CHAPTER 147 Tumors of the Orbit
A variety of lesions can affect the orbit (Table 147-1). These lesions have variable clinical manifestations, operative indications, and treatment options, all of which are determined by the pathology. Orbital tumors can occur in all areas of the orbit, and surgical approaches must be available to provide 360 degrees of access.
TABLE 147-1 Incidence of Orbital Tumors and Pseudotumors at the Senior Author’s Institution over a 12-Year Period
| Spheno-orbital meningioma | 135 |
| Hemangioma | 101 |
| Optic nerve/orbital meningioma | 60 |
| Neurofibroma | 39 |
| Optic nerve glioma | 39 |
| Lymphangioma | 36 |
| Dermoid | 28 |
| Benign mixed lacrimal gland | 21 |
| Hemangiopericytoma | 20 |
| Rhabdomyosarcoma | 12 |
| Adenoid cystic carcinoma | 10 |
| Arteriovenous malformations | 8 |
| Fibrous histiocytoma | 7 |
| Venous varices | 7 |
| Cholesteatoma | 3 |
| Metastatic tumor | 185 |
| Nonspecific orbital inflammation | 180 |
| TOTAL | 891 |
Clinical Manifestations
Tumors that involve the orbit can be classified into two major groups: primary tumors of the orbit and tumors with other sites of origin that extend into the orbit. The most frequent primary orbital tumors in adults include lymphoid tumors, cavernous hemangiomas, and meningiomas, whereas dermoid cysts, capillary hemangiomas, and rhabdomyosarcoma predominate in children (see Table 147-1).1 The most common tumors that extend into the orbit are meningiomas, followed by sinonasal carcinomas. The most frequent initial symptom of an orbital mass is proptosis, which occurs in 44% of patients. Change in visual acuity is often a late finding or indicates a tumor that is close to the orbital apex or optic nerve.1
Orbital tumors can also be divided into three categories based on their location within the orbit: (1) intraconal (within the extraocular muscle cone), (2) extraconal, and (3) intracanalicular (within the optic canal), with differing features based on these locations. Intraconal tumors tend to cause early vision loss and impairment of ocular motility, as well as axial proptosis. These effects result from direct pressure on the optic nerve and impingement on extraocular muscles. Extraconal tumors cause proptosis as an early manifestation. Visual impairment occurs late as a result of tumor involvement of the optic nerve or the individual muscles and deformity of the globe itself. Finally, intracanalicular tumors cause early vision loss, papilledema, and the appearance of optociliary shunt vessels on the surface of the optic discs. These tumors cause minimal or no proptosis.2
Surgical Anatomy
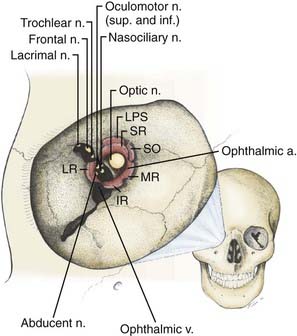
The orbit is a cone-shaped structure with an approximate volume of 30 cm3. The base of the cone is quadrangular, with its widest dimension just posterior to the orbital rim. The apex is formed by the optic canal (containing the optic nerve and ophthalmic artery) and superior orbital fissure (gateway for the superior and inferior divisions of the oculomotor nerve, the trochlear nerve, branches of V1, the abducens nerve, the superior ophthalmic vein, and sympathetic fibers from the cavernous sinus) (Fig. 147-1). The orbit is composed of seven different bones. Its roof is part of the frontal bone, and the floor consists of parts of the maxilla and zygoma. Its lateral wall is formed by the zygoma and greater sphenoid wing, and the medial wall has contributions from the maxilla, lacrimal bone, and ethmoid bone. Medially, the sphenoid and ethmoid sinuses border the inner aspect of the cone, as well as the optic canal.
