CHAPTER 60 Tumors of the Pancreas
PANCREATIC CANCER
Pancreatic cancer is the second most common gastrointestinal malignancy in the United States, and approximately 37,680 new cases are expected to occur in 2008.1 Despite its relatively low incidence compared with other malignancies, it represents the fourth leading cause of cancer death in men and women (34,290 deaths expected in 2008). Overall, pancreatic cancer carries an unfavorable prognosis. For all stages combined, the one- and five-year relative survival rates are 24% and 5%, respectively.1 Early detection, accurate preoperative staging, and better treatment options remain a challenge.
EPIDEMIOLOGY
Incidence
Pancreatic cancer is very rare before the age of 45 years, but its occurrence rises sharply thereafter. It affects men more than women (ratio of 1.3 : 1), and is more common in blacks. The incidence in black men is 14.8 per 100,000, compared with 8.8 in the general population.2
Populations at Risk
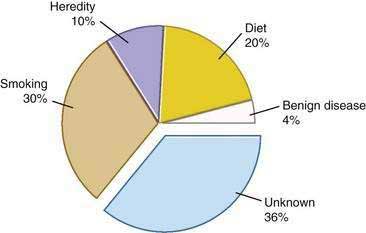
Genetic as well as environmental factors have been found to be associated with the development of pancreatic cancer (Fig. 60-1). Table 60-1 summarizes some of the genetic syndromes associated with an increased risk of pancreatic cancer. One of the most prominent of these syndromes is hereditary pancreatitis, even though it accounts for only a small fraction of pancreatic cancer cases (see Chapter 57). Affected patients have an abnormal trypsin gene that is transmitted as an autosomal dominant trait; their risk for development of pancreatic cancer by age 70 years is estimated at 40%.3 Patients with other, nonhereditary forms of chronic pancreatitis also have a higher likelihood of pancreatic cancer (see Chapter 59). A multinational study found this risk to be 2% per decade, independent of the type of pancreatitis.4
Table 60-1 Historical Features and Germline Genetic Alterations Associated with an Increased Risk of Pancreatic Cancer
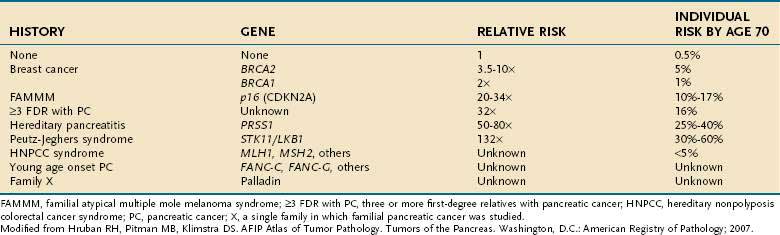
Individuals with familial Peutz-Jeghers syndrome, discussed in Chapter 122, also have an increased risk of pancreatic cancer, with an impressive 132-fold increased risk over the general population.5 Germline mutations in p16 are observed in kindreds with the familial atypical mole-malignant melanoma (FAMMM) syndrome, and these individuals are at risk for pancreatic cancer and melanoma.6 Lastly, patients with BRCA2 gene mutations (which predispose to hereditary breast cancer in women and men), also have a familial predisposition to pancreatic cancer.7
Other unknown genetic abnormalities are yet to be identified. In several population studies, 7% to 8% of patients with pancreatic cancer have a first-degree relative with the disease.8 Patients with three or more first-degree relatives with the disease, have a 32-fold increased risk over the general population; those with two first degree relatives have 6-fold increased risk; and those with one first-degree relative have a 2.3-fold increased risk.9
There are no specific recommendations for screening patients at risk for pancreatic cancer, because available techniques lack sensitivity for detection of very small lesions. The timing and frequency of such screening are also uncertain. The American Gastroenterological Association (AGA) suggests that screening should begin at age 35 in patients with hereditary pancreatitis. Patients with a history of familial pancreatic cancer should begin screening 10 years before the age at which pancreatic cancer has been first diagnosed in their relatives. The AGA also states that such screening is probably best done with spiral computed tomography (CT) and endoscopic ultrasonography (EUS).10,11
Environmental Factors
The most important environmental factor in pancreatic cancer, and possibly the only one that has been firmly established, is cigarette smoking.12 Multiple cohort and case-control studies have found that the relative risk for smokers of developing pancreatic cancer is at least 1.5.13–15 The risk may be particularly elevated in smokers who have homozygous deletions of the gene for glutathione S-transferase T1 (GSTT1), which is a carcinogen metabolizing enzyme.16 Furthermore, the risk rises with the amount of cigarette consumption, and the excess risk level returns to baseline by 15 years after cessation of the habit.15
The second most important environmental factor associated with pancreatic cancer appears to be dietary influences. A high intake of fat or meat has been linked to the development of this neoplasm,17 and a protective effect is ascribed to fresh fruits and vegetables.18 Reduced serum levels of lycopene, a carotenoid present in fruits, and selenium were found in subjects who subsequently had pancreatic cancer.19
Diabetes mellitus is very common in patients with pancreatic cancer. In most cases diabetes has been diagnosed within the preceding two years and may or may not be associated with obesity.20 Thus, recent onset of diabetes may help identify patients with pancreatic cancer, particularly in individuals older than 50 years. Destruction of the pancreas is unlikely to be sufficient to cause endocrine insufficiency in most patients with pancreatic cancer, and it has been proposed that higher production of islet amyloid polypeptide (amylin) by the tumor is responsible for the diabetogenic state. In fact, glucose tolerance frequently improves in patients who have undergone tumor resection.21
PATHOLOGY
As reviewed in Chapter 55, three different epithelial cell types are found in the normal pancreas: (1) acinar cells, which account for about 80% of the gland volume; (2) ductal cells, comprising 10% to 15%; and (3) endocrine (islet) cells, making up about 1% to 2%. More than 95% of the malignant neoplasms of the pancreas arise from the exocrine elements of the gland (ductal and acinar cells) and demonstrate features consistent with adenocarcinoma. Endocrine neoplasms account for only 1% to 2% of pancreatic tumors (see Chapter 32). Nonepithelial malignancies are exceedingly rare.22
The World Health Organization (WHO) has proposed a classification of pancreatic exocrine tumors that is widely used today (Table 60-2).23
Table 60-2 World Health Organization Classification of Primary Tumors of the Exocrine Pancreas
Data from Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000.
Ductal adenocarcinoma accounts for 85% to 90% of pancreatic tumors.22 Autopsy series have shown that 60% to 70% of these tumors are localized in the head of the gland, 5% to 10% in the body, and 10% to 15% in the tail. On gross examination these tumors appear as firm masses with ill-defined margins blending into the surrounding pancreatic parenchyma. The average size of carcinomas in the head of the pancreas is 2.5 to 3.5 cm, compared with 5 to 7 cm for tumors in the body or tail. Differences in tumor size at presentation are related to the earlier development of symptoms and signs in proximal tumors than in distal neoplasms.
Tumors in the head of the gland have a propensity for obstruction of the distal common bile duct and pancreatic duct. Anatomic obstruction of these structures results in jaundice and chronic obstructive pancreatitis. Pancreatic pathologic changes observed include duct dilatation and fibrous atrophy of the pancreatic parenchyma. Some tumors can involve the duodenum or the ampulla of Vater. Extrapancreatic extension into the retroperitoneal tissues is almost always present at the time of diagnosis and can result in invasion of the portal vein or the superior mesenteric vessels and nerves. Neoplasms of the tail of the pancreas do not cause biliary or pancreatic duct obstruction. Extrapancreatic extension in distal tumors causes invasion of the spleen, stomach, splenic flexure of the colon, or left adrenal gland. In patients with advanced disease, metastases to the lymph nodes, liver, and peritoneum are common; the lung, pleura, and bone are less commonly involved.24
Microscopically, ductal adenocarcinomas are graded as well, moderately, or poorly differentiated.22 Well-differentiated tumors show irregular tubular neoplastic glands with mild cellular atypia, low mitotic activity, and significant mucin production. Loss of differentiation results from lack of cellular arrangement into glandular structures, increases in cellular atypia and mitotic figures, and cessation of mucin production. Some studies have demonstrated that histologic grading correlates with survival.25
Several immunohistochemical markers have shown diagnostic usefulness in mucin-producing tumors such as pancreatic adenocarcinoma. Among the better-known markers are MUC1, MUC3, MUC4, carcinoembryonic antigen (CEA), CA 19-9, DuPan 2, and CA 125.22 These markers are unable to differentiate between tumors of pancreatic and extrapancreatic origins, limiting their usefulness in the evaluation of liver metastases of unknown primary. However, they are particularly useful in separating neoplastic from non-neoplastic ductal changes and in distinguishing ductal from acinar or neuroendocrine tumors. Cytokeratins are other useful markers in differentiating among acinar, ductal, and islet cell tumors. Although all ductal adenocarcinomas stain for cytokeratins 7, 8, 18, and 19, most acinar and neuroendocrine tumors do not stain for cytokeratin 7.22
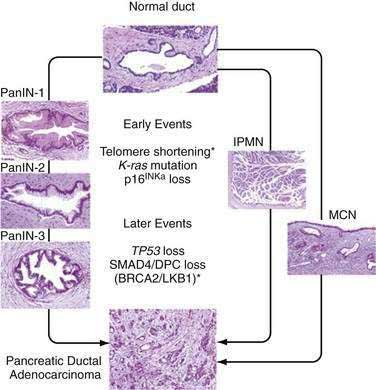
Progression of small pancreatic intraductal lesions to ductal adenocarcinoma has been described, similar to the adenoma-carcinoma model in colorectal cancer.26 These precursor lesions are referred to as pancreatic intraepithelial neoplasia (PanIN). PanINs are microscopic findings that are graded from 1 to 3, the last being equivalent to carcinoma in situ.
Molecular Pathology
Multiple combinations of genetic mutations are commonly found in pancreatic adenocarcinomas. Table 60-3 lists the most common. The accumulation of genetic alterations over time is thought to result in the development of pancreatic cancer. For example, progression of PanIN lesions from grade 1 to 3 has been linked to an increasing number of genetic mutations (Fig. 60-2).27
| GENE (CHROMOSOMAL REGION) | PERCENT OF TUMORS WITH GENETIC MUTATION |
|---|---|
| K-ras (12p)* | >90% |
| p16 CDKN2A (9p)† | >95% |
| PK53 (17p)2 | 50%-70% |
| SMAD4/DPC4 (18q)† | 55% |
| AKT2 (19q)* | 10%-20% |
| MYB (6q)* | 10% |
| AIB1 (20q)* | 10% |
| BRCA2 (13q)† | 7%-10% |
| LKB1/STK11 (19p)† | <5% |
| MKK4 (17p)† | <5% |
| TGF-β-R1 (9q) or TGF-β-R2 (3p) | <5% |
| RB1 (13q) | <5% |
TGF-β, transforming growth factor-β.
Modified from Hruban RH, Wilentz RE. The pancreas. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia: Elsevier; 2005.
