35 Tumors of the Lung, Pleura, and Mediastinum
Epidemiology and Etiology
Lung cancer is the leading cause of cancer mortality for men and women, exceeding the mortality of breast, colon, and prostate cancer combined.1 In the United States, more than 219,000 new cases of lung cancer and 159,000 deaths were anticipated in 2009.1
The epidemiologic data on smoking and lung cancer fulfill the criteria for causal association, including the consistency of results across studies, the strength of relationship, its specificity, the correct temporal sequence between exposure and disease, and the coherence of the association as evidenced by a dose-response relationship.2 It is estimated that cigarette smoking is the cause of 90% of all lung cancers.3 Multiple genetic alterations of tumor suppressor genes and oncogenes can lead to the pathogenesis of lung cancer. Carcinogens in cigarette smoke are involved in these genetic alterations, mainly through the formation of deoxyribonucleic acid (DNA) adducts.4 There is a clear dose-response relationship between the risk of lung cancer and the number of cigarettes smoked per day, the degree of inhalation, and the age at initiation of smoking.5 The relative risk of developing lung cancer decreases as the time from cessation increases.5
Approximately 10% of lung cancers are thought to be caused by carcinogens other than cigarette smoking. Among nonsmokers, 30% of lung cancer deaths are attributed to radon exposure.3 Increases in lung cancer risk also accompany exposure to carcinogens such as asbestos, bis(chloromethyl) ether, polycyclic aromatic hydrocarbons, chromium, nickel and inorganic arsenic compounds.6,7 The association with occupational exposure to these agents appears to be independent of cigarette smoking. In addition, a number of occupations with high smoking prevalence have increased cancer risk. Women who work as waitresses, cashiers, nurses’ aides, and orderlies have smoking prevalences in excess of 40%, as do men who work as drivers, construction workers, painters, mechanics, and watchmen.8
Chemoprevention strategies have failed to demonstrate the ability to reduce the risk of developing lung cancer. Epidemiologic and case control trials previously reported an inverse relationship between the incidence of lung cancer and the intake of many food groups, including dietary carotenoids such as beta-carotene and lycopene.9 The mechanism of this interaction was theorized to be related to scavenging of free radicals or through antioxidative effects. However, the Beta-Carotene and Retinol Efficacy Trial (CARET), which included more than 18,000 participants, found that supplementation with betacarotene actually increased the risk of developing lung cancer by 12% versus patients who received placebo, and even increased all-cause mortality by 8%.10 Likewise, the retinoid isotretinoin was ineffective in reducing the development of second primary tumors in 1166 patients with prior stage I non–small cell lung cancer (NSCLC), and even increased the risk of death for active smokers.11
Pathogenesis and Histologic Findings
Pathogenesis
There is increasing evidence that lung cancer is derived from a pluripotent stem cell that is capable of expressing a variety of phenotypes. This epithelial stem cell, in normal histogenesis, differentiates into those cells found in the tracheobronchial tree, including pseudostratified reserved cells, ciliated goblet columnar cells, and neuroendocrine cells as type I and II pneumocytes seen lining the alveoli. Those cells that are capable of division can express hyperplastic, metaplastic, or neoplastic change. Lung cancer may exhibit two or more histologic patterns; the frequency of this occurrence may depend of the number of sections examined. In one study, when at least 10 blocks from each tumor were examined, 45 of the 100 cases showed elements of both squamous cell carcinoma and adenocarcinoma.12
Clinical Presentation
Routine screening for lung cancer is being investigated once again. Prior trials conducted in the 1970s failed to show any benefit for the use of chest x-ray examination to screen smokers for lung cancer. More recent studies, however, are exploring the use of computed tomography (CT) scans for screening because they can detect smaller lung nodules. The Early Lung Cancer Action Project was a pilot study of 1000 patients older than the age of 60 with greater than 10 pack-years of smoking who underwent routine screening with low-dose thoracic CT scans. It found almost four times as many cancers as chest x-ray examination alone and these lesions were six times as likely to be early stage and therefore more curable.13 A follow-up study, the International Early Lung Cancer Action Project screened 31,567 asymptomatic persons at risk for lung cancer from 1993 through 2005.14 Of these, 27,456 had repeat screenings 7 to 18 months later. Of the 31,567 participants, 484 were diagnosed with lung cancer, 85% of whom were clinical stage I. The estimated survival in this subgroup was 88%. Other groups have concluded that screening increases the rate of lung cancer diagnosis and treatment, but doesn’t reduce the risk of advanced lung cancer or death from lung cancer.15 Currently, a large national trial is underway to rigorously assess the benefit of screening in this population.