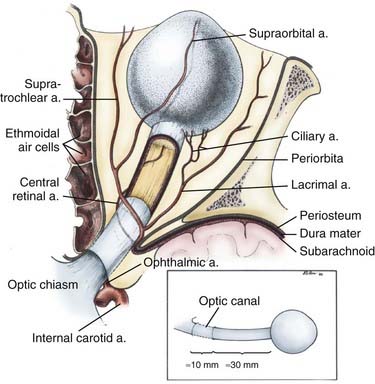
The ophthalmic artery, which provides the major blood supply to the optic nerve, arises from the supraclinoid internal carotid artery (ICA) and passes laterally and inferior to the optic nerve in the optic canal (Fig. 147-2). It then assumes a more medial position as it enters the orbit. Understanding of the course of the ophthalmic artery is critical whenever the orbital apex is approached surgically. It gives rise to a major intraneural branch, the central retinal artery, 8 to 15 mm posterior to the globe, which penetrates the medial midportion of the nerve and provides the sole blood supply to the retina. The intraorbital optic nerve is supplied by a pial plexus derived from the ciliary arteries. The intracranial and intracanalicular portions of the optic nerve are supplied by fine perforators arising from the ICA and superior hypophysial artery. The superior and inferior ophthalmic veins provide the major drainage for the orbit. The superior ophthalmic vein passes over the lateral rectus muscle and through the superior orbital fissure before entering the cavernous sinus. The inferior ophthalmic vein forms from venous channels in the floor and medial wall of the orbit before anastomosing with the superior ophthalmic vein and pterygoid plexus.
Surgical Approaches (Lateral and Medial Corridors)
Endoscopic assistance through standard external approaches was used to improve visualization as early as the 1980s.3 Endoscopic endonasal orbital and optic nerve decompression has become accepted treatment of thyroid eye disease and traumatic optic neuropathy unresponsive to steroids.4–9 Sporadic cases of endoscopic decompression, biopsy, and resection of tumors that involve the orbit have been reported.10–19 The EEA has been extended to permit resection of all types of skull base tumors, including posterior, middle, and anterior fossa intradural tumors.20,21 EEAs provide excellent access for intraconal and extraconal tumors that are medial and inferior to the optic nerve and can be applied to any median intracranial extension, provided that key neurovascular structures (such as the optic nerve) remain lateral to the tumor. In addition, EEAs provide access to most of the orbit, from the posterior globe to the orbital apex.
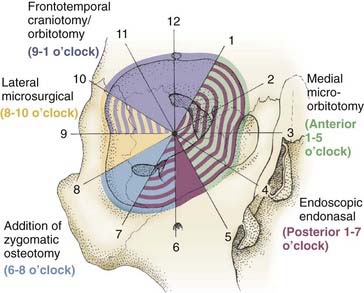
Combined, the aforementioned approaches provide 360 degrees of access to the entire orbit, with selection of the approach guided primarily by avoidance of crossing the optic nerve (Fig. 147-3).
Lateral Corridors
External Approaches
Fronto-orbital Temporal and Pterional Transcranial Approaches
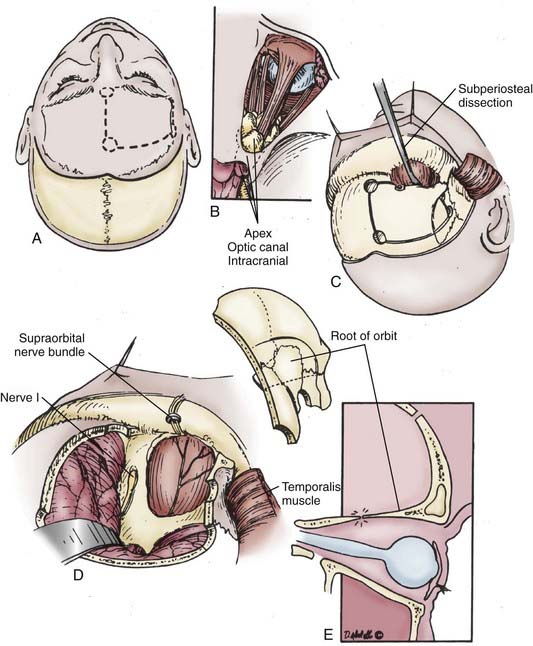
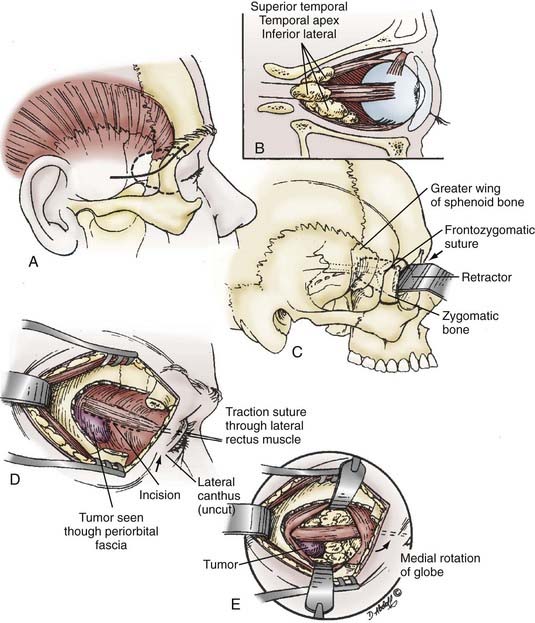
The frontotemporal incisions and scalp flap elevation are designed according to the particular need for orbital access. A standard pterional incision (curvilinear, just anterior to the tragus to the midline apex of the anterior hairline) is usually adequate to access the superolateral orbit. However, to extend the access medially to the superior orbit or inferiorly/laterally, the incision should be at least a modified bicoronal one extending from the tragus on the side ipsilateral to the pathology to the contralateral superior temporal line or even to the contralateral tragus (Fig. 147-4). An imaginary line drawn between one end of the incision and the other should cross the orbital region of interest. The entire scalp flap should be elevated in a subperiosteal plane over the frontoparietal area and the