A long list of oncogenes and their products has been implicated in the pathogenesis of pancreatic cancer.28 Mutations in the K-ras gene are a hallmark of pancreatic adenocarcinoma and appear to be present in more than 90% of tumors. Studies in intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) of the pancreas have shown that the frequency of K-ras mutations correlates with the extent of microscopic dysplasia within tumors.29,30 Evidence suggests that K-ras mutation may be an early genetic event in pancreatic carcinogenesis, but K-ras mutations may be detected even in the setting of chronic pancreatitis without frank neoplasia.31
Loss of function in several tumor suppressor genes has also been found in pancreatic tumors, notably p16 (also called INK4A), TP53, and DPC4 (also called SMAD4). The combination of p16 and K-ras mutations is uncommon in other human tumors and may be a molecular “signature” for pancreatic adenocarcinoma.32 Loss of p16 function is observed in 80% to 95% of sporadic pancreatic cancers; p16 has a critical role in regulation of the cell cycle, and disruption of its function can lead to accelerated cell division and growth.33 Germline mutations in p16 are associated with the FAMMM syndrome, which is characterized by a high incidence of melanoma and pancreatic cancer.34
The most commonly mutated tumor suppressor gene in human cancer, TP53, is also mutated in a high percentage of pancreatic tumors.28,35 Disruption of TP53 function has been linked to alterations in the cell cycle, regulation of transcription, deoxyribonucleic acid (DNA) repair, and apoptosis, leading to the rampant genetic instability that characterizes pancreatic cancer (see Chapter 3). Another tumor suppressor gene involved in pancreatic carcinogenesis is DPC4.36 The current evidence suggests that DPC4 is a key transcription factor involved in the regulation of transforming growth factor-β expression and subsequent growth inhibition.37 Therefore, disruption of DPC4 could have critical effects in cell cycle regulation and cell differentiation.
Besides oncogenes and tumor suppressor genes, other genetic pathways have been implicated in pancreatic carcinogenesis. Up-regulation of epidermal growth factor (EGF) receptors and their ligands has been well established.38 Telomere shortening and dysfunction also has been found.39 More recently, abnormal activation of genes involved in pancreatic organogenesis has been described (see Chapter 55). One study suggests that the sonic hedgehog gene product is abnormally present early as well as late in pancreatic carcinogenesis.40
CLINICAL FEATURES
Most patients with pancreatic cancer experience symptoms late in the course of disease. The lack of early symptomatology leads to delays in diagnosis, and less than 20% of patients present with resectable masses.41 Tumors of the head of the pancreas produce symptoms earlier in the course of disease. In contrast, tumors of the distal gland are characterized by their “silent” presentation, with physical findings appearing only after extensive local growth or widely metastatic disease has developed. Clinical signs and symptoms can offer clues to the resectability of pancreatic tumors (Table 60-4).42
Table 60-4 Demographics and Presenting Symptoms and Signs in Patients with Unresectable (Palliated) and Resected Pancreatic Cancer*
| PALLIATED(N = 256) | RESECTED (N = 512) | |
|---|---|---|
| Demographics | ||
| Age, average (yr) | 64.0 | 65.8 |
| Men/women | 57%/43% | 55%/45% |
| Race | 91% white | 91% white |
| Symptoms and Signs (%) | ||
| Abdominal pain | 64 | 36* |
| Jaundice | 57 | 72* |
| Weight loss | 48 | 43 |
| Nausea/vomiting | 30 | 18* |
| Back pain | 26 | 2* |
* P = 0.001 vs. palliated group.
Modified from Sohn TA, Lillemoe KD, Cameron JL, et al. Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg 1999; 188:658.
Pain can be a major symptom in many patients with pancreatic cancer. Pain is primarily due to invasion of the celiac or superior mesenteric arterial plexus.43 The pain is of low intensity, dull, and vaguely localized to the upper abdomen. In advanced disease, pain may be localized to the middle and upper back. The pain also may be postprandial and lead patients to reduce their caloric intake, a situation that ultimately results in weight loss or cachexia.
Less common symptoms include those of diabetes and pancreatitis. New-onset diabetes mellitus may herald pancreatic cancer and can be observed in 6% to 68% of patients.44 Acute pancreatitis is occasionally the first manifestation of pancreatic cancer,45 and the clinician must keep this fact in mind especially when dealing with an older adult patient who presents with acute pancreatitis of unclear etiology.
DIAGNOSIS
Computed Tomography
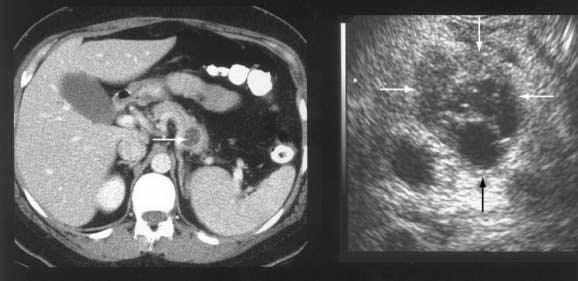
Although transabdominal ultrasonography is frequently the first modality used in many patients with pancreatic cancer (because 50% of them present with jaundice), the method of choice for diagnosis and staging of pancreatic cancer is CT.46–48 The pancreas is ideally imaged by means of the thin-section, pancreatic protocol, helical CT.49,50 In large series, a correct diagnosis of pancreatic cancer using CT can be made in up to 97% of patients.47 Refinements in the CT resolution of peripancreatic blood vessels has rendered routine angiography obsolete for the evaluation of suspected pancreatic masses.46,47
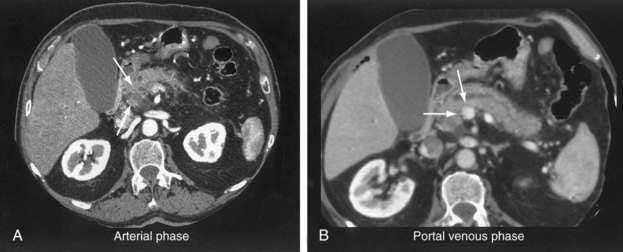
The pancreatic protocol CT consists of dual-phase scanning using intravenous and oral contrast agents. The first, arterial (pancreatic) phase is obtained 40 seconds after administration of intravenous contrast agent. At this time maximum enhancement of the normal pancreas is obtained, allowing identification of nonenhancing neoplastic lesions (Fig. 60-3A). The second, portal venous phase is obtained 70 seconds after injection of intravenous contrast agent and allows accurate detection of liver metastases and assessment of tumor involvement of the portal and mesenteric veins (see Fig. 60-3B).
Current CT criteria for unresectability of a pancreatic tumor are as follows: (1) distant metastasis (e.g., liver, peritoneum, other), (2) arterial encasement of the celiac axis or superior mesenteric artery, and/or (3) occlusion of the portal vein or superior mesenteric vein.46–48 With these criteria, CT has been shown to be almost 100% accurate in predicting unresectable disease.46 However, approximately 25% to 50% of patients predicted to have resectable disease according to these CT criteria are found to have unresectable lesions at laparotomy.46–48 These patients clearly do not benefit from surgical exploration, and their identification by preoperative imaging remains a challenge.
The most common causes of unresectability of a pancreatic tumor are small peritoneal or liver tumor implants and vascular involvement by tumor. The advent of helical pancreatic protocol CT has helped improve the preoperative determination of surgical resectability, particularly in relation to vessel invasion. Later studies have shown that assessment of the degree of circumferential vessel involvement by tumor can help predict unresectability.51–53 Other efforts aimed at detecting small peritoneal and liver metastases beyond the resolution of CT have focused on the development of staging laparoscopy, as discussed later.
Endoscopic Retrograde Cholangiopancreatography
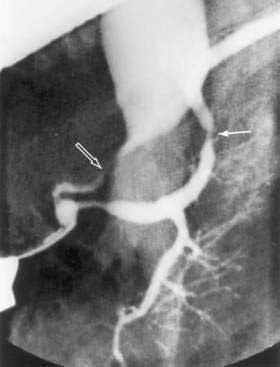
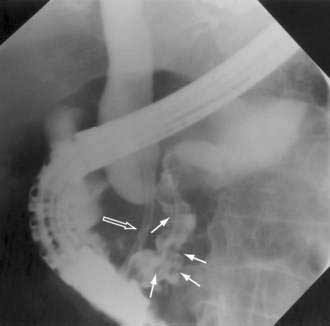
Since its introduction in 1968, endoscopic retrograde cholangiopancreatography (ERCP) has become a mainstay in the differential diagnosis of various tumors of the periampullary region.54 The majority of these tumors originate from the pancreas (85%), and less commonly from the distal bile duct (6%), ampulla (4%), or duodenum (4%). ERCP allows visualization of the pancreatico-biliary tree to distinguish benign (stones) from malignant causes of obstruction. A “double-duct sign,” representing strictures in biliary and pancreatic ducts, is classically found in many patients with pancreatic cancer (Fig. 60-4).
Tissue sampling can also be obtained during ERCP. For tumors of the ampulla or duodenum, biopsy of mucosal lesions is readily obtained with endoscopic forceps. Tumors of the distal bile duct may also be sampled via brush biopsy for routine cytology or genetic analysis.55,56
As discussed earlier, CT allows for identification of pancreatic tumors in the majority of patients with pancreatic cancer, rendering ERCP unnecessary in most cases. In practice, however, many patients with pancreatic cancer undergo ERCP, not for the purpose of diagnosis but rather for stenting of the biliary duct (see Chapters 61 and 70). Routine preoperative biliary duct stenting to relieve jaundice has not been shown to decrease postoperative morbidity and mortality, and the procedure can increase the likelihood of surgical infectious complications.57–59 Therefore, its practice in patients with resectable tumors (as assessed by CT) cannot be recommended, unless it is anticipated that surgery will delayed for more than two weeks. For patients with jaundice and unresectable or metastatic disease, endoscopic biliary stenting, preferably with expandable metal stents, offers excellent palliation.60
Endoscopic Ultrasonography
EUS may be the most accurate test for the diagnosis of pancreatic cancer.61 Several studies have shown that EUS has a higher sensitivity and specificity than CT for detecting pancreatic masses (Fig. 60-5).62,63 EUS also has been found to be more accurate than CT in assessing vascular invasion and predicting tumor resectability. Other advantages of EUS include accurate assessment of peripancreatic nodal disease, and allowance of tumor biopsy by fine-needle aspiration (FNA).64
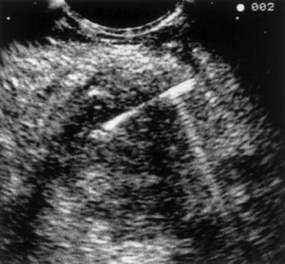
Figure 60-5. Endoscopic ultrasonographic image of pancreatic cancer. The figure shows the needle during biopsy of the tumor.