Diagnostic and Staging Procedures
Imaging Studies
Chest x-ray examination remains an extremely valuable tool in the diagnosis of lung cancer. However, CT scan has enormous advantages in its ability to detect lesions that cannot be visualized on chest x-ray examination. It also plays an important role in staging lung cancer, especially spread to areas of the mediastinum undetected on plain films. Typically, a lymph node is considered normal if it is less than 1 cm in diameter on its short axis. CT may also suggest possible areas of local invasion of the primary tumor to the chest wall, vertebrae, or mediastinal structures. Small pleural effusions or pleural nodules, often undetected on plain films, may be evident on CT. A CT of the chest for lung cancer evaluation should also include part of the upper abdomen, which allows for evaluation of the liver and adrenals for metastatic disease. Scanning with 18-fluorodeoxyglucose positron emission tomography (FDG-PET) scanning can also detect neoplastic activity. Studies have shown CT and PET to be more specific and sensitive than PET alone.16 Generally, the sensitivity, specificity, and accuracy of CT scan alone is approximately 70%. CT and PET together have an accuracy of approximately 90% in single-institution studies, although these results may worsen when the technique is studied across multiple institutions. Magnetic resonance imaging (MRI) has not been shown to be more effective than chest CT for lung tumor, but may be helpful to evaluate invasion of vertebral bodies in the mediastinum.
Bronchoscopy
Endobronchial ultrasonography allows detailed imaging of the bronchial wall and peribronchial mediastinum, and may also add to the evaluation of endobronchial lesions.17
Endobronchial ultrasound-guided biopsy of mediastinal and hilar lymph nodes is becoming more commonly employed in the staging of lung cancer. Yields of this technique are approximately 95% with minimal to no severe toxicity.18
Mediastinoscopy and Mediastinotomy
Mediastinoscopy is the most accurate technique to assess superior mediastinal lymph nodes, which are frequently involved in NSCLC. The procedure is simple, safe, and effective in experienced hands. In one large series, the complication rate from cervical mediastinoscopy was 2.3% and the mortality was 0%.19 In patients suspected of having inoperable disease by virtue of mediastinal involvement as detected by CT scanning, confirmation by mediastinoscopy is indicated. For patients who are potentially operable, it is especially important to sample contralateral mediastinal lymph nodes to determine if N3 disease is present. Involvement of aorticopulmonary lymph nodes are typically assessed by anterior mediastinotomy (Chamberlain procedure).20 It is important to remember that these are first-echelon mediastinal lymph nodes for left lung tumors, especially from the left upper lobe, and they may not be sampled when a cervical mediastinoscopy is performed.
Staging of Lung Cancer
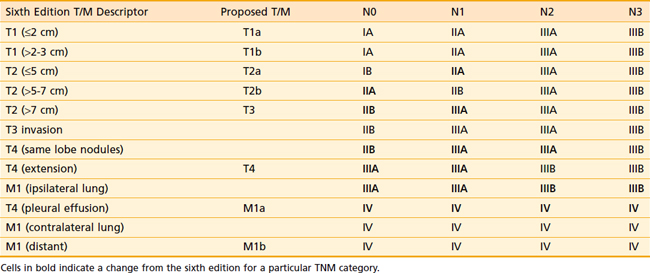
In 1997, the sixth edition of the tumor-node-metastasis (TNM) staging system for NSCLC was published.21 Some criticisms of the staging included that the sample size was relatively small, the data was from few institutions, the staging was mostly surgical, modern imaging was not incorporated into the staging, and there was minimal external validation. In an attempt to improve on these shortcomings, the International Association for the Study of Lung Cancer (IASLC) revised the TNM staging system in 2009.