superficial layer of the deep temporal fascia. We incise this fascia, which is continuous with the periosteum of the orbitozygomatic complex, by following an imaginary line from the superior orbital rim to the root of the zygoma. Elevation of the scalp/facial flap continues deep relative to this plane (i.e., subperiosteally) to protect the frontal branches of the facial nerve. Elevation with inferior retraction of the temporalis muscle exposes the lateral orbit. The muscle can easily be dissected from the underlying squamosal bone by starting the dissection at the root of the zygoma after making a posterior incision in the muscle and dissecting inferiorly to superiorly in the direction of the fascial attachments rather than against them as is traditionally done. This provides a much cleaner dissection and may help preserve some of the microvascular supply to the muscle, thus decreasing atrophy. If any part of the zygoma will be removed for access, the corresponding attachment (origin) of the masseter muscle is transected. Its fascia is incised and elevated from the muscle (parotid-masseteric fascia). Dissection of the muscle is then completed by electrocautery while using a malleable retractor to protect the temporalis muscle, which lies deep relative to the masseter.
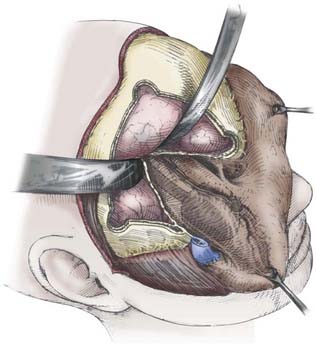
Next, a standard frontotemporal craniotomy is performed. The lateral bone of the greater wing of the sphenoid is then dissected free from the dura and removed with rongeurs and a high-speed drill until flush with the orbit. Bone removal should stop at the depth of the orbitomeningeal artery to prevent inadvertent injury to the contents of the superior orbital fissure. The orbitomeningeal artery is an important landmark marking the “tip of the iceberg,” with the superior orbital fissure lying beneath. At this point the frontal dura can be dissected free from the roof of the orbit so that the orbital bone is freed from dura on one side and periorbita on the other before performing the orbital osteotomies. We prefer to use a reciprocating saw for the orbital osteotomies while protecting the brain and orbit with malleable retractors (“brain ribbons”). The saw is inserted into the orbit and the cut is made from the orbit toward the frontal dura. The medial cut is usually at or just lateral to the supraorbital notch. The lateral cut is made by inserting the tip of the reciprocating saw into the inferior orbital fissure and completing an osteotomy from within the orbit at a level just above or through the zygomatic prominence, as needed. The final posterior osteotomy is completed with a small drill bit from the brain side while protecting the orbit with a ribbon. This cut is made from the posterior aspect of the medial osteotomy across the roof of the orbit, through the remaining sphenoid wing, and connected laterally to the lateral osteotomy in the inferior orbital fissure. These osteotomies should be extended as posterior as possible to prevent loss of orbital bone, which would require reconstruction to prevent enophthalmos. At this point the orbitozygomatic complex can be freed from any remaining soft tissue attachment and removed to provide access to the entire superolateral orbit, as well as the regional frontotemporal dura and related brain region, if needed (Fig. 147-5). It is important that the posterior portion of the superior orbit be removed adequately because this bone can prevent adequate dural retraction and defeat any advantage of the superior orbitotomy.