Limitations of EUS are manifold. EUS is highly operator-dependent, and it is estimated that experience with 100 such examinations is needed to be considered proficient.63 Imaging by EUS can be compromised by the presence of a biliary stent, which results in imaging artifacts and loss of tissue detail. Due to technical and anatomic constraints, imaging of the portal vein and splenic vein is generally superior to imaging of the superior mesenteric artery and vein.65 For this reason EUS may lack accuracy when assessing vascular invasion at the level of the superior mesenteric vessels. Lastly, EUS provides no information regarding metastatic disease, and a complementary CT or magnetic resonance imaging (MRI) scan is required for complete staging of disease.
Magnetic Resonance Imaging
MRI has been increasingly used in the evaluation of pancreatic tumors, and several groups have shown results that rival those of helical CT.66,67 In one study, pancreatic tumor detection was reported in 90% of patients for MRI versus 76% for helical CT.66 Optimal MRI resolution is obtained with T1-weighted images and the use of dynamic gadolinium enhancement. Tumors are viewed as low-signal masses against the high-signal background of normal pancreatic parenchyma. Pancreatic masses, ductal dilation, and liver metastasis can be demonstrated in exquisite detail. Additionally, MR angiography and MR venography techniques using gadolinium contrast enhancement can demonstrate vascular involvement by tumor, obviating conventional angiography. Unlike CT, MRI does not involve radiation and uses an iodine-free contrast agent with rare renal toxicity. Limitations of MRI are related to cost, availability, and clinicians’ familiarity with and predilection for CT imaging.
Magnetic resonance cholangiopancreatography (MRCP) can also be obtained at the time of MRI.68 In a prospective, controlled study, MRCP was found to be as sensitive as ERCP in detecting pancreatic carcinomas.69 MRCP uses heavy T2-weighted images that emphasize fluid-containing structures such as ducts, cysts, and peripancreatic fluid collections. Images obtained are highly comparable with those obtained with ERCP and readily demonstrate pancreatic ductal obstruction, ectasia, and calculi. In contrast to ERCP, MRCP is noninvasive and does not require injection of contrast into the pancreatico-biliary tree, avoiding possible complications such as allergy, pancreatitis, or infection. However, no therapeutic or diagnostic intervention can be performed with MRCP.
Positron Emission Tomography
Positron emission tomography (PET) is a noninvasive imaging tool that provides metabolic rather than morphologic information on tumors.70,71 This diagnostic method is based on greater use of glucose by tumor cells than by normal pancreatic parenchyma. The radioactive glucose analog fluorodeoxyglucose (FDG) F 18 is administered intravenously, followed by detection of FDG uptake by the PET scanner. The normal pancreas is not usually visualized by FDG-PET. In contrast, pancreatic carcinoma appears as a focal area of increased uptake in the pancreatic bed. Hepatic metastases appear as “hot spots” within the liver. Owing to the lack of anatomic detail, PET scanning is not a principal diagnostic modality for pancreatic cancer. However, FDG-PET can be helpful in differentiating benign from malignant pancreatic masses when morphologic data are equivocal.72 It can also be useful in assessing tumor recurrence after pancreatic resection, when scar tissue or postoperative changes may be difficult to differentiate from carcinoma. Finally, FDG-PET can be of benefit in assessing tumor response to neoadjuvant chemoradiation, which may lead to alteration in clinical management.
Percutaneous and Endoscopic Ultrasonography–Guided Aspiration Cytology
FNA cytology of the pancreas has been one of the major advances in the management of patients with pancreatic tumors. CT-guided biopsy has been used for more than 20 years and is regarded as a safe, reliable procedure, with a reported sensitivity of 57% to 96% and virtually no false-positive results.73,74 Experience with EUS-guided FNA shows similar results.64 Whenever a patient is deemed to have unresectable or metastatic pancreatic cancer, CT- or EUS-guided FNA biopsy is indicated for histologic confirmation of disease, unless a palliative surgical procedure is required. Even if the diagnosis of chronic pancreatitis is reasonably eliminated, proof of malignancy will exclude other rare benign diseases of the pancreas, such as tuberculosis and sarcoidosis. Furthermore, FNA cytology can usually distinguish between adenocarcinoma and other pancreatic tumors, such as neuroendocrine neoplasms and lymphomas, which carry a better prognosis (see Chapters 29 and 32).75
Serum Markers
A wide variety of tumor markers have been proposed for pancreatic cancer, but currently the only one with any practical usefulness is CA 19-9. Although not suitable for screening, this marker is a valuable adjunct in pancreatic cancer for diagnosis, prognosis, and monitoring of treatment.76
For diagnosing pancreatic cancer, the sensitivity and specificity of CA 19-9 vary with the threshold value set. One of the major caveats of this tumor marker is that in the presence of jaundice, and especially with cholangitis, very high values can be found in the absence of malignancy (false-positive results). In addition, patients with a Lewis blood group phenotype (-a-b) do not express the CA 19-9 antigen, resulting in false-negative results. Despite these shortcomings, one study reported a sensitivity and specificity of 86% and 87%, respectively, using a cut-off value of 37 U/mL.77
Initial reports of CA 19-9 response to chemotherapy indicated that a decrease in value greater than 20% during treatment correlated with improved survival in patients with locally advanced or metastatic disease.78 A more contemporary study using a larger patient population did not confirm these findings. Based on these latest results, CA 19-9 response to chemotherapy cannot be used as an independent prognostic factor for survival.79
STAGING
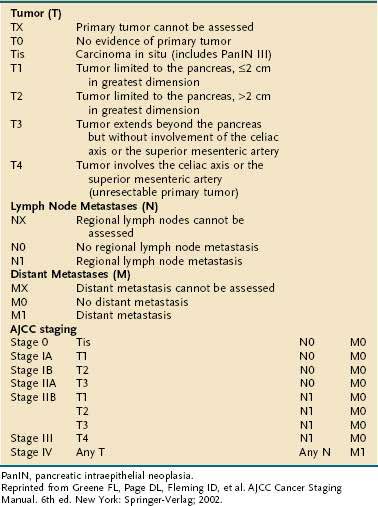
The American Joint Committee on Cancer (AJCC) staging system for pancreatic cancer is shown in Table 60-5.80 The system was last revised in 2002, and modifications were made to better identify unresectable (T4, stages III and IV) from resectable disease (T1-3, stages I and II). Despite these changes, several limitations of the staging system still exist. First, adequate evaluation of lymph node status cannot be performed without surgical intervention; this drawback may lead to understaging of locally advanced disease in patients who are not candidates for laparotomy. Second, the margins of resection, which carry great prognostic significance, are not taken into consideration when assigning clinical stage. Because of these and other shortcomings, the AJCC staging system has found limited clinical applicability.
Staging of pancreatic cancer is predicated on the identification of three distinct patient groups. The first group involves those presenting with metastatic disease. Surgery is best avoided in these patients in view of their short survival, and chemotherapy is their principal treatment modality other than palliative care measures.81 The second group comprises patients who have locally advanced (unresectable) disease but no metastases. These patients can benefit from neoadjuvant chemoradiation and, according to their treatment response, may be candidates for surgical exploration or intraoperative radiation therapy.82–85 A third group consists of patients with resectable disease for whom surgical resection should be advocated.
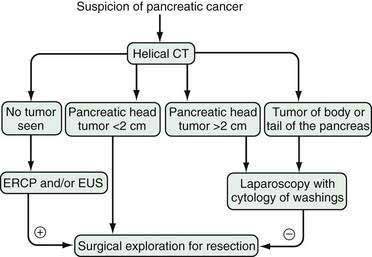
A minimal staging workup for a patient with pancreatic cancer should include a physical exam, and a CT or MRI of the abdomen and pelvis.80 As discussed, contemporary CT or MRI is extremely accurate in identifying unresectable disease.46,47,66 In selected cases, EUS may complement CT scanning by allowing further assessment of vascular invasion and tissue sampling. However, CT imaging fails to correctly predict resectability in 25% to 50% of patients. In most cases, lesions missed are beyond the resolution of current radiologic imaging; they include small implants on the peritoneal surfaces of the liver, abdominal wall, stomach, intestine, or omentum. Additionally, micrometastases only detectable by peritoneal washings are also missed. Successful detection of such tumor dissemination depends on access to the peritoneal cavity and visual inspection, which at present can be achieved only by laparoscopy or laparotomy.
Several large studies have documented the value of staging laparoscopy in the evaluation of patients with pancreatic cancer.86–90 The staging procedure consists of a simple diagnostic laparoscopy with biopsy of suspicious nodules and collection of peritoneal washings for cytologic analysis. Peritoneal cytology results are important for staging because patients with occult metastases detected by this method have been shown to carry a prognosis similar to that of patients with M1 disease (stage IV).91,92 More complicated staging procedures involving extended laparoscopic dissections of the peripancreatic bed and laparoscopic ultrasonography can also be added if necessary.89,90
Published data demonstrate that approximately 25% of patients in whom localized disease is demonstrated by CT also have unsuspected metastatic implants.87,88 A further 10% have positive peritoneal cytology results despite a lack of gross metastatic disease at laparoscopy. For most of these patients, laparoscopy has prevented unnecessary surgical explorations to assess tumor resectability.93 The combination of CT scan and staging laparoscopy has enhanced the identification of patients with metastatic, localized-unresectable, and resectable pancreatic cancer and has helped in stratifying patients to different treatment protocols.88 Our current algorithm for the diagnosis and staging of pancreatic cancer is shown in Figure 60-6. We recommend laparoscopy for all patients with tumors in the body and tail of the pancreas (in which the frequency of unsuspected metastases approaches 50%) and for patients with tumors in the head of the pancreas larger than 2 cm, because the yield of laparoscopy in lesions smaller than 2 cm is less than 10%.
TREATMENT
Surgical Therapy
Surgical resection is the only potentially curative treatment for pancreatic cancer. Because of advanced disease at presentation, only about 15% to 20% of patients are candidates for pancreatectomy.41 Extrapancreatic disease, most commonly presenting as malignant ascites or metastasis to the liver, peritoneum, or periaortic lymph nodes, is an absolute contraindication to resection. Relative contraindications to resection include encasement or occlusion of the superior mesenteric vein (SMV) and portal vein (PV), or direct extension of disease to the celiac axis, superior mesenteric artery (SMA), vena cava (VC), or aorta. Vascular resection and reconstruction may be applied in very selected cases to allow resection of locally advanced disease.94
The most common operation for pancreatic cancer is the Whipple pancreaticoduodenectomy (Fig. 60-7), which removes primarily the head of the pancreas.95 In the past, total pancreatectomy was advocated as a better operation for pancreatic cancer.96 It was thought to provide a more radical resection and allowed avoidance of the pancreaticojejunal anastomosis, which can be a source of considerable morbidity and mortality. However, total pancreatectomy has not been shown to improve survival when compared with the more limited pancreaticoduodenectomy, and results in obligate exocrine insufficiency and brittle diabetes, which are difficult to manage.97–99 Other extensions to the standard Whipple procedure, such as addition of retroperitoneal lymphadenectomy, have shown no significant survival benefit (Fig. 60-8) and may result in additional morbidity (longer hospital stays, increased rates of pancreatic fistula, and higher incidence of delayed gastric emptying).100–102
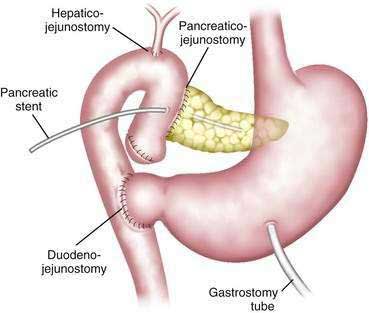
Figure 60-7. Diagram of the pylorus-preserving pancreaticoduodenectomy. A pancreatic stent is shown in the pancreatic duct.