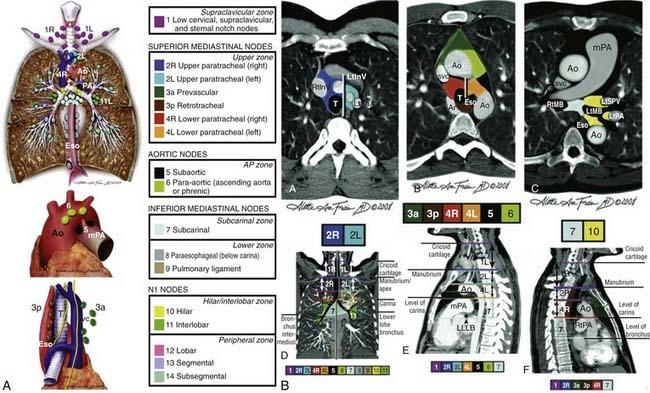
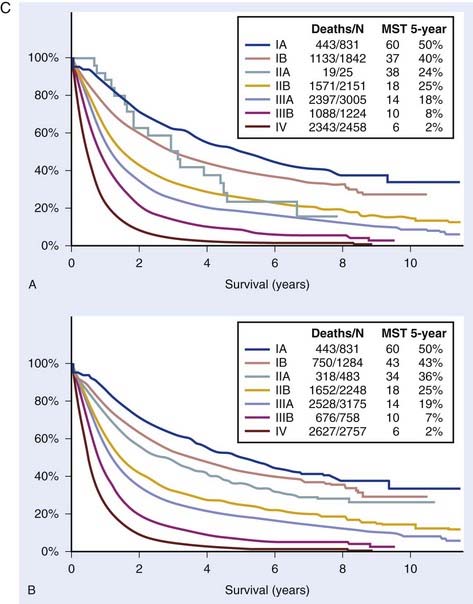
There are a number of changes from the sixth edition. T1 tumors have been subclassified by size into T1a (≤2 cm) and T1b (>2-3 cm). T2 tumors have been subclassified into T2a (>3-5 cm) and T2b (>5-7 cm). T3 classification now includes tumors greater than 7 cm (previously T2 tumors). Two nodules in the same lobe are now classified as T3 (previously T4). An ipsilateral lung tumor is now T4 (previously M1). Malignant pleural effusions, which were generally treated in the same manner as metastatic disease, are now classified as M1a (previously T4). Contralateral lung tumors are also M1a. Distant metastases have been reclassified as M1b. Table 35-1 presents these changes. Table 35-2 presents the new stage groupings. Fig. 35-1A-C shows the mediastinal lymph node mapping, anatomic locations, and survival by the new staging system.22
Table 35-1 Update on the 7th Edition of the American Joint Committee on Cancer Guidelines
| T (Primary Tumor) | |
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus)a |
| T1a | Tumor ≤2 cm in greatest dimension |
| T1b | Tumor >2 cm but ≤3 cm in greatest dimension |
| T2 | |
a The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximally to the main bronchus, is also classified as T1.
b Most pleural (and pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple cytopathologic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element and the patient should be classified as T1, T2, T3, or T4.
Prognostic Factors
Early-Stage (I or II) Non–Small Cell Lung Cancer
The major prognostic determinant for this group is the stage of disease, particularly the size of the tumor and the presence or absence of lymph node spread.23 Sawyer and colleagues reported that in patients with resected N1 disease, positive bronchoscopic findings, smaller tumor size, and a greater number of resected N1 nodes were associated with an improvement in clinical outcome.24
Advanced-Stage (III or IV) Non–Small Cell Lung Cancer
Pretreatment stage, performance status, and weight loss are most important.25 The definitions of weight loss have varied among researchers, but weight loss of greater than 5% during the preceding six months is frequently used. Serum LDH, a predictor of survival in SCLC and other malignancies, also appears to be an independent survival variable. Whether any specific metastatic disease site confers a survival advantage remains controversial. Bone and liver metastases have often been cited as predicting shorter survival. Isolated spleen metastases, although rare, might be associated with a better outcome.26 The histologic subtype of NSCLC has little influence on survival in advanced disease.