This approach is ideal for tumors such as meningiomas with both intracranial and orbital components (see Fig. 147-3). If intracranial access is desired, after opening the frontotemporal dura, multiple retraction stitches can be applied across the internal surface of the dura to retract the orbit inferiorly. If the middle cranial fossa dura is involved or there is significant superior extension, the addition of zygomatic osteotomies can be advantageous. This allows more aggressive removal of subtemporal bone and access to the basal foramina and decreases brain retraction when addressing superiorly extending lesions by allowing a more inferior to superior angle of dissection. We prefer to remove the orbit and zygoma as one piece with a straight osteotomy through the main portion of the zygoma and a diagonal cut flush with its posterior attachment to the temporal bone (root of the zygoma). A variant of the approach just described is a “one-piece cranio-orbitozygomatic approach” wherein the craniotomy and orbitozygotomy are done as one piece; it is reported to potentially improve cosmetic outcomes. We have not found cosmesis to be an issue with the “two-piece” approach, however, because defects are easily reconstructed with titanium mesh and plates.
Lateral Microsurgical Approach
The lateral microsurgical approach can be used for tumors located in the superior temporal or inferior/lateral compartments of the orbit or the orbital apex (see Fig. 147-3). This approach is generally restricted to lesions lacking intracranial extension. Tumor located deeper in the apex necessitates more extensive removal of the greater wing of the sphenoid and may require one of the combined approaches described earlier. The skin incision is 3 to 4 cm in length, extends from the lateral aspect of the eyebrow, and curves posteriorly in a line in the temple that could be covered by eyeglasses (Fig. 147-6). A vertical incision is made in the most anterior lateral orbital rim periosteum, and subperiosteal dissection from the inner wall of the orbit is performed similar to that described previously, with the periorbita dissected posteriorly. The temporalis muscle can now be dissected posteriorly in a subperiosteal plane to expose the external lateral orbital bone.
Medial Corridors
External Approaches
Anterior Medial Micro-orbitotomy Approach
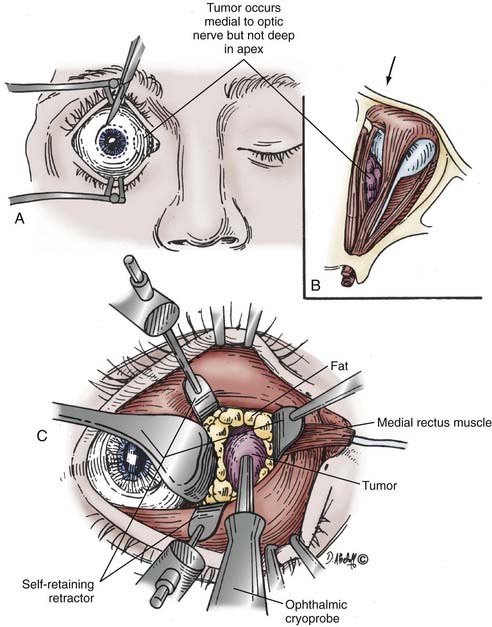
Tumors located anteriorly and medially in the orbit can be accessed by a medial micro-orbitotomy approach. Ophthalmic preparation of the orbit and surrounding area is performed, and a small eyelid retractor is put in place. A conjunctival peritomy is performed 90 degrees around the cornea (Fig. 147-7). The medial rectus muscle is isolated with a double-armed suture at its insertion site into the globe after relaxing conjunctival incisions are made superior and inferior to the muscle. The muscle is then freed from its intermuscular septa and check ligaments distally and severed from the insertion site. The lid retractor is removed and a medial orbital self-retaining retractor inserted. The retractor has an enucleation spoon that is placed medially on the globe for medial-to-lateral retraction (see Fig. 147-7). The orbital fat is dissected while viewed with the operating microscope, aided by more self-retaining retractors. Cottonoids or cotton-tipped applicators are used for retraction of orbital fat. Performing a lateral orbitotomy (as described previously) can provide even more room for lateral globe displacement while avoiding compression of the orbital contents.
Endonasal Approaches
EEAs provide a novel access to the orbit while limiting morbidity. The main advantage of any EEA is its anterior and medial trajectory, which is ideal for skull base lesions that are anterior and medial to critical neurovascular structures (e.g., pituitary tumors relative to the optic chiasm and nerves) because it reduces manipulation of these structures (except when they are already encased in tumor). This advantage holds true for orbital tumors in the medial and inferior half of the orbit (see Fig. 147-3). The key anatomic landmark is the optic nerve. Tumors that displace the optic nerve superiorly and laterally are usually excellent targets for an endonasal approach.