(From Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of the pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg 2000; 231:293.)
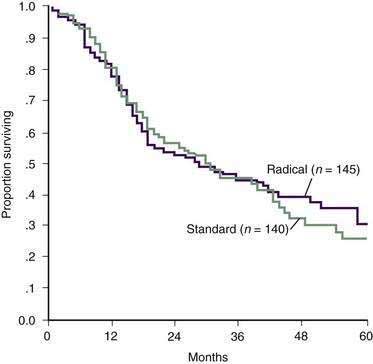
Figure 60-8. Actuarial survival after pancreaticoduodenectomy with standard and radical lymphadenectomy.
(From Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. Part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002; 236:355.)
In the past, pancreaticoduodenectomy was associated with high morbidity and mortality rates. Many contemporary large series now consistently show mortality rates of less than 3%, with a concomitant decrease in complications (Table 60-6).103–108 Pancreatic fistula, the most common and dreaded complication after the Whipple procedure, is observed in only 5% to 10% of patients today. These changes have been attributed to the emergence of intensive care units, as well as advances in surgical technique, anesthesia, antibiotics, and interventional radiology. Improved outcomes have also been linked to the development of centers of excellence for pancreatic surgery, where surgeons can develop greater expertise. A recent study using the Medicare database showed a fourfold increase in mortality when comparing pancreaticoduodenectomy performed in hospitals with an average of less than one case per year to those hospitals performing more than five cases per year.109 A subsequent study suggested that long-term outcomes are likewise improved at high-volume centers.110
Despite improved outcomes after pancreatectomy, surgery is still underused in the United States for treatment of pancreatic cancer. A study on early stage pancreatic cancer by Bilimoria and colleagues using the National Cancer Data Base from 1995 to 2004, identified that 38.2% of patients with potentially resectable disease were not offered surgery.111 Surgery was less likely to be offered to patients who were older than 65 years, black, of low socioeconomic status, less educated, or had cancer involving the head of the pancreas. The study also reported differences in outcomes between patients with stage I disease who underwent pancreatectomy versus those not offered surgery (median survival 19.3 months versus 8.4 months, respectively). These data raise awareness of nihilistic attitudes still linked to pancreatic surgery, and disparities in availability of health care today. This information also presents an opportunity for quality improvement in the future.
Ultimately, prognosis for pancreatic cancer remains poor, even after potentially curative surgery in appropriately selected patients. Five-year actuarial survival rates range from 10.5% to 25% and median survivals between 10.5 and 20 months.108,112–114 Analysis of five-year survivors demonstrate an actual five-year survival rate of approximately 10%.115–117 Among those variables that have been shown by multivariate analysis to be significant predictors of a better outcome are tumor size less than 3 cm, absence of lymph node metastases, negative resection margins, well-differentiated tumors, and intraoperative blood loss of less than 750 mL.108,112–114,118
Palliative Procedures
Large published series of patients with pancreatic cancer show that only between 15% and 20% are resectable. Patients with unresectable disease require some form of palliation to relieve jaundice, duodenal obstruction, or pain. Operations for biliary bypass are very effective, and are often combined with gastrojejunostomy to alleviate duodenal obstruction and celiac plexus block for pain control. However, these operations are not exempt from risk in debilitated patients with pancreatic cancer. A large series shows postoperative mortality of 3.1% and a complication rate of 22%, with a median survival of 6.5 months.42
Relief of jaundice can also be achieved by biliary stents placed percutaneously or endoscopically (see Chapters 61 and 70). Because these procedures are usually well tolerated and perfomed on an outpatient basis, they have become increasingly popular in the management of malignant biliary obstruction. In experienced hands, endoscopic stent placement has a success rate of more than 85%, with a 1% to 2% procedure-related mortality. Several randomized trials have demonstrated no difference in survival between patients palliated endoscopically versus surgically for obstructive jaundice.119,120
Two types of biliary stents are available: plastic and self-expandable metallic stents. The plastic stents are preferred for short-term use, and require exchange every three months to prevent complications from stent occlusion or cholangitis. The metallic self-expandable stents (Wallstent) have improved long-term patency rates when compared with plastic stents, and are preferred for long-term application.60,121,122 In clinical practice, plastic stents are preferred for patients who are surgical candidates, whereas metal stents are used for unresectable patients.
Traditionally, duodenal obstruction has been treated surgically via gastrojejunostomy. The efficacy of this intervention has been demonstrated in the setting of randomized controlled trials.123,124 More recently, reports of the use of expandable metallic stents to relieve duodenal malignant obstruction have shown success, and this modality may be used increasingly in the future.125,126
Pain in pancreatic cancer can be extremely distressing and may respond poorly to oral narcotics. Percutaneous or surgical chemical neurolysis with alcohol is an alternative palliative measure that can help in controlling pain or decreasing narcotic use. EUS can be used for this purpose as well (see Chapter 61). Randomized trials have shown that neurolysis of the celiac ganglion can offer relief to many patients.127,128 Lastly, radiation therapy may also be used for pain management in selected patients.
Chemotherapy and Radiation Therapy
Adjuvant Chemoradiation
From 1980 to 2000, adjuvant chemoradiation was standard of care in the United States and many other countries after potentially curative pancreatic cancer resection. These recommendations were based on the results of a study conducted by the Gastrointestinal Tumor Study Group (GITSG) between 1974 and 1982.129,130 The study randomized 43 patients after surgery to either observation or chemoradiation. Chemoradiation consisted of 4000 cGy of external beam radiation with concurrent bolus of 5-fluorouracil (5-FU) as radiosensitizer. Median survival in the treated group was 20 months, which was significantly longer than the 11-month survival in the untreated group. The GITSG study was criticized for its small sample size and lack of statistical power. However, a separate study by the Norwegian Pancreatic Cancer Trial group showed similar results and supported a survival benefit for adjuvant treatment.131
In 1999 the results of a study by the European Organization for Research and Treatment of Cancer (EORTC) questioned the value of adjuvant chemoradiation in pancreatic cancer.132 Similar to the study design by the GITSG, the EORTC randomized patients after surgery to observation or chemoradiation (4000 cGy external beam radiation and concurrent 5-FU by continuous infusion). This time the study group was relatively large and consisted of 114 patients with pancreatic cancer. The median survival was 4.5 months longer in the treatment group than in the observation group (17.1 vs. 12.6 months, respectively), but this difference was not statistically significant. Likewise, the projected two-year survival was not significantly different between groups (37 vs. 23 months, respectively).
Further controversy ensued after the results of a study by the European Study Group for Pancreatic Cancer (ESPAC-1 trial) were released in 2001.133,134 This is the largest adjuvant treatment trial for pancreatic cancer to date, recruiting 289 patients. The ESPAC-1 study had a complicated design that randomized patients after surgery to four groups: observation, chemotherapy, chemoradiation, or chemoradiation plus chemotherapy. The results did not show a difference in median survival between patients receiving chemoradiotherapy and those who did not (15.5 vs. 16.1 months) (Fig. 60-9). Even for patients with positive resection margins who are thought to be prime candidates for chemoradiation, this treatment did not have a survival impact. Results of the ESPAC-1 trial are validated by its large sample size, but have generated great controversy. Criticism points mainly to the study’s convoluted randomization scheme, interrupted course of radiation, and pooling of data for statistical analysis.
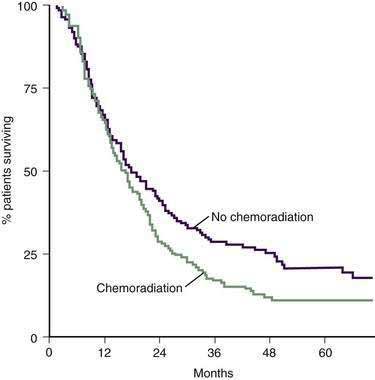
Figure 60-9. Survival after pancreatic resection with or without adjuvant chemoradiation.
(From Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 358:1200.)
Thus, the best available data today are equivocal regarding the use of chemoradiation in adjuvant fashion outside of clinical trials. New chemoradiation trials using gemcitabine instead of 5-FU have generated significant interest. A phase II trial by Small and colleagues showed a disease control rate of 84.6%, and a one-year survival rate of 47% for patients with unresectable disease.135 It remains to be seen whether these results hold ground in the setting of a phase III trial.
Chemotherapy for Metastatic Disease
Patients presenting with stage IV disease (see Table 60-5) as well as those developing distant metastasis after attempted curative resection are candidates for chemotherapy. Chemotherapy in this situation is never curative, and its palliative benefit must be balanced against its potential toxic side effects. Assessment of tumor response to chemotherapy is based primarily on serial imaging studies, serum marker (CA 19-9) trends, and changes in tumor-related symptoms.
Only two chemotherapy agents have been associated with survivals longer than five months in pancreatic cancer: 5-FU and gemcitabine. Up until the mid-1990s, 5-FU was the drug of choice for treatment of advanced or metastatic pancreatic cancer. Monotherapy with 5-FU has a poor objective response rate (0% to 10%) and an average median survival of about five months.136 Combination chemotherapy regimens containing 5-FU have increased objective response rates (15% to 40%) but have failed to show a survival advantage in randomized trials.137–140
Gemcitabine is a deoxycytidine analog that is thought to interfere with DNA synthesis and repair. A well-known randomized trial by Burris and colleagues compared weekly gemcitabine to weekly 5-FU infusion in previously untreated patients.141 Patients treated with gemcitabine had a slightly improved median survival compared with those treated with 5-FU (5.6 vs. 4.4 months, respectively). An impressive one-year survival advantage was also noted for the gemcitabine arm (18% vs. 2%, respectively) (Fig. 60-10). Importantly, improvement in disease-related signs and symptoms (pain, performance status, weight loss) were more likely in the gemcitabine group (23.8%) than in the 5-FU group (4.8%). Gemcitabine has been shown to have clinical benefit even in patients for whom 5-FU treatment has failed.142
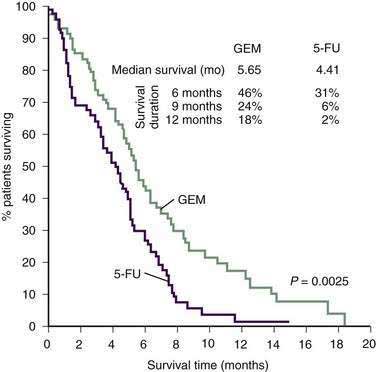
Figure 60-10. Survival in 126 patients with metastatic pancreatic cancer treated with gemcitabine (GEM) or 5-fluorouracil (5-FU).