Operable Non–Small Cell Lung Cancer
Patient Selection
The preoperative assessment of patients considered for surgical treatment of lung cancer includes clinical staging of the disease to assess its resectability, assessment of the cardiopulmonary reserve of the patient to determine whether the intended pulmonary resection is possible, and assessment of the patient with regard to the perioperative risk of the procedure. Traditionally, patients are suitable candidates for pneumonectomy if the ultimate forced expiratory volume in 1 second (FEV 1) is greater than 1.2 L, the patient does not suffer from hypercarbia, and cor pulmonale is not present.27–29 Pulmonary function studies best suited to assess these parameters include spirometry, arterial blood gases, diffusion capacity, and, where indicated, ventilation-perfusion scans to estimate the proportions of functioning pulmonary tissue required to be excised. Patients requiring lobectomy or lesser resections require similar pulmonary function parameters.30,31 Pulmonary complications increase remarkably when the FEV 1 forced vital capacity (FVC) ratio is less than 75% of predicted, indicating significant airways obstruction. FEV 1/FVC ratios of less than 50% of predicted lead to unacceptable postoperative morbidity and mortality.
Technique
If complete excision can be obtained by lobectomy, this has results equal to those of pneumonectomy.32 In proximally situated tumors, in which a pneumonectomy may be required for total excision, lung-conserving operations using bronchoplastic procedures to preserve uninvolved lobes (e.g., sleeve lobectomy) have been demonstrated to be feasible, but may have higher local recurrence rates. The role of mediastinal lymphadenectomy as part of the surgical procedure remains hotly debated. Complete mediastinal lymphadenectomy provides the best possible surgical staging by removing all lymph nodes, which can then be analyzed pathologically for metastatic involvement. This can help to identify patients who may require postoperative adjuvant radiation therapy. At minimum, for accurate final surgical-pathologic staging, lymph node sampling of all draining areas should be performed at the time of surgical resection. Pneumonectomy should be performed with a mortality of less than 6%, lobectomy less than 3%, and lesser resections 1% or less. These mortality figures are significantly affected by the age of the patient, the stage of disease, and the extent of resection.33
Lobectomy has been shown to be superior to more limited surgical resections such as wedge resection or segmentectomy. In a randomized trial conducted by the Lung Cancer Study Group (LCSG), there was improved local control in patients having the more extensive resection.34 A recent trial, Cancer and Leukemia Group B (CALGB) 39802, evaluated the role of video-assisted thoracic surgery in lobectomy. They found that 86.5% of patients with stage I disease were able to undergo a successful procedure with a shorter procedure length and chest tube duration than typically seen.35 With the advent of improved imaging and the possible role of screening CT scans, this issue might need to be re-examined, particularly for tumors that are subcentimeter in size.
Results
According to the recent IASLC lung cancer staging project, following surgical resection for lung cancer, the 5-year survival is 73% for stage IA, 58% for stage IB, 46% for stage IIA and 36% for stage IIB.36
Patterns of Failure Following Surgery
Recognition of prognostic and surgical factors that predict for specific anatomic failure patterns can allow selection of patients for local, systemic, or combined therapy. In general, patients who fail after surgery present with disease outside of the chest 70% of the time, locally 20% of the time, and local and distant simultaneously 10% of the time.37 Therefore, systemic treatment is most likely to yield benefit postoperatively. Because of the relative chemoresistance of NSCLC, any benefit would likely be modest and would take a large trial to detect. Postoperative treatment can also be difficult to tolerate. Unlike breast cancer, in which patients recover quickly after modified radical mastectomy or lumpectomy, thoracic surgery, even if done thoracoscopically, requires significant recovery time. It has been shown in many studies that patients are only able to tolerate 60% of the intended treatment after surgery.
Second primary lung cancers occur frequently in all surviving patients at a rate of approximately 1% per year.38,39
Preoperative Radiation Therapy
In 1975, the National Cancer Institute published two separate but integrated multi-institutional randomized trials addressing the use of preoperative radiotherapy followed by surgery in both operable and inoperable NSCLC without evidence of preoperative radiotherapy advantage.40 It is clear from both the nonrandomized and randomized data that preoperative irradiation alone does not improve long-term survival and has no role as a single-induction modality in the management of marginally resectable or unresectable stage IIIA or IIIB disease. The use of radiation therapy as a single preoperative modality is no longer studied consistently because of the advent of effective chemotherapy agents. Most current trials investigate the use of preoperative concomitant chemoradiotherapy.
