eschmann SM, Horger M, et al. Benefit of anatomical-functional image fusion in the diagnostic work-up of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2003;30:835-43.
59. Filippone A, Cianci R, Milano A, et al. Obscure gastrointestinal bleeding and small intestinal pathology: comparison between wireless capsule endoscopy and multidetector-row CT enteroclysis. Abdom Imaging. 2008;33:398-406.
60. DiMizio R, Rollandi GA, Bellomi M, et al. Multidetector-row helical CT enteroclysis. Radiol Med. 2006;111:1-10.
61. Boudiaf M, Jaff A, Soyer P, et al. Small-intestinal disease: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338-44.
62. Pilleul F, Penigaud M, Milot L, et al. Possible small-intestinal neoplasms: contrast enhanced and water-enhanced multidetector CT enteroclysis. Radiology. 2006;241:796-801.
63. Artifon EL, Couto DJr, Sakai P, da Silveira EB. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290-6.
64. Ito H, Perez A, Brooks DC, et al. Surgical treatment of small intestinal cancer: a 20-year single institution experience. J Gastrointest Surg. 2003;7:925-30.
65. Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-26.
66. Czaykowski P, Hui D. Chemotherapy in small intestinal adenocarcinoma: 10-year experience of the British Columbia cancer agency. Clinical Oncology. 2007;19:143-9.
67. Crawley C, Ross P, Norman A, Cunningham D. The Royal Marsden experience of small intestinal adenocarcinoma treated with protracted venous infusion 5-fluorouracil. Br J Cancer. 1998;78(4):508-10.
68. Locher C, Malka D, Boige V, et al. Combination chemotherapy in advanced small intestinal adenocarcinoma. Oncology. 2005;69:290-4.
69. Gibson MK, Holcraft CA, Kvols LK, Haller D. Phase II study of 5-fluorouracil, doxorubicin and mitomycin-C for metastatic small intestinal adenocarcinoma. Oncologist. 2005;10:132-7.
70. Polyzos A, Kouraklis G, Giannopoulos A, et al. Irinotecan as salvage chemotherapy for advanced small bowel adenocarcinoma: a series of three patients. J Chemother. 2003;15(5):503-6.
71. Jacks SP, Hundley JC, SHen P, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis from small intestinal adenocarcinoma. J Surg Oncol. 2005;91:112-17.
72. Fishman PN, Pond GR, Moore MJ, et al. Natural history and chemotherapy effectiveness for advanced adenocarcinoma of the small bowel: a retrospective review of 113 cases. Am J Clin Oncol. 2006;29:225-31.
73. Veyrieres M, Baillet P, Hay JM, et al. Factors influencing long-term survival in 100 cases of small intestine primary adenocarcinoma. Am J Surg. 1997;173:237-9.
74. Brucher BL, Stein HJ, Roder JD, et al. New aspects of prognostic factors in adenocarcinomas of the small bowel. Hepatogastroenterology. 2001;48(39):727-32.
75. Wu T-J, Yeh C-N, Chao T-C, et al. Prognostic factors of primary small intestinal adenocarcinoma: univariate and multivariate analysis. World J Surg. 2006;30:391-8.