(From Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997; 15:2403.)
On the basis of the results of these studies, gemcitabine is currently the standard of care for patients with metastatic pancreatic cancer. However, single-agent gemcitabine treatment is associated with poor objective response rates (5.4%).141 In an attempt to improve response rates and survival, multiple gemcitabine-based combination chemotherapy regimens have been developed. In these protocols, gemcitabine has been coupled with either cisplatin, fluorouracil, irinotecan, oxaliplatin, pemetrexed, or exatecan.143–148 Objective response rates have shown improvement with combination therapy, but this improvement has not translated into a survival benefit.
Poor results with conventional chemotherapy have led to ongoing development of novel agents against pancreatic cancer. As opposed to the nonselective action of current cytotoxic drugs, these new agents are designed to target specific cellular pathways involved in tumor progression. Examples include inhibitors of farnesyltransferase, epidermal growth factor receptor, and matrix metalloproteinases (Table 60-7).149,150 These new biological agents show impressive results in both in vitro and animal studies.151 However, efficacy in human trials has been more difficult to demonstrate.149,150,152,153
| TARGETED MOLECULE | AGENT(S) | MECHANISM OF ACTION |
|---|---|---|
| Farnesyltransferase | Tipifarnib (Zarnestra) | Inhibit mutant K-ras function |
| Epidermal growth factor receptor | Cetuximab (Erbitux)Trastuzumab (Herceptin) | Block cell membrane receptors for growth factors |
| Gefitinib (Iressa) | ||
| Erlotinib (Tarceva) | ||
| Matrix metalloproteinase | MarimastatBAY 12-9566 | Block extracellular matrix degradation and tumor invasion |
CYSTIC TUMORS OF THE PANCREAS
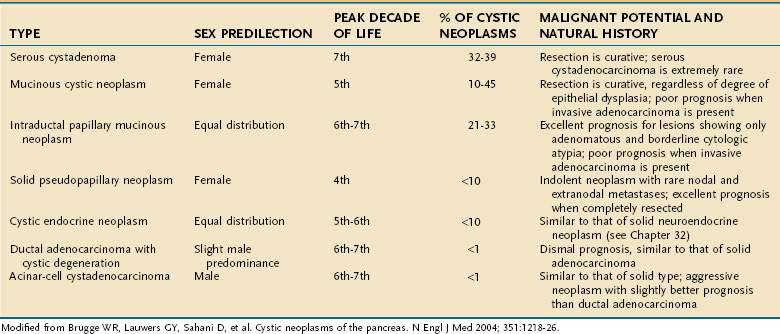
Cystic tumors of the pancreas are relatively uncommon, accounting for less than 10% of pancreatic neoplasms.154 A recent review of 24,000 abdominal CT and MRI studies at one institution during an 8-year period, revealed that pancreatic cysts were present in 1.2% of patients, and 60% of these were likely to be cystic neoplasms.155 MCNs, serous cystadenomas, and IPMNs comprise more than 80% of the primary cystic neoplasms of the pancreas (Table 60-8). Accurate recognition of these lesions is important because of their ability to masquerade as pancreatic pseudocysts, and their high cure rate following surgical treatment. Contemporary trends in pancreatic surgery reveal that cystic neoplasms account for an increasing number of resections.103 A summary of some of the distinguishing features of the most common cystic tumors of the pancreas can be seen in Table 60-8 and is discussed later.
DIFFERENTIAL DIAGNOSIS
Patient evaluation after discovery of a cystic lesion of the pancreas should initially be directed toward exclusion of a pancreatic pseudocyst.154,156 As opposed to cystic neoplasms (see Fig. 59-7), pseudocysts lack an epithelial lining and represent collections of pancreatic secretions that have extravasated from a duct disrupted by inflammation or obstruction (see Chapters 58 and 59). Patients with pseudocysts often have a history of acute or chronic pancreatitis, or abdominal trauma, whereas most of those with cystic tumors lack such antecedent factors. Radiographic characteristics that favor a diagnosis of pseudocyst over cystic neoplasms include lack of septae, loculations, solid components, or cyst wall calcifications on CT or MRI. For pseudocysts, ERCP or MRCP traditionally demonstrates communication between the cyst and the main pancreatic duct. Evaluation of pseudocyst fluid reveals high levels of amylase, which is atypical for cystic tumors.
If a diagnosis of pancreatic pseudocyst can be ruled out, evaluation should subsequently focus on identifying those tumors that require surgical resection due to actual or potential malignancy. As opposed to ductal adenocarcinoma, cystic neoplasms with malignant potential are slow growing, and favorable prognoses have been reported even in the setting of malignant degeneration. Tumors with malignant potential include MCNs, IPMNs, solid pseudopapillary tumors (SPTs), and cystic islet cell tumors. Serous cystadenomas, in contrast, are almost universally benign; they represent approximately one third of all pancreatic cystic neoplasms (see Table 60-8).
The growing popularity of CT and MRI in the evaluation of abdominal complaints has led to increased identification of cystic lesions in the pancreas as incidental findings.155,157 In addition to being asymptomatic, many of these cysts are also very small (<2 cm). Depending on the clinical scenario, it may be prudent to follow some of these patients nonoperatively.155,158 However, malignant cyst transformation is likely in patients older than 70 years, patients with new symptoms, or patients showing cyst growth on serial imaging.155,157,158 Nonoperative management is not adequate in these patients, and they should proceed to pancreatic resection if possible.
DIAGNOSTIC IMAGING
The diagnostic examination of choice is a CT or MRI of the abdomen with intravenous contrast enhancement, which enables tumor localization and, sometimes, discrimination between pseudocysts and cystic neoplasms. ERCP is useful for the diagnosis of IPMNs and allows procurement of biopsy samples by brushings. EUS allows detailed characterization of the cyst wall identifying fine structures such as septa, papilla, or wall nodules (Fig. 60-11). Additionally, FNA of the cyst contents is possible with EUS. Our group and others have demonstrated the value of cyst fluid analysis in the evaluation of cystic neoplasms (Table 60-9).154
Data also suggest that PET can be useful in the evaluation of cystic tumors of the pancreas. This modality detects metabolic activity within pancreatic cysts, which is believed to correlate with the presence or absence of malignancy. In an Italian study of 56 benign and malignant cystic tumors, PET had a sensitivity of 94% and a specificity of 97% in detecting malignant neoplasms.159 Although PET is still not frequently used in the evaluation of pancreatic cystic neoplasms, it may have a role in the nonsurgical follow-up of pancreatic cysts.
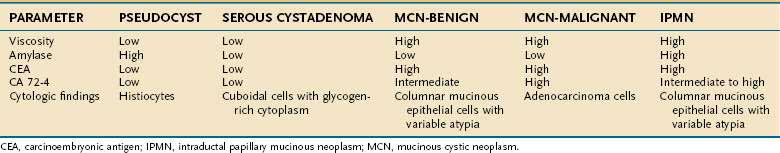
MUCINOUS CYSTIC NEOPLASMS
MCNs are the most frequently encountered cystic tumors of the pancreas, accounting for 10% to 45% of tumors. Most tumors consist of thick-walled cysts with occasional septations that are filled with thick mucous or hemorrhagic material (Fig. 60-12).160 MCNs display a clinical and histologic spectrum ranging from clearly benign to frankly malignant tumors. Current pathologic classification distinguishes between benign, borderline, or malignant (cystadenocarcinoma) tumors based on their maximal degree of dysplasia. This classification scheme correlates with patient prognosis and suggests that all these tumors should be treated as premalignant lesions with eventual evolution to aggressive behavior if left untreated.161
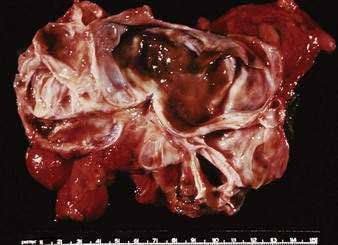
Figure 60-12. Resection specimen of a mucinous cystic neoplasm of the tail of the pancreas. Note the multiloculated cyst.
For many years, MCNs have been misidentified as IPMNs and vice versa. This has led to confusion regarding the true clinical and pathologic features of MCNs. The International Association of Pancreatology put forward guidelines to accurately differentiate an MCN from IPMN.162 These guidelines require the histologic presence of ovarian-type stroma within the tumor to establish the diagnosis of MCN. Using this criterion, several large series of patients with MCN have been published in the English literature and are used herein to describe their clinicopathologic features.163–166
An MCN should be suspected when a CT or MRI of the abdomen shows a cyst within the body or tail of the pancreas in a middle-aged woman. If MRCP is done, there should be no communication between the pancreatic duct and the cyst itself. EUS is indicated when clinical and imaging characteristics deviate from this classic pattern. EUS can identify septations and cyst wall nodules in more detail than MRI or CT, and allows cyst wall biopsy and cyst fluid aspiration for analysis. Cyst fluid analysis generally reveals thick and mucoid material, low fluid amylase, elevated tumor markers (CEA), and mucinous epithelial cells by cytology. These findings may help in differentiating MCN from serous cystadenomas (low fluid CEA), and IPMN (high fluid amylase) (see Table 60-9).
The pathologic classification of tumors in the most recent published series included benign adenomas (72%), borderline neoplasms (10.5%), carcinoma in situ (5.5%), and invasive cancer (12%).163 Similar distribution of tumors can be found in other published series where ovarian stroma was used as a screening criteria. Malignant MCNs were significantly larger than their benign counterparts (80 vs. 45 mm, respectively), and more likely to harbor nodules within their walls. Histologic and mutational analysis suggests that benign tumors can evolve into malignant ones given enough time.29
Five-year survival rates are excellent for benign or borderline MCNs, where the reported cure rate is 100%.161,163,166 Given that MCNs are never multifocal, long-term surveillance is not required for patients with resected noninvasive tumors. For invasive MCNs, five-year survival rates range from 30% to 63% in resected tumors.161,163–166 In our experience, unresectable malignant tumors carry as bad a prognosis as unresected ductal adenocarcinoma. There is no reported experience on the use of adjuvant treatment for MCN.
SEROUS CYSTADENOMAS
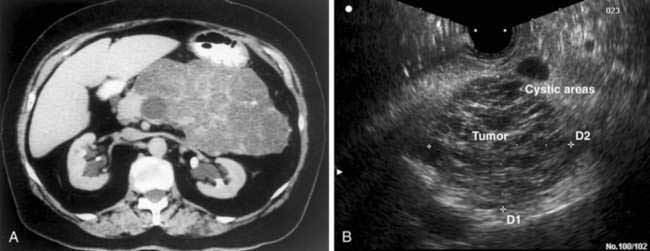
Serous cystadenomas, formerly known as microcystic adenomas, are the second most common cystic tumor of the pancreas.167 The clinical presentation of serous cystadenomas is similar to that of MCNs, occurring mostly in women (75%) with a mean age of 62 years.168 Most (50% to 70%) occur in the body or tail of the pancreas.169 An association with von Hippel-Lindau disease also has been noted. Most patients present with vague abdominal pain or discomfort, but a significant number can present with a palpable mass when the tumor has attained a large size (10 to 25 cm). Increasingly, incidental asymptomatic tumors are being detected during evaluation for other unrelated conditions.