A German trial evaluated the role of preoperative chemoradiation in patients with stage III NSCLC.41 A total of 524 patients were randomly assigned to two treatment groups. The experimental arm consisted of three cycles of cisplatin and etoposide followed by twice-daily radiation with concurrent carboplatin and vindesine and then surgical resection. Patients with positive margins or unresectable disease received more radiation. The control group received three cycles of cisplatin and etoposide followed by surgery and PORT. There was no difference in progression-free survival (9.5 months versus 10 months), the primary endpoint of the study. The preoperative radiation arm did have an increased rate of mediastinal downstaging and pathologic response. However, there was no difference in the rate of pneumonectomy (35% in both arms), which suggests that the there is minimal to no role of the use of preoperative radiation to achieve the goal of minimizing the surgical intervention. If a patient needs a pneumonectomy prior to radiotherapy, it is likely he or she will still need a pneumonectomy after induction chemoradiation. The role of preoperative radiotherapy for superior sulcus tumors is somewhat unique and is discussed later in this chapter.
Postoperative Radiation Therapy
The LCSG investigated the efficacy of postoperative mediastinal irradiation in completely resected stage II and III squamous cell carcinoma of the lung. This trial randomized 210 patients to receive 50 Gy in 25 fractions after surgery versus observation alone. The locoregional failure rate (as first site of failure) was reduced from 41% to 3% with radiotherapy for all node-positive patients. Despite this improvement, the increase in locoregional control with radiotherapy did not translate into a survival benefit for stage II patients because more than two thirds of first failures were distant.42 The LCSG failed to separate patients with N1 and N2 disease but rather combined and analyzed them as a single group.42 A trend toward improved survival was observed in N2 patients receiving radiotherapy. Eastern Cooperative Oncology Group (ECOG) trial 3590 randomized 488 patients with stages II through IIIA disease after surgery to either radiation therapy alone or radiation therapy and concurrent cisplatin-etoposide. Five-year survival was 40% and 38%, respectively (P = not significant).43
The Medical Research Council of the United Kingdom also completed a randomized adjuvant trial in which 308 patients with stage II and III disease were treated with either 40 Gy or no further therapy, although a trend toward improved survival was seen in the N2 subgroup. Again, no overall survival benefit was observed.44
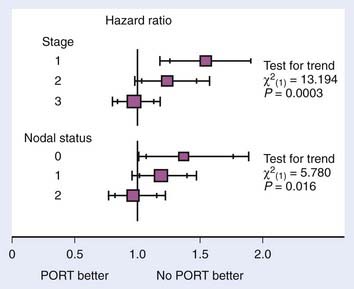
The meta-analysis performed by the Medical Research Council confirms these results. They report local recurrences in 276 patients in the surgery-only arms of the trials. There were 195 local recurrences in patients receiving PORT. In addition, there was a trend toward improved survival in stage III and N2 patients, although it did not reach significance45 (Fig. 35-2). Stage I and II patients had decreased survival from PORT, presumably from increased toxicity of treatment without any benefit from the radiation.
There have been criticisms of the meta-analysis because of its methodologic flaws. The trials studied had variable and unspecified staging, used cobalt-60 or other low-energy photons, and had inadequate treatment planning. Also, the interval between surgery and PORT was inconsistent and sometimes not reported. A Surveillance, Epidemiology and End Results (SEER) analysis showed that the use of PORT decreased after the publication of the metaanalysis.46
Machtay and colleagues investigated whether the excess toxicity observed from PORT in the meta-analysis was evident in their patient population.47 They reviewed 202 patients retrospectively who received PORT for stage II and III disease with a median dose of 55 Gy. They found the actuarial rate of death from intercurrent disease was 13.5% compared with the expected rate of 10%. A re-examination of the ECOG trial E3590 also attempted to evaluate the risk from PORT.48 They found that the risk of death from intercurrent disease was 12.9% in patients receiving PORT or PORT plus chemotherapy versus an expected rate of 10.1%. Therefore, there is some toxicity associated from the PORT treatment, but of a lower magnitude than seen in the meta-analysis.