Medial-Inferior Extraconal Approach
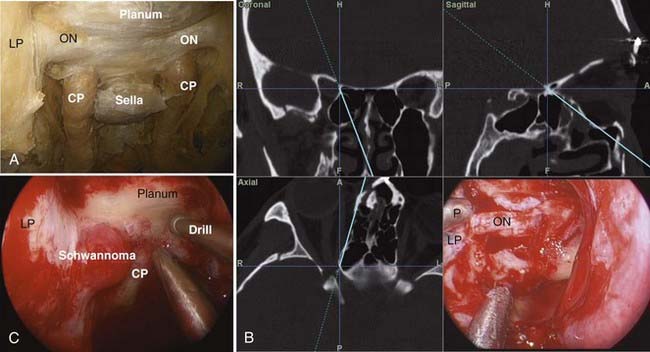
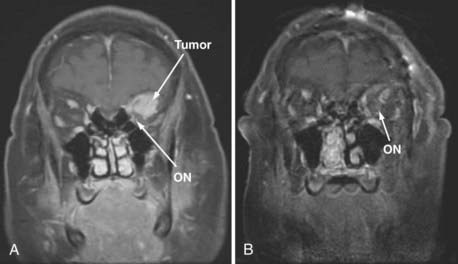
A 0-degree endoscope is used for most of the operation. The sinus phase includes a complete uncinectomy, wide maxillary antrostomy, anterior and posterior ethmoidectomies, and sphenoidotomy to expose the medial and inferior orbital walls. The extent of this phase can be limited, depending on the access needed, to preserve olfaction. The sphenoid sinus is opened from the natural os and extended medially, laterally, and superiorly, as needed, for visualization of the bony landmarks overlying the ICA and optic nerve, the planum and sella (Fig. 147-8). Next, the lamina papyracea is removed in an anterior-to-posterior direction. At this point the periorbita, optic nerve, and carotid artery are well defined (Fig. 147-8). The key anatomic landmarks in this region are the lateral and medial opticocarotid recesses. The lateral opticocarotid recess represents the pneumatization of the optic strut and provides access to the superior orbital fissure. The medial opticocarotid recess is perhaps more critical because it represents the intersection of the optic nerve and ICA at the opticocarotid cistern. This can be valuable for identifying the nerve posteromedially and isolating the optic sheath, which can then be followed anteriorly into the orbit.
For the medial-inferior extraconal technique, a single-nostril approach with preservation of the middle turbinate can often be used, especially when addressing smaller tumors. However, when dissection of the orbital apex or intraconal work is needed, a two-nostril approach provides more flexibility for dissection and manipulation of instruments. During a two-nostril approach, the middle turbinate ipsilateral to the pathology is removed, the posterior nasal septum is resected, and the sphenoid sinus rostrum is opened widely. This transforms the posterior sinonasal cavities into one single space for ease of dissection. It should be noted that if a two-nostril approach is planned and a nasoseptal flap22 is needed for reconstruction (recommended for intradural work and cases involving exposure of the carotid artery), this flap must be raised before transecting the posterior septum or the vascular pedicle will be lost.
If a tumor such as a tuberculum or planum meningioma has extension along the medial optic nerve, the entire tumor can be addressed endonasally with a transplanum approach combined with orbital apex decompression and tumor resection from the canal. It is common with these tumors for the optic/orbital extension to be medial to the optic nerve because their origin is medial. Traditional transcranial approaches require transection of the falciform ligament and retraction of or blind dissection behind an often already tenuous ipsilateral optic nerve. This carries a well-documented risk of vision loss. Such retraction is obviated with an endonasal approach, and early series using this approach show potential for improved outcomes.23–30
Transmaxillary Extraconal Approach
The transmaxillary extraconal approach involves a sinonasal exposure similar to that described for the inferior-medial extraconal approach. In addition, a medial maxillectomy is extended anteriorly, and a portion of the piriform aperture and anterior maxillary sinus wall is removed. This allows more lateral extension inferiorly. The pterygopalatine fossa may be dissected and the branches of the trigeminal nerve identified to avoid inadvertent injury. Once identified, V2 can be traced back to the foramen rotundum and the infraorbital nerve followed into the infraorbital foramen. This provides immediate access to the inferior rectus and orbital contents above. It is very important to preserve the ophthalmic branch (V1) of the trigeminal nerve during the dissection because the vidian nerve is likely to be damaged during the trans–pterygopalatine fossa approach, especially if the vidian canal is used as a guide to the petrous ICA.31 The decreased lacrimation associated with sacrifice of the vidian artery combined with an insensate cornea has the potential for significant corneal morbidity.