Macroscopically, serous cystadenomas consist of well-circumscribed pancreatic neoplasms that on cross section show numerous tiny cysts separated by delicate fibrous septa, giving them a honeycomb appearance (Fig. 60-13).170 The cysts are filled with clear watery fluid and are often arranged around a central stellate scar that may be calcified. The pathognomonic image by CT scan is that of a spongy mass with a central “sunburst” calcification, but this finding occurs in only 10% of patients (Fig. 60-14A).154 EUS may allow better resolution of the honeycomb structure than CT (see Fig. 60-14B). Macrocystic variants are known that may be indistinguishable from MCNs.171,172 Cyst fluid analysis characteristically reveals low viscosity, low levels of CEA, and negative cytology (see Table 60-9).
Unlike MCNs, serous cystadenomas are considered benign tumors. Rare case reports of serous cystadenocarcinomas exist but constitute less than 3% of known cases.173,174 Surgical resection is the treatment of choice for symptomatic lesions. Options for resection depend on tumor location and include distal pancreatectomy with or without splenectomy, Whipple procedure, middle pancreatectomy, or enucleation.
Serous cystadenomas may be safely observed if asymptomatic. Observation carries the risk of continued growth, which may lead to complications such as hemorrhage, obstructive jaundice, pancreatic insufficiency, or gastric outlet obstruction. Recent data suggest that tumor size at presentation may be used to identify patients who may benefit from surgical resection.168 Tumors larger than 4 cm in maximum diameter at presentation were found to have faster growth rates than their smaller counterparts. These investigators recommend using this measurement to select patients for surgical treatment.
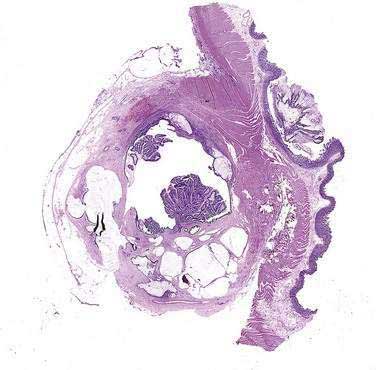
INTRADUCTAL PAPILLARY MUCINOUS NEOPLASMS
Since their initial description in 1982, hundreds of cases of intraductal papillary mucinous neoplasms have been reported in the literature.175 Increased awareness about this disease and its differentiation from chronic pancreatitis, coupled with refinements in diagnostic imaging, have led to an explosion of “new” cases being recognized and reported. A variety of terms have been applied in reference to these tumors and include mucinous ductal ectasia, intraductal mucin-producing tumor, intraductal cystadenoma, pancreatic duct villous adenoma, and intraductal papillary neoplasm. Since 1996, however, both the World Health Organization and the Armed Forces Institute of Pathology have uniformly referred to this entity as IPMNs.24,176
IPMNs represent papillary neoplasms within the main pancreatic duct showing mucin hypersecretion that often leads to duct dilation and chronic obstructive pancreatitis (Fig. 60-15). IPMNs are considered premalignant pancreatic lesions, and histologically their epithelium may demonstrate areas ranging from hyperplasia to carcinoma within a single tumor. Based on their histologic characteristics, IPMNs are generally classified into the following groups: benign (adenoma), borderline, or malignant.176 The malignant group is further subclassified into noninvasive (carcinoma in situ) and invasive carcinoma based on extension beyond the basement membrane. Malignant neoplasms account for 60% of IPMNs in recently published series, and two thirds of these showed invasive carcinoma (Table 60-10). Lymph node metastases are observed in 33% to 51% of invasive tumors.177–179
Clinically, IPMNs occur with equal frequency in men and women, with a median age at diagnosis of about 65 years. Approximately 75% of patients are symptomatic, with abdominal pain and weight loss being the most common complaints. A history of recurrent pancreatitis is given by 20% of patients, and acute pancreatitis is found in about 25% at presentation. No constellation of symptoms allows preoperative identification of the malignant variants of IPMNs. However, patients with malignant neoplasms are more likely to be older and more likely to present with jaundice or new-onset diabetes.177,178
Evaluation of patients with IPMNs includes CT or MRI of the abdomen and MRCP and ERCP. CT often demonstrates dilation of the pancreatic duct with or without an associated cystic mass. Duct dilation is often so impressive that it may mimic MCNs on CT imaging (Fig. 60-16). Evaluation by ERCP typically shows a patulous ampulla of Vater with extruding mucus, a finding that is often pathognomonic for IPMNs. Other findings during pancreatography include main duct dilation, filling defects due to viscid mucus or tumor nodules (Fig. 60-17), and communication between cystic areas and the main pancreatic duct.
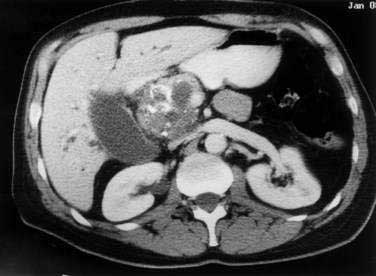
Figure 60-16. Computed tomography scan of an intraductal papillary mucinous neoplasm (IPMN). The IPMN is affecting the head of the pancreas.
Adequate treatment of IPMNs requires pancreatic resection, which successfully relieves symptoms and prevents tumor progression to invasive carcinoma. Simpler procedures such as sphincterotomy may partially treat symptomatology but do not address the malignant potential of these tumors. Pancreaticoduodenectomy is the treatment of choice for most patients given the predominance of IPMNs in the head of the pancreas, but distal pancreatectomy is indicated for lesions in the body or tail of the gland (Table 60-11). Intraoperative frozen sections are necessary to confirm negative resection margins and may mandate extended resection if positive. When IPMNs involve the entire ductal system, total pancreatectomy is the only curative surgical option.
Prognosis after resection of IPMN is excellent, with five-year disease specific survival of 75% or better (Fig. 60-18).177–180 Factors associated with worse outcome include elevated bilirubin, an invasive IPMN, lymph node metastases, and vascular invasion.178 Disease recurrence in the pancreatic remnant is rarely seen after resection of noninvasive IPMNs. Reported recurrences for invasive tumors have ranged from 12% to 65%, most occurring within 3 years of resection.177,178,181 These figures underscore the importance of long-term surveillance and postoperative imaging for those with invasive tumors. Patients with recurrent disease localized to the pancreas can benefit from completion pancreatectomy.177
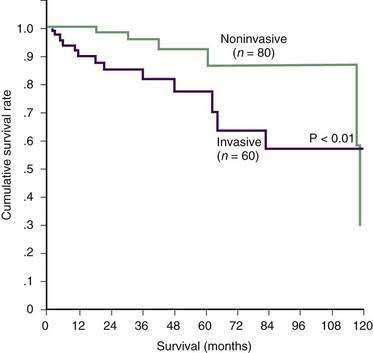
Figure 60-18. Actuarial survival in patients with invasive and noninvasive intraductal papillary mucinous neoplasms.
(From Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: Clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004; 239:678.)
SOLID PSEUDOPAPILLARY TUMORS
SPTs of the pancreas are rare neoplasms first described in 1934. They account for less than 10% of the cystic tumors of the pancreas.154 Although these tumors can occur in both genders, women are more frequently affected than men (10 : 1 ratio). In general, this is a disease of young women in their 30s, with very few cases (5%) reported in adults older than 50 years.182 Several pediatric series can be found in the literature, and these patients (age range 1 to 18 years) account for at least 20% of all known cases (Fig. 60-19).183
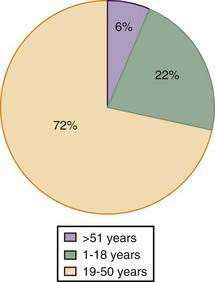
Figure 60-19. Age distribution of patients with solid pseudopapillary tumors.
(From Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: Review of 718 patients reported in English literature. J Am Coll Surg 2005; 200:965.)
The most common clinical presentation is abdominal pain, found in approximately 50% of patients.182 The second most common finding is a large abdominal mass, which is the principal complaint in 35% of patients. At least 15% of tumors are discovered as incidental findings, often during routine physical exams or during evaluation after trauma. As for other cystic lesions of the pancreas, evaluation with CT (or MRI) coupled with biopsy via endoscopic ultrasound usually provides sufficient information to direct clinical management.
Most pseudopapillary tumors are found in the body and tail of the pancreas (60%). The tumors can be quite large at presentation, with 34% of patients having masses larger than 10 cm in diameter. Grossly, small tumors are found to be relatively solid, whereas larger variants show significant cystic degeneration. Microscopically, a mixture of solid, pseudopapillary, and hemorrhagic pseudocystic areas are observed. Uniform neoplastic tumor cells are seen separated by vascular hyalinized stroma.184
Although most SPTs demonstrate benign behavior, these tumors are considered to be lesions of uncertain malignant potential. A large meta-analysis of published patient series worldwide found a 20% frequency of solid-pseudopapillary carcinoma.182 Current criteria for malignancy include perineural invasion, angioinvasion, invasion of adjacent tissues, or metastases.184 The most common site of metastasis is the liver, and metastatic disease is observed in 5% to 10% of patients at presentation.
Despite their female predominance, no association to estrogen receptors has been identified in SPTs. Other common mutated genes in pancreatic carcinogenesis such as K-ras and TP53 are also absent in these tumors. Instead, SPTs show abnormal expression of the E-cadherin protein. Although normal cells show E-cadherin expression within the cell membrane, 100% of SPTs show complete loss of E-cadherin expression in the cells or abnormal localization of E-cadherin to the cell nucleus.185 These findings are not related to mutations in the E-cadherin gene, but rather to abnormal expression of partner molecules such as β-catenin and p120.186 This finding is of diagnostic use, and loss of cell-to-cell adhesion may help to explain the cystic degeneration observed in this neoplasm.
Overall, SPTs are very slow-growing neoplasms. Complete resection is the treatment of choice, and resection of synchronous metastases is also recommended when possible.187 Prognosis is usually excellent with five-year survival around 95%. Prolonged survival has also been reported even in the presence of metastatic disease.182,188 No significant data are available about adjuvant treatment for SPTs.