A SEER analysis of the association between PORT and survival in patients with stage II or stage III disease was performed.49 It showed that for patients with N2 disease, the use of PORT was associated with a significant increase in survival and that for patients with N0 disease, it was associated with significant decrease in survival. A subgroup analysis of the Adjuvant Navelbine International Trial Association (ANITA) trial of adjuvant chemotherapy evaluated the role of PORT.50 Of the 840 patients in the ANITA trial, 232 received PORT. In univariate analysis, PORT had a deleterious effect on the overall population survival. Patients with pN1 disease had an improved survival from PORT in the observation arm (median survival [MS] 25.9 versus 50.2 months), whereas PORT had a detrimental effect in the chemotherapy group (MS 93.6 months and 46.6 months). In contrast, survival was improved in patients with pN2 disease who received PORT, both in the chemotherapy (MS 23.8 versus 47.4 months) and observation arm (MS 12.7 versus 22.7 months).
Preoperative Chemotherapy
Initial studies using a variety of chemotherapy regimens suggested that preoperative chemotherapy could be administered for selected stage IIIA and a few stage IIIB patients and may be beneficial in this setting.51,52 Combination chemotherapy with high-dose cisplatin (120 mg/m2), vinca alkaloids, and mitomycin (MVP) was used preoperatively in the stage IIIA patients with clinically apparent ipsilateral mediastinal spread.53 In a group of 73 patients, the objective major response rate to MVP chemotherapy was 77% with a 10% complete-response rate. Overall, 60% of patients underwent complete resections and the median survival was 19 months.53
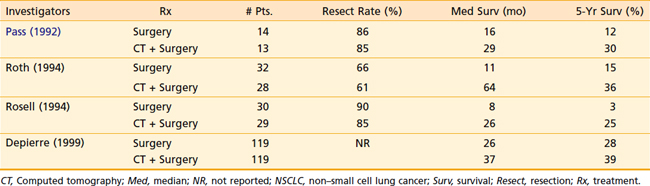
There are multiple trials in which patients undergoing surgical resection were randomized to induction chemotherapy or no treatment.54,55 The first trial reported by Pass et al.54 was a small trial that suggested a survival benefit in patients treated with preoperative cisplatin and etoposide. In 1994, two trials were published, each of which was closed due to early stopping rules after enrollment of approximately 60 patients. Both trials used induction regimens containing cisplatin and showed a significant increase in survival with chemotherapy and surgery as opposed to surgery alone. The largest trial, from Depierre et al.,55 enrolled 355 patients and treated them with three cycles of induction mitomycin, ifosfamide, and cisplatin, and two cycles postoperatively. Median survival improved from 26 months to 37 months, but was not statistically significant (Table 35-3).
Adjuvant Chemotherapy Following Complete Resection of Non–Small Cell Lung Cancer
In 1995, the Non–Small Cell Lung Cancer Collaborative Group published a meta-analysis of 17 adjuvant chemotherapy trials spanning the years 1965 through 1991 and involving 4467 patients.56 The use of chemotherapy regimens that incorporated alkylating agents worsened survival, and the use of 5-fluorouracil–based regimens had no effect. However, treatment with a cisplatin-based chemotherapy regimen resulted in a nonsignificant (P = 0.08) trend toward improved survival of 5% over 5 years. At that time, most oncologists chose not to administer adjuvant chemotherapy because the toxicity of the treatment outweighed the benefit.
This sentiment has now shifted since the publication of multiple other larger phase III trials showing more consistent benefit. The first of these was the International Adjuvant Lung Cancer Trial, which randomized 1867 patients to chemotherapy or no chemotherapy following surgical resection for patients with stage I through IIIA disease.57 All patients received cisplatin (80-120 mg/m2) and a second agent chosen by the investigator, either etoposide or a vinca alkaloid. PORT was given at the discretion of the institution. With a median follow-up of 56 months, 5-year survival significantly improved from 40.4% to 44.5%. In a study conducted by the National Cancer Institute of Canada, 482 patients with resected stage IB or II disease were randomized to treatment with vinorelbine and cisplatin or observation.58 The treatment effect was even larger in this trial, with median survivals of 94 and 73 months (P = 0.04) and 5-year survival rates of 69% and 54% (P = 0.03) respectively. The ANITA study also employed vinorelbine-cisplatin for patients with stage IB through IIIA disease.59 The median survival improved from 44 to 66 months and the 5-year survival improved by 8.6%. Not all studies showed a benefit to adjuvant chemotherapy, however, with negative results reported in the Adjuvant Lung Cancer Project Italy60 and the Big Lung Trial. Only CALGB studied adjuvant carboplatin, in combination with paclitaxel, and this was tested selectively in stage IB disease.61 This smaller study with 344 patients demonstrated no improvement in survival (HR 0.83, P = 0.12). An exploratory analysis from that trial did show a benefit for patients with tumors greater than 4 cm (HR 0.69, P = 0.043).