Medial Intraconal Approach
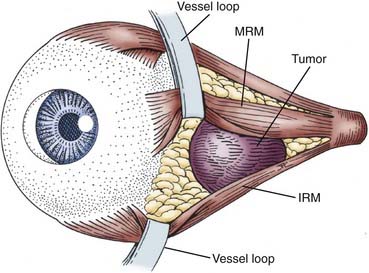
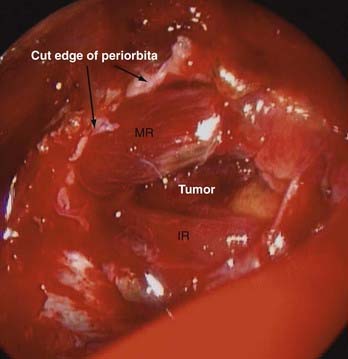
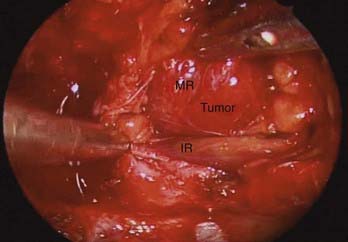
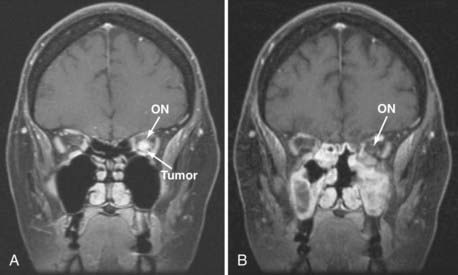
Adding an oculoplastic surgeon to the EEA team has allowed us to develop a technique that includes retraction of the rectus muscle for intraconal access. From an external approach, the medial and inferior rectus muscles are identified at their insertion on the globe and controlled with vessel loops (Fig. 147-9). This allows passive movement of the muscles, which aids in endoscopic identification of them. It also allows retraction of the muscles, which widens the space between them and makes them easier to dissect (Fig. 147-10). Once this intraconal corridor is developed, the tumor can be identified and removed with limited bipolar cauterization and extensive sharp dissection (Fig. 147-11). A cotton-tipped applicator held by one surgeon provides excellent retraction of orbital fat. Again, standard microsurgical techniques are applied. Internal tumor debulking becomes even more important because the tumor mass itself can obscure visualization of the dissection margins. It is helpful to have one surgeon retract the mass inferiorly and medially while the other uses bimanual dissection to clear the lateral tumor margin. It is critical that the same microsurgical techniques used for open approaches be strictly adhered to when operating endonasally. Because the optic nerve is almost always lateral and superior to the tumor, once the lateral margin is cleared, the nerve is out of the field of dissection. As the dissection approaches the orbital apex, special care must be taken to avoid injury to the ophthalmic and central retinal arteries, which assume a medial trajectory with respect to the optic nerve within the orbit. The remaining soft tissue tumor attachments can be cut and the tumor removed en bloc.
Reconstruction can consist of the application of fibrin glue, acellular dermis, abdominal free fat graft, mucosal grafts, or (in most cases) a nasoseptal flap. Given the extensive dissection required for intraconal tumors and the possibility of rectus muscle scarring in the sinus with resultant diplopia, we often reconstruct the medial orbital wall with a pedicled nasoseptal mucoperichondrial flap.22 This flap is raised at the beginning of the procedure and pedicled on the posterior nasoseptal artery. It is placed in the nasopharynx during resection of the orbital tumor. Once the tumor is removed, the rectus muscle vessel loops are released, and extraocular movements are tested. The rectus muscles are placed back into their native positions, and orbital fat is pulled over them to prevent scarring. When a septal flap is used, it is placed over the defect, and Surgicel and a fibrin sealant are used to hold it in position during initial healing. No packing is used because of the potential for increasing intraocular pressure by putting pressure on a large orbital defect. We have not had a problem with enophthalmos when using the nasal septal flap for endonasal reconstruction of large medial orbital defects, and we believe that this flap recapitulates the periorbital fascia nicely.