OTHER NONENDOCRINE PANCREATIC TUMORS
Other nonendocrine tumors of the pancreas include acinar cell carcinomas, lymphomas, and sarcomas. Acinar cell carcinomas are extremely rare, representing 1% to 2% of pancreatic tumors. Clinical presentation may be indistinguishable from pancreatic ductal adenocarcinoma, and some variants of acinar cell carcinomas can show cystic components. A minority of patients (10% to 15%) may present with the lipase hypersecretion syndrome, with high serum lipase levels and peripheral fat necrosis observed clinically.189 The lipase hypersecretion syndrome is often present in the setting of liver metastasis and is considered an unfavourable prognostic factor. Overall, acinar cell carcinomas are considered an aggressive malignancy, and 50% of cases present with liver metastasis. However, prognosis appears to be better than for ductal adenocarcinoma. In a recent study of acinar cell carcinomas using the Surveillance, Epidemiology, and End Results (SEER) database, five-year survival was 22% for patients with unresectable disease and 72% for those with resectable disease.190
Pancreatic lymphoma (see also Chapter 29) represents less than 1% to 2% of all pancreatic malignancies, and less than 1% of all extranodal non-Hodgkin’s lymphomas.191 Whenever a large mass is identified in the pancreas without biliary obstruction, pain, or weight loss, the diagnosis should be contemplated. An elevated serum lactate dehydrogenase level supports a diagnosis of lymphoma. FNA biopsy with flow cytometry is highly accurate in establishing the diagnosis.192 Identification of pancreatic lymphoma is important because treatment is primarily nonsurgical, and prognosis is much better than for pancreatic adenocarcinoma. Treatment usually consists of a combination of chemotherapy and radiation therapy, and cure rates near 30% are reported in the literature.192 A few patients with small tumors, thought to represent carcinomas, have been treated with surgery alone and have had excellent survival.191 Pancreatic endocrine tumors are discussed in Chapter 32.
Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391-8. (Ref 103.)
Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173-80. (Ref 111.)
Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250-6. (Ref 109.)
Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-26. (Ref 154.)
Burris HAIII, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403-13. (Ref 141.)
Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: Lessons from 163 resected patients. Ann Surg. 2008;247:571-9. (Ref 163.)
Ductal adenocarcinoma. In: Hruban RH, Pitman MB, Klimstra DS, editors. AFIP Atlas of Tumor Pathology. Tumors of the Pancreas. Washington, D.C.: American Registry of Pathology; 2007:111-64. (Ref 22.)
Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: A large single-center experience. Gastrointest Endosc. 1999;50:786-91. (Ref 63.)
Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218-49. (Ref 28.)
Jimenez RE, Warshaw AL, Rattner DW, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409-14. (Ref 88.)
Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442-6. (Ref 3.)
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-10. (Ref 134.)
O’Malley ME, Boland GW, Wood BJ, et al. Adenocarcinoma of the head of the pancreas: Determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513-18. (Ref 52.)
Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: Clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-85. (Ref 177.)
Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355-66. (Ref 102.)
1. American Cancer Society. Cancer Facts and Figures 2008. Atlanta: American Cancer Society; 2008.
2. Ries LAG, Eisner MP, Kosary CL. SEER Cancer Statistics Review, 1973-1996. Bethesda, Md: National Cancer Institute; 2000.
3. Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442-6.
4. Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-7.
5. Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-53.
6. Lynch HT, Fusaro RM, Lynch JF, et al. Pancreatic cancer and the FAMMM syndrome. Fam Cancer. 2008;7:103-12.
7. Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342-6.
8. Hruban RH, Petersen GM, Goggins M, et al. Familial pancreatic cancer. Ann Oncol. 1999;10:69-73.
9. Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634-8.
10. DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117:1464-84.
11. Klapman J, Malafa MP. Early detection of pancreatic cancer: Why, who, and how to screen. Cancer Control. 2008;15:280-7.
12. Ahlgren JD. Epidemiology and risk factors in pancreatic cancer. Semin Oncol. 1996;23:241-50.
13. Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: A case-control study based on direct interviews. J Natl Cancer Inst. 1994;86:1510-16.
14. Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156:2255-60.
15. Ghadirian P, Simard A, Baillargeon J. Tobacco, alcohol, and coffee and cancer of the pancreas. A population-based, case-control study in Quebec, Canada. Cancer. 1991;67:2664-70.
16. Duell EJ, Holly EA, Bracci PM, et al. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J Natl Cancer Inst. 2002;94:297-306.
17. Farrow DC, Davis S. Diet and the risk of pancreatic cancer in men. Am J Epidemiol. 1990;132:423-31.
18. Howe GR, Jain M, Miller AB. Dietary factors and risk of pancreatic cancer: Results of a Canadian population-based case-control study. Int J Cancer. 1990;45:604-8.
19. Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: Serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989;49:895-900.
20. Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. Italian Pancreatic Cancer Study Group. N Engl J Med. 1994;331:81-4.
21. Permert J, Larsson J, Westermark GT, et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994;330:313-18.
22. Ductal adenocarcinoma. In: Hruban RH, Pitman MB, Klimstra DS, editors. AFIP Atlas of Tumor Pathology. Tumors of the Pancreas. Washington, D.C.: American Registry of Pathology; 2007:111-64.
23. Kloppel G, Hruban RH, Longnecker DS, et al. Tumours of the exocrine pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000:219-51.
24. Tumors of the exocrine pancreas. In: Solcia E, Capella C, Kloppel G, editors. Tumors of the Pancreas. Washington, D.C.: Armed Forces Institute of Pathology; 1997:31-144.
25. Luttges J, Schemm S, Vogel I, et al. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol. 2000;191:154-61.
26. Hruban RH, Adsay NV, Bores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579-86.
27. Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002-6.
28. Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218-49.
29. Jimenez RE, Warshaw AL, Z’Graggen K, et al. Sequential accumulation of K-ras mutations and p53 overexpression in the progression of pancreatic mucinous cystic neoplasms to malignancy. Ann Surg. 1999;230:501-9.
30. Z’Graggen K, Rivera JA, Compton CC, et al. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491-8.
31. Rivera JA, Rall CJ, Graeme-Cook F, et al. Analysis of K-ras oncogene mutations in chronic pancreatitis with ductal hyperplasia. Surgery. 1997;121:42-9.
32. Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140-3.
33. Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731-7.
34. Goldstein AM, Fraser MC, Struewing JP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970-4.
35. Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731-4.
36. Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350-3.
37. Chiao PJ, Hunt KK, Grau AM, et al. Tumor suppressor gene Smad4/DPC4, its downstream target genes, and regulation of cell cycle. Ann N Y Acad Sci. 1999;880:31-7.
38. Friess H, Berberat P, Schilling M, et al. Pancreatic cancer: The potential clinical relevance of alterations in growth factors and their receptors. J Mol Med. 1996;74:35-42.
39. Van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541-7.
40. Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851-6.
41. Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049-57.
42. Sohn TA, Lillemoe KD, Cameron JL, et al. Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg. 1999;188:658-66.
43. Fasanella KE, Davis B, Lyons J, et al. Pain in chronic pancreatitis and pancreatic cancer. Gastroenterol Clin North Am. 2007;36:335-64. ix
44. Yalniz M, Pour PM. Diabetes mellitus: A risk factor for pancreatic cancer? Langenbecks Arch Surg. 2005;390:66-72.
45. Mujica VR, Barkin JS, Go VL. Acute pancreatitis secondary to pancreatic carcinoma. Study group participants. Pancreas. 2000;21:329-32.
46. Coley SC, Strickland NH, Walker JD, et al. Spiral CT and the pre-operative assessment of pancreatic adenocarcinoma. Clin Radiol. 1997;52:24-30.
47. Freeny PC, Traverso LW, Ryan JA. Diagnosis and staging of pancreatic adenocarcinoma with dynamic computed tomography. Am J Surg. 1993;165:600-6.
48. Megibow AJ, Zhou XH, Rotterdam H, et al. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability—Report of the Radiology Diagnostic Oncology Group. Radiology. 1995;195:327-32.
49. Boland GW, O’Malley ME, Saez M, et al. Pancreatic-phase versus portal vein-phase helical CT of the pancreas: Optimal temporal window for evaluation of pancreatic adenocarcinoma. AJR Am J Roentgenol. 1999;172:605-8.
50. Lu DS, Vedantham S, Krasny RM, et al. Two-phase helical CT for pancreatic tumors: Pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697-701.
51. Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: Criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439-43.
52. O’Malley ME, Boland GW, Wood BJ, et al. Adenocarcinoma of the head of the pancreas: Determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513-18.
53. Raptopoulos V, Steer ML, Sheiman RG, et al. The use of helical CT and CT angiography to predict vascular involvement from pancreatic cancer: Correlation with findings at surgery. AJR Am J Roentgenol. 1997;168:971-7.
54. Sahani DV, Shah ZK, Catalano OA, et al. Radiology of pancreatic adenocarcinoma: Current status of imaging. J Gastroenterol Hepatol. 2008;23:23-33.
55. Glasbrenner B, Ardan M, Boeck W, et al. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999;31:712-17.
56. Levy MJ, Baron TH, Clayton AC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263-73.
57. Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47-55.
58. Povoski SP, Karpeh MSJr, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230:131-42.
59. Sohn TA, Yeo CJ, Cameron JL, et al. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg. 2000;4:258-67.
60. Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7.
61. Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: Prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492-501.
62. DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-63.
63. Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: A large single-center experience. Gastrointest Endosc. 1999;50:786-91.
64. Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459-64.
65. Rosch T, Dittler HJ, Strobel K, et al. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: A blind reevaluation of videotapes. Gastrointest Endosc. 2000;52:469-77.
66. Ichikawa T, Haradome H, Hachiya J, et al. Pancreatic ductal adenocarcinoma: Preoperative assessment with helical CT versus dynamic MR imaging. Radiology. 1997;202:655-62.
67. Lopez HE, Amthauer H, Hosten N, et al. Prospective evaluation of pancreatic tumors: Accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology. 2002;224:34-41.
68. Barish MA, Yucel EK, Ferrucci JT. Magnetic resonance cholangiopancreatography. N Engl J Med. 1999;341:258-64.
69. Adamek HE, Albert J, Breer H, et al. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: A prospective controlled study. Lancet. 2000;356:190-3.
70. Inokuma T, Tamaki N, Torizuka T, et al. Value of fluorine-18-fluorodeoxyglucose and thallium-201 in the detection of pancreatic cancer. J Nucl Med. 1995;36:229-35.
71. Zimny M, Bares R, Fass J, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: A report of 106 cases. Eur J Nucl Med. 1997;24:678-82.
72. Rose DM, Delbeke D, Beauchamp RD, et al. 18-Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surg. 1999;229:729-37.
73. DelMaschio A, Vanzulli A, Sironi S, et al. Pancreatic cancer versus chronic pancreatitis: Diagnosis with CA 19-9 assessment, US, CT, and CT-guided fine-needle biopsy. Radiology. 1991;178:95-9.
74. Parsons LJr, Palmer CH. How accurate is fine-needle biopsy in malignant neoplasia of the pancreas? Arch Surg. 1989;124:681-3.
75. Webb TH, Lillemoe KD, Pitt HA, et al. Pancreatic lymphoma. Is surgery mandatory for diagnosis or treatment? Ann Surg. 1989;209:25-30.
76. Ritts RE, Pitt HA. CA 19-9 in pancreatic cancer. Surg Oncol Clin North Am. 1998;7:93-101.
77. Safi F, Schlosser W, Falkenreck S, et al. CA 19-9 serum course and prognosis of pancreatic cancer. Int J Pancreatol. 1996;20:155-61.