Ultimately, another meta-analysis compiled the results of the trials that used cisplatin-based chemotherapy regimens. Coined the Lung Adjuvant Cisplatin Evaluation Meta-analysis, this basically showed the same level of benefit noted in the 1995 analysis: 5% over 5 years. However, with larger patient numbers, the benefit was statistically significant (HR 0.89, P = 0.005).62 The degree of benefit was affected by stage: For stage IA, the HR was 1.40; for stage IB, the HR was 0.93; for stage II, the HR was 0.83; and for stage III, the HR was 0.83. Patients with a good performance status also benefitted more, but gender, type of surgery, and the choice of chemotherapy agent given with cisplatin had no effect.
In summary, administration of adjuvant cisplatin-based chemotherapy has become the standard of care for patients with resected stage II and IIIA disease. The benefit for patients with stage IB disease is less clear, although this may be appropriate for fit patients with large tumors. Toxicity remains a roadblock, with only 50% to 60% of patients completing all planned therapy in the studies mentioned. Substitution of carboplatin for patients who cannot tolerate cisplatin has not been studied in appropriately powered phase III trials. Various pathologic markers, such as expression of the DNA repair enzyme, ERCC-1, or metagene profiles may identify patients most likely to benefit from adjuvant chemotherapy, but these have not completed prospective assessment.63,64
Preoperative Chemoradiotherapy
The Southwest Oncology Group (SWOG) reported on 126 patients in a phase II trial who received 45 Gy with concurrent cisplatin and etoposide prior to surgical resection. There was 59% response rate and 89 patients (71%) underwent resection and 58% had a pathologic complete response or minimal microscopic disease. Despite this, patients with advanced disease (T2-3N2, T4N2-3) had only a 12 month median survival. Patients with more limited disease (T1N2, T4N0-1) had a 32-month median survival.65
Superior Sulcus Tumors
The use of preoperative radiation therapy was pioneered by Paulson.66 Preoperative doses of 3000 to 3500 R were used to reduce the volume of tumor, thus facilitating complete resection. Operations were performed 1 month after the completion of radiation therapy to allow time for pain relief with an accompanying improvement in performance status, resolution of skin changes facilitating good wound healing, and in some patients to exclude the emergence of distant metastases that would make radical local therapy inappropriate. In the initial report of this procedure, five of nine patients followed for more than 1 year were disease-free.67 in a number of series a variety of preoperative doses of radiation have been used and survival figures range from 20% to 34% at 5 years.
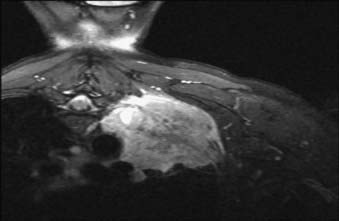
Generally accepted contraindications to surgery in patients with superior sulcus tumors include extensive involvement of the brachial plexus, involvement of the subclavian artery, vertebral bodies, esophagus, mediastinal node metastases, or distant metastases. Fig. 35-3 shows a CT scan of a right-lung T4 Pancoast tumor. The patient was treated with chemoradiation therapy followed by surgical resection. Patients with unresectable T4 tumors or mediastinal nodal metastases can be treated with radical radiation therapy. In general, fields are designed as described in the section on radiation techniques and total doses of 60 Gy or greater are used.
The current standard treatment of superior sulcus tumors generally consists of preoperative chemoradiotherapy to 45 Gy. This is followed 4 weeks later with surgical resection. This regimen was studied in a phase II SWOG protocol that enrolled 111 patients and reported a 65% rate of pathologic complete response or minimal microscopic disease and a 55% 2-year survival.68