Complications and Their Management
Through our first 37 EEAs for orbital tumors, we have seen no cases of optic nerve injury or permanent worsening of diplopia (Table 147-2). However, this technique is evolving, and complication rates have yet to be well defined.
TABLE 147-2 Endoscopic Endonasal Approach for Resection of Orbital Tumors at the Authors’ Institution
| Meningioma—orbital | 9 |
| Squamous cell carcinoma | 4 |
| Fibrous dysplasia | 3 |
| Trauma | 3 |
| Meningioma—anterior base, extending to the orbit | 2 |
| Hemangioma | 2 |
| Adenoid cystic carcinoma | 2 |
| Schwannoma | 2 |
| Inverted papilloma | 2 |
| Osteoma | 2 |
| Glioma | 1 |
| Rhabdomyosarcoma | 1 |
| Sinonasal undifferentiated carcinoma | 1 |
| Mucocele | 1 |
| Ossifying fibroma | 1 |
| Osteosarcoma | 1 |
| TOTAL | 37 |
Choice of Approach
Tumor location rather than tumor type determines the type of approach selected (Figs. 147-12 and 147-13), as evidenced by the similarity among the pathologies resected via standard approaches (see Table 147-1) and endonasal approaches (see Table 147-2).
Selection of approaches is based on the meridian of the optic nerve (a vertical line drawn perpendicular to the optic nerve) (see Fig. 147-3). Lesions that are located primarily lateral (8 to 10 o’clock, Fig. 147-3) to the optic nerve are approached via a lateral orbitotomy (lateral microsurgical approach) corridor. If the lesion has an inferior lateral extension, a zygomatic osteotomy can be added (6 to 8 o’clock, Fig. 147-3). If the lesion has superior lateral (9 to 1 o’clock, Fig. 147-3) or intracranial extension, one of the transcranial approaches is used. Lesions located medial to the optic nerve need to have their anterior/posterior extension considered. Medial lesions located in the anterior orbit (1 to 5 o’clock, Fig. 147-3) can be accessed via the anterior medial micro-orbitotomy approach. However, medial lesions that extend posteriorly are more challenging and are ideally suited for an EEA (1 to 7 o’clock, Fig. 147-3). In the end, the approaches should not be considered in isolation but often need to be combined to provide 360-degree access to the entire intraconal and extraconal orbit.
Disadvantages of the endonasal approach include the added morbidity of endonasal dissection and potential sequelae related to sinus dysfunction. The endonasal approach requires two experienced endoscopic surgeons who are both familiar with the orbital anatomy, and for intraconal lesions, most cases even require three surgeons: two endoscopic surgeons and an ophthalmologist for external control of the extraocular muscles (see Fig. 147-4A). This approach also requires specialized endoscopic instrumentation and angled dissectors. Finally, controlling the rectus muscles during endonasal surgery is currently evolving, and effective and uncomplicated muscle retractors are currently not available. As endoscopic endonasal technology and instrumentation advance, the ability to approach orbital pathology with less morbidity will also continue to advance.
Couldwell WT, Weiss MH, Rabb C, et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539-550.
de Divitiis E, Cavallo LM, Cappabianca P, et al. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors. Part 2. Neurosurgery. 2007;60:46-58.
Dusick JR, Esposito F, Kelly DF, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832-841.
Dutton JJ. Optic nerve sheath meningioma. Surg Ophthalmol. 1992;37:167-183.
Gardner PA, Kassam AB, Thomas A, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63:36-52.
Hadad G, Bassagasterguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882-1886.
Har-El G. Combined endoscopic transmaxillary-transnasal approach to the pterygoid region, lateral sphenoid sinus, and retrobulbar orbit. Ann Otol Rhinol Laryngol. 2005;114:439-442.
Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4.
Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3.
Kassam AB, Vescan AD, Carrau RL, et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108:177-183.
Kennerdell JS, Amsbaugh GA, Myers EN. Transantral-ethmoidal decompression of optic canal fracture. Arch Ophthalmol. 1976;94:1040-1043.
Kitano M, Taneda M. Extended transsphenoidal approach with submucosal posterior ethmoidectomy for parasellar tumors. Technical note. J Neurosurg. 2001;94:999-1004.
Lund VJ, Rose GE. Endoscopic transnasal orbital decompression for visual failure due to sphenoid wing meningioma. Eye. 2006;20:1213-1219.