78. Halm U, Schumann T, Schiefke I, et al. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013-16.
79. Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132-8.
80. Exocrine pancreas. In: Greene FL, Page DL, Fleming ID, et al, editors. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002:157-62.
81. Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1-7.
82. Czito BG, Willett CG, Clark JW, et al. Current perspectives on locally advanced pancreatic cancer. Oncology (Huntingt). 2000;14:1535-45.
83. Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705-10.
84. Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295-99.
85. Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-46.
86. Bemelman WA, de Wit LT, van Delden OM, et al. Diagnostic laparoscopy combined with laparoscopic ultrasonography in staging of cancer of the pancreatic head region. Br J Surg. 1995;82:820-4.
87. Fernandez-del Castillo C, Rattner DW, Warshaw AL. Further experience with laparoscopy and peritoneal cytology in the staging of pancreatic cancer. Br J Surg. 1995;82:1127-9.
88. Jimenez RE, Warshaw AL, Rattner DW, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409-14.
89. John TG, Wright A, Allan PL, et al. Laparoscopy with laparoscopic ultrasonography in the TNM staging of pancreatic carcinoma. World J Surg. 1999;23:870-81.
90. Minnard EA, Conlon KC, Hoos A, et al. Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg. 1998;228:182-7.
91. Makary MA, Warshaw AL, Centeno BA, et al. Implications of peritoneal cytology for pancreatic cancer management. Arch Surg. 1998;133:361-5.
92. Merchant NB, Conlon KC, Saigo P, et al. Positive peritoneal cytology predicts unresectability of pancreatic adenocarcinoma. J Am Coll Surg. 1999;188:421-6.
93. Espat NJ, Brennan MF, Conlon KC. Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg. 1999;188:649-55.
94. Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: Margin status and survival duration. J Gastrointest Surg. 2004;8:935-49.
95. Jimenez RE, Warshaw AL. Pancreaticoduodenectomy for pancreatic cancer: Results after Kausch-Whipple and pylorus-preserving resection. In: Beger H, Buchler M, Kozarek RA, et al, editors. The pancreas: An integrated textbook of basic science, medicine and surgery. Oxford, UK: Blackwell Publishing, 2008.
96. ReMine WH, Priestley JT, Judd ES, et al. Total pancreatectomy. Ann Surg. 1970;172:595-604.
97. Brooks JR, Brooks DC, Levine JD. Total pancreatectomy for ductal cell carcinoma of the pancreas. An update. Ann Surg. 1989;209:405-10.
98. van Heerden JA, ReMine WH, Weiland LH, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas. Mayo Clinic experience. Am J Surg. 1981;142:308-11.
99. Andren-Sandberg A, Ihse I. Factors influencing survival after total pancreatectomy in patients with pancreatic cancer. Ann Surg. 1983;198:605-10.
100. Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. Lymphadenectomy study group. Ann Surg. 1998;228:508-17.
101. Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: Comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613-22.
102. Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355-66.
103. Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391-8.
104. Buchler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883-9.
105. Kazanjian KK, Hines OJ, Eibl G, et al. Management of pancreatic fistulas after pancreaticoduodenectomy: Results in 437 consecutive patients. Arch Surg. 2005;140:849-54.
106. Mullen JT, Lee JH, Gomez HF, et al. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg. 2005;9:1094-104.
107. Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: A 20-year experience in 516 patients. Arch Surg. 2004;139:718-25.
108. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-79.
109. Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250-6.
110. Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178-83.
111. Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173-80.
112. Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68-72.
113. Benassai G, Mastrorilli M, Quarto G, et al. Survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Chir Ital. 2000;52:263-70.
114. Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618-23.
115. Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-9.
116. Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: Analysis of 5-year survivors. Ann Surg. 1998;227:821-31.
117. Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549-58.
118. Meyer W, Jurowich C, Reichel M, et al. Pathomorphological and histological prognostic factors in curatively resected ductal adenocarcinoma of the pancreas. Surg Today. 2000;30:582-7.
119. Shepherd HA, Royle G, Ross AP, et al. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: A randomized trial. Br J Surg. 1988;75:1166-8.
120. Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344:1655-60.
121. Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-82.
122. Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: A prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-95.
123. Lillemoe KD, Cameron JL, Hardacre JM, et al. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322-8.
124. Van Heek NT, de Castro SM, van Eijck CH, et al. The need for a prophylactic gastrojejunostomy for unresectable periampullary cancer: A prospective randomized multicenter trial with special focus on assessment of quality of life. Ann Surg. 2003;238:894-902.
125. Graber I, Dumas R, Filoche B, et al. The efficacy and safety of duodenal stenting: A prospective multicenter study. Endoscopy. 2007;39:784-7.
126. Maire F, Hammel P, Ponsot P, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735-42.
127. Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217:447-55.
128. Polati E, Finco G, Gottin L, et al. Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 1998;85:199-201.
129. Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899-903.
130. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987;59:2006-10.
131. Bakkevold KE, Arnesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater—Results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A:698-703.
132. Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776-82.
133. Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576-85.
134. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-10.
135. Small WJr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: A multicenter phase II trial. J Clin Oncol. 2008;26:942-7.
136. Ahlgren JD. Chemotherapy for pancreatic carcinoma. Cancer. 1996;78:654-63.
137. Phase II studies of drug combinations in advanced pancreatic carcinoma: Fluorouracil plus doxorubicin plus mitomycin C and two regimens of streptozotocin plus mitomycin C plus fluorouracil. The Gastrointestinal Tumor Study Group. J Clin Oncol. 1986;4:1794-8.
138. Cullinan S, Moertel CG, Wieand HS, et al. A phase III trial on the therapy of advanced pancreatic carcinoma. Evaluations of the Mallinson regimen and combined 5-fluorouracil, doxorubicin, and cisplatin. Cancer. 1990;65:2207-12.
139. Cullinan SA, Moertel CG, Fleming TR, et al. A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric carcinoma. Fluorouracil vs fluorouracil and doxorubicin vs fluorouracil, doxorubicin, and mitomycin. JAMA. 1985;253:2061-7.
140. Oster MW, Gray R, Panasci L, et al. Chemotherapy for advanced pancreatic cancer. A comparison of 5-fluorouracil, adriamycin, and mitomycin (FAM) with 5-fluorouracil, streptozotocin, and mitomycin (FSM). Cancer. 1986;57:29-33.
141. Burris HAIII, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403-13.
142. Rothenberg ML, Moore MJ, Cripps MC, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347-53.
143. Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol. 2006;24:4441-7.
144. Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-5.
145. Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946-52.
146. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-16.
147. Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639-45.
148. Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776-83.
149. Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430-8.
150. Bramhall SR, Schulz J, Nemunaitis J, et al. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-7.
151. Jimenez RE, Hartwig W, Antoniu BA, et al. Effect of matrix metalloproteinase inhibition on pancreatic cancer invasion and metastasis: An additive strategy for cancer control. Ann Surg. 2000;231:644-54.
152. Moore MJ, Hamm J, Dancey J, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-302.
153. Bramhall SR, Rosemurgy A, Brown PD, et al. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: A randomized trial. J Clin Oncol. 2001;19:3447-55.
154. Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-26.
155. Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: Observe or operate. Ann Surg. 2004;239:651-7.
156. Martin I, Hammond P, Scott J, et al. Cystic tumours of the pancreas. Br J Surg. 1998;85:1484-6.
157. Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: Clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-3.
158. Allen PJ, Jaques DP, D’Angelica M, et al. Cystic lesions of the pancreas: Selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7:970-7.
159. Sperti C, Pasquali C, Chierichetti F, et al. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann Surg. 2001;234:675-80.
160. Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573-80.
161. Wilentz RE, Bores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320-7.
162. Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32.
163. Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: Lessons from 163 resected patients. Ann Surg. 2008;247:571-9.
164. Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: Demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026-31.
165. Thompson LD, Becker RC, Przygodzki RM, et al. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: A clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1-16.
166. Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: Clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410-22.
167. Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): A clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69:289-98.
168. Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: Tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413-19.
169. Pyke CM, van Heerden JA, Colby TV, et al. The spectrum of serous cystadenoma of the pancreas. Clinical, pathologic, and surgical aspects. Ann Surg. 1992;215:132-9.
170. Albores-Saavedra J, Gould EW, Angeles-Angeles A, et al. Cystic tumors of the pancreas. Pathol Ann. 1990;25(Pt 2):19-50.
171. Lewandrowski K, Warshaw A, Compton C. Macrocystic serous cystadenoma of the pancreas: A morphologic variant differing from microcystic adenoma. Hum Pathol. 1992;23:871-5.
172. Lee SE, Kwon Y, Jang JY, et al. The morphological classification of a serous cystic tumor (SCT) of the pancreas and evaluation of the preoperative diagnostic accuracy of computed tomography. Ann Surg Oncol. 2008;15:2089-95.
173. Abe H, Kubota K, Mori M, et al. Serous cystadenoma of the pancreas with invasive growth: Benign or malignant? Am J Gastroenterol. 1998;93:1963-6.
174. Strobel O, Z’Graggen K, Schmitz-Winnenthal FH, et al. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion. 2003;68:24-33.
175. Ohashi K, Murakami Y, Maruyama M. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348-51.
176. Longnecker DS, Adler G, Hruban RH, et al. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press; 2000:237-40.
177. Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: Clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678-85.
178. D’Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: An analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400-8.
179. Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: An updated experience. Ann Surg. 2004;239:788-97.
180. Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261-5.
181. Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500-7.
182. Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: Review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-72.
183. Choi SH, Kim SM, Oh JT, et al. Solid pseudopapillary tumor of the pancreas: A multicenter study of 23 pediatric cases. J Pediatr Surg. 2006;41:1992-5.
184. Kloppel G, Luttges J, Klimstra D, et al. Solid-pseudopapillary neoplasm. In: Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press; 2000:246-8.
185. Serra S, Salahshor S, Fagih M, et al. Nuclear expression of E-cadherin in solid pseudopapillary tumors of the pancreas. J Pancreas. 2007;8:296-303.
186. Chetty R, Jain D, Serra S. p120 catenin reduction and cytoplasmic relocalization leads to dysregulation of E-cadherin in solid pseudopapillary tumors of the pancreas. Am J Clin Pathol. 2008;130:71-6.
187. Martin RC, Klimstra DS, Brennan MF, et al. Solid-pseudopapillary tumor of the pancreas: A surgical enigma? Ann Surg Oncol. 2002;9:35-40.
188. Tipton SG, Smyrk TC, Sarr MG, et al. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733-7.
189. Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006;12:3180-5.
190. Wisnoski NC, Townsend CMJr, Nealon WH, et al. 672 patients with acinar cell carcinoma of the pancreas: A population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141-8.
191. Koniaris LG, Lillemoe KD, Yeo CJ, et al. Is there a role for surgical resection in the treatment of early-stage pancreatic lymphoma? J Am Coll Surg. 2000;190:319-30.
192. Saif MW. Primary pancreatic lymphomas. JOP. 2006;7:262-73.