McMains KC, Kountakis SE. Contemporary diagnosis and approaches toward optic nerve decompression. Oper Tech Otolaryngol. 2006;17:178-183.
Sethi DS, Lau DP. Endoscopic management of orbital apex lesions. Am J Rhinol. 1997;11:449-455.
1 Mercandetti M. Tumors, Orbital. eMedicine Chapter. Last Updated 2/7/2007 http://www.emedicine.com/oph/topic758.htm
2 Dutton JJ. Optic nerve sheath meningioma. Surg Ophthalmol. 1992;37:167-183.
3 Norris JL, Cleasby GW. Endoscopic orbital surgery. Am J Ophthalmol. 1981;91:249-252.
4 Takahashi R. Exposure of the optic canal. Operation. 1951;5:300-302.
5 Niho S, Yasuda K, Sato T, et al. Decompression of the optic canal by the transethmoidal route. Am J Ophthalmol. 1961;51:659-665.
6 Kennerdell JS, Amsbaugh GA, Myers EN. Transantral-ethmoidal decompression of optic canal fracture. Arch Ophthalmol. 1976;94:1040-1043.
7 Sofferman RA. An extracranial microsurgical approach to the optic nerve. J Microsurg. 1979;1:195-202.
8 Sofferman RA. Sphenoethmoid approach to the optic nerve. Laryngoscope. 1981;91:184-196.
9 McMains KC, Kountakis SE. Contemporary diagnosis and approaches toward optic nerve decompression. Oper Tech Otolaryngol. 2006;17:178-183.
10 Miller NR, Agrawal N, Sciubba JJ, et al. Image-guided transnasal endoscopic resection of an orbital solitary fibrous tumor. Ophthal Plast Reconstr Surg. 2008;24:65-67.
11 Gerencer RZ, Patel U, Hunter C, et al. The role of endoscopic sinus surgery in the diagnosis and treatment of metastatic orbital carcinoid tumors. Ear Nose Throat J. 2007;86:157-161.
12 Lund VJ, Rose GE. Endoscopic transnasal orbital decompression for visual failure due to sphenoid wing meningioma. Eye. 2006;20:1213-1219.
13 Karaki M, Kobayashi R, Mori N. Removal of an orbital apex hemangioma using an endoscopic transethmoidal approach: technical note. Neurosurgery. 2006;59(1 suppl 1):ONSE159-160.
14 Luu QC, Lasky JL, Moore TB, et al. Treatment of embryonal rhabdomyosarcoma of the sinus and orbit with chemotherapy, radiation, and endoscopic surgery. J Pediatr Surg. 2006;41:e15-e17.
15 Har-El G. Combined endoscopic transmaxillary-transnasal approach to the pterygoid region, lateral sphenoid sinus, and retrobulbar orbit. Ann Otol Rhinol Laryngol. 2005;114:439-442.
16 Naraghi M, Kashfi A. Endonasal endoscopic resection of ethmoido-orbital osteoma compressing the optic nerve. Am J Otolaryngol. 2003;24:408-412.
17 Herman P, Lot G, Silhouette B, et al. Transnasal endoscopic removal of an orbital cavernoma. Ann Otol Rhinol Laryngol. 1999;108:147-150.
18 Senior BA, Lanza DC, Kennedy DW, et al. Computer-assisted resection of benign sinonasal tumors with skull base and orbital extension. Arch Otolaryngol Head Neck Surg. 1997;123:706-711.
19 Sethi DS, Lau DP. Endoscopic management of orbital apex lesions. Am J Rhinol. 1997;11:449-455.
20 Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4.
21 Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3.
22 Hadad G, Bassagasterguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882-1886.
23 Gardner PA, Kassam AB, Thomas A, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63:36-52.
24 Jho HD. Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invasive Neurosurg. 2001;44:190-193.
25 Couldwell WT, Weiss MH, Rabb C, et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539-550.
26 Cook SW, Smith Z, Kelly DF. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery. 2004;55:239-246.
27 de Divitiis E, Cavallo LM, Cappabianca P, et al. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors. Part 2. Neurosurgery. 2007;60:46-58.
28 Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400-406.
29 Dusick JR, Esposito F, Kelly DF, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832-841.
30 Kitano M, Taneda M. Extended transsphenoidal approach with submucosal posterior ethmoidectomy for parasellar tumors. Technical note. J Neurosurg. 2001;94:999-1004.
31 Kassam AB, Vescan AD, Carrau RL, et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108:177-183.